Abstract
Perturbations in the prenatal and early life environment can contribute to the development of offspring stress dysregulation, a pervasive symptom in neuropsychiatric disease. Interestingly, the vertical transmission of maternal microbes to offspring and the subsequent bacterial colonization of the neonatal gut overlap with a critical period of brain development. Therefore, environmental factors such as maternal stress that are able to alter microbial populations and their transmission can thereby shape offspring neurodevelopment. As the neonatal gastrointestinal tract is primarily inoculated at parturition through the ingestion of maternal vaginal microflora, disruption in the vaginal ecosystem may have important implications for offspring neurodevelopment and disease risk. Here, we discuss alterations that occur in the vaginal microbiome following maternal insult and the subsequent effects on bacterial assembly of the neonate gut, the production of neuromodulatory metabolites, and the developmental course of stress regulation.
Keywords: Maternal Stress, Vaginal microbiome, Microbiota, Fetal programming, Neurodevelopment, HPA stress axis
1. Introduction
Early life perturbations such as stress, inflammation, or infection produce long-term effects on the developing brain, increasing subsequent risk of neuropsychiatric disorders throughout life. Despite advances in understanding the mechanistic roles of the maternal milieu in normal and pathological neurodevelopment, significant progress in biomarker discovery and the treatment of neuropsychiatric disorders has not been made. This is in part due to the multifactorial presentation of neuropsychiatric conditions and common comorbidities, including chronic gastrointestinal (GI) dysfunction. As a growing body of evidence suggests that a critical window for neurodevelopment overlaps with microbial colonization of the gastrointestinal tract, it is likely that environmental perturbations could similarly impact both systems (Borre et al., 2014, Stilling et al., 2014).
In particular, maternal stress during pregnancy has been associated with an increased incidence of neurodevelopmental disorders and gastrointestinal dysfunction (Chrousos, 2009, Mawdsley and Rampton, 2006, O'Mahony et al., 2009). Among the many maladaptive effects it exhibits on the mother, chronic stress during pregnancy alters vaginal host immunity and resident bacteria composition (Culhane et al., 2001, Wadhwa et al., 2001, Witkin et al., 2007). The vaginal ecosystem is a dynamic community shown to be sensitive to a variety of factors such as body composition, diet, infection, antibiotic treatment and stress (Bennet et al., 2002, Cho et al., 2012, Turnbaugh et al., 2009, Ravel et al., 2011, Koenig et al., 2011), and is poised to communicate information about the state of the pending external environment. Maternal vaginal microflora is ingested into the neonatal gut during parturition, establishing the initial microbial population. Therefore, perturbations to the vaginal ecosystem could have significant consequences for offspring development and disease risk. For example, dysbiosis of vaginal microflora can impact the microbial assembly of the neonatal gut where decreased diversity and stability of microbial populations could promote disruption of key processes involved in host metabolism, immune function, and neurodevelopment (Round and Mazmanian, 2009, Nicholson et al., 2012, Maslowski and Mackay, 2011, Cryan and Dinan, 2012). The hypothalamic-pituitary-adrenal (HPA) stress axis may be particularly sensitive to gut microbial disruption as its development overlaps with the initial colonization of the neonatal gut (Borre et al., 2014, Walker et al., 1986). Critically, HPA axis dysregulation has long been recognized as a hallmark of inflammatory and psychiatric disorders, where both hyper- and hypo-responsivity have been reported (Bale et al., 2010, Howerton and Bale, 2012, Moghaddam, 2002, Lupien et al., 2009).
In this review, we discuss the influence of maternal-infant microbial transmission on early life programming, and the ability for stress to alter this process (Fig. 1). Specifically, we will highlight a potential mechanistic role for the neonate gut microbiome to contribute to nutrient metabolism, thereby linking itself to the developing brain. We outline the bidirectional communication between the HPA stress axis and gut microbiota, and consider the implication of early microbial dysbiosis during critical neurodevelopmental windows, emphasizing potential sex-specific consequences across a number of behavioral domains. We conclude by providing some perspectives on future directions in this area.
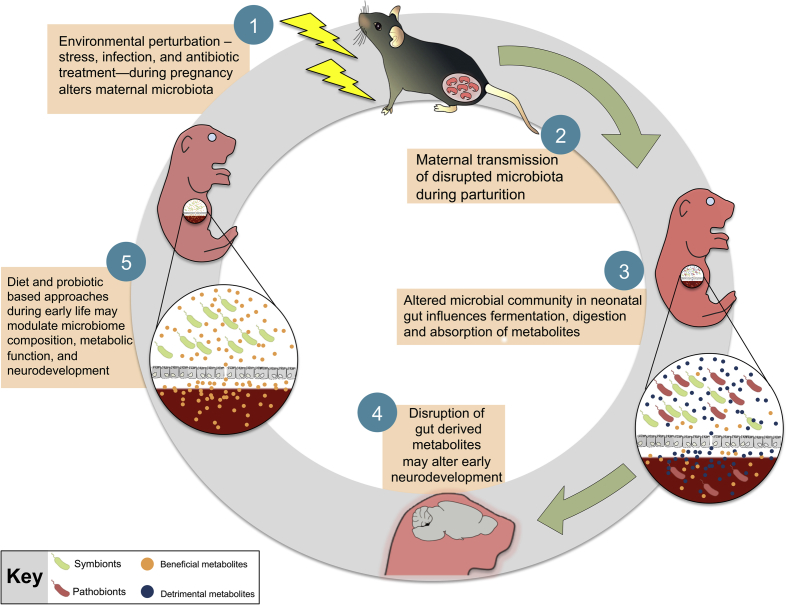
Fig. 1.
A proposed model for the role of maternal microbial transmission in early life programming and neurodevelopment. Environmental perturbations, such as stress or infection, during pregnancy destabilize the vaginal ecosystem that may lead to dysbiosis of the vaginal flora characterized by a shift from a Lactobacillus-dominant (symbionts) environment to overgrowth of opportunistic pathogens (pathobionts). Vertical transmission of a disrupted microbiota may compromise key developmental processes of the neonate, including the synthesis and absorption of microbe-derived metabolites, maturation of the gastrointestinal tract, and immune function. Outcompetition by pathobionts in the neonatal gut may increase production of detrimental metabolites and alter downstream neurodevelopmental events, including development of the hypothalamic-pituitary-adrenal (HPA) stress axis, as its development overlaps with early colonization patterns of the neonatal gut. Disruption during this critical window may result in long-term programming that persists even after stable core microbiota has been established. Administration of probiotics or dietary factors that promote maturation of the neonatal gut provide a promising avenue of therapeutic treatments by which to modulate microbiota composition, metabolic function, and neurodevelopment of the host.
2. The vaginal microbiome
The female reproductive tract and its microflora form a dynamic ecosystem, with the vaginal mucosal environment determining the survival of specific bacterial species, and the microflora in turn contributing to the vaginal environment. The hormonal control of vaginal glycogen content is believed to be a major factor shaping the microbial composition and stability within the female reproductive tract. Upon estradiol stimulation, glycogen is deposited onto mature vaginal epithelium where it is metabolized to glucose by the epithelial cells and bacterial enzymes (Linhares et al., 2011, Redondolopez et al., 1990). Lactobacillus was the first bacterial genus identified with the capacity to metabolize vaginal glucose into lactic acid and hydrogen peroxide, and it is predominantly these H2O2-producing strains that thrive in low vaginal pH conditions. By maintaining low vaginal pH and producing H2O2, as well as by stimulating the immune system and preventing further colonization through competitive exclusion, healthy Lactobacillus populations protect the female reproductive tract from infection by opportunistic pathogens. Indeed, overgrowth of Gardnerella vaginalis, a harmful toxin-producing bacterium, has been associated with increased vaginal pH and loss of H2O2-producing Lactobacillus (Hawes et al., 1996, Mijac et al., 2006, Soper, 1997, Tomas et al., 2003, Vallor et al., 2001).
During pregnancy, steroid hormones such as progesterone and estradiol stimulate high levels of glycogen deposition onto vaginal epithelium further promoting the growth of favorable acidophilic vaginal bacteria like Lactobacillus. However, these hormones also play a significant role in immunosuppression during pregnancy. While this effect is adaptive as it allows tolerance of the developing offspring, it may also increase maternal vulnerability to environmental challenges (Trowsdale and Betz, 2006, Zuk and Stoehr, 2002). Stress during pregnancy can exaggerate the normal physiological immunosuppression, thereby increasing maternal vulnerability to genitourinary infection and its related obstetrical risks including associations with neurodevelopmental disorders. For instance, in a recent epidemiological study, mothers of children with autism spectrum disorder reported greater frequency and severity of vaginal bacterial infections during pregnancy (Zerbo et al., 2013). Importantly, recurrent vaginal bacterial and fungal infections can trigger a variety of local and global responses that may result in the eventual loss of the beneficial Lactobacillus-dominant vaginal ecosystem (Gupta et al., 1998, Ehrstrom et al., 2005). The downstream effects of stress-related Lactobacillus depletion on maternal-infant microbial transmission, host metabolism, and immune function remain to be examined, but likely include important consequences for the developing brain.
3. Maternal-infant microbial transmission
Two different modes of maternal-infant transmission have been proposed: 1) horizontal, where the infant's predominant microbial acquisition is from the external environment, and 2) vertical, where there is maternal transmission of vaginal microbes during parturition (Bright and Bulgheresi, 2010). Emerging evidence, however, suggests that vertical transmission primarily accounts for the initial colonization of the infant gut, which can influence maturation of the gastrointestinal tract and ensure the proper extraction of energy and macromolecules essential for normal development (Bright and Bulgheresi, 2010, Cilieborg et al., 2012, Collado et al., 2012, Mackie et al., 1999). Recent appreciation for the influence of this mother-infant microbial transmission on offspring development has sparked new interest in understanding the potential connection between perturbations during pregnancy and early life programming.
3.1. Vertical transmission during parturition
At the turn of the twentieth century, French pediatrician Henry Tissier proposed that human infants develop within a sterile environment, with primary microbial exposure occurring through contact of the newborn with maternal vaginal microbiota (Tissier, 1900). However, recent studies have cast some reservations on the ‘sterile’ womb hypothesis (Funkhouser and Bordenstein, 2013). Maternal gut bacteria have been isolated in umbilical cord blood, amniotic fluid, meconium, and placental and fetal membranes of infants from uncomplicated and otherwise healthy pregnancies (Jimenez et al., 2005, Rautava et al., 2012, Steel et al., 2005, Gosalbes et al., 2013, Aagaard et al., 2014). However, the mechanism by which the maternal gut bacteria gain access to the developing fetus is not well understood and needs to be further characterized. Nevertheless, during vaginal delivery, the amniotic fluid is exposed to a complex microbial world within the birth canal and ingestion of this fluid by offspring likely serves as a primary mode of widespread maternal microbial transmission (Mackie et al., 1999). Notably, the gastric content and bacterial serotypes isolated from the nasopharynxes of newborns were similar to those of their mothers' vagina immediately before birth (Bettelheim et al., 1974, Brook et al., 1979). Additionally, Streptococcus or Lactobacillus dominance in the maternal vagina has been associated with a similar predominance pattern in her offspring's gut (Mändar and Mikelsaar, 1996), and Lactobacillus species of maternal origin (e.g., L. crispatus, L. fermentum, L. gasseri, and L. vaginalis) have been isolated from infant fecal samples (Matsumiya et al., 2002, Carlsson and Gothefors, 1975).
Importantly, a variety of environmental factors may disrupt the vertical transmission of microbiota with potential impacts on early development (Wopereis et al., 2014). Widespread obstetric practices such as vaginal cleansing with disinfectants and application of antiseptic creams shortly before birth have been shown to reduce maternal transmission of Streptococcus agalactiae, a bacteria involved in group B streptococcal (GBS) sepsis in the newborn (Stray-Pederson et al., 1999). However, the spectrum of activity of these disinfectants includes many beneficial microbes such as Lactobacillus and its use has been attributed in preventing colonization of the newborn with commensal bacteria from the maternal vagina (Tannock et al., 1990). Moreover, administration of intrapartum antibiotics as a preemptive prophylaxis against GBS infection leads to dysbiosis of the vaginal flora characterized by a shift from a Lactobacillus-dominant environment to an antibiotic-resistant polymicrobial mixture such as Klebsiella, Citrobacter, Enterobacter, and Escherichia coli (Tanaka et al., 2009, Keski-Nisula et al., 2013, Fallani et al., 2010, Newton and Wallace, 1998). Vertical transmission of these antibiotic-resistant coliforms influences early colonization patterns of the neonate and the effects of maternal antibiotic treatment on offspring gut microbiota persist well after cessation of treatment (Tanaka et al., 2009, Keski-Nisula et al., 2013, Fallani et al., 2010, Newton and Wallace, 1998). More recent rodent studies have shown that maternal exposure to low dose antibiotics during lactation depleted Lactobacillus abundance, increased fat mass, and altered metabolic hormones in offspring (Cox et al., 2014, Cox and Blaser, 2013). Further, transfer of these disrupted microbiota to germ-free mice was sufficient to recapitulate the obesity phenotype, supporting that microbiota disruption was sufficient for long-term programing of host metabolic dysfunction (Bruce-Keller et al., 2014). These results exhibit strong translational value in light of a recent report drawing associations between antibiotic exposure during the first 6 months following birth and an increased body mass (Trasande et al., 2013).
Early colonization of a stable core microbiota is also influenced by mode of delivery (Salminen et al., 2004, Rouphael et al., 2008, Rousseau et al., 2011, Cooperstock et al., 1983). Vaginal bacteria from the mother initially colonize the intestine of vaginally delivered infants, whereas bacteria from the mother's skin and the local environment (e.g., healthcare workers, air, other newborns) colonize infants born via caesarean section. Newborns delivered by caesarean section show delayed colonization by Bacteroides and Bifidobacterium, as well as an overgrowth of Clostridium difficile. The resulting differences in colonizing microbiota for vaginally and caesarean delivered children persist well into childhood and are associated with increased body mass and childhood obesity (Salminen et al., 2004, Blustein et al., 2013). Taken together, environmental factors exhibit great influence on vertical transmission of microbiota, early colonization patterns, and long-term programming of metabolic function.
4. Microbe-derived metabolites
The mutualistic nature of the host-microbe relationship relies on interactions between microbial metabolite production and the host immune, endocrine, and neural systems. Bacterial colonization of the neonatal gut beginning with beneficial pioneer species is critical during the early developmental window, and provides an important source of metabolites for the neonate. The relative composition, diversity and abundance of beneficial bacteria modulates the level of synthesis of a vast array of neuromodulatory molecules and neurotransmitters, including catecholamines, gamma-aminobutyric acid (GABA), serotonin, tryptophan, glutamate, acetylcholine and histamine (Iyer et al., 2004, Higuchi et al., 1997, Wikoff et al., 2009, LeBlanc et al., 2013, Ross et al., 2010). The microbial control of GABA, tryptophan, and serotonin metabolism within the context of neurodevelopmental risk and resilience has been exquisitely reviewed elsewhere (Forsythe et al., 2010, O'Mahony et al., 2014a).
Invertebrate model systems, such as Caenorhabditis elegans and Drosophila melanogaster, have revealed that the activity of the microbiome and its metabolic products directly influence host development and physiology (Cabreiro et al., 2013, Ridley et al., 2012, Shin et al., 2011, Storelli et al., 2011, Sharon et al., 2010). More recent advances in rodent models are beginning to elucidate the physiological roles of gut metabolites in mammals. Commensal bacteria in the mammalian gut actively ferment soluble dietary carbohydrates and produce a variety of biologically active metabolites, commonly referred to short chain fatty acids (SCFA's), including butyrate, acetate and propionate. Of these metabolites, propionate and butyrate readily cross the gut-blood and blood–brain barriers via a monocarboxylate transporter (Karuri et al., 1993, Bergersen et al., 2002, Conn et al., 1983). In the brain, propionate and other SCFAs impact neuronal metabolism as well as the synthesis and release of neurotransmitters during early neurodevelopment (Peinado et al., 1993, Rafiki et al., 2003). Importantly, a careful balance of brain SCFAs must be achieved, as excessive levels have been associated with neural mitochondrial dysfunction and severe behavioral deficits in rodents (Macfabe, 2012, de Theije et al., 2014a, de Theije et al., 2014b, de Theije et al., 2011).
In addition to their direct role in fermentation, commensal gut microbiota express many enzymes with immunomodulatory and neuromodoulatory implications. For example, the gene encoding histidine decarboxylase (HDC), which catalyzes the conversion of l-histidine to histamine, was recently identified in Lactobacillus reuteri, a beneficial microbe found in the gut of rodents and humans (Thomas et al., 2012). Critically, circulating histidine availability is also directly proportional to histidine content and histamine synthesis in the brain (Schwartz et al., 1972, Taylor and Snyder, 1971). Histaminergic fibers originate from the tuberomamillary region of the posterior hypothalamus and project widely to most regions of the developing brain, including the hippocampus, dorsal raphe, cerebellum, and neighboring nuclei of the hypothalamus (Panula et al., 1989). The ability of microbiota to modulate synthesis of a vast array of neuromodulatory molecules highlight the need for additional studies characterizing of the role of microbiota-derived metabolites on broad neurodevelopmental events.
Accumulating evidence draws associations between microbe-generated metabolites during early development and endophenotypes of neuropsychiatric disease. Studies in GF mice revealed that microbial exposure during early life modulated dopamine signaling, neuronal mitochondrial function, neuroplasticity, and motivational behaviors in adult animals (Diaz Heijtz et al., 2011, Matsumoto et al., 2013). Further, in a mouse model of maternal immune activation during pregnancy, decreased abundance of the beneficial Bacteroides fragilis and increased serum levels of microbe-derived metabolites 4-ethylphenylsulfate and indolepyruvate were observed in exposed offspring. Direct administration of these metabolites to unexposed offspring increased adult anxiety-like behaviors similar to those observed following maternal immune activation, supporting that microbe-generated metabolites may affect brain programming (Hsiao et al., 2013). The findings are consistent with available epidemiological studies where disruption of microbe-generated metabolites has been reported in children with comorbid presentation of gastrointestinal dysfunction and autism spectrum disorders (ASD) (Wang and Kasper, 2014, Marques et al., 2010). Reduced urinary levels of carnosine, glycine, serine, threonine, alanine and histidine have also been observed in children with ASD, suggesting an imbalance of resident gut bacteria involved in both amino acid and carbohydrate metabolism may be present (Williams et al., 2011, Ming et al., 2012). A reduced capacity for nutrient digestion and transport in children with ASD has been related to increased levels of Clostridium species, Bacteriodetes depletion, and loss of metabolites related to energy homeostastis (e.g disaccharidases, hexose transporters) (Williams et al., 2011). Future efforts should focus on putative mechanisms by which microbe-dependent production of neuromodulatory metabolites can result in neurodevelopmental dysregulation predictive of disease.
5. Stress and microbiota
The consequence of environmental stressors on gut microbiome composition in adults has been established for nearly four decades (Tannock and Savage, 1974). This association was first developed from observations that short-term environmental challenges – deprivation from food, water, and bedding – decreased the abundance of beneficial bacteria, such as Lactobacillus, and increased the susceptibility to opportunistic pathogens in mice (Tannock and Savage, 1974). However, quantification of bacteria in these early studies was limited to phyla that could be cultured in the lab, failing to account for >99% of microorganisms that could not be cultivated by standard techniques (Hugenholtz et al., 1998). Recent advances in metagenomic analyses have identified microbial communities not previously cataloged, and captured a more complete representation of the microbial composition in the intestine (Leser et al., 2002, Dinan and Cryan, 2012, Lutgendorff et al., 2008, Bendtsen et al., 2012). With these improved technologies, reduced microbial richness and opportunistic overgrowth of bacteria have been subsequently reported in animal models where adult chronic stress was examined, and where long-term programming changes in the HPA stress axis were found (Bailey et al., 2010). Additionally, social stress-mediated depletion of Lactobacillus was associated with increased translocation of cutaneous-derived microflora to the inguinal and mesenteric lymph nodes (Bailey et al., 2010, Bailey et al., 2006, Bailey et al., 2011). Although the mechanistic significance of bacteria translocation in these lymphoid organs on HPA axis reprogramming is not clear, sympathetic and noradrenergic innervation of lymphoid organs plays a critical role in the neuroimmune modulation of the HPA axis (Elenkov et al., 2000).
5.1. Early life stress and neonate gut ecology
Stress pathway dysregulation is the most common symptom in neuropsychiatric disorders, yet mechanisms involved in determining potential developmental windows of susceptibility are not fully understood. Animal models of maternal stress have provided insight into the long-term programming of offspring outcomes, but characterization of the role of maternal stress on bacterial colonization patterns and early life programming is only emerging (O'Mahony et al., 2009, Bailey and Coe, 1999, Bailey et al., 2004). Maternal stress during pregnancy has been shown to alter the microbial composition of the offspring gut (Bailey et al., 2004). Pregnant rhesus macaques were exposed to acoustic startle stress during a period of either early (days 50–92) or late (days 105–147) gestation and then the offspring gut microbiota characterized postnatally at 2 days and 2, 8, 16, and 24 weeks. Offspring exposed to early gestational stress exhibited Lactobacillus depletion, while Bifidobacteria and Lactobacillus abundance were depleted in offspring exposed to stress during late gestation, suggesting a temporal specificity of stress impact on microbiota. Infants exposed to stress during gestation also exhibited subclinical colonization with the opportunistic pathogen Shigella flexneri during the first 24 weeks of life.
Similar to prenatal stress, maternal separation reduced fecal Lactobacillus abundance in separated offspring relative to nonseparated cohorts in rhesus macaques (Macaca mulatta) (Bailey and Coe, 1999). Lactobacillus depletion was associated with increased distress-related behaviors and increased susceptibility to bacterial infection three days post-separation (Bailey and Coe, 1999). Maternal separation also elicited elevated cortisol levels in separated offspring relative to non-separated cohorts, although this increase in stress responsivity was not correlated with Lactobacillus levels. More recently, an investigation of maternal separation in a rodent model reported long-term disruption of offspring microbial communities, which may contribute to the increased stress reactivity and anxiety-like behaviors observed in these animals as adults (O'Mahony et al., 2009). Interestingly, concurrent treatment with Lactobacillus probiotics during the early phase of maternal separation mitigated maternal separation-mediated corticosterone release in pups, a direct measure of HPA axis responsivity (Gareau et al., 2007), illustrating the potential therapeutic benefit of microbial populations. Potential mechanisms by which stress-mediated changes in early gut microflora may affect brain development are discussed below.
5.2. Microbial programming of the brain
The role of the early gut microbiota in neurodevelopmental programming and stress-related risk and resilience has been largely established through the use of germ-free (GF) mice that are born and raised under axenic conditions, devoid of all microorganisms. For example, exposure of GF male and female mice to restraint stress was associated with aberrant ACTH and corticosterone profiles, downregulation of brain derived neurotrophic factor (BDNF) expression in the hippocampus and glucocorticoid receptor and N-methyl-d-aspartate receptor subunit 2a (NR2a) expression in the cortex (Sudo et al., 2004, Clarke et al., 2013). However, similar changes were not observed following restraint of conventionally housed mice suggesting that the absence of the early microbiota influences stress responsivity into adulthood. Further, monoassociation with Bifidobacterium infantis, a bacterium commonly isolated from the neonate gut, partially rescued the HPA stress activation, and gnotobiotic mice reconstituted with normal specific pathogen-free microbiota exhibited decreased anxiety-like behaviors (Sudo et al., 2004, Clarke et al., 2013, Nishino et al., 2013).
Further evidence of the role of microbiota in shaping stress pathway regulation comes from the study of serotonergic dysregulation, a common feature in sex-specific affective disorders (Ressler and Nemeroff, 2000, Goel and Bale, 2010). Consistent with previous reports of sex differences in serotonergic neurocircuitry and established sex differences in the HPA axis stress response (Goel and Bale, 2010), hippocampal serotonin and 5-HIAA, the main metabolite of serotonin, concentrations were higher in conventionally colonized (CC) female mice than in males (Clarke et al., 2013). Interestingly, serotonin and 5-HIAA levels remain unchanged in GF females relative to CC females, while concentrations of these monoamines and metabolites were increased to female-typical levels in GF male mice (Clarke et al., 2013), suggesting potential dysmasculinization of hippocampal serotonergic neurocircuitry in GF males. Consistent with previous work on early life stress and sex-specific dysregulation of neuroplasticity (Mueller and Bale, 2008), BDNF expression was decreased in the hippocampus of GF male, but not GF female mice (Clarke et al., 2013). While bacterial colonization of GF males during the post-weaning period did not rescue hippocampal serotonergic alterations, this treatment successfully rescued altered anxiety-like behaviors observed in male GF mice (Clarke et al., 2013). This demonstration of the absence of a normal gut microbiota exhibiting consequences on neurodevelopment and adult behavior in males but not females introduces the possibility that the microbiome may also contribute to a larger extent to sex differences in the susceptibility to disease.
Of great importance to stress pathway regulation, a direct interaction between gonadal hormones and microbial exposure in mediating sex-specific disease risk has been recently illustrated (Markle et al., 2013, Yurkovetskiy et al., 2013). The incidence of autoimmune disorders such as type 1 diabetes (T1D) displays a strong female bias, with nearly twice as many females affected as males (Pozzilli et al., 1993). Similar sex-specific susceptibility is observed in the non-obese diabetes (NOD) mouse model where female NOD mice exhibit increased incidence of T1D pathogenesis relative to NOD males (Pozzilli et al., 1993). To examine if phenotypes are related to the sex-specific microbiome profiles observed in NOD mice, which emerge at the onset of puberty and remain stable well into adulthood, researchers transferred male cecal contents into pubertal NOD female mice. Remarkably, this transfer resulted in masculinization of the microbial composition, increased testosterone levels, and metabolite profile of glycerophospholipids and sphingolipids in female recipients, demonstrating, amazingly, that male microbiota provides sex-specific protective effects against T1D pathogenesis (Markle et al., 2013). Notably, commensal bacteria may be directly responsible for testosterone production and its effects on metabolism, as both male and female NOD mice exhibited altered testosterone profiles and T1D-like pathology when reared under germ-free conditions. These studies are among the first to demonstrate the ability of microbial transfer to impact disease risk and resilience. Behavioral phenotypes also appear to be transmissible via the microbiota, as germ-free NIH Swiss mice inoculated with cecal contents from BALB/c mice, an innately anxious strain of mice, displays a behavioral phenotype similar to the donor species (Bercik et al., 2011). These combined results have important implications for the etiology and potential treatment of functional gastrointestinal intestinal disorders, which are female biased in presentation and comorbid with psychiatric disorders, including anxiety and depression (Chang et al., 2006, Mikocka-Walus et al., 2008, O'Mahony et al., 2014b). Thus, microbiota transfer studies across a variety of experimental conditions will undoubtedly expand our understanding of the role of the microbiota in biological processes, including brain development, immunity, and metabolic function.
6. Conclusions and future directions
The quality of the early postnatal environment influences the course of development, which in turn determines the health of the individual across the life span. Transmission of individual differences in behavioral and physiological responses to environmental stimuli is a key factor in predicting stress-related disorders. To date, alterations in maternal care, diet, and stress are known influences on sex-specific outcomes related to offspring disease vulnerability (Bale et al., 2010). Vertical transmission of maternal microbes to offspring is emerging as a factor in transgenerational disease risk and resilience. The vaginal microbiome influences early-host microbe interactions in the neonate, and therefore affects long-term programming of microbial colonization patterns, immune function, metabolic status, neurodevelopment, and disease risk into adulthood. From a clinical perspective, screening of the vaginal flora during late pregnancy may also provide critical insight into the early colonization patterns of the newborn gastrointestinal tract and associated disease risk.
The mechanisms underlying the communication between the developing central nervous system and gastrointestinal tract likely involve microbial production of neuromodulatory metabolites, vagal nerve innervation, and innate immunity. Emerging reports suggest that microbe derived metabolites can be both beneficial and detrimental to host development, although more research is needed to identify and characterize the downstream targets of these metabolites (Hsiao et al., 2013, Dorrestein et al., 2014). Finally, vaginal microbial communities are plastic, and can be rapidly altered following dietary, probiotic, and environmental interventions. This gives rise to the intriguing possibility that therapeutic treatment of vaginal microbiota may be a viable target for maternal stress and immune related neurodevelopmental disorder prevention.
Acknowledgments
This work was supported by the National Institutes of Health Grants MH104184, MH091258, and MH087597. We would like to thank C. Howard for insightful discussion.
References
- Aagaard K., Ma J., Antony K.M., Ganu R., Petrosino J., Versalovic J. The placenta harbors a unique microbiome. Sci. Trans. Med. 2014;6:237–265. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M.T., Coe C.L. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev. Psychobiol. 1999;35:146–155. [PubMed] [Google Scholar]
- Bailey M.T., Lubach G.R., Coe C.L. Prenatal stress alters bacterial colonization of the gut in infant monkeys. J. Pediatr. Gastroenterol. Nutr. 2004;38:414–421. doi: 10.1097/00005176-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Bailey M.T., Engler H., Sheridan J.F. Stress induces the translocation of cutaneous and gastrointestinal microflora to secondary lymphoid organs of C57BL/6 mice. J. Neuroimmunol. 2006;171:29–37. doi: 10.1016/j.jneuroim.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Bailey M.T., Dowd S.E., Parry N.M., Galley J.D., Schauer D.B., Lyte M. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infect. Immun. 2010;78:1509–1519. doi: 10.1128/IAI.00862-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M.T., Dowd S.E., Galley J.D., Hufnagle A.R., Allen R.G., Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav. Immun. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale T.L., Baram T.Z., Brown A.S., Goldstein J.M., Insel T.R., McCarthy M.M. Early life programming and neurodevelopmental disorders. Biol. Psychiatry. 2010;68:314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendtsen K.M.B., Krych L., Sorensen D.B., Pang W.Y., Nielsen D.S., Josefsen K. Gut microbiota composition is correlated to Grid Floor induced stress and behavior in the BALB/c mouse. PloS one. 2012:7. doi: 10.1371/journal.pone.0046231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet R., Eriksson M., Nord C.E. The fecal microflora of 1–3-month-old infants during treatment with eight oral antibiotics. Infection. 2002;30:158–160. doi: 10.1007/s15010-002-2140-z. [DOI] [PubMed] [Google Scholar]
- Bercik P., Denou E., Collins J., Jackson W., Lu J., Jury J. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. doi: 10.1053/j.gastro.2011.04.052. e1-3. [DOI] [PubMed] [Google Scholar]
- Bergersen L., Rafiki A., Ottersen O.P. Immunogold cytochemistry identifies specialized membrane domains for monocarboxylate transport in the central nervous system. Neurochem. Res. 2002;27:89–96. doi: 10.1023/a:1014806723147. [DOI] [PubMed] [Google Scholar]
- Bettelheim K.A., Breadon A., Faiers M.C., O'Farrell S.M., Shooter R.A. The origin of O serotypes of Escherichia coli in babies after normal delivery. J. Hyg. 1974;72:67–70. doi: 10.1017/s0022172400023226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blustein J., Attina T., Liu M., Ryan A.M., Cox L.M., Blaser M.J. Association of caesarean delivery with child adiposity from age 6 weeks to 15 years. Int. J. Obes. 2013;37:900–906. doi: 10.1038/ijo.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borre Y.E., O'Keeffe G.W., Clarke G., Stanton C., Dinan T.G., Cryan J.F. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol. Med. 2014;20:509–518. doi: 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Bright M., Bulgheresi S. A complex journey: transmission of microbial symbionts. Nat. Rev. Microbiol. 2010;8:218–230. doi: 10.1038/nrmicro2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook I., Barrett C.T., Brinkman C.R., 3rd, Martin W.J., Finegold S.M. Aerobic and anaerobic bacterial flora of the maternal cervix and newborn gastric fluid and conjunctiva: a prospective study. Pediatrics. 1979;63:451–455. [PubMed] [Google Scholar]
- Bruce-Keller A.J., Salbaum J.M., Luo M., Et Blanchard, Taylor C.M., Welsh D.A. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol. Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabreiro F., Au C., Leung K.Y., Vergara-Irigaray N., Cocheme H.M., Noori T. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153:228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Gothefors L. Transmission of Lactobacillus jensenii and Lactobacillus acidophilus from mother to child at time of delivery. J. Clin. Microbiol. 1975;1:124–128. doi: 10.1128/jcm.1.2.124-128.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Toner B.B., Fukudo S., Guthrie E., Locke G.R., Norton N.J. Gender, age, society, culture, and the patient's perspective in the functional gastrointestinal disorders. Gastroenterology. 2006;130:1435–1446. doi: 10.1053/j.gastro.2005.09.071. [DOI] [PubMed] [Google Scholar]
- Cho I., Yamanishi S., Cox L., Methe B.A., Zavadil J., Li K. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Cilieborg M.S., Boye M., Sangild P.T. Bacterial colonization and gut development in preterm neonates. Early Hum. Dev. 2012;88(1):S41–S49. doi: 10.1016/j.earlhumdev.2011.12.027. [DOI] [PubMed] [Google Scholar]
- Clarke G., Grenham S., Scully P., Fitzgerald P., Moloney R.D., Shanahan F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- Collado M.C., Cernada M., Bauerl C., Vento M., Perez-Martinez G. Microbial ecology and host-microbiota interactions during early life stages. Gut microbes. 2012;3:352–365. doi: 10.4161/gmic.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn A.R., Fell D.I., Steele R.D. Characterization of alpha-keto acid transport across blood-brain barrier in rats. Am. J. Physiol. 1983;245:E253–E260. doi: 10.1152/ajpendo.1983.245.3.E253. [DOI] [PubMed] [Google Scholar]
- Cooperstock M., Riegle L., Woodruff C.W., Onderdonk A. Influence of age, sex, and diet on asymptomatic colonization of infants with Clostridium-Difficile. J. Clin. Microbiol. 1983;17:830–833. doi: 10.1128/jcm.17.5.830-833.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox L.M., Blaser M.J. Pathways in microbe-induced obesity. Cell. Metab. 2013;17:883–894. doi: 10.1016/j.cmet.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox L.M., Yamanishi S., Sohn J., Alekseyenko A.V., Leung J.M., Cho I. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J.F., Dinan T.G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Culhane J.F., Rauh V., McCollum K.F., Hogan V.K., Agnew K., Wadhwa P.D. Maternal stress is associated with bacterial vaginosis in human pregnancy. Maternal Child Health J. 2001;5:127–134. doi: 10.1023/a:1011305300690. [DOI] [PubMed] [Google Scholar]
- de Theije C.G., Wu J., da Silva S.L., Kamphuis P.J., Garssen J., Korte S.M. Pathways underlying the gut-to-brain connection in autism spectrum disorders as future targets for disease management. Eur. J. Pharmacol. 2011;668 (1):S70–S80. doi: 10.1016/j.ejphar.2011.07.013. [DOI] [PubMed] [Google Scholar]
- de Theije C.G., Wopereis H., Ramadan M., van Eijndthoven T., Lambert J., Knol J. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain, Behav. Immun. 2014;37:197–206. doi: 10.1016/j.bbi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- de Theije C.G., Koelink P.J., Korte-Bouws G.A., Lopes da Silva S., Korte S.M., Olivier B. Intestinal inflammation in a murine model of autism spectrum disorders. Brain, Behav. Immun. 2014;37:240–247. doi: 10.1016/j.bbi.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R., Wang S., Anuar F., Qian Y., Bjorkholm B., Samuelsson A. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. U. S. A. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan T.G., Cryan J.F. Regulation of the stress response by the gut microbiota: Implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369–1378. doi: 10.1016/j.psyneuen.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Dorrestein P.C., Mazmanian S.K., Knight R. Finding the missing links among metabolites, microbes, and the host. Immunity. 2014;40:824–832. doi: 10.1016/j.immuni.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrstrom S.M., Kronfeld D., Thuresson J., Rylander E. Signs of chronic stress in women with recurrent candida vulvovaginitis. Am. J. Obstet. Gynecol. 2005;193:1376–1381. doi: 10.1016/j.ajog.2005.03.068. [DOI] [PubMed] [Google Scholar]
- Elenkov I.J., Wilder R.L., Chrousos G.P., Vizi E.S. The sympathetic nerve - an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Fallani M., Young D., Scott J., Norin E., Amarri S., Adam R. Intestinal microbiota of 6-week-old infants across Europe: geographic influence beyond delivery mode, breast-feeding, and antibiotics. J. Pediatr. Gastroenterol. Nutr. 2010;51:77–84. doi: 10.1097/MPG.0b013e3181d1b11e. [DOI] [PubMed] [Google Scholar]
- Forsythe P., Sudo N., Dinan T., Taylor V.H., Bienenstock J. vol. 24. 2010. pp. 9–16. (Mood and Gut Feelings. Brain, Behavior, and Immunity). [DOI] [PubMed] [Google Scholar]
- Funkhouser L.J., Bordenstein S.R. Mom knows best: the universality of maternal microbial transmission. PLoS Biol. 2013;11:e1001631. doi: 10.1371/journal.pbio.1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau M.G., Jury J., MacQueen G., Sherman P.M., Perdue M.H. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007;56:1522–1528. doi: 10.1136/gut.2006.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N., Bale T.L. Sex differences in the serotonergic influence on the hypothalamic-pituitary-adrenal stress axis. Endocrinology. 2010;151:1784–1794. doi: 10.1210/en.2009-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosalbes M.J., Llop S., Valles Y., Moya A., Ballester F., Francino M.P. Meconium microbiota types dominated by lactic acid or enteric bacteria are differentially associated with maternal eczema and respiratory problems in infants. Clin. Exp. Aller. 2013;43:198–211. doi: 10.1111/cea.12063. [DOI] [PubMed] [Google Scholar]
- Gupta K., Stapleton A.E., Hooton T.M., Roberts P.L., Fennell C.L., Stamm W.E. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli colonization in women with recurrent urinary tract infections. J. Infect. Dis. 1998;178:446–450. doi: 10.1086/515635. [DOI] [PubMed] [Google Scholar]
- Hawes S.E., Hillier S.L., Benedetti J., Stevens C.E., Koutsky L.A., WolnerHanssen P. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J. Infect. Dis. 1996;174:1058–1063. doi: 10.1093/infdis/174.5.1058. [DOI] [PubMed] [Google Scholar]
- Higuchi T., Hayashi H., Abe K. Exchange of glutamate and gamma-aminobutyrate in a Lactobacillus strain. J. Bacteriol. 1997;179:3362–3364. doi: 10.1128/jb.179.10.3362-3364.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howerton C.L., Bale T.L. Prenatal programming: at the intersection of maternal stress and immune activation. Hormones Behav. 2012;62:237–242. doi: 10.1016/j.yhbeh.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao E.Y., McBride S.W., Hsien S., Sharon G., Hyde E.R., McCue T. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz P., Goebel B.M., Pace N.R. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J. Bacteriol. 1998;180:4765–4774. doi: 10.1128/jb.180.18.4765-4774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer L.M., Aravind L., Coon S.L., Klein D.C., Koonin E.V. Evolution of cell-cell signaling in animals: did late horizontal gene transfer from bacteria have a role? Trends Genet. TIG. 2004;20:292–299. doi: 10.1016/j.tig.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Jimenez E., Fernandez L., Marin M.L., Martin R., Odriozola J.M., Nueno-Palop C. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr. Microbiol. 2005;51:270–274. doi: 10.1007/s00284-005-0020-3. [DOI] [PubMed] [Google Scholar]
- Karuri A.R., Dobrowsky E., Tannock I.F. Selective cellular acidification and toxicity of weak organic acids in an acidic microenvironment. Br. J. Cancer. 1993;68:1080–1087. doi: 10.1038/bjc.1993.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keski-Nisula L., Kyynarainen H.R., Karkkainen U., Karhukorpi J., Heinonen S., Pekkanen J. Maternal intrapartum antibiotics and decreased vertical transmission of Lactobacillus to neonates during birth. Acta Paediatr. 2013;102:480–485. doi: 10.1111/apa.12186. [DOI] [PubMed] [Google Scholar]
- Koenig J.E., Spor A., Scalfone N., Fricker A.D., Stombaugh J., Knight R. vol. 108 (1) 2011. Succession of Microbial Consortia in the Developing Infant Gut Microbiome; pp. 4578–4585. (Proceedings of the National Academy of Sciences of the United States of America). [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc J.G., Milani C., de Giori G.S., Sesma F., van Sinderen D., Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr. Opin. Biotech. 2013;24:160–168. doi: 10.1016/j.copbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Leser T.D., Amenuvor J.Z., Jensen T.K., Lindecrona R.H., Boye M., Moller K. Culture-independent analysis of gut bacteria: the pig gastrointestinal tract microbiota revisited. Appl. Environ. Microbiol. 2002;68:673–690. doi: 10.1128/AEM.68.2.673-690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhares I.M., Summers P.R., Larsen B., Giraldo P.C., Witkin S.S. Contemporary perspectives on vaginal pH and lactobacilli. Am. J. Obstetrics Gynecol. 2011:204. doi: 10.1016/j.ajog.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lutgendorff F., Akkermans L.M.A., Soderholm J.D. The role of microbiota and probiotics in stress-induced gastrointestinal damage. Curr. Mol. Med. 2008;8:282–298. doi: 10.2174/156652408784533779. [DOI] [PubMed] [Google Scholar]
- Macfabe D.F. Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb. Ecol. Health Dis. 2012:23. doi: 10.3402/mehd.v23i0.19260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie R.I., Sghir A., Gaskins H.R. Developmental microbial ecology of the neonatal gastrointestinal tract. Am. J. Clin. Nut. 1999;69:1035S–1045S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- Mändar R., Mikelsaar M. vol. 69. 1996. pp. 30–35. (Transmission of Mother's Microflora to the Newborn at Birth Biology of the Neonate). [DOI] [PubMed] [Google Scholar]
- Markle J.G., Frank D.N., Mortin-Toth S., Robertson C.E., Feazel L.M., Rolle-Kampczyk U. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- Marques T.M., Wall R., Ross R.P., Fitzgerald G.F., Ryan C.A., Stanton C. Programming infant gut microbiota: influence of dietary and environmental factors. Curr. Opin. Biotechnol. 2010;21:149–156. doi: 10.1016/j.copbio.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Maslowski K.M., Mackay C.R. Diet, gut microbiota and immune responses. Nat. Immunol. 2011;12:5–9. doi: 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]
- Matsumiya Y., Kato N., Watanabe K., Kato H. Molecular epidemiological study of vertical transmission of vaginal Lactobacillus species from mothers to newborn infants in Japanese, by arbitrarily primed polymerase chain reaction. J. Infect. Chemother. Official J. Jpn. Soc. Chemother. 2002;8:43–49. doi: 10.1007/s101560200005. [DOI] [PubMed] [Google Scholar]
- Matsumoto M., Kibe R., Ooga T., Aiba Y., Sawaki E., Koga Y. Cerebral low-molecular metabolites influenced by intestinal microbiota: a pilot study. Front. Syst. Neurosci. 2013;7:9. doi: 10.3389/fnsys.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawdsley J.E., Rampton D.S. The role of psychological stress in inflammatory bowel disease. Neuroimmunomodulation. 2006;13:327–336. doi: 10.1159/000104861. [DOI] [PubMed] [Google Scholar]
- Mijac V.D., Dukic S.V., Opavski N.Z., Dukic M.K., Ranin L.T. Hydrogen peroxide producing lactobacilli in women with vaginal infections. Eur. J. Obstet. Gyn. R. B. 2006;129:69–76. doi: 10.1016/j.ejogrb.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Mikocka-Walus A., Turnbull D., Moulding N., Wilson I., Andrews J.M., Holtmann G. Psychological comorbidity and complexity of gastrointestinal symptoms in clinically diagnosed irritable bowel syndrome patients. J. Gastroenterol. Hepatol. 2008;23:1137–1143. doi: 10.1111/j.1440-1746.2007.05245.x. [DOI] [PubMed] [Google Scholar]
- Ming X., Stein T.P., Barnes V., Rhodes N., Guo L. Metabolic perturbance in autism spectrum disorders: a metabolomics study. J. Proteome Res. 2012;11:5856–5862. doi: 10.1021/pr300910n. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol. Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- Mueller B.R., Bale T.L. Sex-specific programming of offspring emotionality after stress early in pregnancy. J. Neurosci. Official J. Soc. Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton E.R., Wallace P.A. Effects of prophylactic antibiotics on endometrial flora in women with postcesarean endometritis. Obstet. Gynecol. 1998;92:262–268. doi: 10.1016/s0029-7844(98)00164-1. [DOI] [PubMed] [Google Scholar]
- Nicholson J.K., Holmes E., Kinross J., Burcelin R., Gibson G., Jia W. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- Nishino R., Mikami K., Takahashi H., Tomonaga S., Furuse M., Hiramoto T. Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. Neurogastroenterol. Motil. Official J. Eur. Gastrointest. Motil. Soc. 2013;25:521–528. doi: 10.1111/nmo.12110. [DOI] [PubMed] [Google Scholar]
- O'Mahony S.M., Marchesi J.R., Scully P., Codling C., Ceolho A.M., Quigley E.M. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- O'Mahony S.M., Clarke G., Borre Y.E., Dinan T.G., Cryan J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome Axis. Behav. Brain Res. 2014 doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- O'Mahony S.M., Felice V.D., Nally K., Savignac H.M., Claesson M.J., Scully P. Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience. 2014;277:885–901. doi: 10.1016/j.neuroscience.2014.07.054. [DOI] [PubMed] [Google Scholar]
- Panula P., Pirvola U., Auvinen S., Airaksinen M.S. Histamine-immunoreactive nerve fibers in the rat brain. Neuroscience. 1989;28:585–610. doi: 10.1016/0306-4522(89)90007-9. [DOI] [PubMed] [Google Scholar]
- Peinado A., Yuste R., Katz L.C. Extensive dye coupling between rat neocortical neurons during the period of circuit formation. Neuron. 1993;10:103–114. doi: 10.1016/0896-6273(93)90246-n. [DOI] [PubMed] [Google Scholar]
- Pozzilli P., Signore A., Williams A.J., Beales P.E. NOD mouse colonies around the world–recent facts and figures. Immunol. Today. 1993;14:193–196. doi: 10.1016/0167-5699(93)90160-M. [DOI] [PubMed] [Google Scholar]
- Rafiki A., Boulland J.L., Halestrap A.P., Ottersen O.P., Bergersen L. Highly differential expression of the monocarboxylate transporters MCT2 and MCT4 in the developing rat brain. Neuroscience. 2003;122:677–688. doi: 10.1016/j.neuroscience.2003.08.040. [DOI] [PubMed] [Google Scholar]
- Rautava S., Collado M.C., Salminen S., Isolauri E. Probiotics modulate host-microbe interaction in the placenta and fetal gut: a randomized, double-blind, placebo-controlled trial. Neonatology. 2012;102:178–184. doi: 10.1159/000339182. [DOI] [PubMed] [Google Scholar]
- Ravel J., Gajer P., Abdo Z., Schneider G.M., Koenig S.S., McCulle S.L. vol. 108 (1) 2011. Vaginal Microbiome of Reproductive-age Women; pp. 4680–4687. (Proceedings of the National Academy of Sciences of the United States of America). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondolopez V., Cook R.L., Sobel J.D. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev. Infect. Dis. 1990;12:856–872. doi: 10.1093/clinids/12.5.856. [DOI] [PubMed] [Google Scholar]
- Ressler K.J., Nemeroff C.B. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress. Anxiety. 2000;12(1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Ridley E.V., Wong A.C., Westmiller S., Douglas A.E. Impact of the resident microbiota on the nutritional phenotype of Drosophila melanogaster. PloS one. 2012;7:e36765. doi: 10.1371/journal.pone.0036765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R.P., Mills S., Hill C., Fitzgerald G.F., Stanton C. Specific metabolite production by gut microbiota as a basis for probiotic function. Int. Dairy J. 2010;20:269–276. [Google Scholar]
- Round J.L., Mazmanian S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouphael N.G., O'Donnell J.A., Bhatnagar J., Lewis F., Polgreen P.M., Beekmann S. Clostridium difficile-associated diarrhea: an emerging threat to pregnant women. Am. J. Obstet. Gynecol. 2008:198. doi: 10.1016/j.ajog.2008.01.062. [DOI] [PubMed] [Google Scholar]
- Rousseau C., Levenez F., Fouqueray C., Dore J., Collignon A., Lepage P. Clostridium difficile colonization in early infancy is accompanied by changes in intestinal microbiota composition. J. Clin. Microbiol. 2011;49:858–865. doi: 10.1128/JCM.01507-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen S., Gibson G.R., McCartney A.L., Isolauri E. Influence of mode of delivery on gut microbiota composition in seven year old children. Gut. 2004;53:1388–1389. doi: 10.1136/gut.2004.041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J.C., Lampart C., Rose C. Histamine formation in rat brain in vivo: effects of histidine loads. J. Neurochem. 1972;19:801–810. doi: 10.1111/j.1471-4159.1972.tb01394.x. [DOI] [PubMed] [Google Scholar]
- Sharon G., Segal D., Ringo J.M., Hefetz A., Zilber-Rosenberg I., Rosenberg E. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20051–20056. doi: 10.1073/pnas.1009906107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S.C., Kim S.H., You H., Kim B., Kim A.C., Lee K.A. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science. 2011;334:670–674. doi: 10.1126/science.1212782. [DOI] [PubMed] [Google Scholar]
- Soper D.E. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J. Womens Health. 1997;6:590. doi: 10.1093/infdis/174.5.1058. [DOI] [PubMed] [Google Scholar]
- Steel J.H., Malatos S., Kennea N., Edwards A.D., Miles L., Duggan P. Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr. Res. 2005;57:404–411. doi: 10.1203/01.PDR.0000153869.96337.90. [DOI] [PubMed] [Google Scholar]
- Stilling R.M., Dinan T.G., Cryan J.F. Microbial genes, brain & behaviour – epigenetic regulation of the gut-brain axis. Genes, Brain, Behav. 2014;13:69–86. doi: 10.1111/gbb.12109. [DOI] [PubMed] [Google Scholar]
- Storelli G., Defaye A., Erkosar B., Hols P., Royet J., Leulier F. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell. Metab. 2011;14:403–414. doi: 10.1016/j.cmet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Stray-Pederson B., Bergan T., Hafstad A., Normann E., Grogaard J., Vangdal M. Vaginal disinfection with chlorhexidine during childbirth. Int. J. Antimicrob. Ag. 1999;12:245–251. doi: 10.1016/s0924-8579(99)00068-0. [DOI] [PubMed] [Google Scholar]
- Sudo N., Chida Y., Aiba Y., Sonoda J., Oyama N., Yu X.N. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Kobayashi T., Songjinda P., Tateyama A., Tsubouchi M., Kiyohara C. Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol. Med. Microbiol. 2009;56:80–87. doi: 10.1111/j.1574-695X.2009.00553.x. [DOI] [PubMed] [Google Scholar]
- Tannock G.W., Savage D.C. Influences of dietary and environmental stress on microbial-populations in murine gastrointestinal-tract. Infect. Immun. 1974;9:591–598. doi: 10.1128/iai.9.3.591-598.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G.W., Fuller R., Smith S.L., Hall M.A. Plasmid profiling of members of the family Enterobacteriaceae, lactobacilli, and bifidobacteria to study the transmission of bacteria from mother to infant. J. Clin. Microbiol. 1990;28:1225–1228. doi: 10.1128/jcm.28.6.1225-1228.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K.M., Snyder S.H. Histamine in rat brain: sensitive assay of endogenous levels, formation in vivo and lowering by inhibitors of histidine decarboxylase. J. Pharmacol. Exp. Ther. 1971;179:619–633. [PubMed] [Google Scholar]
- Thomas C.M., Hong T., van Pijkeren J.P., Hemarajata P., Trinh D.V., Hu W. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PloS one. 2012;7:e31951. doi: 10.1371/journal.pone.0031951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissier H. University of Paris; Paris, France: 1900. Recherches sur la flore intestinale des nourrissons: état normal et pathologique. [Google Scholar]
- Tomas M.S.J., Bru E., Nader-Macias M.E. Comparison of the growth and hydrogen peroxide production by vaginal probiotic lactobacilli under different culture conditions. Am. J. Obstet. Gynecol. 2003;188:35–44. doi: 10.1067/mob.2003.123. [DOI] [PubMed] [Google Scholar]
- Trasande L., Blustein J., Liu M., Corwin E., Cox L.M., Blaser M.J. Infant antibiotic exposures and early-life body mass. Int. J. Obes. 2013;37:16–23. doi: 10.1038/ijo.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowsdale J., Betz A.G. Mother's little helpers: mechanisms of maternal-fetal tolerance. Nat. Immunol. 2006;7:241–246. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- Turnbaugh P.J., Ridaura V.K., Faith J.J., Rey F.E., Knight R., Gordon J.I. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009;1:6–14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallor A.C., Antonio M.A., Hawes S.E., Hillier S.L. Factors associated with acquisition of, or persistent colonization by, vaginal lactobacilli: role of hydrogen peroxide production. J. Infect. Dis. 2001;184:1431–1436. doi: 10.1086/324445. [DOI] [PubMed] [Google Scholar]
- Wadhwa P.D., Culhane J.F., Rauh V., Barve S.S., Hogan V., Sandman C.A. Stress, infection and preterm birth: a biobehavioural perspective. Paediatr. Perinat. Ep. 2001;15:17–29. doi: 10.1046/j.1365-3016.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- Walker C.D., Perrin M., Vale W., Rivier C. Ontogeny of the stress response in the rat: role of the pituitary and the hypothalamus. Endocrinology. 1986;118:1445–1451. doi: 10.1210/endo-118-4-1445. [DOI] [PubMed] [Google Scholar]
- Wang Y., Kasper L.H. The role of microbiome in central nervous system disorders. Brain, Behav. Immun. 2014;38:1–12. doi: 10.1016/j.bbi.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikoff W.R., Anfora A.T., Liu J., Schultz P.G., Lesley S.A., Peters E.C. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B.L., Hornig M., Buie T., Bauman M.L., Cho Paik M., Wick I. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PloS one. 2011;6:e24585. doi: 10.1371/journal.pone.0024585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin S.S., Linhares I.M., Giraldo P., Ledger W.J. An altered immunity hypothesis for the development of symptomatic bacterial vaginosis. Clin. Infect. Dis. Official Publ. Infect. Dis. Soc. Am. 2007;44:554–557. doi: 10.1086/511045. [DOI] [PubMed] [Google Scholar]
- Wopereis H., Oozeer R., Knipping K., Belzer C., Knol J. The first thousand days – intestinal microbiology of early life: establishing a symbiosis. Pediatr. allergy Immunol. Official Publ. Eur. Soc. Pediatr. Allergy Immunol. 2014;25:428–438. doi: 10.1111/pai.12232. [DOI] [PubMed] [Google Scholar]
- Yurkovetskiy L., Burrows M., Khan A.A., Graham L., Volchkov P., Becker L. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39:400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbo O., Qian Y., Yoshida C., Grether J.K., Van de Water J., Croen L.A. Maternal infection during pregnancy and autism spectrum disorders. J. Autism Dev. Disord. 2013 doi: 10.1007/s10803-013-2016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk M., Stoehr A.M. Immune defense and host life history. Am. Nat. 2002;160(4):S9–S22. doi: 10.1086/342131. [DOI] [PubMed] [Google Scholar]