Abstract
Genes and environment interact to influence cognitive and emotional functions throughout life. Early-life experiences in particular contribute to vulnerability or resilience to a number of emotional and cognitive illnesses in humans. In rodents, early-life experiences directly lead to resilience or vulnerability to stress later in life, and influence the development of cognitive and emotional deficits. The mechanisms for the enduring effects of early-life experiences on cognitive and emotional outcomes are not completely understood. Here, we present emerging information supporting experience-dependent modulation of the number and efficacy of synaptic inputs onto stress-sensitive neurons. This synaptic ‘rewiring’, in turn, may influence the expression of crucial neuronal genes. The persistent changes in gene expression in resilient versus vulnerable rodent models are likely maintained via epigenetic mechanisms. Thus, early-life experience may generate resilience by altering synaptic input to neurons, which informs them to modulate their epigenetic machinery.
Keywords: Synaptic plasticity, Resilience, Stress, Corticotropin releasing hormone (CRH), Maternal care, Epigenetics
1. Introduction
Resilience is defined as an active and adaptive biological, psychological, and social response to an event that may otherwise impair one's normal function (McEwen, 2007, Dudley et al., 2011, Russo et al., 2012). Resilience typically implies the presence of insult-related pathologies that are overcome by molecular, cellular, synaptic, and finally behavioral changes that enable coping and normal function.
Much has been written about the origins of resilience (Barker, 1989, Yehuda et al., 2006, Gluckman et al., 2007, Feder et al., 2009, Russo et al., 2012). There is clear evidence that resilience and vulnerability are influenced by genetic factors (Caspi et al., 2003, Binder et al., 2008) and gene-environment interactions (Caspi et al., 2003, Bale et al., 2010, Dincheva et al., 2014). In addition, a large body of work has supported strong correlations of early-life experience/environment and resilience to cognitive and emotional illnesses later in life (Schmidt et al., 2011, Baram et al., 2012, Lucassen et al., 2013, Huang, 2014, Insel, 2014, Santarelli et al., 2014). Several theories have been put forth that strongly suggest a causal and adaptive relationship between early-life experience and lifetime vulnerability or resilience to disease (Barker, 1989, McEwen, 2000, Gluckman et al., 2007, Baram et al., 2012, Sandman et al., 2012).
Whereas human studies produce associations which can strongly suggest a causal relationship between early-life experience and vulnerability or resilience to disease, direct manipulations of early-life experience in animal models have been shown to lead to persistent changes in aspects of brain function, including resilience to subsequent insults such as stress. Indeed, a large number of primate and rodent models have been created to directly manipulate early-life experience, in order to generate resilience or vulnerability (see Maras and Baram, 2012, Huang, 2014 for recent reviews). Broadly categorized, these paradigms aim to model early-life adversity such as chronic stress (Schmidt et al., 2011, Molet et al., 2014), or to create a nurturing early-life environment, typically based on optimized maternal care or novelty (see Akers et al., 2008, Champagne et al., 2008, Korosi and Baram, 2009, Baram et al., 2012, Tang et al., 2014). Indeed, rodents raised in these distinct environments generally develop vulnerability (Huot et al., 2002, Romeo et al., 2004, Brunson et al., 2005, Champagne et al., 2008, van Hasselt et al., 2012) or resilience (Liu et al., 1997, Fenoglio et al., 2005, van Hasselt et al., 2012) to future stress and to cognitive and/or emotional deficits.
Although the influence of early-life experience on life-time resilience and vulnerability are well established, the underlying mechanisms are not fully understood. It is now generally agreed that enduring changes in the expression of important genes might be involved, and that these changes might persist via epigenetic mechanisms including histone and DNA modifications (Meaney and Szyf, 2005, Borrelli et al., 2008, Roth et al., 2009, McClelland et al., 2011, Sun et al., 2013, Morrison et al., 2014). However, fundamental and crucial questions remain unanswered. For examples, what is the essence of the experience or environmental-signal that is perceived by the developing brain? How does the signal reach important neurons that change in response to the early-life experience? What are these neurons that are re-programmed to enable the structural and functional plasticity that underlies resilience? How do these neurons know to modulate their epigenetic machinery?
We attempt to address these questions here.
2. Early-life experience, maternal signals, and brain programming
As mentioned above, direct manipulation of maternal care patterns has yielded long-lasting resilience or vulnerability to cognitive and emotional deficits. We briefly describe the frameworks for bi-directional manipulation of maternal signals to young rodents that have been employed by our group, because the robust outcomes enable examination of the underlying mechanisms.
2.1. Controlled manipulation to augment maternal care
The handling paradigm (Levine, 1957, Plotsky and Meaney, 1993, Avishai-Eliner et al., 2001a), which involves brief (15 min) daily separation of rat pups from the mother during the first weeks of life, was used as a model of enhanced maternal care. These brief separations promoted increased maternal-derived sensory input upon reunion with their mothers (Fig. 1) (Liu et al., 1997, Fenoglio et al., 2006). This paradigm led to increased resilience to depressive-like behavior (Meaney et al., 1991) and improved learning and memory (Liu et al., 2000, Fenoglio et al., 2005).
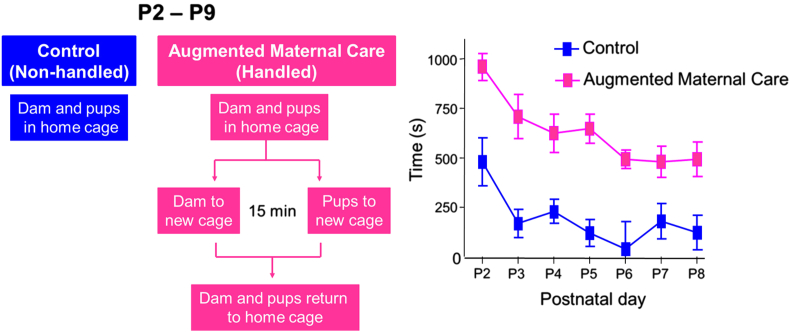
Fig. 1.
Brief daily separations of rat pups from their mother lead to increased sensory input from the mother to the pups upon their reunion. A. A schematic of the handling paradigm: during postnatal day 2–9, the mother and the pups were separated for 15 min in different cages, and then reunited in the home cage. Control mother and pups remained in the home cage. B. Maternal sensory stimulation of the pups, specifically licking and grooming, was observed and quantified daily during the 30 min after the mothers and the pups were returned to home cages (n = 6 mothers per group). Adapted from Fenoglio et al. (2006) with permission.
2.2. Controlled manipulation to disrupt maternal care
Commonly, early-life stress is generated by maternal separation (MS), a manipulation believed to be stressful. Extended absence of the mother provokes hypothermia and starvation, so many models use intermittent maternal deprivation and hence intermittent stress. In the human condition, when infants and children grow up in famine, war, or in the presence of drug-abusing mothers, the stress is typically chronic rather than intermittent, and the mother is typically present. Maternal care behaviors during these conditions might be the source of stress in the infant (Whipple and Webster-Stratton, 1991, Koenen et al., 2003, Kendall-Tackett, 2007, Baram et al., 2012), as is particularly well documented in neglect/abuse situations, where maternal care is unpredictable and fragmented (Whipple and Webster-Stratton, 1991, Gaudin et al., 1996).
Aiming to recapitulate the human condition, we generated a model of chronic early-life stress (CES) where the mother is continuously present. The paradigm involves limiting the bedding and nesting material in the cage (for a detailed review, see Molet et al., 2014). This impoverished cage environment resulted in abnormal maternal care, i.e., fragmented maternal-derived sensory input to the pups. The latter, as reported in humans, provoked chronic uncontrollable early-life “emotional stress” (Gilles et al., 1996, Avishai-Eliner et al., 2001b, Ivy et al., 2008, Baram et al., 2012). There was minimal change in the overall duration of maternal care or of specific aspects of care (licking and grooming, nursing, etc) (Ivy et al., 2008). However, in both mice and rats, maternal care was fragmented and unpredictable: each bout of behavior is shorter and the sequence of nurturing behaviors is unpredictable (Rice et al., 2008, Baram et al., 2012). In some cases, especially when cage environment was altered later in the development of the pups (postnatal days 3–8 and 8–12 rather than 2–9), rough handling of the pups by the mother was noted (Moriceau et al., 2009, Raineki et al., 2010, Raineki et al., 2012). The CES model of aberrant maternal care and early-life experience led to emotional and cognitive vulnerabilities, and eventually overt pathology, including early cognitive aging (for a detailed review, see Molet et al., 2014). For example, Raineki et al., found depressive-like symptoms measured as increased immobility time in the forced swim test (FST) in adolescent rats that experienced CES. When tested during adolescence and young adulthood using paradigms such as novelty induced hypophagia, open-field, and elevated plus maze, rodents stressed early in life showed anxiety-like behaviors (Wang et al., 2012; Dalle Molle et al., 2012, Malter Cohen et al., 2013). Finally, memory deficits were unveiled by the Morris water maze and novel object recognition tasks in adult rats and mice that were exposed to fragmented maternal care using the CES model (Brunson et al., 2005, Rice et al., 2008; Ivy et al., 2010, Wang et al., 2011).
The ability to manipulate early-life experience in both adverse and salubrious directions provides powerful frameworks for examining the mechanisms for the resulting vulnerability and resilience.
3. What are the neurons that are re-programmed to enable the structural and functional plasticity that underlies resilience?
A significant body of work has established a molecular signature of the resilience or vulnerability phenotypes generated by early-life experience in rodents. In adult rats experiencing augmented maternal care, an enduring upregulation of glucocorticoid receptor (GR) expression in hippocampus, and a repression of corticotropin releasing hormone (CRH) expression in hypothalamic paraventricular (PVN) neurons was reported (Plotsky and Meaney, 1993, Avishai-Eliner et al., 2001a). The epigenetic basis of the enduring enhancement of hippocampal GR expression was uncovered by pioneering studies by the Meaney group (Weaver et al., 2004). Examination of the temporal evolution of the molecular signature of rats experiencing augmented maternal care revealed that repression of CRH expression in hypothalamus preceded the increased GR expression in hippocampus, and was directly dependent on recurrent predictable barrages of maternal care (Avishai-Eliner et al., 2001a, Fenoglio et al., 2006). These data suggested that the CRH neuron in the hypothalamus may be an early locus of maternal care-induced brain programming.
Notably, it is unlikely that changes in CRH or GR expression in themselves explain the remarkable resilient phenotype of rats experiencing augmented maternal care early in life. Whereas the GR and CRH are likely important mediators of long-lasting effects of maternal care, they may also serve as marker genes, a tool to study mechanisms of broad, enduring gene expression changes. In addition, determining the locations of the changes in gene and protein expression helps to identify specific ‘target neurons’ that are re-programmed to enable the structural and functional plasticity that underlies resilience.
4. How do the CRH neurons in the hypothalamus ‘know’ to modulate their epigenetic machinery?
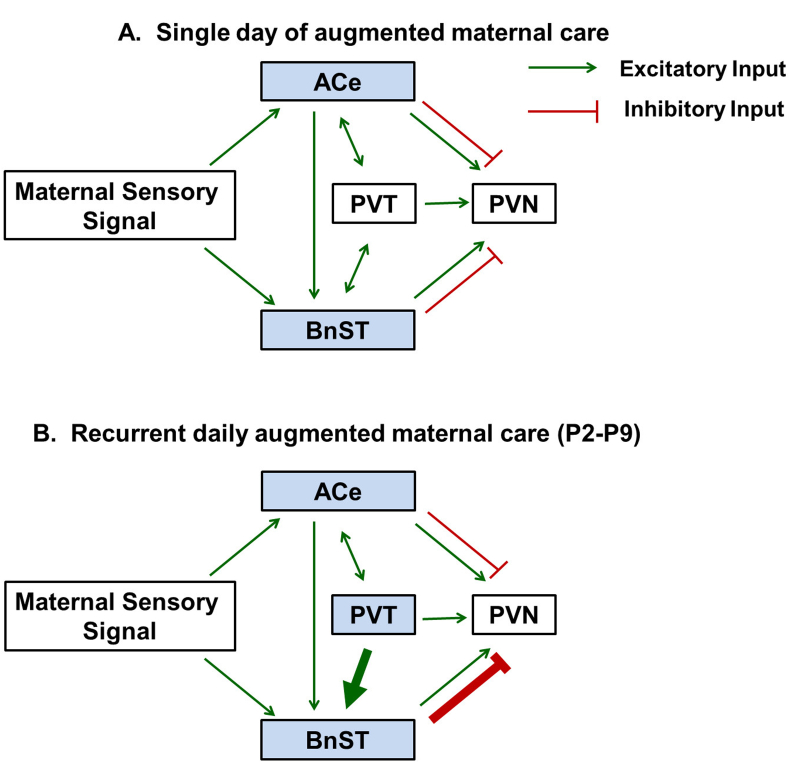
As mentioned above, the repression of gene expression in CRH neurons occurred early and was already present after a week of ‘handling’, i.e., on postnatal day 9 in the pups (Avishai-Eliner et al., 2001a, Fenoglio et al., 2006, Korosi et al., 2010). In addition, the CRH-expressing neurons in the hypothalamus were identified as a component of a neuronal network activated by maternal care (Fenoglio et al., 2006). The latter finding emerged from Fos-labeling and mapping studies that queried which neurons were activated at several time points after returning of pups to their mothers following brief (15 min) separations. The Fos mapping studies demonstrated that the maternal signal traveled via the central nucleus of the amygdala (ACe) and bed nucleus of the stria terminalis (BnST) to the hypothalamic PVN (Fenoglio et al., 2006). Interestingly, while single augmented maternal care activated these structures and did not result in repressed CRH expression, a week of recurrent daily barrages of maternal care activated also the ‘stress-memory’ center of the brain, the paraventricular nucleus of the thalamus (PVT) (Bhatnagar and Dallman, 1998, Fenoglio et al., 2006). The combinatorial output of the signal to the hypothalamic CRH cells emerging from activation of PVT, ACe, and BnST of recurrently handled pups differed from that of single-handled pups, and resulted in robust and enduring suppression of CRH gene expression in these neurons (Fig. 2) (Fenoglio et al., 2006, Karsten and Baram, 2013). This reduction in CRH expression in hypothalamic PVN, together with the apparent network changes involving this neuronal population, led us to focus on the CRH-expressing cells in the PVN as important mediators of molecular changes associated with resilience.
Fig. 2.
Proposed network changes to the corticotropin releasing hormone –expressing, stress-sensitive hypothalamic neurons following single or recurrent episode of augmented maternal care. The paraventricular nucleus of the hypothalamus (PVN) receives excitatory (in green) and inhibitory (in red) synaptic inputs from the central nucleus of the amygdala (ACe) and bed nucleus of the stria terminalis (BnST), and excitatory projections from the paraventricular nucleus of the thalamus (PVT). Excitatory bidirectional afferents exist between the PVT to both ACe and BnST. A. ACe and BnST (but not PVT) are activated by a single episode of augmented maternal care. B. Activation of PVT requires recurrent daily augmented maternal care. This is thought to lead to increased activation of the areas of BnST that send inhibitory synaptic input onto the CRH neurons in the PVN. Adapted from Karsten and Baram (2013) with permission. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Neurons receive information mainly by synaptic contact, so that altered excitatory and/or inhibitory synaptic input onto CRH neurons as a result of maternal care might be a plausible mechanism for the alteration of molecular machinery in these neurons that enduringly reduces CRH expression. Synaptic innervation of neurons is now known to be dynamic and modulated by experience (Brunson et al., 2001, Verkuyl et al., 2004, Horvath, 2005). For CRH neurons, the majority of input is mediated by GABAergic and glutamatergic synapses (Aubry et al., 1996, Boudaba et al., 1997, Cullinan, 2000, Miklos and Kovacs, 2002, Ziegler et al., 2012), via GABAA (Cullinan, 2000) and glutamate receptors (Aubry et al., 1996, Kiss et al., 1996, Cullinan, 2000, Di et al., 2003, Ulrich-Lai et al., 2011, Ziegler et al., 2012). Combining electrophysiology, quantitative analyses of vesicular transporters and quantitative confocal and electron microscopy, Korosi et al., studied if enhanced early-life experience reduced excitation to CRH neurons or augmented their inhibition (Korosi et al., 2010). Using similar methodologies, Gunn et al., examined the excitatory and inhibitory input onto CRH-expressing hypothalamic neurons of mice experiencing aberrant, fragmented maternal care in cages with limited bedding and nesting material (Gunn et al., 2013).
5. Early-life experience leads to synaptic ‘rewiring’ of hypothalamic CRH neurons involved in resilience
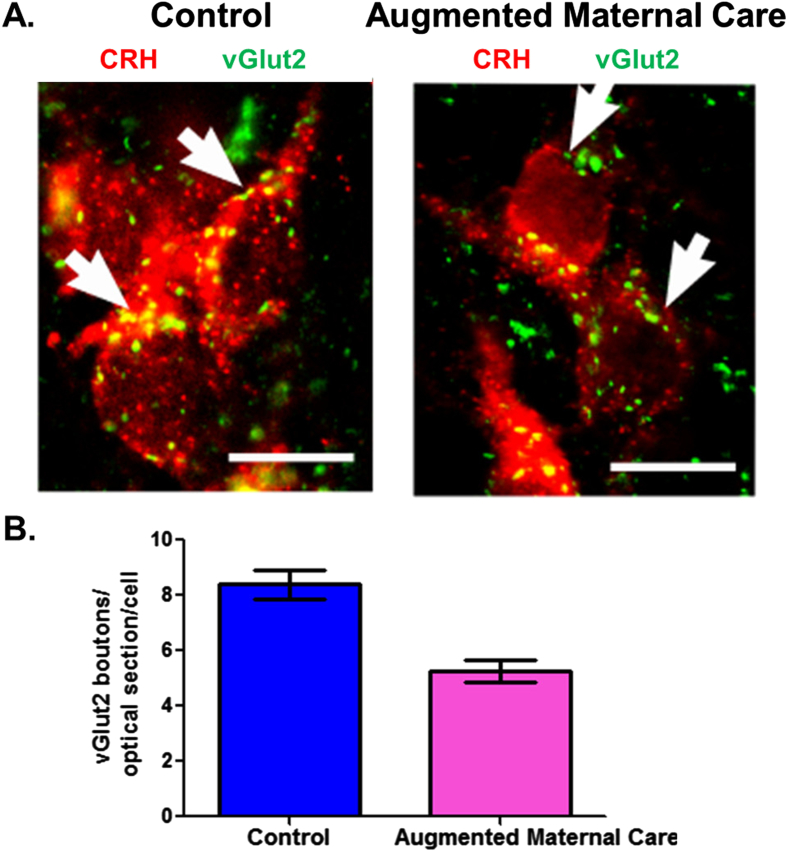
Using several different methods, Korosi et al., discovered reduced number and function of excitatory synapse that abut onto CRH-expressing neurons in pups experiencing a week of recurrent augmented maternal care (Korosi et al., 2010). While enhanced maternal care resulted in reduced levels of the glutamatergic transporter vGlut2 via Western blot, no change in the levels of the GABA-A transporter vGAT was detected. Dual-label confocal microscopy revealed a reduced number of vGlut2-positive puncta (presynaptic terminals) abutting identified CRH neurons (Fig. 3). Quantitative electron microscopy revealed reduced number of asymmetric (excitatory) synapses onto CRH neurons in pups experiencing augmented maternal care. Finally, the frequency of miniature post synaptic excitatory currents (EPSCs), a functional measure of excitatory synapses, was reduced in presumed CRH cells of rats experiencing recurrent increased maternal care (Korosi et al., 2010).
Fig. 3.
Fewer excitatory (vGlut2-positive) boutons contact CRH-ir neurons in parvocellular PVN of pups that experienced augmented maternal care as compared to the controls. A. Merged confocal microscope images of sections at the level of the paraventricular nucleus (PVN) of the hypothalamus double labeled for CRH (red) and vGlut2 (green) in P9 rats that had either control or augmented early-life experience. B. Quantification of the vGlut2-positive boutons contacting CRH-ir soma shows a 36% reduction in the vGlut-2 abutting CRH neurons in pups that experienced enhanced maternal care (5.4 ± 0.9) as compared with controls (8.4 ± 0.9). Scale bars, 10 μm. Adapted from Korosi et al. (2010) with permission. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
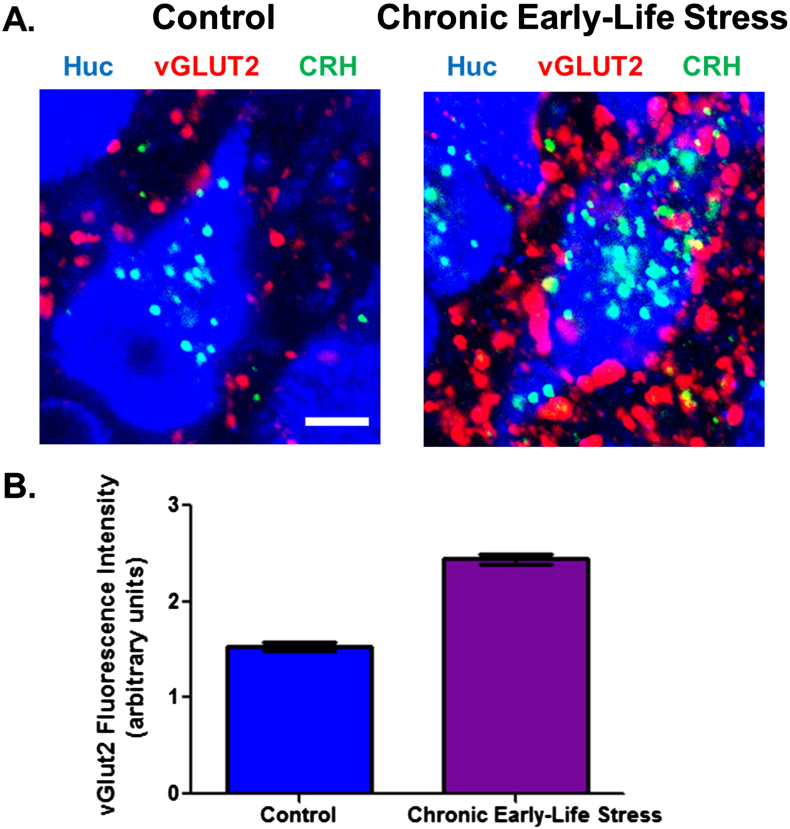
Obviously, if ‘optimal’ early-life experience and specifically maternal signals reduce excitatory synapses, then aberrant maternal care should increase excitatory synapses onto CRH neurons. Indeed, a recent study by Gunn et al. (2013) found that mice experiencing the limited bedding and nesting cage environment, which provokes fragmented maternal care and chronic stress, had increased levels of CRH expression in the PVN (Gunn et al., 2013). Remarkably, immunohistochemical and electrophysiological approaches demonstrated a robust increase in excitatory input onto the stress-sensitive CRH-expressing neurons, in direct contrast to the observation following enhanced early-life experience (Fig. 4).
Fig. 4.
Increased CRH levels and greater glutamatergic output (more vGlut2 positive boutons) onto CRH-expressing neurons in the PVN of rats that experienced early-life stress as compared to controls. Merged confocal microscope images of sections at the level of the paraventricular nucleus of the hypothalamus (PVN) double labeled for CRH (green) and vGlut2 (red) in P22–P28 animals exposed to chronic early-life stress as compared with controls. B. Quantification of fluorescence intensity of vGlut2 in the PVN reveals a 60% increase in vGlut2 expression in early-life stressed animals (1.53 ± 0.08) relative to controls (2.44 ± 0.09). Scale bars, 100 μm. Adapted from Gunn et al. (2013) with permission. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Together, these findings support the idea that early-life experience influences resilience via tuning of the level of excitatory input into stress-sensitive neuronal populations, which in turn affects intracellular programs. Notably, at least in the case of optimal early-life experience, the synaptic changes were transient. Hence, they likely serve as a trigger of neurons to ‘turn on’ or ‘tweak’ gene expression regulatory pathways and epigenetic mechanisms that maintain the expression changes enduringly. Whereas we do not understand how the transient synaptic changes modulate downstream intracellular signaling, we propose that the decrease in the excitatory drive onto the CRH neurons following augmented maternal care leads to reduced calcium influx into the CRH cells, which can potentially initiate transcriptional programs, resulting in decreased CRH expression. Once initiated, the transcriptional changes may then be stably maintained via epigenetic mechanisms (McClelland et al., 2011, Karsten and Baram, 2013).
6. Summary and current questions
Early-life experience interacts with genetic factors to shape cognitive and emotional outcomes. Specifically, early-life experiences influence resilience or vulnerability to emotional and cognitive illnesses. Salient ‘signals’ by which early-life experiences program the brain include recurrent sensory inputs from the mother. Fragmentation and unpredictability of maternal-derived signals might promote vulnerability to mental illness, whereas consistency and predictability might promote resilience. The salient signal from the early-life environment is transported to stress-sensitive neurons via neuronal networks, and it modulates the numbers and function of synapses impinging on these neurons. Optimal early-life experience seems to reduce excitation to CRH-expressing hypothalamic neurons whereas chronic early-life stress and fragmented maternal care increases excitation onto these same neurons. The plasticity of synaptic wiring of crucial stress-responsive neurons likely initiate gene expression programs within these neurons, and the resulting changes of gene expression may persist-perhaps to subsequent generations-via epigenetic mechanisms.
Acknowledgments
This work was supported by the National Institute of Health grants NS28912, MH73136, and P50 MH096889. We thank Barbara Cartwright for editorial help.
References
- Akers K.G., Yang Z., DelVecchio D.P., Reeb B.C., Romeo R.D., McEwen B.S. Social competitiveness and plasticity of neuroendocrine function in old age: influence of neonatal novelty exposure and maternal care reliability. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002840. e2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry J.M., Bartanusz V., Pagliusi S., Schulz P., Kiss J.Z. Expression ofionotropic glutamate receptor subunit mRNAs by paraventricular corticotropinreleasingfactor (CRF) neurons. Neurosci. Lett. 1996;205:95–98. doi: 10.1016/0304-3940(96)12380-6. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S., Eghbal-Ahmadi M., Tabachnik E., Brunson K.L., Baram T.Z. Down-regulation of hypothalamic corticotropin-releasing hormone messenger ribonucleic acid (mRNA) precedes early-life experience-induced changes in hippocampal glucocorticoid receptor mRNA. Endocrinology. 2001;142:89–97. doi: 10.1210/endo.142.1.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S., Gilles E.E., Eghbal-Ahmadi Y., Bar-El Y., Baram T. Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. J. Neuroendocrinol. 2001;13:799–807. doi: 10.1046/j.1365-2826.2001.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale T.L., Baram T.Z., Brown A.S., Goldstein J.M., Insel T.R., McCarthy M.M. Early life programming and neurodevelopmental disorders. Biol. Psychiatry. 2010;68:314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram T.Z., Davis E.P., Obenaus A., Sandman C.A., Small S.L., Solodkin A. Fragmentation and unpredictability of early-life experience in mental disorders. Am. J. Psychiatry. 2012;169:907–915. doi: 10.1176/appi.ajp.2012.11091347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D.J. Rise and fall of Western diseases. Nature. 1989;338:371–372. doi: 10.1038/338371a0. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S., Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Binder E.B., Bradley R.G., Liu W., Epstein M.P., Deveau T.C., Mercer K.B. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli E., Nestler E.J., Allis C.D., Sassone-Corsi P. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60:961–974. doi: 10.1016/j.neuron.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudaba C., Schrader L.A., Tasker J.G. Physiological evidence for local excitatory synaptic circuits in the rat hypothalamus. J. Neurophysiol. 1997;77:3396–3400. doi: 10.1152/jn.1997.77.6.3396. [DOI] [PubMed] [Google Scholar]
- Brunson K.L., Avishai-Eliner S., Hatalski C.G., Baram T.Z. Neurobiology of the stress response early in life: evolution of a concept and the role of corticotropin releasing hormone. Mol. Psychiatr. 2001;6:647–656. doi: 10.1038/sj.mp.4000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson K.L., Kramár E., Lin B., Chen Y., Colgin L.L., Yanagihara T.L. Mechanisms of late-onset cognitive decline after early life stress. J. Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A., Sugden K., Moffitt T.E., Taylor A., Craig I.W., Harrington H. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Champagne D.L., Bagot R.C., van Hasselt F., Ramakers G., Meaney M.J., de Kloet E.R. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J. Neurosci. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan W.E. GABA(A) receptor subunit expression within hypophysiotropic CRH neurons: a dual hybridization histochemical study. J. Comp. Neurol. 2000;419:344–351. doi: 10.1002/(sici)1096-9861(20000410)419:3<344::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Dalle Molle R., Portella A.K., Goldani M.Z., Kapczinski F.P., Leistner-Segal S., Salum G.A., Manfro G.G., Silveira P.P. Associations between parenting behavior and anxiety in a rodent model and a clinical sample: relationship to peripheral BDNF levels. Transl. Psychiatry. 2012;2:e195. doi: 10.1038/tp.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S., Malcher-Lopes R., Halmos K.C., Tasker J.G. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J. Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dincheva I., Pattwell S.S., Tessarollo L., Bath K.G., Lee F.S. BDNF modulates contextual fear learning during adolescence. Dev. Neurosci. 2014;36:269–276. doi: 10.1159/000358824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley K.J., Li X., Kobor M.S., Kippin T.E., Bredy T.W. Epigenetic mechanisms mediating vulnerability and resilience to psychiatric disorders. Neurosci. Biobehav. Rev. 2011;35:1544–1551. doi: 10.1016/j.neubiorev.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Feder A., Nestler E.J., Charney D.S. Psychobiology and molecular genetics of resilience. Nat. Rev. Neurosci. 2009;2009(10):446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio K.A., Brunson K.L., Baram T.Z. Hippocampal neuroplasticity induced by early-life stress: functional and molecular aspects. Front. Neuroendocrinol. 2006;27:180–192. doi: 10.1016/j.yfrne.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio K.A., Brunson K.L., Avishai-Eliner S., Stone B.A., Kapadia B.J., Baram T.Z. Enduring, handling-evoked enhancement of hippocampal memory function and glucocorticoid receptor expression involves activation of the corticotropin-releasing factor type 1 receptor. Endocrinology. 2005;146:4090–4096. doi: 10.1210/en.2004-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudin J.M., Jr., Polansky N.A., Kilpatrick A.C., Shilton P. Family functioning in neglectful families. Child Abuse Negl. 1996;20:363–377. doi: 10.1016/0145-2134(96)00005-1. [DOI] [PubMed] [Google Scholar]
- Gilles E.E., Schultz L., Baram T.Z. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr. Neurol. 1996;15:114–119. doi: 10.1016/0887-8994(96)00153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman P.D., Hanson M.A., Beedle A.S. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am. J. Hum. Biol. 2007;19:1–19. doi: 10.1002/ajhb.20590. [DOI] [PubMed] [Google Scholar]
- Gunn B.G., Cunningham L., Cooper M.A., Corteen N.L., Seifi M., Swinny J.D. Dysfunctional astrocytic and synaptic regulation of hypothalamic glutamatergic transmission in a mouse model of early-life adversity: relevance to neurosteroids and programming of the stress response. J. Neurosci. 2013;33:19534–19554. doi: 10.1523/JNEUROSCI.1337-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath T.L. The hardship of obesity: a soft-wired hypothalamus. Nat. Neurosci. 2005;8:561–565. doi: 10.1038/nn1453. [DOI] [PubMed] [Google Scholar]
- Huang L.T. Early-life stress impacts the developing hippocampus and primes seizure occurrence: cellular, molecular, and epigenetic mechanisms. Front. Mol. Neurosci. 2014;7:8. doi: 10.3389/fnmol.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot R.L., Plotsky P.M., Lenox R.H., McNamara R.K. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002;950:52–63. doi: 10.1016/s0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- Insel T.R. Mental disorders in childhood: shifting the focus from behavioral symptoms to neurodevelopmental trajectories. JAMA. 2014;311:1727–1728. doi: 10.1001/jama.2014.1193. [DOI] [PubMed] [Google Scholar]
- Ivy A.S., Brunson K.L., Sandman C., Baram T.Z. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154:1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy A., Rex C., Chen Y., Dubé C., Maras P., Grigoriadis D., Gall C., Lynch G., Baram T.Z. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J. Neurosci. 2010;30:13005–13015. doi: 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten C.A., Baram T.Z. How does a neuron “know” to modulate its epigenetic machinery in response to early-life environment/experience? Front. Psychiatry. 2013;4:89. doi: 10.3389/fpsyt.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall-Tackett K.A. Violence against women and the perinatal period: the impact of lifetime violence and abuse on pregnancy, postpartum, and breastfeeding. Trauma Violence Abuse. 2007;8:344–353. doi: 10.1177/1524838007304406. [DOI] [PubMed] [Google Scholar]
- Kiss J., Gorc T.J., Kuhn R., Knopfel T., Csaky A., Halasz B. Distribution of metabotropic glutamate receptor 1a in the rat hypothalamus: an immunocytochemical study using monoclonal and polyclonal antibody. Acta Biol. Hung. 1996;47:221–237. [PubMed] [Google Scholar]
- Koenen K.C., Moffitt T.E., Caspi A., Taylor A., Purcell S. Domestic violence is associated with environmental suppression of IQ in young children. Dev. Psychopathol. 2003;15:297–311. doi: 10.1017/s0954579403000166. [DOI] [PubMed] [Google Scholar]
- Korosi A., Baram T.Z. The pathways from mother's love to baby's future. Front. Behav. Neurosci. 2009;3:27. doi: 10.3389/neuro.08.027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi A., Shanabrough M., McClelland S., Liu Z.W., Borok E., Gao X.B. Early-life experience reduces excitation to stress-responsive hypothalamic neurons and reprograms the expression of corticotropin-releasing hormone. J. Neurosci. 2010;30:703–713. doi: 10.1523/JNEUROSCI.4214-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Liu D., Diorio J., Day J.C., Francis D.D., Meaney M.J. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat. Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Liu D., Diorio J., Tannenbaum B., Caldji C., Francis D., Freedman A. Maternal care, hippocampal glucocorticoid receptors and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lucassen P.J., Naninck E.F., van Goudoever J.B., Fitzsimons C., Joels M., Korosi A. Perinatal programming of adult hippocampal structure and function; emerging roles of stress, nutrition and epigenetics. Trends Neurosci. 2013;36:621–631. doi: 10.1016/j.tins.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Malter Cohen M., Jing D., Yang R.R., Tottenham N., Lee F.S., Casey B.J. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc. Natl. Acad. Sci. U. S. A. 2013;110:18274–18278. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maras P.M., Baram T.Z. Sculpting the hippocampus from within: stress, spines, and CRH. Trends Neurosci. 2012;35:315–324. doi: 10.1016/j.tins.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland S., Korosi A., Cope J., Ivy A., Baram T.Z. Emerging roles of epigenetic mechanisms in the enduring effects of early-life stress and experience on learning and memory. Neurobiol. Learn. Mem. 2011;96:79–88. doi: 10.1016/j.nlm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Allostasis, allostatic load, and the aging nervous system: role of excitatory amino acids and excitotoxicity. Neurochem. Res. 2000;25:1219–1231. doi: 10.1023/a:1007687911139. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Meaney M.J., Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin. Neurosci. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney M.J., Viau V., Bhatnagar S., Betito K., Iny L.J., O'Donnell D. Cellular mechanisms underlying the development and expression of individual differences in the hypothalamic-pituitary-adrenal stress response. J. Steroid Biochem. Mol. Biol. 1991;39:265–274. doi: 10.1016/0960-0760(91)90072-d. [DOI] [PubMed] [Google Scholar]
- Miklos I.H., Kovacs K.J. GABAergic innervation of corticotropin-releasing hormone (CRH)-secreting parvocellular neurons and its plasticity as demonstrated by quantitative immunoelectron microscopy. Neuroscience. 2002;113:581–592. doi: 10.1016/s0306-4522(02)00147-1. [DOI] [PubMed] [Google Scholar]
- Molet J., Maras P.M., Avishai-Eliner S., Baram T.Z. Naturalistic rodent models of chronic early-life stress. Dev. Psychobiol. 2014 doi: 10.1002/dev.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Shionoya K., Jakubs K., Sullivan R.M. Early-life stress disrupts attachment learning: the role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. J. Neurosci. 2009;29:15745–15755. doi: 10.1523/JNEUROSCI.4106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison K.E., Rodgers A.B., Morgan C.P., Bale T.L. Epigenetic mechanisms in pubertal brain maturation. Neuroscience. 2014;264:17–24. doi: 10.1016/j.neuroscience.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky P.M., Meaney M.J. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res. Mol. Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Raineki C., Cortes M.R., Belnoue L., Sullivan R.M. Effects of early-life abuse differ across development: infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J. Neurosci. 2012;32:7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C., Holman P.J., Debiec J., Bugg M., Beasley A., Sullivan R.M. Functional emergence of the hippocampus in context fear learning in infant rats. Hippocampus. 2010;20:1037–1046. doi: 10.1002/hipo.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C.J., Sandman C.A., Lenjavi M.R., Baram T.Z. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo R.D., Fossella J.A., Bateup H.S., Sisti H.M., Brake W.G., McEwen B.S. Maternal separation suppresses TGF alpha mRNA expression in the prefrontal cortex of male and female neonatal C57BL/6 mice. Brain Res. Dev. Brain Res. 2004;152:73–77. doi: 10.1016/j.devbrainres.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Roth T.L., Lubin F.D., Funk A.J., Sweatt J.D. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol. Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo S.J., Murrough J.W., Han M.H., Charney D.S., Nestler E.J. Neurobiology of resilience. Nat. Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman C.A., Davis E.P., Glynn L.M. Prescient human fetuses thrive. Psychol. Sci. 2012;23:93–100. doi: 10.1177/0956797611422073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli S., Lesuis S.L., Wang X.D., Wagner K.V., Hartmann J., Labermaier C., Scharf S.H., Müller M.B., Holsboer F., Schmidt M.V. Evidence supporting the match/mismatch hypothesis of psychiatric disorders. Eur. Neuropsychopharmacol. 2014;24:907–918. doi: 10.1016/j.euroneuro.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Schmidt M.V., Wang X.D., Meijer O.C. Early life stress paradigms in rodents:potential animal models of depression? Psychopharmacol. (Berl) 2011;214:131–140. doi: 10.1007/s00213-010-2096-0. [DOI] [PubMed] [Google Scholar]
- Sun H., Kennedy P.J., Nestler E.J. Epigenetics of the depressed brain: role of histone acetylation and methylation. Neuropsychopharmacology. 2013;38:124–137. doi: 10.1038/npp.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A.C., Reeb-Sutherland B.C., Romeo R.D., McEwen B.S. On the causes of early life experience effects: evaluating the role of mom. Front. Neuroendocrinol. 2014;35:245–251. doi: 10.1016/j.yfrne.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai Y.M., Jones K.R., Ziegler D.R., Cullinan W.E., Herman J.P. Forebrain origins of glutamatergic innervation to the rat paraventricular nucleus of the hypothalamus: differential inputs to the anterior versus posterior subregions. J. Comp. Neurol. 2011;519:1301–1319. doi: 10.1002/cne.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hasselt F.N., Cornelisse S., Zhang T.Y., Meaney M.J., Velzing E.H., Krugers H.J., Joels M. Adult hippocampal glucocorticoid receptor expression and dentate synaptic plasticity correlate with maternal care received by individuals early in life. Hippocampus. 2012;22:255–266. doi: 10.1002/hipo.20892. [DOI] [PubMed] [Google Scholar]
- Verkuyl J.M., Hemby S.E., Joëls M. Chronic stress attenuates GABAergic inhibition and alters gene expression of parvocellular neurons in rat hypothalamus. Eur. J. Neurosci. 2004;20:1665–1673. doi: 10.1111/j.1460-9568.2004.03568.x. [DOI] [PubMed] [Google Scholar]
- Wang X.D., Rammes G., Kraev I., Wolf M., Liebl C., Scharf S.H., Rice C.J., Wurst W., Holsboer F., Deussing J.M., Baram T.Z., Stewart M.G., Müller M.B., Schmidt, M.V. Forebrain CRF(1) modulates early-life stress-programmed cognitive deficits. J. Neurosci. 2011;31:13625–13634. doi: 10.1523/JNEUROSCI.2259-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.D., Labermaier C., Holsboer F., Wurst W., Deussing J.M., Muller M.B., Schmidt M.V. Early-life stress-induced anxiety-related behavior in adult mice partially requires forebrain corticotropin-releasing hormone receptor 1. Eur. J. Neurosci. 2012;36:2360–2367. doi: 10.1111/j.1460-9568.2012.08148.x. [DOI] [PubMed] [Google Scholar]
- Weaver I.C., Cervoni N., Champagne F.A., D'Alessio A.C., Sharma S., Seckl J.R. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Whipple E.E., Webster-Stratton C. The role of parental stress in physically abusive families. Child Abuse Negl. 1991;15:279–291. doi: 10.1016/0145-2134(91)90072-l. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Flory J.D., Southwick S., Charney D.S. Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. nn. N. Y. Acad. Sci. 2006;1071:379–396. doi: 10.1196/annals.1364.028. [DOI] [PubMed] [Google Scholar]
- Ziegler D.R., Edwards M.R., Ulrich-Lai Y.M., Herman J.P., Cullinan W.E. Brainstem origins of glutamatergic innervation of the rat hypothalamic paraventricular nucleus. J. Comp. Neurol. 2012;520:2369–2394. doi: 10.1002/cne.23043. [DOI] [PMC free article] [PubMed] [Google Scholar]