Abstract
Implantable intracortical microelectrodes face an uphill struggle for widespread clinical use. Their potential for treating a wide range of traumatic and degenerative neural disease is hampered by their unreliability in chronic settings. A major factor in this decline in chronic performance is a reactive response of brain tissue, which aims to isolate the implanted device from the rest of the healthy tissue. In this review we present a discussion of materials approaches aimed at modulating the reactive tissue response through mechanical and biochemical means. Benefits and challenges associated with these approaches are analyzed, and the importance of multimodal solutions tested in emerging animal models are presented.
Introduction
Many traumatic injuries and degenerative diseases in the central nervous system (CNS) are insufficiently or completely untreatable, and have a tremendous personal and societal impact. For example, spinal cord injuries (SCI) occur at a rate of 12,000 cases per year in the US, currently affect up to 400,000 Americans (NSCISC, 2010). Additionally, there are at least 3.3 million Americans suffering from visual impairment (In et al., 2004) and about 750,000 affected by severe to profound hearing impairment (Mohr et al., 2001). The lack of sufficient treatments for sensory impairment and CNS injuries has stimulated research into using external devices to interface with the remaining healthy parts of the CNS as a means to restore or enhance lost neural function. Devices interfacing with the CNS can be categorized by size into macroelectrodes and microelectrodes. Deep brain stimulation (DBS) using macroelectrodes is FDA-approved and relatively widespread for the treatment of Parkinson disease (Lozano et al., 2002; Woods et al., 2002; Rodriguez-Oroz et al., 2005; Benabid et al., 2009; Bronstein et al., 2011), and is under investigation for the treatment of major depression (Jiménez et al., 2005; Lozano et al., 2008; Malone Jr et al., 2009; Bewernick et al., 2010; Anderson et al., 2012) and chronic pain (Levy et al., 1987; Coffey, 2001; Bittar et al., 2005). While DBS macroelectrodes are subject biological reactions of brain tissue, they exhibit satisfactory long-term stability due to their size scale and exclusive operation in the stimulation paradigm (Henderson et al., 2002; Umemura et al., 2003; Moss et al., 2004; Butson et al., 2006; Nielsen et al., 2007; Lempka et al., 2009; Hughes et al., 2011). On the other hand, intracortical microelectrodes have been used with varying degrees of success to treat blindness (Nicolelis, 2003) and deafness (Loeb, 1990; Lenarz et al., 2006) as well as enable bladder and muscle control in paralyzed patients (Grill, 2000). The smaller size scale and the desired operation in both the recording and stimulation paradigms, however, mean that the quest for widespread clinical adoption of implantable intracortical microelectrodes faces a host of formidable obstacles. As such, intracortical microelectrodes and their mechanical and biochemical design considerations will be the subject of this review.
Intracortical microelectrodes are a major focus of research for implantable neural interfaces due to their ability to isolate smaller neuronal populations for recording and/or stimulation, as well as their ability to selectively target different cortical depths. While intracortical microelectrodes show great promise, they are unreliable in chronic settings, displaying a drop in signal to noise ratio (SNR), an increase in impedance, and a loss of neuronal discrimination with time post-implant (Vetter et al., 2004; Williams et al., 2007). This degradation in intracortical device performance is generally correlated with a reactive response of brain tissue. A recent retrospective analysis indicates that while acute mechanical failure is responsible for a large proportion of current device failures (Barrese et al., 2013), the reactive tissue response remains a major factor in the decline of chronic device performance if acute failure is otherwise controlled.
This reactive tissue response is a complex aggregate of interdependent responses involving multiple native and invading cell types, including microglia, astrocytes, neurons, and dural fibroblasts, and blood borne cells. The immune response typically presents two distinct phases. An acute phase starts immediately following the insertion of the device, which causes the breach of the blood brain barrier (BBB), and the introduction and accumulation of blood borne components and cells (Saxena et al. 2013). Consequent edema, accumulation of proteins, and secretion of inflammatory cytokines results in the activation and recruitment of microglia to the surface of the inserted device, followed by reactive astrocytes (Biran et al., 2005; He and Bellamkonda, 2008). This volatile stage transitions into a more stable chronic phase characterized by the encapsulation of the device in a tightly bound glial sheath accompanied by the loss of recording sensitivity over time. Recordings can be recovered temporarily by the application of DC pulses, which are hypothesized to disrupt the tight junctions of the astrocytes in the glial sheath (Johnson et al., 2005; Otto et al., 2006), but maintaining reliably high fidelity chronic recordings remains challenging.
The exact biological pathways governing the progression of the reactive tissue response to intracortical devices are not well characterized. An important finding from recent years is the confirmation of the biphasic nature of the reactive tissue response (Potter et al., 2012), where a highly volatile acute stage that appears to respond well to various interventions transitions to a chronic stage that is more or less impervious to current treatment approaches. Changes in electrophysiological characteristics of the implanted electrode generally do not correspond well with observed cellular responses during the acute stage (Prasad and Sanchez, 2012; Prasad et al., 2012), questioning the efficacy of impedance monitoring in predicting the progression of the tissue response. Chronic damage to the BBB resulting from the indwelling of the implanted device appears to be one of the dominant factors in the extent of device failure (Saxena et al., 2013; Karumbaiah et al., 2012). Additionally, the reactive tissue response has been shown to be non-uniform and depth related (Woolley et al., 2013c), with stronger scarring closer to the surface of the brain. Transdural implants elicit a much higher response than subdural implants that are inserted completely below the meninges (Markwardt et al., 2013), and a recent retrospective analysis of intracortical microelectrode failure revealed widely variable meningeal reactions in non-human primates (Barrese et al., 2013). These findings collectively suggest that the introduction of dural fibroblasts and blood borne cells and proteins into the brain activates inflammatory pathways, and that this activation is strongest at the site of injury to these structures. Potentially possible mechanisms include accumulation of reactive oxygen species and activation of toll-like receptor 4 (TL4) precipitating microglial activation and neuronal degeneration (Potter et al., 2013; Ravikumar et al., 2013).
While these recent findings shed more light on the biological mechanisms governing the progression of the reactive tissue response, they suggest the importance of simultaneously examining multiple components of the reactive tissue response at multiple time scales. Reductionist approaches to understanding and mitigating the reactive tissue response have had limited success. This suggests that the problem is likely multi-dimensional, and likely non-linear. Thus, single-factor or other limited experimental designs are unlikely to provide meaningful data, particularly in the chronic phase of the tissue response. Despite the limitations of these reductionist approaches, several factors have shown to modulate the reactive tissue response, particularly the acute phase. Two of the most significant factors have been: 1) the mechanical structure and size of the substrate of the device, and 2) modulation of the local biochemical environement around the device. In the following sections we will review these mechanical and biochemical intervention strategies with respect to the reactive tissue response and device performance.
1. Mechanical Approaches
1.1 Substrate Material
1.1.1 Mechanical mismatch between device and tissue
A significant difference in stiffness exists between brain tissue and electrode materials. Conventionally used substrate materials such as tungsten, silicon, platinum, steel, and glass have a Young’s modulus (i.e., a measure of stiffness) of 6–7 orders of magnitude greater than that of the brain tissue (Lee et al., 2004; Takeuchi et al., 2004). Due to this mismatch, micromotion generated by respiratory and pulsatile movement can perturb the surrounding tissue over a long period of time by exerting shear stress on the tissue and/or by cutting off the tissue with sharp edges (Lee et al. 2005; Gilletti & Muthuswamy 2006; Harris & Tyler 2014).
To identify the effect of rigid electrode materials to brain tissue, several parameters including geometry, material properties, buckling load, and estimated force were taken into consideration (Goldstein and Salcman, 1973). Mathematical modeling studies using finite-element model (FEM) were conducted to evaluate the extent of shear stress induced by micromotion (Subbaroyan et al., 2005; Lee et al., 2005; Zhu et al., 2011). These results suggest that the implanted devices indeed generate strong enough stress on surrounding cells to stretch-activate ion channels and cause deformation. In vitro cell culture studies verified that an increased level of astrogliosis and neuronal death may happen in excessive strain conditions (Cullen et al., 2007). Furthermore, the use of soft materials can potentially reduce the shear stress by several orders of magnitude and minimize the possibility of damaging the surrounding tissue, leading to a healthy neuronal environment. In addition, neurons were found to extend their projections more actively to a preferred range of stiffness, mostly at lower moduli than that of conventional materials, and this further strengthens the idea of using soft materials (Leach et al., 2007; Georges et al., 2006; Kerstein et al., 2013).
1.1.2 Flexible substrates
Early generation electrodes specifically designed to meet the flexibility constraint were polyimide-based microelectrodes (Stieglitz et al., 2000; Rousche et al., 2001; Lee et al., 2004; Takeuchi et al., 2004). The fabrication of these devices involved metal sites sandwiched by thin film of polyimide using standard planar photolithographic techniques. Parylene-C is also considered to be a suitable material which can serve as a flexible substrate backbone (Suzuki et al., 2003; Pellinen et al., 2005; Rodger et al., 2008; Kim et al., 2014). These types of electrodes reduced the strain forces between the tissue and the devices caused by micromotion, which can potentially enhance the devices’ functional longevity. However, these materials did not show an improved surface chemistry over silicon as evidenced by cytoarchitectural examinations (Winslow et al., 2010). While flexible substrate materials offer advantages in providing a lower Young’s modulus and ease of prototyping/fabrication, their major disadvantage is their need for aid in insertion. Since the stiffness of the electrodes made out of compliant materials is targeted to match with the brain tissue after insertion, the electrodes are likely to buckle on the surface of the cortex when attempting to penetrate into the meninges (Lee et al., 2005).
Insertion of flexible devices has been enabled by using insertion platforms that provide increased rigidity to push the electrode through the pia mater. The device surface can be coated with an agent that is rigid, biocompatible, and rapidly biodegradable while breaking down into biocompatible metabolites. Coating the device with carboxyl monolayer, poly(ethylene-glycol) (PEG), gelatin, or tyrosine-derived resorbing polymers has been proposed to provide enough rigidity for insertion (Kozai and Kipke, 2009; Chen et al., 2010; Lind et al., 2010; Lewitus et al., 2011). With such insertion aids, the mechanical properties of electrodes were successfully modulated within physiological conditions. Side effects, however, may exist as these carriers increase the surface area of the shank, evoking greater tissue damage. Dissolvable insertion shuttles might also subject their cargo to substantial fluid forces (Rakuman et al., 2013). In addition, flexible electrodes that are insertion-guided by carriers will need to consider the long-term effects of the carrier need to be considered in order to accurately examine the performance of flexible substrate material itself.
A solution to such potential pitfalls might be the use of mechanically adaptive nanocomposite materials. One example uses rigid cellulose nanofibers embedded within a soft polymeric matrix, resulting in a substrate material that is rigid when dry but becomes flexible upon hydration in the brain. (Harris et al., 2011b; Hess et al., 2011). Shape memory polymers could also potentially be used as substrate materials exhibiting rigid-soft state transition upon hydration (Ware et al., 2014). For applications requiring access to deeper regions of the brain, such as the thalamus, ductile metals such as titanium offer a unique combination of robustness and flexibility in spite of their relatively high Young’s modulus (McCarthy et al. 2011a; McCarthy et al. 2011b)
While large leaps have been made with flexible substrate materials, multiple concerns remain. Recent findings indicate that material density might be as important as its stiffness (Lind et al., 2013), reaffirming the need for further studies to understand the role of mechanotransduction in the brain. Moreover, while highly flexible electrode material may be beneficial in reducing tissue responses, their tendency of moving and flexing according to the brain movement may be detrimental to locating and fixing the electrode at the target position. Site-substrate modulus mismatch can potentially be another issue that needs further investigation.
1.2 Form Factor
Size and architecture of the electrode are often tightly bound to materials, as different fabrication processes result in different shapes of devices. Nonetheless, it is not solely the material itself that determines the functionality of a newly design electrode. Material, size/architecture, and insertion/anchoring techniques need to be rigorously discussed at the same time in the design of an intracortical electrode. A trade-off also needs to be considered between single-shank and multi-shank devices. Increasing the number of shanks per implanted device provides redundancy which insulated against the risk of total device encapsulation of single-shank devices, but increases the likelihood of striking a major blood vessel.
1.2.1 Size and Architecture
Intuitively, electrodes with smaller cross-sectional area are likely to cause a smaller mechanical injury. A small amount of exposed surface area to the brain tissue reduces the macrophage recruitment to the electrode, which in turn mitigates the total acute phase of the reactive tissue response, including the neuronal kill zone (Bernatchez et al., 1996; Szarowski et al., 2003; Skousen et al., 2011; Thelin et al., 2011). Recent research utilizing small size electrodes has histologically verified that smaller electrodes cause less severe astrogliosis and blood brain barrier (BBB) instability (Seymour and Kipke, 2007; Kozai et al., 2012; Karumbaiah et al., 2013). However, a major disadvantage of using such small electrode sites is the corresponding compromise of the sensitivity of neural recordings or charge carrying capacity of microstimulation (Merrill and Tresco, 2004; Ludwig et al., 2006).
In addition to size, electrode architecture is another important design factor to be considered. A physical interpretation of architecture modulation is to make the sites undergo less severe foreign body response (FBR) than normally expected with the shank size. Seymour et al. evaluated an electrode design that put sites on a lateral wing structure so that sites are spatially separated from the bulky shank (Seymour and Kipke, 2007; Seymour et al., 2011). Their work demonstrated the effectiveness of modulating site placement and introduced the concept of lattice structure (i.e. open architecture) to intracortical electrodes. Early use of electrode perforation to maintain electrode-tissue interface integrity was mostly done for planar surface arrays (Boppart et al., 1992). For intracortical electrodes, lattice structures are expected to reestablish the cell-to-cell network severed during the insertion and enhance isotropic diffusion of cell signaling molecules (Seymour and Kipke, 2007). Lattice designs also exhibit a small cross-section, reducing the area that glial cells can react to (Skousen et al., 2011). This design is an active research area (Hara et al., 2013) and chronic electrophysiological recordings and cytokine analysis must be evaluated in future studies in order to validate immunohistological findings.
1.2.2 Insertion/Anchoring
The sharpness of the electrode tip becomes less crucial as electrodes gets smaller, but cannot be neglected since blunt tips evoke a more severe tissue response as they result in higher tissue strain and vascular damage during device insertion (Bjornsson et al., 2006). As long as the tip is sharpened, however, differently shaped tapered tips did not result in different degrees of tissue response, whether it was a sword- or chisel-pointed tips (Edell et al., 1992). Higher insertion speeds can compensate for blunt tips by enabling the devices to neatly penetrate the tissue without causing tissue distortion due to vascular severing, rupturing, or dragging (Rousche and Normann, 1992; Rennaker et al., 2005; Welkenhuysen et al., 2011). Different shank shapes were also found to elicit different tissue response, such that cylindrical shafts caused less tissue response than planar shafts (Karumbaiah et al., 2013). As shank shape has little to do with insertion-related injury, sharper planar shanks might induce more tissue damage from micromotion.
The degree of micromotion effect is also tightly related to anchoring method. Functional electrodes are normally anchored to the skull directly or via tethering cables to protect the device from external forces. This makes them more prone to induce injuries during micromotion, as opposed to non-functional free-floating electrodes that are more likely to move along with brain tissues. Histological studies have verified that anchored devices evoke significantly greater immune response than un-anchored devices (Kim et al., 2004b; Biran et al., 2007; Thelin et al., 2011). Nonetheless, current technologies do not allow for functionally active and fully free-floating electrodes, completely free of tethering forces due to cabling. Even putative wireless electronics will have significant momentum that will likely result in none-zero tethering forces.
All in all, a number of studies have provided insights to the mechanical factors influencing the chronic functionality of electrodes. However, some studies combined their approach with several other paradigms such as surface coatings, suggesting that the results may not be solely due to the mechanical factors. Better understanding of biochemical perspectives is also required to address the complicated nonlinear multimodal problem.
2. Drug delivery and biomimetic approaches
A twin approach to mechanical design considerations is the biochemical manipulation of the local microenvironment using biomolecules that act through either endocytosis or surface interactions. CNS injury results in the breach of the blood brain barrier (BBB), causing infiltration of blood borne macrophages, leukocytes, dural fibroblasts, and other cells which are otherwise foreign to the brain. Additionally, the persistent presence of an implanted device in the brain activates microglia causing secretion of various inflammatory cytokines (Polikov et al., 2005; Karumbaiah et al., 2012; Prasad and Sanchez, 2012). While some of these secreted molecules are neuroprotective in nature, many of them are pro-inflammatory and neurotoxic. Hence, a plausible strategy is to deliver anti-inflammatory drugs and other biomolecules to promote neuronal health, and/or to inhibit the production of inhibitory molecules like chondroitin sulfate proteoglycans to the implant site. As per this approach, researchers have used various anti-inflammatory drugs, antibiotics, anti-oxidants, cell-cycle inhibitors and neuropeptides that act via various mechanisms and were delivered with a range of techniques (Shain et al., 2003; He et al., 2007; Zhong and Bellamkonda, 2007; Rennaker et al., 2007; Potter et al., 2013, 2014). In this section we will compare and contrast the drug-acting mechanism as well as the delivery technique in the development of neuroengineered materials.
2.1 Systemic Delivery: Benefits and challenges
Systemic delivery of drugs/molecules is by far the most common method owing to its feasibility and relative non-invasiveness. The majority of these drugs target various stages of known inflammatory pathways. The spinal cord and traumatic brain injury literature provide us with a wide variety of potential therapeutics that are good candidates to be screened for use with implantable neural microelectrodes. One example, hydralazine, can scavenge particular molecular byproducts of spinal cord injury, and induce some degree of behavioral recovery (Leung et al., 2011). Other polymeric molecules like poly(ethylene glycol) (PEG) have shown potential in treating spinal cord and traumatic brain injury. PEG can induce cellular recovery following peripheral injection due to its hydrophilicity, which allow it to induce membrane sealing, and consequently, behavioral recovery (Luo et al., 2002; Borgens et al., 2002; Koob et al., 2005; Koob and Borgens, 2006; Koob et al., 2008). PEG has also been shown to prevent changes in microelectrode impedance associated with protein adsorption in vitro (Sommakia et al., 2009) and in vivo (Sommakia, 2013) One of the most commonly used anti-inflammatory drugs in the context of implantable neural microelectrodes is dexamethasone. Dexamethasone is a potent anti-inflammatory drug that acts on glucocorticoid receptors, and has been shown to result in reduced glial scar formation when injected systematically for six days compared to a single injection(Shain et al., 2003; Spataro et al., 2005). Administration of minocycline, an antibiotic, for 2 days before and 5 days after implantation correlated with better quality of neural recordings from rats for 1 month (Rennaker et al., 2007). Natural antioxidants that act by scavenging reactive oxygen species, such as resveratrol and curcumin, have been shown to reduce the intensity of glial scarring and BBB permeability (Potter et al., 2013, 2014). The success reported in these studies, however, manifested primarily during the acute phase, but dematerialized once the chronic response emerged. Thus, there is a strong need to further study the genetic and biochemical mechanisms governing the transition between the two injury phases. Additional obstacles facing systemically administered drugs include BBB permeability, multiple injections, peripheral metabolism, the need for higher dosages, and other side effects. For example, dexamethasone overdosing results in side effects in multiple organs (Schacke et al. 2002). These aforementioned disadvantages to peripheral, systemic delivery have stimulated research into more localized forms of delivery.
2.2 Local delivery
2.2.1 Microfluidics
Initial attempts were centered on the use of microfluidic technology to pump drug-carrying solutions through microfluidic channels built into micromachined implantable probes. The first reported design of such a device in a silicon-based planar device featured buried microfluidic channels with fluid outlet ports located cross-sectionally (Chen et al., 1997). Improvements on this design included shuttered release ports to reduce the effects of passive diffusion from open ports (Papageorgiou et al., 2001, 2006). Alternative designs used open channels where the drug would diffuse out of the entire length of the channel (Retterer et al., 2004, 2008), and a flexible microcatheter affixed to the back of a regular Michigan array (Rohatgi et al., 2009). Microfluidic technology has also been attempted in flexible polymer-based devices, such as polyimide (Metz et al., 2004) and parylene (Takeuchi et al., 2005; Ziegler et al., 2006). These microfluidic approaches are feasible in acute settings, but practical challenges concerning channel patency, biofouling during insertion, and chronic attachment to a pump have led to the development of alternative drug delivery approaches. However, successes of microfluidic technology in other biomedical applications (Parker et al., 2007) suggest that further improvements of this approach may be feasible.
2.2.2 Drug release and coatings
The most prevalent drug delivery platform that has been used in conjunction with implantable neural electrodes is hydrogels. Hydrogels are networks of hydrophilic polymeric molecules, held in shape by molecular entanglement, secondary forces, or covalent crosslinking (Hoffman, 2002). Engineering hydrogels for biomedical applications continues to be an extensive and extremely active area of research (Peppas, 1997; Qiu and Park, 2001; Park et al., 2011; Hoffman, 2012). Hydrogels feature prominently in neural repair & tissue engineering strategies (Schmidt and Leach, 2003; Willerth and Sakiyama-Elbert, 2007; Nisbet et al., 2008), making research into their uses with implantable neural microelectrodes a logical next step. Hydrogels can be based on a wide variety of synthetic and naturally-occurring polymeric molecules, including, but not limited to, hyaluronic acid (HA), poly(lactic-co-glycolic acid) (PLGA), poly(ethylene glycol) (PEG), agarose, alginate, fibrin, and chitosan (Katz and Burdick, 2009). Attempts have been made at improving neuronal interfacing with devices by releasing neurotrophic factors, such as NGF, from various hydrogels, such as PEG/PLA (Winter et al., 2007), and pHEMA (Cadotte and DeMarse, 2005; Jhaveri et al., 2008). Alginate hydrogels have been used to improve electrical properties of deposited polypyrrole films (Kim et al. 2004). Such alginate hydrogels were shown to be capable of releasing dexamethasone-loaded nanoparticles, resulting in reduced glial inflammation around the electrodes for up to a few weeks (Shain et al., 2003; Kim and Martin, 2006). Dexamethasone-releasing hydrogels are able to provide sensitive neural recordings, but only in conjunction with conductive polymers deposited on the electrode site, due to spatial displacement of neurons because of the size scale and swelling of the hydrogels (Kim et al., 2010). This size scale, reaching up to 360um (Abidian and Martin, 2009), has led to interest in drug release from smaller scale films.
Micron-scale evaporation-deposited nitrocellulose films have been successfully used to release alpha-melanocyte stimulating hormone (α-MSH), reducing microglial activation in vitro) (Zhong and Bellamkonda, 2005), and dexamethasone (Zhong and Bellamkonda, 2007), attenuating glial responses and decreasing neuronal loss in vivo. Conducting polymers (CP), which have been shown to be excellent materials for neural recording (Ludwig et al., 2006, 2011) and microstimulation (Wilks et al., 2009), offer an interesting potential as sub-micron scale platforms for electrochemically-actuated drug release. Passive dexamethasone release from polypyrrole showed reduced microglial activation in vitro (Wadhwa et al., 2006), while improved release characteristics were realized through electrochemical actuation of PLGA-templated PEDOT nanotubes (Abidian et al., 2006). The biggest challenge facing CP films as a drug release platform is their localization to the microelectrode site through an electrochemical deposition process, indicating the potential superiority of films with higher surface coverage.
Even thinner films can be achieved by dip-coating layers of non-swelling molecules. One such example is a prodrug synthesized by linking poly(N-vinyl pyrrolidone) with prednisolone via hydrazone linkage to form a biocompatible nanoscale film. This nanoscale film was shown to be capable of releasing prednisolone at physiological pH conditions and resulted in the reduction of microglial activation markers in vitro (Cao and He, 2010), but it is unclear how such films would affect the electrical characteristics of neural microelectrodes. Other nanoscale films under investigation for use in neural microelectrodes are silica thin films produced using a sol-gel process. As a potential drug release platform, silica thin films do not have negative effects on the electrical properties of neural microelectrodes (Pierce et al., 2009), and have been shown to be capable of releasing a variety of therapeutic molecules (Radin and Ducheyne, 2005, 2007; Radin et al., 2009). Recent work with flexible shank materials (reviewed in the previous section) avoids coatings and instead involves incorporating drugs into the polymeric components of these flexible shanks (Potter et al., 2014). Figure 4 shows an example in the difference in size between hydrogel and sol-gel thin film coatings.
Figure 4.
An example in the difference in the size scale between sub-millimeter to micrometer scale hydrogels (A, (Abidian and Martin, 2009)) in the hydrated state (left column) versus the dehydrated state (right column), and nanoscale sol-gel silica thin-films (B, (Pierce et al., 2009)). An examination of potential tradeoffs between the two coatings should be conducted when designing a solution to modulate the reactive tissue response.
2.2.3 Surface mediated interactions
Similarly drawing inspiration from the tissue engineering literature is the concept of modifying the surface of implantable neural microelectrodes to mimic healthy extracellular matrix of the brain parenchyma. This biomimetic approach has two main branches. The first approach aims to encourage neuron attachment to the electrode, while the second approach aims to manipulate glial cells in an attempt to attenuate glial scar formation (Polikov et al., 2005).
Several groups have attempted to create surfaces more conducive to neural growth by incorporating or immobilizing cell adhesion molecules. A common approach is the use of multipeptide laminin fragments for the purpose of modulating neural attachment and growth. The coating methods employed include covalent immobilization on dextran-coated surfaces (Massia et al., 2004), covalent immobilization on amino-modified glass (Kam et al., 2002), self-assembling peptide nanofibers (Wu et al., 2006; Tysseling-Mattiace et al., 2008), copolymerization with conductive polymers (Cui et al., 2001; Stauffer and Cui, 2006), electrostatic layer-by-layer deposition (He et al., 2006), microcontact printing (James et al., 2000; St. John et al., 1997), fiber templating within a hydrogel (Yu et al., 2005), and covalent binding to silica sol-gel (Jedlicka et al. 2006; Jedlicka et al. 2007a; Jedlicka et al. 2007b).
In vitro results generally show that surfaces functionalized with whole laminin(He and Bellamkonda, 2005), laminin sequences YIGSR (Cui et al., 2001; Yu et al., 2005), or IKVAV result in improved neuron and neuron-like cell (PC12) growth. The incorporation of the laminin fragment RGD, however, results at best in a non-specific effect on multiple cell types (Zhang et al., 2005) and at worst a preferential adhesion affinity for non-neuronal cells (Kam et al., 2002). More recently, the immobilization of a neuron specific adhesion molecule, L1, on silicon electrodes was investigated and showed improved neural adhesion in vitro (Azemi et al., 2008) and in vivo (Azemi et al., 2010, 2011).
The use of peptide adhesion sequences targeting astrocytes has shown that YIGSR and IKVAV have no effect on astrocyte growth and adhesion in vitro, but that the Neural Cell Adhesion Molecule (NCAM) sequence KHIFSDDSSE causes increased astrocyte growth compared to fibroblasts (Kam et al., 2002). Since NCAM is known to have an inhibitory effect on astrocyte proliferation following injury (Sporns et al., 1995), Kam et al. argue that immobilizing the KHIFSDDSSE sequence to a neural electrode could attenuate the intensity of the resulting glial scar in vivo.
While these in vitro studies show potential for biomimetic coatings, the results have not translated quite as successfully in vivo. Cui at al. have shown that the incorporation of YIGSR in polypyrrole coatings result in more neuron survival at the electrode site compared to control sites (Cui et al., 2003). He et al. demonstrate that electrostatically deposited laminin (He et al., 2006) and α-MSH functionalized using silane chemistry (He et al., 2007) result in a less intense glial scar as measured by immunohistochemistry and cytokine measurements Recent insights into the factors involved in the reactive tissue response suggest a potentially wider range of biomimetic approaches, such as superoxide dismutase mimetic surfaces to reduce accumulation of reactive oxygen species (Potter-Baker et al., 2014).
3. Summary: Challenges and Future Directions
Despite some of the approaches discussed in this review reporting improvements in the acute reactive tissue response, very few have shown significant and consistent results in mitigating the chronic reactive tissue response and improving device performance reliability. Within the scope of the review presented here, there are several new directions for design and evaluation that deserve investigation. Specifically: 1) adaptive, hybrid substrates/composite materials, 2) improved device-tissue evaluation techniques, 3) more appropriate animal models, and 4) assessment of the multi-dimensional interaction in the reactive tissue response.
Hybrid substrates or composite materials can be designed to possess the desired properties of a brain-matched Young’s modulus with an appropriate form factor and specifically targeted surface chemistries. Using composite materials, Kozai et al. showed that ultrasmall devices may exhibit a “stealth-like” property in the tissue (Kozai et al., 2012). Meanwhile, other devices that adapt their mechanical properties post-insertion should allow for sufficient strength for penetration of the pia mater, but a relaxation of stiffness after a certain period of time (Rakuman et al., 2013; Ware et al., 2014; Harris et al., 2011a, 2011b). Finally, advancements in micro- and nano-printing technologies will provide the ability to tailor the mitigation strategy to the spatially-patterned tissue response (Woolley et al., 2013c). Specifically, novel ultrasonic on-demand microplotting technology should provide the ability to spatially deliver neuroactive compounds on the surface of implanted electrode arrays.
The field will also benefit from the development of better and more consistent evaluation techniques. The ability to image detailed cellular and sub-cellular anatomy in thick sections that contain devices, or in fully intact biological systems (Woolley et al., 2011, 2013a, 2013b, 2013c) are major technological improvements. The ability to image device-tissue interactions in vivo provides another step forward, allowing longitudinal observations (Schendel et al., 2013). Better evaluation techniques will also enable a deeper examination of the contributions of surgical technique variability to the overall variations in tissue responses.
Ultimately, neural microelectrodes require validation via pre-clinical (i.e. animal) studies, and finally clinical studies. Current clinical studies offer unprecedented evaluation of device performance (Hochberg et al., 2006; Perge et al., 2013; Hochberg and Cochrane, 2013); however, pre-clinical evaluation must still be conducted for new or modified devices. Patrick Tresco conducted a retrospective literature analysis of the neural-implanted microdevice literature, and his findings suggested that rodents are not an appropriate testing model; only feline models or larger should be chosen (Patrick Tresco, personal communication). The non-human primate model system has provided valuable information about overall device performance (Barrese et al., 2013), especially under challenging mechanical circumstances, such as a 3G acceleration event (Santhanam et al., 2007); however, non-human primates are expensive test-beds for initial studies of newly designed device modifications. The swine is an emerging pre-clinical model, specifically, the Yucatan mini-pig (Borton et al., 2011; Agha et al., 2013). The swine model offers the advantages of sulcogyral topography, as well as considerably better human anatomical and physiological semblance than rodents or felines. Furthermore, swine are considerably cheaper and easier to acquire than non-human primates. While swine present challenges to consistent stereotactic application due to their pneumatized frontal sinuses and oblique ear canals (Lind et al., 2007; Gizewski et al., 2007; Sauleau et al., 2009), thus making them more attractive as a peripheral model, robust steps are being taken to overcome this challenge through the use of improved swine-specific stereotactic apparatuses (Bjarkam et al., 2004).
Finally, future approaches need to assess and mitigate the multi-dimensional interaction in the reactive tissue response. Simultaneous collection of multi-parametric data, including local and systemic biochemistry, electrophysiology, impedance spectroscopy, as well in vivo imaging will allow for high-dimensional analysis of correlation. Recent reports from the Bellamkonda lab provide exemplars of this approach (Karumbaiah et al., 2012, 2013; Saxena et al., 2013). Alternative approaches that attempt to determine causality through pro-inflammatory challenges and accelerated lifetime experiments are expected to shed light on the multi-dimensionality of the problem (Otto and Williams, 2012).
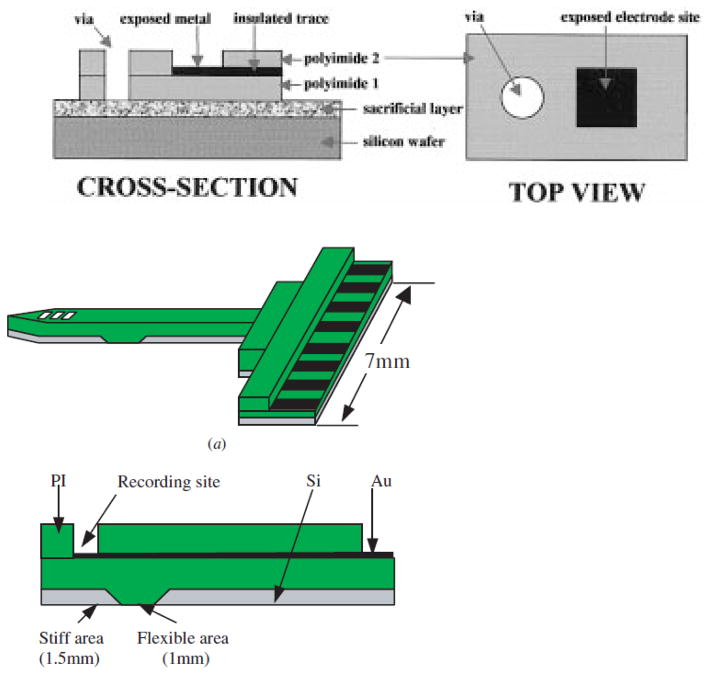
Fig. 1.
Schematic views of polyimide-based electrodes: (a) A standard version of polyimide electrodes (Rousche et al., 2001), and (b) A silicon backbone layer attached version with partially flexible area (Lee et al., 2004). Electrode sites are shown in black.
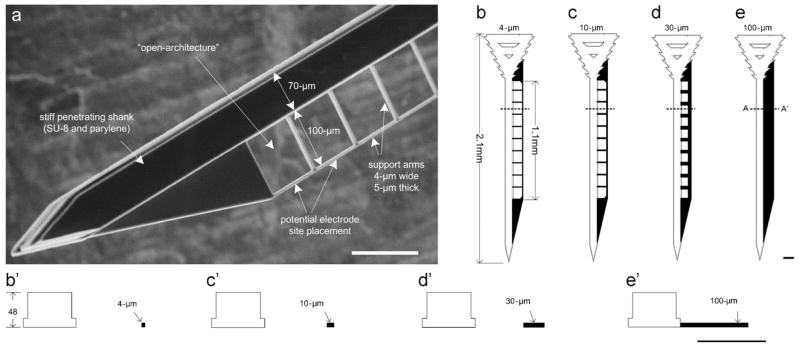
Fig. 2.
A parylene-based electrode design with a flexible lateral wing: (a) a SEM view, and (b)–(e) CAD drawings of lattice structure electrodes with different perforation sizes (Seymour and Kipke, 2007).
Fig. 3.
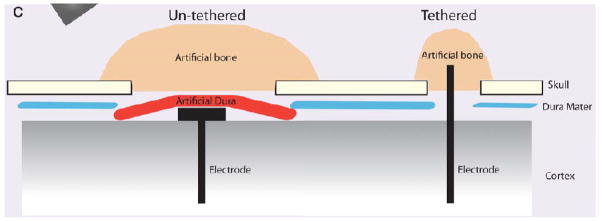
An illustration of tethered vs un-tethered (or anchored vs un-anchored) implantation techniques. Un-tethered implant is completely sealed beneath artificial dura, leaving no connection to the artificial bone or skull. In contrast, tethered implant is glued to the sealant materials. From (Thelin et al., 2011)
Highlights.
Key aspects of brain tissue response to implanted microelectrodes are introduced.
Mechanical design factors, including material and form factor, are discussed.
Biochemical design approaches are reviewed.
Challenges and future directions for the field are proposed.
Acknowledgments
This work was sponsored by the Defense Advanced Research Projects Agency (DARPA) Microsystems Technology Office (MTO), under the auspices of Dr. Jack W. Judy (jack.judy@darpa.mil) and Dr. Doug Weber (douglas.weber@darpa.mil) as part of the Reliable Neural Technology Program, through the Space and Naval Warfare Systems Command (SPAWAR) Systems Center (SSC) Pacific grant No. N66001-11-1-4013). Portions of this work were also supported by the NIH (NIDCD R03DC009339-02), and the Purdue Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abidian MR, Kim DH, Martin DC. Conducting-polymer nanotubes for controlled drug release. Adv Mater. 2006;18:405–409. doi: 10.1002/adma.200501726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abidian MR, Martin DC. Multifunctional Nanobiomaterials for Neural Interfaces. Adv Funct Mater. 2009;19:573–585. doi: 10.1002/adfm.200801473. [DOI] [Google Scholar]
- Agha NS, Komar J, Yin M, Borton DA, Nurmikko A. A fully wireless platform for correlating behavior and neural data from an implanted, neural recording device: Demonstration in a freely moving swine model. Neural Engineering (NER), 2013 6th International IEEE/EMBS Conference on; 2013. pp. 989–992. [Google Scholar]
- Anderson RJ, Frye MA, Abulseoud OA, Lee KH, McGillivray JA, Berk M, Tye SJ. Deep brain stimulation for treatment-resistant depression: Efficacy, safety and mechanisms of action. Neurosci Biobehav Rev. 2012;36:1920–1933. doi: 10.1016/j.neubiorev.2012.06.001. doi: http://dx.doi.org/10.1016/j.neubiorev.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Azemi E, Gobbel GT, Cui XT. Seeding neural progenitor cells on silicon-based neural probes. J Neurosurg. 2010;113:673–681. doi: 10.3171/2010.1.JNS09313. [DOI] [PubMed] [Google Scholar]
- Azemi E, Lagenaur CF, Cui XT. The surface immobilization of the neural adhesion molecule L1 on neural probes and its effect on neuronal density and gliosis at the probe/tissue interface. Biomaterials. 2011;32:681–692. doi: 10.1016/j.biomaterials.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azemi E, Stauffer WR, Gostock MS, Lagenaur CF, Cui XT. Surface immobilization of neural adhesion molecule L1 for improving the biocompatibility of chronic neural probes: In vitro characterization. Acta Biomater. 2008 doi: 10.1016/j.actbio.2008.02.028. [DOI] [PubMed] [Google Scholar]
- Barrese JC, Rao N, Paroo K, Triebwasser C, Vargas-Irwin C, Franquemont L, Donoghue JP. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J Neural Eng. 2013;10:66014. doi: 10.1088/1741-2560/10/6/066014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol. 2009;8:67–81. doi: 10.1016/S1474-4422(08)70291-6. [DOI] [PubMed] [Google Scholar]
- Bernatchez SF, Parks PJ, Gibbons DF. Interaction of macrophages with fibrous materials<i> in vitro</i>. Biomaterials. 1996;17:2077–2086. doi: 10.1016/0142-9612(96)00014-2. [DOI] [PubMed] [Google Scholar]
- Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, Axmacher N, Lemke M, Cooper-Mahkorn D, Cohen MX, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67:110–116. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Biran R, Martin DC, Tresco PA. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp Neurol. 2005;195:115–126. doi: 10.1016/j.expneurol.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Biran R, Martin DC, Tresco PA. The brain tissue response to implanted silicon microelectrode arrays is increased when the device is tethered to the skull. J Biomed Mater Res Part A. 2007;82:169–178. doi: 10.1002/jbm.a.31138. [DOI] [PubMed] [Google Scholar]
- Bittar RG, Kar-Purkayastha I, Owen SL, Bear RE, Green A, Wang S, Aziz TZ. Deep brain stimulation for pain relief: a meta-analysis. J Clin Neurosci. 2005;12:515–519. doi: 10.1016/j.jocn.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Bjarkam CR, Cancian G, Larsen M, Rosendahl F, Ettrup KS, Zeidler D, Blankholm AD, Østergaard L, Sunde N, Sørensen JC. A MRI-compatible stereotaxic localizer box enables high-precision stereotaxic procedures in pigs. J Neurosci Methods. 2004;139:293–298. doi: 10.1016/j.jneumeth.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Bjornsson CS, Oh SJ, Al-Kofahi YA, Lim YJ, Smith KL, Turner JN, De S, Roysam B, Shain W, Kim SJ. Effects of insertion conditions on tissue strain and vascular damage during neuroprosthetic device insertion. J Neural Eng. 2006;3:196–207. doi: 10.1088/1741-2560/3/3/002. [DOI] [PubMed] [Google Scholar]
- Boppart SA, Wheeler BC, Wallace CS. A flexible perforated microelectrode array for extended neural recordings. Biomed Eng IEEE Trans. 1992;39:37–42. doi: 10.1109/10.108125. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Shi R, Bohnert D. Behavioral recovery from spinal cord injury following delayed application of polyethylene glycol. J Exp Biol. 2002;205:1–12. doi: 10.1242/jeb.205.1.1. [DOI] [PubMed] [Google Scholar]
- Borton D, Yin M, Aceros J, Agha N, Minxha J, Komar J, Patterson W, Bull C, Nurmikko A. Developing implantable neuroprosthetics: A new model in pig. Engineering in Medicine and Biology Society, EMBC, 2011 Annual International Conference of the IEEE; 2011. pp. 3024–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, Horak FB, Okun MS, Foote KD, Krack P, et al. Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol. 2011;68:165. doi: 10.1001/archneurol.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butson CR, Maks CB, McIntyre CC. Sources and effects of electrode impedance during deep brain stimulation. Clin Neurophysiol. 2006;117:447–454. doi: 10.1016/j.clinph.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadotte AJ, DeMarse TB. Poly-HEMA as a drug delivery device for in vitro neural networks on micro-electrode arrays. J Neural Eng. 2005;2:114. doi: 10.1088/1741-2560/2/4/007. [DOI] [PubMed] [Google Scholar]
- Cao Y, He W. Synthesis and characterization of glucocorticoid functionalized poly (N-vinyl pyrrolidone): a versatile prodrug for neural interface. Biomacromolecules. 2010;11:1298–1307. doi: 10.1021/bm100095t. [DOI] [PubMed] [Google Scholar]
- Chen CH, Chang YC, Yeh SR, Yao DJ, Chuang SC, Lee YT. Three-dimensional flexible microprobe for recording the neural signal. J Micro/Nanolithography, MEMS, MOEMS. 2010;9:31007. [Google Scholar]
- Chen J, Wise KD, Hetke JF, Bledsoe SC. A multichannel neural probe for selective chemical delivery at the cellular level. Biomed Eng IEEE Trans. 1997;44:760–769. doi: 10.1109/10.605435. [DOI] [PubMed] [Google Scholar]
- Coffey RJ. Deep brain stimulation for chronic pain: results of two multicenter trials and a structured review. Pain Med. 2001;2:183–192. doi: 10.1046/j.1526-4637.2001.01029.x. [DOI] [PubMed] [Google Scholar]
- Cui XT, Lee Va, Raphael Y, Wiler Ja, Hetke JF, Anderson DJ, Martin DC. Surface modification of neural recording electrodes with conducting polymer/biomolecule blends. J Biomed Mater Res. 2001;56:261–72. doi: 10.1002/1097-4636(200108)56:2<261::aid-jbm1094>3.0.co;2-i. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11340598. [DOI] [PubMed] [Google Scholar]
- Cui XT, Wiler J, Dzaman M, Altschuler RA, Martin DC. In vivo studies of polypyrrole/peptide coated neural probes. Biomaterials. 2003;24:777–787. doi: 10.1016/s0142-9612(02)00415-5. [DOI] [PubMed] [Google Scholar]
- Cullen DK, Lessing MC, LaPlaca MC. Collagen-Dependent Neurite Outgrowth and Response to Dynamic Deformation in Three-Dimensional Neuronal Cultures. Ann Biomed Eng. 2007;35:835–846. doi: 10.1007/s10439-007-9292-z. [DOI] [PubMed] [Google Scholar]
- Edell DJ, Toi VV, McNeil VM, Clark LD. Factors influencing the biocompatibility of insertable siliconmicroshafts in cerebral cortex. Biomed Eng IEEE Trans. 1992;39:635–643. doi: 10.1109/10.141202. [DOI] [PubMed] [Google Scholar]
- Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J. 2006;90:3012–3018. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilletti A, Muthuswamy J. Brain micromotion around implants in the rodent somatosensory cortex. Neural Eng. 2006;3:189–195. doi: 10.1088/1741-2560/3/3/001. [DOI] [PubMed] [Google Scholar]
- Gizewski ER, Schanze T, Bolle I, de Greiff A, Forsting M, Laube T. Visualization of the visual cortex in minipigs using fMRI. Res Vet Sci. 2007;82:281–286. doi: 10.1016/j.rvsc.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Goldstein SR, Salcman M. Mechanical factors in the design of chronic recording intracortical microelectrodes. Biomed Eng IEEE Trans. 1973:260–269. doi: 10.1109/TBME.1973.324190. [DOI] [PubMed] [Google Scholar]
- Grill WM. Electrical Activation of Spinal Neural Circuits: Application to Motor-System Neural Prostheses. Neuromodulation. 2000;3:97–106. doi: 10.1046/j.1525-1403.2000.00097.x. [DOI] [PubMed] [Google Scholar]
- Hara SA, Kim BJ, Kuo JTW, Lee CD, Gutierrez CA, Hoang T, Pikov V, Meng E. Perforated 2x2 Parylene sheath electrode array for chronic intracortical recording. Neural Engineering (NER), 2013 6th International IEEE/EMBS Conference on; 2013. pp. 645–648. [Google Scholar]
- Harris JP, Capadona JR, Miller RH, Healy BC, Shanmuganathan K, Rowan SJ, Weder C, Tyler DJ. Mechanically adaptive intracortical implants improve the proximity of neuronal cell bodies. J Neural Eng. 2011a;8:66011. doi: 10.1088/1741-2560/8/6/066011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JP, Hess AE, Rowan SJ, Weder C, Zorman CA, Tyler DJ, Capadona JR. In vivo deployment of mechanically adaptive nanocomposites for intracortical microelectrodes. J Neural Eng. 2011b;8:46010. doi: 10.1088/1741-2560/8/4/046010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J, Tyler D. Biological, Mechanical, and Technological Considerations Affecting the Longevity of Intracortical Electrode Recordings. Crit Rev Biomed Eng. [PubMed] [Google Scholar]
- He W, Bellamkonda RV. Indwelling Neural Implants. In: Reichert Wm., editor. Frontiers in Neuroengineering. CRC; 2008. pp. 151–175. [Google Scholar]
- He W, Bellamkonda RV. Nanoscale neuro-integrative coatings for neural implants. Biomaterials. 2005;26:2983–2990. doi: 10.1016/j.biomaterials.2004.08.021. [DOI] [PubMed] [Google Scholar]
- He W, McConnell GC, Bellamkonda RV. Nanoscale laminin coating modulates cortical scarring response around implanted silicon microelectrode arrays. J Neural Eng. 2006;3:316–326. doi: 10.1088/1741-2560/3/4/009. [DOI] [PubMed] [Google Scholar]
- He W, McConnell GC, Schneider TM, Bellamkonda RV. A novel anti-inflammatory surface for neural electrodes. Adv Mater. 2007;19:3529. [Google Scholar]
- Henderson JM, Pell M, O’Sullivan DJ, McCusker EA, Fung VSC, Hedges P, Halliday GM. Postmortem analysis of bilateral subthalamic electrode implants in Parkinson’s disease. Mov Disord. 2002;17:133–137. doi: 10.1002/mds.1261. [DOI] [PubMed] [Google Scholar]
- Hess AE, Capadona JR, Shanmuganathan K, Hsu L, Rowan SJ, Weder C, Tyler DJ, Zorman CA. Development of a stimuli-responsive polymer nanocomposite toward biologically optimized, MEMS-based neural probes. J Micromechanics Microengineering. 2011;21:54009. [Google Scholar]
- Hochberg LR, Cochrane T. Implanted Neural Interfaces. Neuroethics Pract. 2013:235. [Google Scholar]
- Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2012;64:18–23. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- Hughes PS, Krcek JP, Hobson DE, Del Bigio MR. An unusual inflammatory response to implanted deep brain electrodes. Can J Neurol Sci. 2011;38:168–170. [PubMed] [Google Scholar]
- In S, Congdon N, O’Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P. Causes and Prevalence of Visual Impairment Among Adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- James CD, Davis R, Meyer M, Turner A, Turner S, Withers G, Kam L, Banker G, Craighead HG, Issacson M, et al. Aligned microcontact printing of micrometer-scale poly-L-Lysinestructures for controlled growth of cultured neurons on planar microelectrode arrays. Biomed Eng IEEE Trans. 2000;47:17–21. doi: 10.1109/10.817614. [DOI] [PubMed] [Google Scholar]
- Jedlicka SS, Little KM, Nivens DE, Zemlyanov DY, Rickus JL. Peptide ormosils as cellular substrates. J Mater Chem. 2007a;17:5058–5067. [Google Scholar]
- Jedlicka SS, McKenzie JL, Leavesley SJ, Little KM, Webster TJ, Robinson JP, Nivens DE, Rickus JL. Sol-gel derived materials as substrates for neuronal differentiation: effects of surface features and protein conformation. J Mater Chem. 2006;16:3221–3230. [Google Scholar]
- Jedlicka SS, Rickus JL, Zemlyanov DY. Surface analysis by X-ray photoelectron spectroscopy of sol-gel silica modified with covalently bound peptides. J Phys Chem B. 2007b;111:11850–7. doi: 10.1021/jp0744230. [DOI] [PubMed] [Google Scholar]
- Jhaveri SJ, Hynd MR, Dowell-Mesfin N, Turner JN, Shain W, Ober CK. Release of nerve growth factor from HEMA hydrogel-coated substrates and its effect on the differentiation of neural cells. Biomacromolecules. 2008;10:174–183. doi: 10.1021/bm801101e. [DOI] [PubMed] [Google Scholar]
- Jiménez F, Velasco F, Salin-Pascual R, Hernández JA, Velasco M, Criales JL, Nicolini H. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57:585–593. doi: 10.1227/01.neu.0000170434.44335.19. [DOI] [PubMed] [Google Scholar]
- St John PM, Kam L, Turner SW, Craighead HG, Issacson M, Turner JN, Shain W. Preferential glial cell attachment to microcontact printed surfaces. J Neurosci Methods. 1997;75:171–177. doi: 10.1016/S0165-0270(97)00069-1. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Otto KJ, Kipke DR. Repeated voltage biasing improves unit recordings by reducing resistive tissue impedances. Neural Syst Rehabil Eng IEEE Trans. 2005;13:160–165. doi: 10.1109/TNSRE.2005.847373. [DOI] [PubMed] [Google Scholar]
- Kam L, Shain W, Turner JN, Bizios R. Selective adhesion of astrocytes to surfaces modified with immobilized peptides. Biomaterials. 2002;23:511–515. doi: 10.1016/s0142-9612(01)00133-8. [DOI] [PubMed] [Google Scholar]
- Karumbaiah L, Norman SE, Rajan NB, Anand S, Saxena T, Betancur M, Patkar R, Bellamkonda RV. The upregulation of specific interleukin (IL) receptor antagonists and paradoxical enhancement of neuronal apoptosis due to electrode induced strain and brain micromotion. Biomaterials. 2012;33:5983–5996. doi: 10.1016/j.biomaterials.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Karumbaiah L, Saxena T, Carlson D, Patil K, Patkar R, Gaupp EA, Betancur M, Stanley GB, Carin L, Bellamkonda RV. Relationship between intracortical electrode design and chronic recording function. Biomaterials. 2013;34:8061–8074. doi: 10.1016/j.biomaterials.2013.07.016. [DOI] [PubMed] [Google Scholar]
- Katz JS, Burdick JA. Hydrogel mediated delivery of trophic factors for neural repair. Wiley Interdiscip Rev Nanomedicine Nanobiotechnology. 2009;1:128–139. doi: 10.1002/wnan.10. [DOI] [PubMed] [Google Scholar]
- Kerstein PC, Jacques-Fricke BT, Rengifo J, Mogen BJ, Williams JC, Gottlieb Pa, Sachs F, Gomez TM. Mechanosensitive TRPC1 channels promote calpain proteolysis of talin to regulate spinal axon outgrowth. J Neurosci. 2013;33:273–85. doi: 10.1523/JNEUROSCI.2142-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Abidian MR, Martin DC. Conducting polymers grown in hydrogel scaffolds coated on neural prosthetic devices. J Biomed Mater Res. 2004a;71:577–585. doi: 10.1002/jbm.a.30124. [DOI] [PubMed] [Google Scholar]
- Kim DH, Martin DC. Sustained release of dexamethasone from hydrophilic matrices using PLGA nanoparticles for neural drug delivery. Biomaterials. 2006;27:3031–3037. doi: 10.1016/j.biomaterials.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Kim DH, Wiler JA, Anderson DJ, Kipke DR, Martin DC. Conducting polymers on hydrogel-coated neural electrode provide sensitive neural recordings in auditory cortex. Acta Biomater. 2010;6:57–62. doi: 10.1016/j.actbio.2009.07.034. [DOI] [PubMed] [Google Scholar]
- Kim EGR, John JK, Tu H, Zheng Q, Loeb J, Zhang J, Xu Y. A hybrid silicon-parylene neural probe with locally flexible regions. Sensors Actuators B Chem 2014 [Google Scholar]
- Kim YT, Hitchcock RW, Bridge MJ, Tresco PA. Chronic response of adult rat brain tissue to implants anchored to the skull. Biomaterials. 2004b;25:2229–2237. doi: 10.1016/j.biomaterials.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Koob AO, Borgens RB. Polyethylene glycol treatment after traumatic brain injury reduces beta-amyloid precursor protein accumulation in degenerating axons. J Neurosci Res. 2006;83:1558–1563. doi: 10.1002/jnr.20837. [DOI] [PubMed] [Google Scholar]
- Koob AO, Colby JM, Borgens RB. Behavioral recovery from traumatic brain injury after membrane reconstruction using polyethylene glycol. J Biol Eng. 2008;2:9. doi: 10.1186/1754-1611-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob AO, Duerstock BS, Babbs CF, Sun Y, Borgens RB. Intravenous Polyethylene Glycol Inhibits the Loss of Cerebral Cells after Brain Injury. J Neurotrauma. 2005;22:1092–1111. doi: 10.1089/neu.2005.22.1092. [DOI] [PubMed] [Google Scholar]
- Kozai TDY, Kipke DR. Insertion shuttle with carboxyl terminated self-assembled monolayer coatings for implanting flexible polymer neural probes in the brain. J Neurosci Methods. 2009;184:199–205. doi: 10.1016/j.jneumeth.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozai TDY, Langhals NB, Patel PR, Deng X, Zhang H, Smith KL, Lahann J, Kotov NA, Kipke DR. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat Mater. 2012;11:1065–1073. doi: 10.1038/nmat3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach JB, Brown XQ, Jacot JG, DiMilla PA, Wong JY. Neurite outgrowth and branching of PC12 cells on very soft substrates sharply decreases below a threshold of substrate rigidity. J Neural Eng. 2007;4:26. doi: 10.1088/1741-2560/4/2/003. [DOI] [PubMed] [Google Scholar]
- Lee H, Bellamkonda RV, Sun W, Levenston ME. Biomechanical analysis of silicon microelectrode-induced strain in the brain. J Neural Eng. 2005;2:81–89. doi: 10.1088/1741-2560/2/4/003. [DOI] [PubMed] [Google Scholar]
- Lee K, Singh A, He J, Massia SP, Kim B, Raupp GB. Polyimide based neural implants with stiffness improvement. Sensors Actuators B Chem. 2004;102:67–72. [Google Scholar]
- Lempka SF, Miocinovic S, Johnson MD, Vitek JL, McIntyre CC. In vivo impedance spectroscopy of deep brain stimulation electrodes. J Neural Eng. 2009;6:46001. doi: 10.1088/1741-2560/6/4/046001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenarz T, Lim HH, Reuter G, Patrick JF, Lenarz M. The Auditory Midbrain Implant: A New Auditory Prosthesis for Neural Deafness-Concept and Device Description. Otol Neurotol. 2006;27:838. doi: 10.1097/01.mao.0000232010.01116.e9. [DOI] [PubMed] [Google Scholar]
- Leung G, Sun W, Zheng L, Brookes S, Tully M, Shi R. Anti-acrolein treatment improves behavioral outcome and alleviates myelin damage in experimental autoimmune enchephalomyelitis mouse. Neuroscience. 2011;173:150–155. doi: 10.1016/j.neuroscience.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy RM, Lamb S, Adams JE. Treatment of chronic pain by deep brain stimulation: long term follow-up and review of the literature. Neurosurgery. 1987;21:885–893. doi: 10.1227/00006123-198712000-00017. [DOI] [PubMed] [Google Scholar]
- Lewitus D, Smith KL, Shain W, Kohn J. Ultrafast resorbing polymers for use as carriers for cortical neural probes. Acta Biomater. 2011;7:2483–2491. doi: 10.1016/j.actbio.2011.02.027. [DOI] [PubMed] [Google Scholar]
- Lind G, Linsmeier CE, Schouenborg J. The density difference between tissue and neural probes is a key factor for glial scarring. Sci Rep. 2013:3. doi: 10.1038/srep02942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind G, Linsmeier CE, Thelin J, Schouenborg J. Gelatine-embedded electrodes–a novel biocompatible vehicle allowing implantation of highly flexible microelectrodes. J Neural Eng. 2010;7:46005. doi: 10.1088/1741-2560/7/4/046005. [DOI] [PubMed] [Google Scholar]
- Lind NM, Moustgaard A, Jelsing J, Vajta G, Cumming P, Hansen AK. The use of pigs in neuroscience: modeling brain disorders. Neurosci Biobehav Rev. 2007;31:728–751. doi: 10.1016/j.neubiorev.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Loeb GE. Cochlear Prosthetics. Annu Rev Neurosci. 1990;13:357–371. doi: 10.1146/annurev.ne.13.030190.002041. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Dostrovsky J, Chen R, Ashby P. Deep brain stimulation for Parkinson’s disease: disrupting the disruption. Lancet Neurol. 2002;1:225–231. doi: 10.1016/s1474-4422(02)00101-1. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Ludwig KA, Langhals NB, Joseph MD, Richardson-Burns SM, Hendricks JL, Kipke DR. Poly(3,4-ethylenedioxythiophene) (PEDOT) polymer coatings facilitate smaller neural recording electrodes. J Neural Eng. 2011;8:14001. doi: 10.1088/1741-2560/8/1/014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig KA, Uram JD, Yang J, Martin DC, Kipke DR. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. J Neural Eng. 2006;3:59–70. doi: 10.1088/1741-2560/3/1/007. [DOI] [PubMed] [Google Scholar]
- Luo J, Borgens R, Shi R. Polyethylene glycol immediately repairs neuronal membranes and inhibits free radical production after acute spinal cord injury. J Neurochem. 2002;83:471–480. doi: 10.1046/j.1471-4159.2002.01160.x. [DOI] [PubMed] [Google Scholar]
- Malone DA, Jr, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, Rauch SL, Rasmussen SA, Machado AG, Kubu CS, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65:267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwardt NT, Stokol J, RLR Sub-meninges implantation reduces immune response to neural implants. J Neurosci Methods. 2013 doi: 10.1016/j.jneumeth.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massia SP, Holecko MM, Ehteshami GR. In vitro assessment of bioactive coatings for neural implant applications. J Biomed Mater Res. 2004;68:177–186. doi: 10.1002/jbm.a.20009. [DOI] [PubMed] [Google Scholar]
- McCarthy PT, Otto KJ, Rao MP. Robust penetrating microelectrodes for neural interfaces realized by titanium micromachining. Biomed Microdevices. 2011a;13:503–515. doi: 10.1007/s10544-011-9519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy PT, Rao MP, Otto KJ. Simultaneous recording of rat auditory cortex and thalamus via a titanium-based, microfabricated, microelectrode device. J Neural Eng. 2011b;8:046007. doi: 10.1088/1741-2560/8/4/046007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill DR, Tresco PA. Impedance Characterization of Microarray Recording Electrodes In Vitro. In Vivo (Brooklyn) 2004:4349–4352. doi: 10.1109/IEMBS.2004.1404210. [DOI] [PubMed] [Google Scholar]
- Metz S, Bertsch A, Bertrand D, Renaud P. Flexible polyimide probes with microelectrodes and embedded microfluidic channels for simultaneous drug delivery and multi-channel monitoring of bioelectric activity. Biosens Bioelectron. 2004;19:1309–1318. doi: 10.1016/j.bios.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Mohr PE, Feldman JJ, Dunbar JL, McConkey-Robbins A, Niparko JK, Rittenhouse RK, Skinner MW. The Societal Costs of severe to profound hearing loss in the United States. Int J Technol Assess Health Care. 2001;16:1120–1135. doi: 10.1017/s0266462300103162. [DOI] [PubMed] [Google Scholar]
- Moss J, Ryder T, Aziz TZ, Graeber MB, Bain PG. Electron microscopy of tissue adherent to explanted electrodes in dystonia and Parkinson’s disease. Brain. 2004;127:2755–2763. doi: 10.1093/brain/awh292. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA. Brain-machine interfaces to restore motor function and probe neural circuits. Nat Rev Neurosci. 2003;4:417–422. doi: 10.1038/nrn1105. [DOI] [PubMed] [Google Scholar]
- Nielsen MS, Bjarkam CR, Sørensen JC, Bojsen-Møller M, Sunde NA, Østergaard K. Chronic subthalamic high-frequency deep brain stimulation in Parkinson’s disease--a histopathological study. Eur J Neurol. 2007;14:132–138. doi: 10.1111/j.1468-1331.2006.01569.x. [DOI] [PubMed] [Google Scholar]
- Nisbet DR, Crompton KE, Horne MK, Finkelstein DI, Forsythe JS. Neural tissue engineering of the CNS using hydrogels. J Biomed Mater Res Part B Appl Biomater. 2008;87:251–263. doi: 10.1002/jbm.b.31000. [DOI] [PubMed] [Google Scholar]
- NSCISC. NSCISC Spinal cord injury facts and figures at a glance. J Spinal Cord Med. 2010;33:439–40. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21061906. [PubMed] [Google Scholar]
- Otto KJ, Johnson MD, Kipke DR. Voltage Pulses Change Neural Interface Properties and Improve Unit Recordings With Chronically Implanted Microelectrodes. Engineering. 2006;53:333–340. doi: 10.1109/TBME.2005.862530. [DOI] [PubMed] [Google Scholar]
- Otto K, Williams J. Novel in vitro and in vivo neural interfaces: normal and accelerated failure assessment. IEEE Pulse. 2012;3:27–29. doi: 10.1109/MPUL.2011.2175631. [DOI] [PubMed] [Google Scholar]
- Papageorgiou D, Bledsoe SC, Gulari M, Hetke JF, Anderson DJ, Wise KD. A shuttered probe with in-line flowmeters for chronic in-vivo drug delivery. Micro Electro Mechanical Systems, 2001. MEMS 2001. The 14th IEEE International Conference on; 2001. pp. 212–215. [Google Scholar]
- Papageorgiou DP, Shore SE, Bledsoe SC, Wise KD. A shuttered neural probe with on-chip flowmeters for chronic in vivo drug delivery. Microelectromechanical Syst J. 2006;15:1025–1033. [Google Scholar]
- Park H, Park K, Shalaby WSW. Biodegradable hydrogels for drug delivery. CRC Press; 2011. [Google Scholar]
- Parker ER, Rao MP, Turner KL, Meinhart CD, MacDonald NC. Bulk micromachined titanium microneedles. Microelectromechanical Syst J. 2007;16:289–295. [Google Scholar]
- Pellinen D, Moon T, Vetter R, Miriani R, Kipke D. Multifunctional flexible parylene-based intracortical microelectrodes. Conf Proc IEEE Eng Med Biol Soc. 2005;5:5272–5275. doi: 10.1109/IEMBS.2005.1615669. [DOI] [PubMed] [Google Scholar]
- Peppas NA. Hydrogels and drug delivery. Curr Opin Colloid Interface Sci. 1997;2:531–537. [Google Scholar]
- Perge JA, Homer ML, Malik WQ, Cash S, Eskandar E, Friehs G, Donoghue JP, Hochberg LR. Intra-day signal instabilities affect decoding performance in an intracortical neural interface system. J Neural Eng. 2013;10:36004. doi: 10.1088/1741-2560/10/3/036004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce AL, Sommakia S, Rickus JL, Otto KJ. Thin-film silica sol-gel coatings for neural microelectrodes. J Neurosci Methods. 2009;180:106–110. doi: 10.1016/j.jneumeth.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005;148:1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Potter KA, Buck AC, Self WK, Callanan ME, Sunil S, Capadona JR. The effect of resveratrol on neurodegeneration and blood brain barrier stability surrounding intracortical microelectrodes. Biomaterials. 2013 doi: 10.1016/j.biomaterials.2013.05.035. [DOI] [PubMed] [Google Scholar]
- Potter KA, Buck AC, Self WK, Capadona JR. Stab injury and device implantation within the brain results in inversely multiphasic neuroinflammatory and neurodegenerative responses. J Neural Eng. 2012;9:046020. doi: 10.1088/1741-2560/9/4/046020. [DOI] [PubMed] [Google Scholar]
- Potter KA, Jorfi M, Householder KT, Johan Foster E, Weder C, Capadona JR. Curcumin-releasing mechanically-adaptive intracortical implants improve the proximal neuronal density and blood-brain barrier stability. Acta Biomater. 2014 doi: 10.1016/j.actbio.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Potter-Baker KA, Nguyen JK, Kovach KM, Gitomer MM, Srail TW, Stewart WG, Skousen JL, Capadona JR. Development of superoxide dismutase mimetic surfaces to reduce accumulation of reactive oxygen species for neural interfacing applications. J Mater Chem B. 2014;2:2248–2258. doi: 10.1039/C4TB00125G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A, Sanchez JC. Quantifying long-term microelectrode array functionality using chronic in vivo impedance testing. J Neural Eng. 2012;9:026028. doi: 10.1088/1741-2560/9/2/026028. [DOI] [PubMed] [Google Scholar]
- Prasad A, Xue QS, Sankar V, Nishida T, Shaw G, Streit WJ, Sanchez JC. Comprehensive characterization and failure modes of tungsten microwire arrays in chronic neural implants. J Neural Eng. 2012;9:056015. doi: 10.1088/1741-2560/9/5/056015. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2001;53:321–339. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- Radin S, Chen T, Ducheyne P. The controlled release of drugs from emulsified, sol gel processed silica microspheres. Biomaterials. 2009;30:850–858. doi: 10.1016/j.biomaterials.2008.09.066. [DOI] [PubMed] [Google Scholar]
- Radin S, Ducheyne P. Controlled release of vancomycin from thin sol-gel films on titanium alloy fracture plate material. Biomaterials. 2007;28:1721–1729. doi: 10.1016/j.biomaterials.2006.11.035. [DOI] [PubMed] [Google Scholar]
- Radin S, Ducheyne P. Learning from Nature How to Design New Implantable Biomaterialsis: From Biomineralization Fundamentals to Biomimetic Materials and Processing Routes. Springer; 2005. Nanostructural control of implantable xerogels for the controlled release of biomolecules; pp. 59–74. [Google Scholar]
- Rakuman H, Ferose W, Ong XC, Tetikol HS, Khilwani R, Cui XT, Ozdoganlar OB, Fedder GK, Gilgunn PJ. Ultra-compliant neural probes are subject to fluid forces during dissolution of polymer delivery vehicles. in. Engineering in Medicine and Biology Society (EMBC), 2013 35th Annual International Conference of the IEEE; 2013. pp. 1550–1553. [DOI] [PubMed] [Google Scholar]
- Ravikumar M, Hageman DJ, Tomaszewski WH, Chandra GM, Skousen JL, Capadona JR, et al. The Effect of Residual Endotoxin Contamination on the Neuroinflammatory Response to Sterilized Intracortical Microelectrodes. J Mater Chem B. 2013 doi: 10.1039/C3TB21453B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennaker RL, Miller J, Tang H, Wilson DA. Minocycline increases quality and longevity of chronic neural recordings. J Neural Eng. 2007;4:L1. doi: 10.1088/1741-2560/4/2/L01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennaker RL, Street S, Ruyle AM, Sloan AM. A comparison of chronic multi-channel cortical implantation techniques: manual versus mechanical insertion. J Neurosci Methods. 2005;142:169–176. doi: 10.1016/j.jneumeth.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Retterer ST, Smith KL, Bjornsson CS, Neeves KB, Spence AJH, Turner JN, Shain W, Isaacson MS. Model neural prostheses with integrated microfluidics: a potential intervention strategy for controlling reactive cell and tissue responses. Biomed Eng IEEE Trans. 2004;51:2063–2073. doi: 10.1109/TBME.2004.834288. [DOI] [PubMed] [Google Scholar]
- Retterer ST, Smith KL, Bjornsson CS, Turner JN, Isaacson MS, Shain W. Constant pressure fluid infusion into rat neocortex from implantable microfluidic devices. J Neural Eng. 2008;5:385. doi: 10.1088/1741-2560/5/4/003. [DOI] [PubMed] [Google Scholar]
- Rodger DC, Fong AJ, Li W, Ameri H, Ahuja AK, Gutierrez C, Lavrov I, Zhong H, Menon PR, Meng E, et al. Flexible parylene-based multielectrode array technology for high-density neural stimulation and recording. Sensors Actuators, B Chem. 2008;132:449–460. [Google Scholar]
- Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto JL, Pollak P, Rehncrona S, Kulisevsky J, Albanese A, Volkmann J, Hariz MI, et al. Bilateral deep brain stimulation in Parkinson’s disease: a multicentre study with 4 years follow-up. Brain. 2005;128:2240–2249. doi: 10.1093/brain/awh571. [DOI] [PubMed] [Google Scholar]
- Rohatgi P, Langhals NB, Kipke DR, Patil PG. In vivo performance of a microelectrode neural probe with integrated drug delivery: Laboratory investigation. Neurosurg Focus. 2009;27:E8. doi: 10.3171/2009.4.FOCUS0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousche PJ, Normann RA. A method for pneumatically inserting an array of penetrating electrodes into cortical tissue. Ann Biomed Eng. 1992;20:413–422. doi: 10.1007/BF02368133. [DOI] [PubMed] [Google Scholar]
- Rousche PJ, Pellinen DS, Pivin DP, Jr, Williams JC, Vetter RJ, Pivin DP, Kipke DR. Flexible polyimide-based intracortical electrode arrays with bioactive capability. Biomed Eng IEEE Trans. 2001;48:361–371. doi: 10.1109/10.914800. [DOI] [PubMed] [Google Scholar]
- Santhanam G, Linderman MD, Gilja V, Afshar A, Ryu SI, Meng TH, Shenoy KV. HermesB: a continuous neural recording system for freely behaving primates. Biomed Eng IEEE Trans. 2007;54:2037–2050. doi: 10.1109/TBME.2007.895753. [DOI] [PubMed] [Google Scholar]
- Sauleau P, Lapouble E, Val-Laillet D, Malbert CH. The pig model in brain imaging and neurosurgery. animal. 2009;3:1138–1151. doi: 10.1017/S1751731109004649. [DOI] [PubMed] [Google Scholar]
- Saxena T, Karumbaiah L, Gaupp EA, Patkar R, Patil K, Betancur M, Stanley GB, Bellamkonda RV. The impact of chronic blood--brain barrier breach on intracortical electrode function. Biomaterials. 2013 doi: 10.1016/j.biomaterials.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Schcke H, Docke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- Schendel Aa, Thongpang S, Brodnick SK, Richner TJ, Lindevig BDB, Krugner-Higby L, Williams JC. A cranial window imaging method for monitoring vascular growth around chronically implanted micro-ECoG devices. J Neurosci Methods. 2013;218:121–30. doi: 10.1016/j.jneumeth.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- Seymour JP, Kipke DR. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials. 2007;28:3594–3607. doi: 10.1016/j.biomaterials.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Seymour JP, Langhals NB, Anderson DJ, Kipke DR. Novel multi-sided, microelectrode arrays for implantable neural applications. Biomed Microdevices. 2011;13:441–451. doi: 10.1007/s10544-011-9512-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shain W, Spataro L, Dilgen J, Haverstick K, Retterer S, Isaacson M, Saltzman M, Turner JN. Controlling cellular reactive responses around neural prosthetic devices using peripheral and local intervention strategies. Neural Syst Rehabil Eng IEEE Trans. 2003;11:186–188. doi: 10.1109/TNSRE.2003.814800. [DOI] [PubMed] [Google Scholar]
- Skousen JL, Merriam SME, Srivannavit O, Perlin G, Wise KD, Tresco PA. Reducing surface area while maintaining implant penetrating profile lowers the brain foreign body response to chronically implanted planar silicon microelectrode arrays. Prog Brain Res. 2011;194:167. doi: 10.1016/B978-0-444-53815-4.00009-1. [DOI] [PubMed] [Google Scholar]
- Sommakia S. Effects of dip-coated films on the properties of implantable intracortical microelectrodes. 2013. [Google Scholar]
- Sommakia S, Rickus JL, Otto KJ. Effects of adsorbed proteins, an antifouling agent and long-duration DC voltage pulses on the impedance of silicon-based neural microelectrodes. Proceedings of the IEEE Engineering in Medicine and Biology Conference. 2009:7139–42. doi: 10.1109/IEMBS.2009.5332456. [DOI] [PubMed] [Google Scholar]
- Spataro L, Dilgen J, Retterer S, Spence AJ, Isaacson M, Turner JN, Shain W. Dexamethasone treatment reduces astroglia responses to inserted neuroprosthetic devices in rat neocortex. Exp Neurol. 2005;194:289–300. doi: 10.1016/j.expneurol.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Sporns O, Edelman GM, Crossin KL, Krushel LA, Cunningham BA. The neural cell adhesion molecule (N-CAM) inhibits proliferation in primary cultures of rat astrocytes. Proc Natl Acad Sci U S A. 1995;92:4323. doi: 10.1073/pnas.92.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer WR, Cui XT. Polypyrrole doped with 2 peptide sequences from laminin. Biomaterials. 2006;27:2405–2413. doi: 10.1016/j.biomaterials.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Stieglitz T, Schuettler M, Meyer JU, et al. Micromachined, polyimide-based devices for flexible neural interfaces. Biomed Microdevices. 2000;2:283–294. [Google Scholar]
- Subbaroyan J, Martin DC, Kipke DR. A finite-element model of the mechanical effects of implantable microelectrodes in the cerebral cortex. Neural Eng. 2005;2:103–113. doi: 10.1088/1741-2560/2/4/006. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Mabuchi K, Takeuchi S. A 3D flexible parylene probe array for multichannel neural recording. First Int IEEE EMBS Conf Neural Eng 2003 Conf Proceedings. 2003 doi: 10.1109/CNE.2003.1196780. [DOI] [Google Scholar]
- Szarowski DH, Andersen MD, Retterer S, Spence AJ, Isaacson M, Craighead HG, Turner JN, Shain W. Brain responses to micro-machined silicon devices. Brain Res. 2003;983:23–35. doi: 10.1016/s0006-8993(03)03023-3. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Suzuki T, Mabuchi K, Fujita H. 3D flexible multichannel neural probe array. J micromechanics microengineering. 2004;14:104. [Google Scholar]
- Takeuchi S, Ziegler D, Yoshida Y, Mabuchi K, Suzuki T. Parylene flexible neural probes integrated with microfluidic channels. Lab Chip. 2005;5:519–523. doi: 10.1039/b417497f. [DOI] [PubMed] [Google Scholar]
- Thelin J, Jörntell H, Psouni E, Garwicz M, Schouenborg J, Danielsen N, Linsmeier CE. Implant size and fixation mode strongly influence tissue reactions in the CNS. PLoS One. 2011;6:e16267. doi: 10.1371/journal.pone.0016267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tysseling-Mattiace VM, Sahni V, Niece KL, Birch D, Czeisler C, Fehlings MG, Stupp SI, Kessler JA. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci. 2008;28:3814–3823. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura A, Jaggi JL, Hurtig HI, Siderowf AD, Colcher A, Stern MB, Baltuch GH. Deep brain stimulation for movement disorders: morbidity and mortality in 109 patients. J Neurosurg. 2003;98:779–784. doi: 10.3171/jns.2003.98.4.0779. [DOI] [PubMed] [Google Scholar]
- Vetter RJ, Williams JC, Hetke JF, Nunamaker EA, Kipke DR. Chronic neural recording using silicon-substrate microelectrode arrays implanted in cerebral cortex. Biomed Eng IEEE Trans. 2004;51:896–904. doi: 10.1109/TBME.2004.826680. [DOI] [PubMed] [Google Scholar]
- Wadhwa R, Lagenaur CF, Cui XT. Electrochemically controlled release of dexamethasone from conducting polymer polypyrrole coated electrode. J Control Release. 2006;110:531–541. doi: 10.1016/j.jconrel.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Ware T, Simon D, Liu C, Musa T, Vasudevan S, Sloan A, Keefer EW, Rennaker RL, Voit W. Thiol-ene/acrylate substrates for softening intracortical electrodes. J Biomed Mater Res Part B Appl Biomater. 2014;102:1–11. doi: 10.1002/jbmb.32946. [DOI] [PubMed] [Google Scholar]
- Welkenhuysen M, Andrei A, Ameye L, Eberle W, Nuttin B. Effect of insertion speed on tissue response and insertion mechanics of a chronically implanted silicon-based neural probe. Biomed Eng IEEE Trans. 2011;58:3250–3259. doi: 10.1109/TBME.2011.2166963. [DOI] [PubMed] [Google Scholar]
- Wilks SJ, Richardson-Burns SM, Hendricks JL, Martin DC, Otto KJ. Poly (3, 4-ethylenedioxythiophene) as a micro-neural interface material for electrostimulation. Front Neuroeng. 2009:2. doi: 10.3389/neuro.16.007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerth SM, Sakiyama-Elbert SE. Approaches to neural tissue engineering using scaffolds for drug delivery. Adv Drug Deliv Rev. 2007;59:325–338. doi: 10.1016/j.addr.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JC, Hippensteel JA, Dilgen J, Shain W, Kipke DR. Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants. J Neural Eng. 2007;4:410–423. doi: 10.1088/1741-2560/4/4/007. [DOI] [PubMed] [Google Scholar]
- Winslow BD, Christensen MB, Yang WK, Solzbacher F, Tresco PA. A comparison of the tissue response to chronically implanted Parylene-C-coated and uncoated planar silicon microelectrode arrays in rat cortex. Biomaterials. 2010;31:9163–9172. doi: 10.1016/j.biomaterials.2010.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter JO, Cogan SF, Rizzo JF. Neurotrophin-eluting hydrogel coatings for neural stimulating electrodes. J Biomed Mater Res B Appl Biomater. 2007;81:551–563. doi: 10.1002/jbmb. [DOI] [PubMed] [Google Scholar]
- Woods SP, Fields JA, Tröster AI. Neuropsychological sequelae of subthalamic nucleus deep brain stimulation in Parkinson’s disease: a critical review. Neuropsychol Rev. 2002;12:111–126. doi: 10.1023/a:1016806711705. [DOI] [PubMed] [Google Scholar]
- Woolley AJ, Desai HA, Gaire J, Ready AL, Otto KJ. A Systemic Triple Label Strategy for Fluorescent Microscopy of Inflammation in CNS and Non-CNS Tissue. Microsc Microanal. 2013a;19:196–197. [Google Scholar]
- Woolley AJ, Desai HA, Gaire J, Ready AL, Otto KJ. Intact Histological Characterization of Brain-implanted Microdevices and Surrounding Tissue. J Vis Exp JoVE. 2013b doi: 10.3791/50126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley AJ, Desai HA, Otto KJ. Chronic intracortical microelectrode arrays induce non-uniform, depth-related tissue responses. J Neural Eng. 2013c;10:026007. doi: 10.1088/1741-2560/10/2/026007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley AJ, Desai HA, Steckbeck MA, Patel NK, Otto KJ. In situ characterization of the brain-microdevice interface using device-capture histology. J Neurosci Methods. 2011;201:67–77. doi: 10.1016/j.jneumeth.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zheng Q, Du J, Song Y, Wu B, Guo X. Self-assembled IKVAV peptide nanofibers promote adherence of PC12 cells. J Huazhong Univ Sci Technol Med. 2006;26:594–596. doi: 10.1007/s11596-006-0530-7. [DOI] [PubMed] [Google Scholar]
- Yu TT, Shoichet MS, TTYU Guided cell adhesion and outgrowth in peptide-modified channels for neural tissue engineering. Biomaterials. 2005;26:1507–1514. doi: 10.1016/j.biomaterials.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Yoo R, Wells M, Beebe TP, Biran R, Tresco PA. Neurite outgrowth on well-characterized surfaces: preparation and characterization of chemically and spatially controlled fibronectin and RGD substrates with good bioactivity. Biomaterials. 2005;26:47–61. doi: 10.1016/j.biomaterials.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Bellamkonda RV. Controlled release of anti-inflammatory agent α-MSH from neural implants. J Control Release. 2005;106:309–318. doi: 10.1016/j.jconrel.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Bellamkonda RV. Dexamethasone-coated neural probes elicit attenuated inflammatory response and neuronal loss compared to uncoated neural probes. Brain Res. 2007;1148:15–27. doi: 10.1016/j.brainres.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu R, Yoon H, Varadan VK, Smith CS, Huang GL. Biomechanical strain analysis at the interface of brain and nanowire electrodes on a neural probe. J Nanotechnol Eng Med. 2011;2:31001. [Google Scholar]
- Ziegler D, Suzuki T, Takeuchi S. Fabrication of flexible neural probes with built-in microfluidic channels by thermal bonding of parylene. Microelectromechanical Syst J. 2006;15:1477–1482. [Google Scholar]