Abstract
pH sensing at the single-cell level without negatively affecting living cells is very important but still a remaining issue in the biomedical studies. A 70 μm reflection-mode fiber-optic micro-pH sensor was designed and fabricated by dip-coating thin layer of organically modified aerogel onto a tapered spherical probe head. A pH sensitive fluorescent dye 2′, 7′-Bis (2-carbonylethyl)-5(6)-carboxyfluorescein (BCECF) was employed and covalently bonded within the aerogel networks. By tuning the alkoxide mixing ratio and adjusting hexamethyldisilazane (HMDS) priming procedure, the sensor can be optimized to have high stability and pH sensing ability. The in vitro real-time sensing capability was then demonstrated in a simple spectroscopic way, and showed linear measurement responses with a pH resolution up to an average of 0.049 pH unit within a narrow, but biological meaningful pH range of 6.12–7.81. Its novel characterizations of high spatial resolution, reflection mode operation, fast response and high stability, great linear response within biological meaningful pH range and high pH resolutions, make this novel pH probe a very cost-effective tool for chemical/biological sensing, especially within the single cell level research field.
Keywords: pH sensing, tapered optical fiber spherical sensor, ORMOSILs ultra-thin layer coating, microliter pH sensing
1. Introduction
Localized, real-time sensing of extra- or intra-cellular pH remains a pressing issue for the optical sensing research field [1–5]. Intracellular pH indictors have been developed in recent years [6, 7] with higher signal-to-noise ratio (S/N), long term stability and specific capability of subcellular localization [8]. However, all these dye based pH sensing products can be degraded by cells once applied, which makes it almost impossible to be used as a long-term pH probe for cells undergoing different treatment conditions. Moreover, the necessity of equipment with high quality inverted microscopic device such as confocal system would further prevent such labelling techniques from being readily used for real-time and/or remote monitoring of the cellular pH variations, and thus highly limited their potential applications in biomedical fields.
Sol-gel derived materials have been widely used in optical sensing areas [9–11]. The relatively fast and easy steps in either acidic or basic catalysis of alkoxides under room temperature, combined with multiple choices of lately introduced [12, 13] ORganically MOdified SILicates (ORMOSILs) in sol-gel formula provide very good optical transparency, stability, adjustable hydrophobicity and porosity [14–19], which make it feasible to impart desired chemicals or properties in sensor fabrication. However, the introduction and entrapment of a specific sensing material into it would then be the key issue [20]. Majorities of sol-gel based pH sensors are derived from tetraethoxysilane (TEOS) based aerogel but mostly suffer from long response time and limited long-term stability [21–23]. Later development focused on covalent immobilization of the indicator molecules [24–26] and surface hydrophobicity modification with organic end-groups, such as trimethylchlorosilane (TMCS), methyltriethoxysilane (MTES) or hexamethyldisiloxane (HMDSO), lead to appropriate replacement of –OH with –CH3 [27, 28], and thus a better performance was achieved with improved reproducibility, shorter response time and enhanced chemical stability. Surfactants such as Sodium dodecyl sulfate (SDS) and hexadecyltrimethylammonium bromide (CTAB) have also been introduced in recent years to form mesoporous structure of silica matrix to obtain efficient host of the sensing molecules [29, 30]. Together, initiation of these ORMOSILs-based sol-gel thin films appeared to be a promising structure in developing compact micro- or nano-scale sensing probes, which can well be integrated with many types of lab-on-chip-based devices [20, 31, 32].
The ORMOSILs based aerogel thin layer coating can be combined with fiber optic devices for small or remote sample pH sensing. Ultra-thin layer sol-gel can be directly dip-coated onto the end face of either as-synthesized or surface modified optical fiber probes. Sub-micron thickness pH sensing films have been developed on platforms like microfluidic device [33–35] as well as fiber-optics based pH sensor [36–38]. However, most of the reported devices were operated in relatively large scale (hundred micrometers to centimeter level), and cannot provide sufficient detection sensitivity, pH resolution, and/or real-time monitoring ability. In addition, some weaknesses of these pH sensors, such as fabrication difficulties, high cost and relatively poor repeatability and reproducibility, were also observed in previous fiber-optic based pH sensors.
In this study, a 70 μm reflection mode fiber-optic micro-pH sensor was fabricated by carbon dioxide (CO2) laser stretching system combined with fusion splicer device, and was functionalized by amide covalent bonding of a pH specific dye BCECF within a sol-gel dip-coated ultra-thin layer on a spherical probing head. The surface hydrophobicity was specifically modified and evaluated. The newly fabricated probes were tested in vitro for real-time pH measurement. The sensitivity, the pH resolution, the stability and reproducibility were investigated. The developed pH sensor provide a promising technique for real-time pH monitoring in many chemical/biological systems, especially in cases of both in vitro and in vivo single-cell level non-invasive detections.
2. Experiments
The spherical-headed tapered fiber-optic pH probe was fabricated from a single mode optical fiber (Corning SMF-28, USA) by using a homemade CO2 laser fiber-stretching system combined with optical fiber fusion splicer (Fujikura, Japan). A sol-gel dip-coating method was employed in this study to form an ultra-thin aerogel layer onto the probe head surface, covalently bond with molecules of a specific pH-sensitive fluorescent dye 2′, 7′-Bis (2-carbonylethyl)-5(6)-carboxyfluorescein (BCECF) (Life technologies, New York) through amide bonds (as shown in Figure 1 and Figure 2).
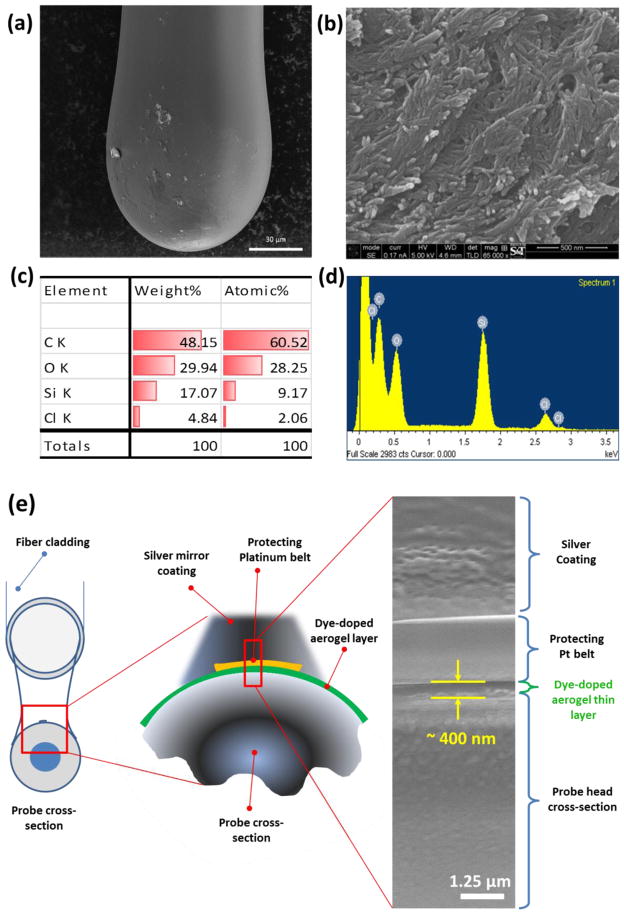
Figure 1.
SEM images and EDS characterization of fabricated spherical head fiber-optic pH probe. Overview of the pH sensor probe structure was shown in low (a) and high (b) magnification. Probe surface elemental characterization of carbon, oxygen, silica and chlorine was done by EDS scanning and showed both in chart (c) and energy distribution plot (d). Scale bar showed in SEM images are 30 μm (a) 500 nm (b).
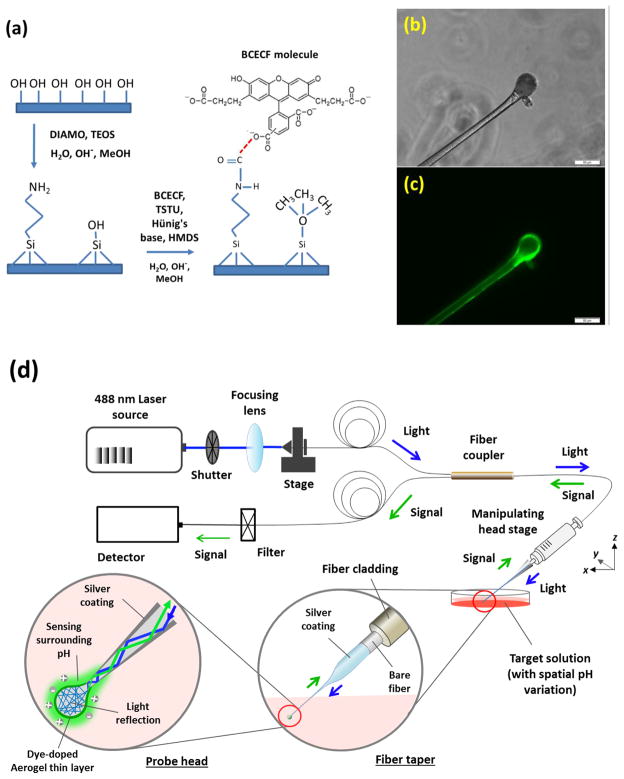
Figure 2.
Configuration and microscopic images of BCECF dye covalent-bonded aerogel thin-layer on probing head with schematic diagram of whole system setup. (a) Brief configuration of BCECF dye molecules covalently bond onto the ultrathin ORMOSILs network thin-layer, followed by HMDS priming for hydrophobicity modification; Tapered spherical head pH probe images under inverted fluorescent microscope with (b) white light and (c) 488 nm (FITC channel) illumination; and (d) Schematic diagram of the optical spectrum measurement setup.
A homemade CO2 laser fiber-stretching system was used to programmably control the taper length and waist diameter. Normally a length of 1.3 ± 0.2 cm “V” shape tapered optical fiber tip was firstly obtained with a waist diameter of around 50 ± 8 μm and tip size around 2–5 μm. A spherical head was specifically designed for maximizing the contacting surface as well as signal reflection. It was fabricated using a distance-melting method on a fusion splicer device with a final diameter of around 70 ± 10 μm.
For ultrathin aerogel coating and BCECF dye covalent bonding, the silanol groups on the surface of the spherical probing head were activated through treatments with a) concentrated nitric acid for 12 hours and b) copious amounts of distilled water and ethanol, followed by drying at 100°C for 3 hours. Fluorescent dye BCECF (1 mg/mL) in Dimethyl sulfoxide (DMSO) was added with excess molar amount of 2-succinimido-1, 1, 3, 3 tetramethyluronium tetrafluoroborate (TSTU) that pre-dissolved in ethanol and small amount of N-ethyldiisopropylamine (Hünigs base), and stir for 10 minutes. Let the reaction proceed for one hour at room temperature. Then the amino-functionalized n-(3-(trimethoxysilyl) propyl)-ethylenediamine (DIAMO) in ethanol was added into the succinimidyl-ester activated dye solution (BCECF: DIAMO = 1:10). Stir it for 10 min to generate amide bonds between dye and DIAMO. Then a mixture of alkoxides, which include tetraethoxysilane (TEOS), DIAMO and methyltrimethoxysilane (MTES) dissolved in methanol (TEOS:DIAMO:MTES = 4:1:1, v/v) and hydrochloric acid (0.1M), were combined with the BCECF - DIAMO - ethanol solution at a v/v ratio of 5:3 and magnetically stirred for 30 minutes, followed by stewing at room temperature in closed vial for 12 hours. Pre-heated tapered probe head were then cooled and dip-coated with the sol-gel solution with a drawing rate of 1 mm/s. Curing process was divided into two steps for gradual heating. Dip-coated probes were set in 50°C for 6 hours and then in 80°C for 24 hours. A surface hydrophobicity modification was finally carried out with a homemade hexamethyldisilazane (HMDS) priming chamber. A 100°C priming temperature was acquired in vacuum and an optimized 3 hours priming time was employed in this study. All BCECF dye related procedure was done in dark condition. Chemicals were purchased from Sigma-Aldrich (St. Louis, Missouri) unless otherwise specified.
The whole probe head area that coated with dye doped aerogel was observed under inverted fluorescent microscope (Figure 1). Both in-general and zoom-in view of the aerogel coated surface (Figure 1a and Figure 1b) show a uniform mesoporous network with protrude nano-scale sensing tails. Correspondingly, the energy-dispersive X-ray spectroscopy (EDS) scanning shows typical peaks of carbon, oxygen and silica within the aerogel surface, which represented successful fabrication of the dye-doped ORMOSILs thin layer onto the probe tip with uniform distribution (Figure 1c, 1d)). Further investigation of the aerogel layer thickness was also done through cross-section cutting by using a focused ion beam system (SEM/FIBs) (FEI, Hillsboro, Oregon) of the coated probe head as shown in Figure 1e acquired an averaged coating layer thickness of ~ 400 nm.
A sol-gel thin layer coating as well as BCECF dye covalent bonding principle is shown in Figure 2a. Brief schematic diagrams of the optical setup combined with probe head image are also shown in Figure 2b – d. The pH probe was excited using a solid-state 488 nm laser source to obtain the best excitation wavelength of the BCECF dye molecules. Laser intensity has been adjusted to acquire an optimized fluorescent signal while minimizing photo bleaching of the dye molecules. A colorimetric method was employed to acquire a normalized fluorescent signal ratio between the peak intensity (560 ± 5 nm) and a constant irrelevant background at 640 nm. A USB2000 spectrometer (Ocean optics, Dunedin, Florida) was used to collect reflected fluorescent signals. In order to acquire a remote sensing capability as well as label free detection under real circumstance, a reflection based sensing method was used in this study as the configuration shown in Figure 2d. During spectrum measurement, fabricated probe was backward fuse-connected with the sensor branch SMF optical fiber cleaved end, so that the using and changing of different probes did not influence the connector and detector interfaces during measurement.
3. Results and discussions
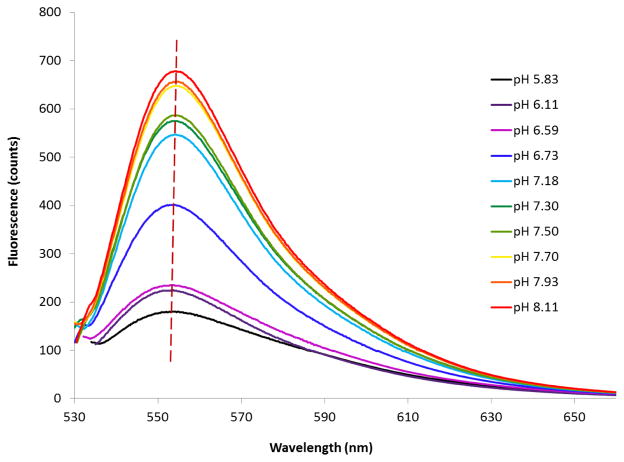
An aerogel based pH sensing ultra-thin film has been fabricated and the fluorescent spectra at different pHs are shown in Figure 3. A bathochromic shift of the fluorescent peak up to 560 nm was observed when compared with the original 535 nm emission peak of BCECF dye in solution. This may due to an increased dielectric constant compared to a solution environment, and thus a changed polarity of the microenvironment of the dye molecules after covalent bonding with the aerogel macromolecule network [25]. A series of 0.1M phosphate buffered saline (PBS) buffer solutions were freshly prepared and were calibrated by a pH meter. The fluorescent responses of the fabricated pH probe to these solutions as changes of pHs while maintaining a relatively constant peak wavelength. This intensity change closely correlates with the probing sensitivity and resolution, and it can theoretically be influenced by testing time and surface property, due to the kinetic saturation rate of the aerogel thin film.
Figure 3.
Reflection spectrum (fluorescent emission) from a pH sensitive thin film covered by BCECF dye doped ORMOSILs ultrathin layer.
The pH sensing capability of the dye doped aerogel thin film is closely correlated with dye concentration and surface properties. Higher dye concentrations are assumed to boost increased signal intensity, however, a large excess of the dye would enhance self-quenching as well as possible leaching of unbound dye molecules. Aerogel surface hydrophobicity is another key factor affecting the pH sensing capability of the probe. Because of the abundant –OH and –NH2 groups on the surface, TEOS/DIAMO derived aerogel thin layer without further hydrophobic modification is vulnerable to water based solution, even at pH values around neutral conditions. An appropriate mixing of MTES and an extra HMDS priming process would highly reduce the risk due to the compensation of sufficient methylsilyl or trimethylsilyl groups.
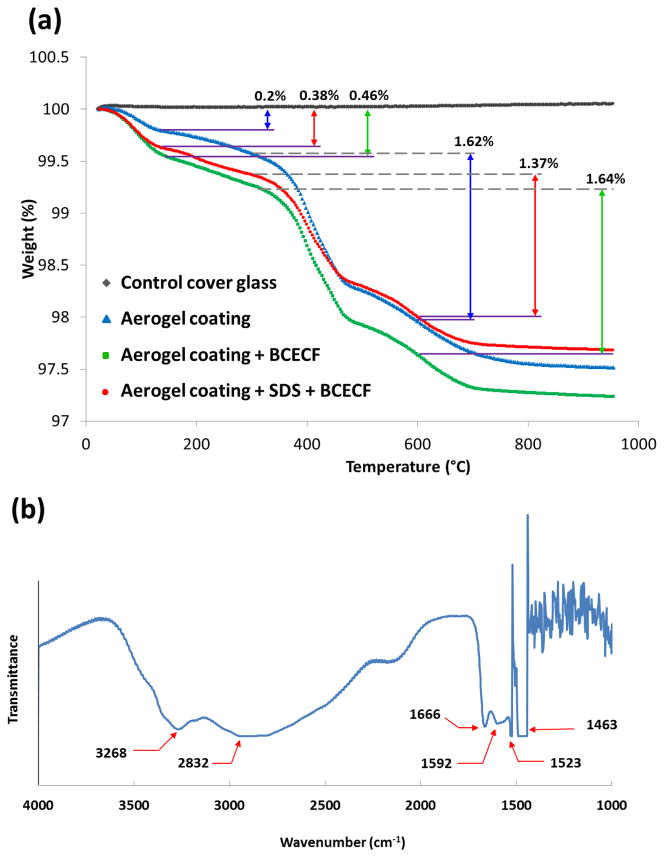
A thermal gravimetric analysis (TGA) (Q50, TA instruments, New Castle, Delaware) was carried out for the ORMOSILs thin layer under nitrogen atmosphere with different surface modifications and the results are shown in Figure 4a. First main weight loss (0.2% in blue, 0.38% in red and 0.46% in green) around 70–160°C is attributed to evaporation of residual solvent and water molecules adsorbed by exposed OH groups. With BCECF dye doped in the sol-gel formula, about 0.25% more weight loss downfall was observed through comparison between the blue and green lines, indicating a successful immobilization of the dye molecules. It was noticed that there was no significant weight loss at temperature less than 200°C, indicating a high degree of silanol poly-condensation in the sensor material [39, 40]. The second weight loss (1.62% in blue, 1.37% in red and 1.64%) at 300–600°C was believed to be attributed to the combustion of organic species, and the wide range of degradation line was due to the pore size distribution [41]. Furthermore, these enhanced mesoporous silica networks was highly due to the introduction of SDS for its ability to host sensing molecules more efficiently [42].
Figure 4.
Chemical characterization of optimized fabrication procedure of the ultrathin BCECF dye doped ORMOSILs layer by (a) TGA and (b) FT-IR.
The FT-IR spectrum of optimized representative sample is shown in Figure 4b. A characteristic feature of the spectrum is the presence of -CH2 related aliphatic chains of TEOS, MTES and DIAMO, indicated by a broad band in the region of 2882–2937 cm−1, which is attributed to stretching modes of -CH2 and partially -CH3 groups. A wide band covers the region from 3100 to 3600 cm−1 arises from the stretching -OH mode of the physically adsorbed water and/or residual silanol groups [43]. The representative primary amino groups that exist in DIAMO to form amide bond also showed characteristic peaks between 3500–3300 cm−1 as well as 1650–1550 cm−1 [25]. Despite peaks are mostly overlapped by other peaks, a peak at 1592 cm−1 explicitly shows the presence of primary amino groups [25]. Moreover, the containing of carboxylates or amino acid zwitterion was also proved as a C=O stretching at 1550–1610 cm−1 region.
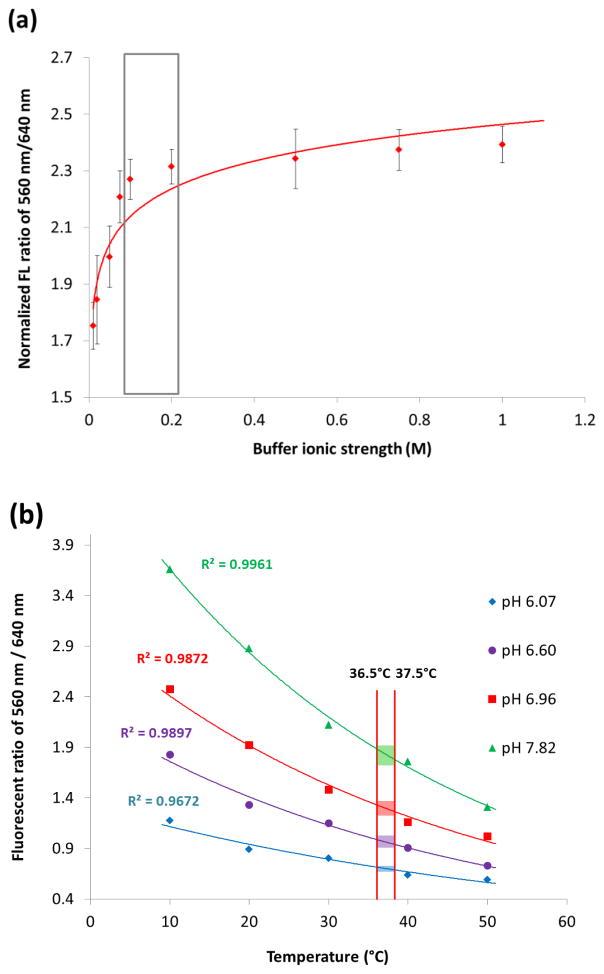
In addition, ionic strength can influence the protonation/deprotonation processes of pH sensors. Excessive ionic strength can also impact the osmotic pressure applied on a porous sensing network [44, 45]. Negative effect of ionic strength in pH measuring has been reported with a sensing material shrunken accompanied by marginal sensitivity [46]. Biosensors for both in vitro and in vivo environment detections will be confronted to lots of variation of ionic strength change and thus the influences have to be evaluated carefully. Figure 5(a) shows a drastic impact of pH variation in pH sensing capability. The extra- and intra-cellular ionic strength range normally from 0.08 to 0.2 M [47] which is shown in the labelled box. Below or above this range, it is not recommended to measure pH with currently proposed probe, otherwise poor sensitivity and large error would be expected. The proper storage of unused probe is also important prior immersion into solutions of high ionic strength because it can induce/form complexes that would hardly be dissolved and thus may irreversibly damage the probe [46].
Figure 5.
The effect of ionic strengths and temperature on pH measurement of the fabricated pH sensor. Other experimental conditions were the same as those described in the text.
The pH sensing capability of the probe can also be influenced by temperature. The temperature impact on pH sensing was observed within a relatively wider temperature range from 10 to 50°C, which is shown in Figure 5(b). The results show that the measured pH signals change significantly at five different temperature conditions. Temperature variation was found exponentially correlated with pH values, and an averaged 21.5% pH decrease was observed with every 10°C temperature increasing. This result is in accordance with previously claimed similar phenomenon [48]. However, since our ultimate goal was to monitor the pH in a single cell where a drastic change of temperature would not be appeared, such thermal influence will not be a significant factor in practical use, e.g., within the range of 37 ± 0.5°C, which is the upper limitation of our incubating system, a pH measurement variation would be expected around 0.047 (with buffer pH of 7.82, green region) to 0.012 (with buffer pH of 6.07, blue region) in normalized fluorescent (FL) ratio of 560nm / 640nm. This variation is way smaller than the standard deviation of each pH measurement and thus would have much less impact on the final pH measurement resolution.
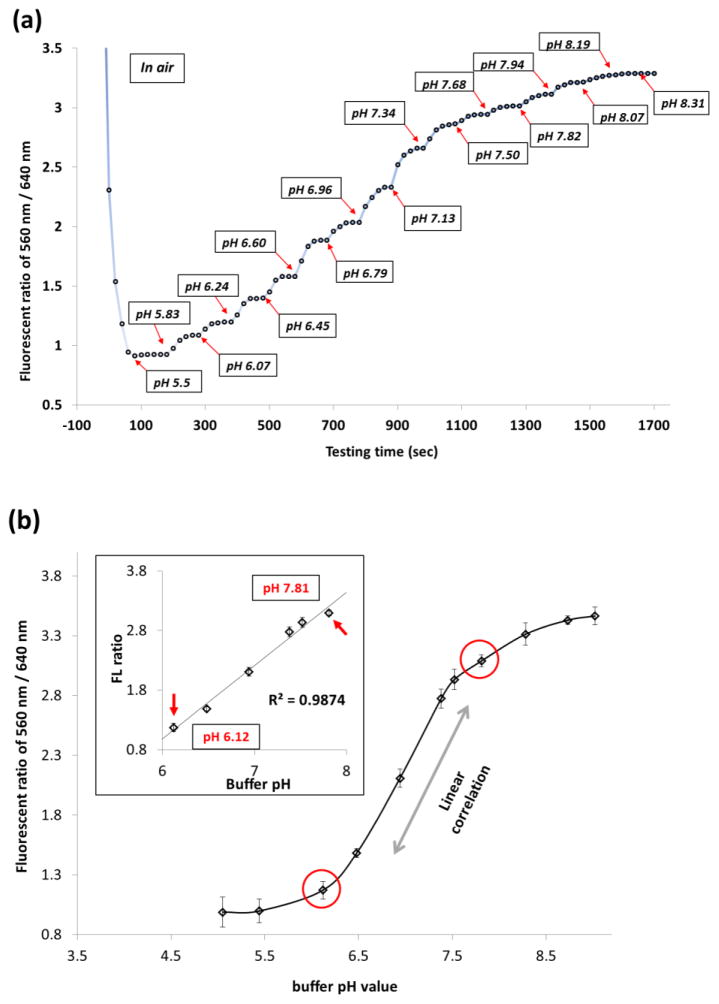
A series of pH measurement with gradient buffer solutions are shown in Figure 6(a). An average equilibrating time was about 40–60 seconds. Measurements in each pH buffer solution were kept still until a plateau of signal had been achieved. A period of 100 seconds was sufficient to reach to such a plateau status at all tested conditions.
Figure 6.
Fluorescent ratio (560/640 nm) vs. pH changes of the fabricated fiber-optic pH sensor. Measured pH range: pH 5.0 to pH 9.0. (a) fluorescence intensity change with time (seconds) in each buffer solution. (b) pH sensitive region of the sensor and linearity of fluorescent ratio changes as a function of buffer pHs.
Figure 6(b) plots the fluorescent signal ratio of 560 nm / 640 nm as a function of buffer pH values, where a linear correlation within the pH ranges from 6.12 to 7.81 (R2 = 0.9874). The linearity fitting of the experimental data indicates a pH sensing resolution of ~ 1.13 fluorescent ratio value (560 nm/640 nm) per pH unit. For each pH buffer solution, the standard deviations were between 0.038 and 0.084 in fluorescence ratio of 560 nm/640 nm based on triplicate measurements. Thus, any two measured signals that have a higher intervals will be well differentiated. Therefore, a pH resolution can theoretically have a range of 0.031 to 0.068 pH unit based on the resolution of fluorescence signals. This good linearity within such a focused pH working range can not only allow wide applications of a simple two-point calibration method [49], but also provide a sensitive and practical measurement capability of monitoring subtle pH change of ~0.049 (on average) pH units.
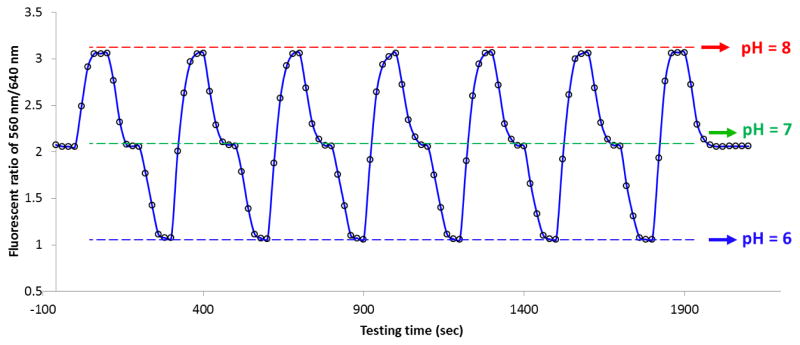
The fabricated 70-μm pH probe was then evaluated for its reproducibility in measurement under real circumstance. Cell culture grade 0.1 M PBS buffer solutions were prepared in three pH condition ranging from pH 6 to pH 8. The pH probe was continually inserted into three buffer solutions in the order of pH 7 → pH8 → pH7 → pH6 → pH7 (and so on) with 100 seconds interval between two pH buffers to acquire equilibration. Figure 7 shows a partial record of the pH measurements that lasts for about half an hour to demonstrate the repeatability and signal stability of the fabricated pH probe. The signal intensity variations are less than 2% during the measurements which is comparable to the traditional pH measurements. In addition, the probes that were similarly fabricated showed good reproducibility as well (with less than 5 % variations). The effect of long term storage (three months) on the probes in a sealed desiccator has been tested and the results showed that it did not change the sensing capability of the fabricated probes. However, after each measurement, the fiber probe needs to be rinsed with ethanol, followed by heat-drying right after rinsing. Fully removal of attached ions and vapor molecules from the surface aerogel networks is highly recommended to maintain its sensing ability and reproducibility.
Figure 7.
Repeatability and signal stability of the fabricated pH probe in cycling measurements of pH 6 (bottom), pH 7 (middle) and pH 8 (upper) buffer solutions for 35 minutes.
In summary, the sensitivity of the newly developed fiber-optic based pH sensor is comparable with other developed or commercialized pH sensors but with a much smaller size (~70 μm) and simplified sensing principle, operation procedure, and low cost. The high spatial resolution and high sensitivity (~0.049 pH unit) that focused only on bio-sample related pH region (pH 6 to pH 8) can potentially measure the pH variations at a surface of a single cell while cells are exposed to different environment such as nanomaerials. The fast sensing rate (within 40–60 seconds) and continuous sensing ability, the reflection-based remote-sensing capability (several meters) combining with significantly simplified calibration process (due to the linear correlation of fluorescence ratio and pH), provide promising application potentials of this novel pH probes in many biological and biomedical fields.
4. Conclusions
A fiber-optic reflection-mode probe based on dye-doped ultra-thin aerogel dip-coating technique has been developed for pH sensing. The fabrication of tapered optical fiber with spherical head was assisted by carbon dioxide laser stretching system and optical fiber fusion splicer device. The aerogel ultra-thin layer coating was implemented by sol-gel dip-coating technique combined with pH sensitive dye covalent-bonding through introduction of ORMOSILs. The pH sensing capability can be modified through adjusting the sol-gel formula with different ratio of alkoxides as well as surface hydrophobicity through HMDS priming. In this study, an optimized fabricating procedure was developed, and the fabricated pH probe was evaluated and demonstrated to have in vitro pH sensing capability in a real-time manner. A good linear response to pH variations was observed with a resolution down to ~0.049 pH unit, within a biological meaningful pH range of 6.12–7.81. The sensing signals were influenced by extreme changes in ionic strengths and temperatures, but no significant interference was observed within normal biological environment. To conclude, its micron-scale size with high spatial resolution, reflection mode operation, fast and repeatable recording, specific linear response within pH 6 to 8 region and a high pH resolution, make this novel pH probe a very cost-effective tool for chemical/biological sensing, especially within the single cell level research field.
Highlights.
A 70 μm reflection mode fiber-optic micro-pH sensor was fabricated;
ORMOSILs thin layer for covalent bonding with pH sensitive dye was employed;
A spherical-headed probe showed a high pH spatial resolution;
This novel probe has superior real-time pH sensing ability and repeatability;
A pH resolution up to 0.049 pH unit was achieved within the pH range of 6.12–7.81.
Acknowledgments
The authors thank Clarissa A. Wisner for assistance with SEM and EDS imaging. This project was supported by National Institute of Health (NIH) (Grant No: R21GM104696).
Abbreviations
- BCECF
2′, 7′-Bis (2-carbonylethyl)-5(6)-carboxyfluorescein
- CTAB
hexadecyltrimethylammonium bromide
- DIAMO
n-(3-(trimethoxysilyl) propyl)-ethylenediamine
- DMSO
Dimethyl sulfoxide
- EDS
energy-dispersive X-ray spectroscopy
- FL
fluorescent
- FT-IR
fourier transform infrared spectroscopy
- FWHM
full width at half maximum
- HMDS
hexamethyldisilazane
- HMDS
hexamethyldisilazane
- HMDSO
hexamethyldisiloxane
- Hünigs base
N-ethyldiisopropylamine
- MTES
methyltriethoxysilane
- ORMOSILs
ORganically MOdified SILicates
- PBS
phosphate buffer solution
- S/N
signal-to-noise ratio
- SDS
Sodium dodecyl sulfate
- SEM/FIBs
scanning electronic microscope/focused ion beams
- TEOS
tetraethoxysilane
- TGA
thermal gravimetric analysis
- TMCS
trimethylchlorosilane
- TSTU
2-succinimido-1, 1, 3, 3 tetramethyluronium tetrafluoroborate
Biographies
Qingbo Yang received his BS degree in Department of Bio-Engineering at Zhengzhou University in China, and received his MS in molecular biology and biochemistry from Shanghai University in China. He joined Dr. Yinfa Ma’s group since September, 2010 and currently is a PhD candidate in Analytical Chemistry at Missouri University of Science and Technology. His research interests include nanomaterial safety and cytotoxicity, single cell and single molecule level imaging and analysis, enhanced Raman spectroscopy, soft tissue wound-healing effect of bioactive glass nano-fibers. He is currently working as graduate research assistant in Chemistry Department and Environmental Research Center at Missouri University of Science and Technology.
Hanzheng Wang is a PhD candidate in Electrical Engineering Department at Clemson University. He received his MS in Electrical Engineering in University of Missouri-Rolla in 2009. His research projects include fiber optics and optical micro-resonator-based chemical, temperature sensors. He is working as a graduate research assistant in Clemson University. He has two patents and more than ten journal publications. He was awarded Newport Spectra-Physics Research Excellence Awards in 2012. He is a member of SPIE, OSA, IEEE and ISA.
Sisi Chen is a graduate student in Chemistry Department at Missouri University of Science and Technology. She received her BS degree in school of chemical biology and pharmaceutical sciences at Capital Medical University in 2012 in Beijing, China. She joined Dr. Yinfa Ma’s research group in 2012 and her research interests including analytical chemistry and bioactive material characterization and evaluation. She is currently focusing on cancer biomarker analysis using a UPLC-MS/MS system. Efforts has also been put into the wound healing mechanism study of different types of bioactive glass nanofibers as well as an NIH funded bio-sensor development project.
Baokai Cheng received his Bachelor’s and Master’s degree in Tsinghua University in 2002 and 2006, respectively. He is now a PhD candidate in electrical engineering at Clemson University. His major research interests focus on development of optical fiber and coaxial cable based devices for sensing applications.
Xinwei Lan received his PhD degree in electrical engineering from Missouri University of Science and Technology in 2013. He is currently a research associate at the Center for Optical Materials Science and Engineering Technologies (COMSET), Clemson University. His research efforts have been dedicated to developing novel optical and microwave sensors for harsh environment sensing, chemical/biological sensing and structure health monitoring. He is a member of the Optical Society (OSA), the Institute of Electrical and Electronics Engineers (IEEE), the American Chemical Society (ACS), and the International Society for Optical Engineers (SPIE).
Honglan Shi received her PhD in analytical chemistry from Missouri University of Science and Technology in May, 2010. She is an associate research professor in Department of Chemistry at Missouri University of Science and Technology. Her research mainly focus on development of advanced analytical techniques and methods for bioanalytical and environmental applications including state-of-the-art instrument development and manufacturing, bioactive glass-biofluid-bioorganism interaction study by advance analytical technologies, advanced test kit developments and manufacturing, method development for rapid characterization and quantification of engineered nanomaterials, development of novel economical and green technologies for water treatment, development of trace emerging pollutants analysis and control in natural and drinking water.
Hai Xiao received his PhD in electrical engineering from Virginia Tech in 2000. He is the Samuel Lewis Bell Distinguished Professor of Electrical and Computer Engineering, jointly affiliated with COMSET, at Clemson University. His research interests mainly focus on photonic and microwave sensors and instrumentation for applications in energy, intelligent infrastructure, clean-environment, biomedical sensing/imaging, and national security.
Yinfa Ma received his PhD in analytical chemistry and minor PhD in biochemistry from Iowa State University. He is a Curators’ Teaching Professor in Department of Chemistry at Missouri University of Science and Technology. His research focuses on bio-analysis and bio-separations, environmental monitoring, and single molecule and single cell imaging, by using variety of state-of-art instruments, such as high performance liquid chromatography (HPLC), high performance capillary electrophoresis (HPCE), GC–MS, HPLC–MS, HPCE–MS, gel electrophoresis, size-exclusion chromatography, and home-built single molecule and single cell imaging system.
Footnotes
Conflicting Interests
The authors declared no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paciorek T, Zažímalová E, Ruthardt N, Petrášek J, Stierhof YD, Kleine-Vehn J, et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;435:1251–6. doi: 10.1038/nature03633. [DOI] [PubMed] [Google Scholar]
- 2.Rosenzweig Z, Kopelman R. Development of a submicrometer optical fiber oxygen sensor. Analytical chemistry. 1995;67:2650–4. doi: 10.1021/ac00111a024. [DOI] [PubMed] [Google Scholar]
- 3.Tan W, Shi ZY, Kopelman R. Development of submicron chemical fiber optic sensors. Analytical chemistry. 1992;64:2985–90. [Google Scholar]
- 4.Vo-Dinh T, Kasili P. Fiber-optic nanosensors for single-cell monitoring. Analytical and bioanalytical chemistry. 2005;382:918–25. doi: 10.1007/s00216-005-3256-7. [DOI] [PubMed] [Google Scholar]
- 5.Rosenzweig Z, Kopelman R. Analytical properties of miniaturized oxygen and glucose fiber optic sensors. Sensors and Actuators B: Chemical. 1996;36:475–83. [Google Scholar]
- 6.Kneen M, Farinas J, Li Y, Verkman A. Green fluorescent protein as a noninvasive intracellular pH indicator. Biophysical journal. 1998;74:1591–9. doi: 10.1016/S0006-3495(98)77870-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seksek O, Henry-Toulmé N, Sureau F, Bolard J. SNARF-1 as an intracellular pH indicator in laser microspectrofluorometry: a critical assessment. Analytical biochemistry. 1991;193:49–54. doi: 10.1016/0003-2697(91)90042-r. [DOI] [PubMed] [Google Scholar]
- 8.Han J, Burgess K. Fluorescent indicators for intracellular pH. Chemical reviews. 2009;110:2709–28. doi: 10.1021/cr900249z. [DOI] [PubMed] [Google Scholar]
- 9.Collinson MM. Analytical applications of organically modified silicates. Microchimica Acta. 1998;129:149–65. [Google Scholar]
- 10.MacCraith BD, McDonagh C. Enhanced fluorescence sensing using sol-gel materials. Journal of Fluorescence. 2002;12:333–42. [Google Scholar]
- 11.Chaudhury N, Gupta R, Gulia S. Sol-gel Technology for Sensor Applications (Review Paper) Defence Science Journal. 2007;57:241–53. [Google Scholar]
- 12.Lintner B, Arfsten N, Dislich H, Schmidt H, Philipp G, Seiferling B. A first look at the optical properties of ormosils. Journal of Non-Crystalline Solids. 1988;100:378–82. [Google Scholar]
- 13.Schmidt H. Organic modification of glass structure new glasses or new polymers? Journal of Non-Crystalline Solids. 1989;112:419–23. [Google Scholar]
- 14.Higgins C, Wencel D, Burke CS, MacCraith BD, McDonagh C. Novel hybrid optical sensor materials for in-breath O2 analysis. Analyst. 2008;133:241–7. doi: 10.1039/b716197b. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez C, Julián B, Belleville P, Popall M. Applications of hybrid organic–inorganic nanocomposites. Journal of Materials Chemistry. 2005;15:3559–92. [Google Scholar]
- 16.McDonagh C, MacCraith B, McEvoy A. Tailoring of sol-gel films for optical sensing of oxygen in gas and aqueous phase. Analytical chemistry. 1998;70:45–50. doi: 10.1021/ac970461b. [DOI] [PubMed] [Google Scholar]
- 17.Bukowski RM, Davenport MD, Titus AH, Bright FV. O2-Responsive Chemical Sensors Based on Hybrid Xerogels that Contain Fluorinated Precursors. Applied spectroscopy. 2006;60:951–7. doi: 10.1366/000370206778397489. [DOI] [PubMed] [Google Scholar]
- 18.Guo L, Zhang W, Xie Z, Lin X, Chen G. An organically modified sol–gel membrane for detection of mercury ions by using 5, 10, 15, 20-tetraphenylporphyrin as a fluorescence indicator. Sensors and Actuators B: Chemical. 2006;119:209–14. [Google Scholar]
- 19.Korent ŠM, Lobnik A, Mohr GJ. Sol-gel-based optical sensor for the detection of aqueous amines. Analytical and bioanalytical chemistry. 2007;387:2863–70. doi: 10.1007/s00216-007-1146-x. [DOI] [PubMed] [Google Scholar]
- 20.Wencel D, Barczak M, Borowski P, McDonagh C. The development and characterisation of novel hybrid sol–gel-derived films for optical pH sensing. Journal of Materials Chemistry. 2012;22:11720–9. [Google Scholar]
- 21.Grant SA, Glass RS. A sol–gel based fiber optic sensor for local blood pH measurements. Sensors and Actuators B: Chemical. 1997;45:35–42. [Google Scholar]
- 22.Makote R, Collinson MM. Organically modified silicate films for stable pH sensors. Analytica chimica acta. 1999;394:195–200. [Google Scholar]
- 23.Ismail F, Malins C, Goddard NJ. Alkali treatment of dye-doped sol–gel glass films for rapid optical pH sensing. Analyst. 2002;127:253–7. [Google Scholar]
- 24.Scott BJ, Wirnsberger G, Stucky GD. Mesoporous and mesostructured materials for optical applications. Chemistry of materials. 2001;13:3140–50. doi: 10.1016/s1386-1425(01)00503-0. [DOI] [PubMed] [Google Scholar]
- 25.Kriltz A, Löser C, Mohr GJ, Trupp S. Covalent immobilization of a fluorescent pH-sensitive naphthalimide dye in sol–gel films. Journal of sol-gel science and technology. 2012;63:23–9. [Google Scholar]
- 26.Nivens DA, Zhang Y, Angel SM. A fiber-optic pH sensor prepared using a base-catalyzed organo-silica sol–gel. Analytica chimica acta. 1998;376:235–45. [Google Scholar]
- 27.Hegde ND, Venkateswara Rao A. Organic modification of TEOS based silica aerogels using hexadecyltrimethoxysilane as a hydrophobic reagent. Applied Surface Science. 2006;253:1566–72. [Google Scholar]
- 28.Nadargi DY, Rao AV. Methyltriethoxysilane: New precursor for synthesizing silica aerogels. Journal of alloys and compounds. 2009;467:397–404. [Google Scholar]
- 29.Wright JD, Sommerdijk N. Sol-Gel Materials: Chemistry and Applications. Gordon and Breach Science Publishers; Amsterdam, The Netherlands: 2001. [Google Scholar]
- 30.El-Nahhal IM, Zourab SM, Kodeh FS, Al-Bawab A. Behaviour of phenol red pH-sensors in the presence of different surfactants using the sol-gel process. International Journal of Environmental Analytical Chemistry. 2010;90:644–56. [Google Scholar]
- 31.Budunoglu H, Yildirim A, Guler MO, Bayindir M. Highly transparent, flexible, and thermally stable superhydrophobic ORMOSIL aerogel thin films. ACS applied materials & interfaces. 2011;3:539–45. doi: 10.1021/am101116b. [DOI] [PubMed] [Google Scholar]
- 32.Chauhan SS, Jasra R, Sharma A. Phenol Red Dye Functionalized Nanostructured Silica Films as Optical Filters and pH Sensors. Industrial & Engineering Chemistry Research. 2012;51:10381–9. [Google Scholar]
- 33.Yan D, Lu J, Ma J, Wei M, Evans DG, Duan X. Fabrication of an anionic polythiophene/layered double hydroxide ultrathin film showing red luminescence and reversible pH photoresponse. AIChE Journal. 2011;57:1926–35. [Google Scholar]
- 34.Lin P, Yan F. Organic Thin-Film Transistors for Chemical and Biological Sensing. Advanced Materials. 2012;24:34–51. doi: 10.1002/adma.201103334. [DOI] [PubMed] [Google Scholar]
- 35.Ozaydin-Ince G, Coclite AM, Gleason KK. CVD of polymeric thin films: applications in sensors, biotechnology, microelectronics/organic electronics, microfluidics, MEMS, composites and membranes. Reports on Progress in Physics. 2012;75:016501. doi: 10.1088/0034-4885/75/1/016501. [DOI] [PubMed] [Google Scholar]
- 36.Zhang S, Tanaka S, Wickramasinghe Y, Rolfe P. Fibre-optical sensor based on fluorescent indicator for monitoring physiological pH values. Medical and Biological Engineering and Computing. 1995;33:152–6. doi: 10.1007/BF02523033. [DOI] [PubMed] [Google Scholar]
- 37.Ganesh AB, Radhakrishnan T. Fiber-optic pH sensor. Fiber and integrated optics. 2006;25:403–9. [Google Scholar]
- 38.Gu B, Yin M, Zhang AP, Qian J, He S. Biocompatible Fiber-Optic pH Sensor Based on Optical Fiber Modal Interferometer Self-Assembled With Sodium Alginate/Polyethylenimine Coating. Sensors Journal, IEEE. 2012;12:1477–82. [Google Scholar]
- 39.Liu YL, Wu CS, Chiu YS, Ho WH. Preparation, thermal properties, and flame retardance of epoxy–silica hybrid resins. Journal of Polymer Science Part A: Polymer Chemistry. 2003;41:2354–67. [Google Scholar]
- 40.Menaa B, Takahashi M, Innocenzi P, Yoko T. Crystallization in hybrid organic-inorganic materials induced by self-organization in basic conditions. Chemistry of materials. 2007;19:1946–53. [Google Scholar]
- 41.Mirshafiei-Langari SA, Roghani-Mamaqani H, Sobani M, Khezri K. In situ atom transfer radical polymerization of styrene in the presence of nanoporous silica aerogel: Kinetic study and investigation of thermal properties. Journal of Polymer Research. 2013;20:1–11. [Google Scholar]
- 42.Taya SA. Sol-Gel Thin Films Immobilized with Bromocresol Purple pH-Sensitive Indicator in Presence of Surfactants. ISRN Analytical Chemistry. 2012
- 43.Takahashi M, Figus C, Kichob T, Enzo S, Casula M, Valentini M, et al. Self-Organized Nanocrystalline Organosilicates in Organic-Inorganic Hybrid Films. Advanced Materials. 2009;21:1732–6. [Google Scholar]
- 44.English AE, Tanaka T, Edelman ER. Equilibrium and non-equilibrium phase transitions in copolymer polyelectrolyte hydrogels. The Journal of chemical physics. 1997;107:1645–54. [Google Scholar]
- 45.Tanaka T. Experimental methods in polymer science: modern methods in polymer research and technology. Academic Press; 2000. [Google Scholar]
- 46.Richter A, Paschew G, Klatt S, Lienig J, Arndt KF, Adler HJP. Review on hydrogel-based pH sensors and microsensors. Sensors. 2008;8:561–81. doi: 10.3390/s8010561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mouat M, Manchester K. The intracellular ionic strength of red cells and the influence of complex formation. Comparative Haematology International. 1998;8:58–60. [Google Scholar]
- 48.Herzog M. Thermophoresis and cooperative binding of nucleotides: lmu. 2012 [Google Scholar]
- 49.Sheppard NF, Jr, Lesho MJ, McNally P, Shaun Francomacaro A. Microfabricated conductimetric pH sensor. Sensors and Actuators B: Chemical. 1995;28:95–102. [Google Scholar]