Abstract
Objective
To assess the effects of rapid voluntary counselling and testing (VCT) for HIV on HIV incidence and uptake of HIV/AIDS services in people at high risk for HIV exposure.
Design
Cochrane systematic review and meta-analysis.
Data sources
We searched PubMed, EMBASE, AIDSearch, LILACS, Global Health, Medline Africa, PsychInfo, CINAHL, Cochrane CENTRAL, Cochrane HIV/AIDS Group Specialized Register and grey literature from 1 January 2001 to 5 June 2014 without language restriction.
Data selection
We included controlled studies that compared rapid VCT with conventional testing among people at risk for HIV exposure.
Data extraction
Two reviewers extracted data. We used Cochrane risk of bias tool and GRADE criteria: risk of bias, inconsistency, indirectness, imprecision and publication bias. For observational studies we used the Newcastle-Ottawa Scale. We used the PRISMA-Equity reporting guideline.
Results
From 2441 articles, we included 8 randomised controlled trials and 5 observational studies. Rapid VCT was associated with a threefold increase in HIV-testing uptake (relative risk (RR)=2.95 95% CI 1.69 to 5.16) and a twofold increase in the receipt of test results (RR=2.14, 95% CI 1.08 to 4.24). Women accepted testing more often than men in rapid VCT arm, but no differences in effect for age or socioeconomic status. Observational studies also showed rapid VCT led to higher rates of uptake of testing. Heterogeneity was high. A cluster-randomised trial reported an 11% reduction in HIV incidence in intervention communities (RR=0.89, 95% CI=0.63 to 1.24) over 3 years trial.
Conclusions
Rapid VCT in health facilities and communities was associated with a large increase in HIV-testing uptake and receipt of results. This has implications for WHO guidelines. The routine use of rapid VCT may also help avoid human rights violations among marginalised populations where testing may occur without informed consent and where existing stigma may create barriers to testing.
Keywords: HIV Testing, Rapid VCT, HIV Services
Strengths and limitations of this study.
This Cochrane systematic review included randomised controlled trials (RCTs) and observational studies from four continents and included a range of groups at high risk for HIV exposure.
This review included rapid voluntary counselling and testing (VCT) interventions from health facilities and community-based interventions.
RCTs showed that rapid VCT was associated with a large increase in HIV-testing uptake and receipt of results but these studies did not report on antiretroviral treatment.
Observational studies showed increased acceptance of HIV testing and did not show age, sex or income differences.
Across the studies there was significant heterogeneity likely due to variations in settings and implementation.
We found only a small number of RCTs (seven) and comparisons were limited for the various rapid VCT interventions with significant heterogeneity likely due to setting and implementation setting differences.
Introduction
HIV counselling and testing is the starting point for treatment and care and play a key role in the UNAIDS’ ‘Getting to zero’ strategy.1 According to 2012 UNAIDS data, about 50% of people living with HIV are unaware of their diagnosis.1–3 Delays in diagnosis result in lost opportunity for prevention and treatment, resulting in poorer health outcomes.4–6 While early diagnosis and treatment has been shown to improve clinical outcomes, quality of life and economic productivity.7–9
HIV remains a disease of public health importance.10 Recently, outbreaks have been identified in people who inject drugs in North America, Europe and parts of Australia.11 12 A disparate proportion of new infections in the USA is accounted for by youth, African-American, Latino as well as Aboriginal populations who are also less likely to get tested, receive results, access and remain in HIV care.1 13–15 The disease continues to be fuelled by unsafe sexual practice between and within sexes.16 These inequities are associated with HIV-related stigma, fear, financial constraints, transportation and system barriers, and a lack of supports within marginalised communities.17–22
Conventional testing, ordering an HIV blood test and having the patient return for results, has not performed well in marginalised communities.13 14 Persons at high risk for HIV exposure include persons who inject drugs, men who have sex with men, persons from HIV epidemic countries (prevalence >1%), street youth, pregnant women, sex workers, low-income and socially disadvantaged people, Aboriginal persons, and other minorities.18 19 23 Alternative HIV counselling and testing strategies have emerged to improve uptake of services in these populations. These include home-based, work-based and parole office-based testing, peer-based and community-based (CB) voluntary counselling and testing (VCT), mobile testing and universal population testing.24 25 Improved update was documented in a Cochrane review on home-based testing and a trial on workplace testing.26 27
The accuracy of rapid HIV tests is now approaching that of laboratory-based ELISA and western blot testing.28 A variety of rapid-test kits exist ranging from oral kits to single use blood drop-based kits. In high-income countries CB rapid VCT may cost up to four times more than facility-based testing.29 Research however, from low-income, high-prevalence settings suggests CB rapid VCT is cost-effective.30 31 Greater cost-effectiveness is associated with outreach-based programmes that use rapid VCT rather than conventional testing.30 32 Others have argued that rapid VCT approaches linked to treatment programmes optimise uptake of treatment for high-risk populations.33–35 Very few systematic review explicitly report on equity. In order to study the effect of rapid VCT on high-risk populations we used an equity-focused systematic review approach to identify, extract and synthesise evidence on equity.
Rapid VCT is a complex intervention aimed to increase the participation of marginalised populations in HIV testing and treatment programmes. Rapid VCT consists of three components: (1) voluntary enrolment, (2) rapid testing (results within 24 h) and (3) counselling and delivery of results and treatment options. A recent systematic review on home-based testing synthesised 19 observational studies from sub-Saharan Africa found the vast majority of participants accepted testing, however comparison groups were limited.36 Another systematic review of mainly observational studies showed 66% increase in uptake of testing among pregnant Kenyan women in antenatal clinics with rapid VCT.37 Thornton et al38 assessed feasibility, acceptability and effectiveness of HIV-testing strategies in high-income countries and reported high overall client satisfaction and positive staff attitudes towards CB testing but called for more data to evaluate the actual strategies, confidentiality concerns and post-test counselling. The 2013 WHO HIV guidelines recommend CB and other HIV testing be done in conjunction with treatment and counselling, but they do not define the critical components of the testing to treatment intervention.39 In order to effectively scale up HIV testing, treatment and viral load suppression more precise knowledge is needed to guide interventions for people at high risk for HIV exposure.
Methods
Primary objective
The aim of our review was to assess the effects of rapid VCT on the following HIV-related outcomes for populations at high risk for HIV exposure: (1) uptake, (2) receipt of results, (3) repeat testing, (4) HIV incidence compared with conventional laboratory testing approaches and (5) stigma.
This review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses-Equity Extension (PRISMA-E) 2012 reporting guideline for equity-focused reviews.40 The review protocol was peer reviewed and published on the Cochrane Database of Systematic Reviews.41
Search methods
We searched PubMed via NLM, EMBASE via OVID, AIDSearch via the web, LILACS via the web, Global Health, Medline Africa, PsychInfo via OVID, CINAHL via EbscoHost, Cochrane CENTRAL via Wiley, Cochrane HIV/AIDS Group Specialized Register, abstracts of important meetings (eg, International AIDS Conference) and AIDS specialty journals. We also contacted experts for unpublished research, trials and dissertations along with trial registers of HIV/AIDS Cochrane Centre and the Cochrane Infectious Diseases review group. All database searches were from 1 January 2001 to 5 June 2014. Details of the search strategy are listed in online supplementary appendices 1–3.
Study selection
After identification of relevant studies and removal of duplicates, two reviewers screened titles and abstracts. Two reviewers then screened full text of relevant articles to determine whether they met eligibility criteria. When disagreements arose, they were resolved with a third reviewer. We contacted authors for additional information when needed.
Data abstraction and selection
Two authors using pretested standard forms independently extracted data including study details, study characteristics, interventions and intervention effects (HIV uptake of testing, HIV incidence and uptake into treatment programmes including the reported measures of association). In addition, we sought information on age, sex, minority status and socioeconomic status (SES).
Eligibility criteria
We included studies that met the following criteria.
Population: Those focused on marginalised populations at high risk for HIV exposure (as defined earlier).
Intervention: We included studies that met the criteria for rapid VCT with three main components: (1) facilitated voluntary enrolment; (2) use of a rapid-testing approach (providing results within 24 h) and (3) outreach counselling, delivery of results and treatment options. Use of the rapid test alone was not sufficient to be considered a rapid VCT.
Comparison: We included ‘conventional approaches’, which could include one or more of the above elements, but not all three. ‘Conventional approaches’ refers to HIV testing in health facilities using traditional laboratory testing approaches where the client has to wait for more than 24 h before results are received.
Study designs: Randomised controlled trials (RCTs), interrupted time series, prospective or retrospective cohort studies and controlled before and after designs that met the above eligibility criteria.
Outcomes of interest include uptake of HIV testing, receipt of HIV tests, repeat HIV testing or retesting,42 HIV incidence and HIV-related stigma.
Assessment of study quality and data synthesis
We assessed risk of bias for the randomised trials using the Cochrane risk of bias tool.43 44 Studies were judged to be low, high or unclear risk of bias.
The included studies vary with respect to the intervention duration, type and settings. We undertook the analysis using the intention-to-counsel and screen principles including all participants in the study arm to which they were originally allocated. We used Review Manager V.5.2 (The Cochrane Collaboration, Oxford, UK) to aggregate data for each outcome using a random effects model. We chose the random effects model to control for unobserved heterogeneity that we assumed would exist among the included studies. We present all pooled effect estimates as relative risks (RR) with 95% CIs. We tested for study heterogeneity using the I2 statistic. We did a sensitivity analysis for gender, SES and education level. A summary of findings table was produced showing relative and absolute effects using GRADE Pro (V.3.6 for Windows). In addition, we conducted a GRADE (Grading of Recommendations Assessment, Development and Evaluation) evidence assessment and profile for each selected outcome.
For cluster-randomised studies, we checked whether studies were adjusted for clustering in the statistical analysis. If results were not adjusted, we adjusted them using the variance inflation factor, as described in section 8.11.2.3 of the Cochrane Handbook.45 We used an intracluster correlation coefficient (ICC) factor of 0.026 obtained from a previous HIV-related study conducted in Zambia.46 The design effect was calculated using the formula: 1+ (cluster size 1)×ICC).
For the observational studies, we appraised studies using the Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies and report the individual study cohort star template.47
Results
Study selection
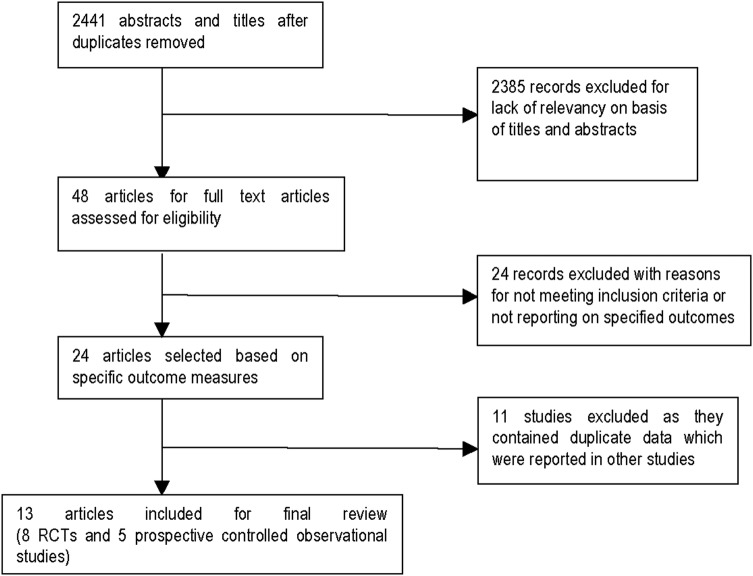
The search resulted in 2441 records and 13 met our inclusion criteria (see figure 1).20 29 48–57 All randomised trials and observational studies were conducted among populations at high risk for exposure and compared rapid VCT with conventional testing. Intervention descriptions and quality assessment of the randomised trials included in the quantitative analysis are shown in tables 1–3.20 48–52 57
Figure 1.
Selection of studies for inclusion in the review (RCT, randomised controlled trial).
Table 1.
Risk of bias as assessed using the Cochrane risk of bias tool
| Study | Random sequence generation | Allocation concealment | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome date (attrition bias) |
|---|---|---|---|---|---|
| Anaya et al49 | Unclear risk | Unclear risk | Low risk | Unclear risk | Low risk |
| Coates et al59 | Low risk | High risk | Low risk | Unclear risk | Low risk |
| Lugada et al51 | Low risk | Unclear risk | Low risk | Unclear risk | Low risk |
| Malonza et al50 | Low risk | Unclear risk | Low risk | Unclear risk | Low risk |
| Read et al58 | Low risk | High risk | High risk | Unclear risk | Low risk |
| Spielberg et al52 | Low risk | High risk | Low risk | Unclear risk | Low risk |
| Sweat et al20 | Low risk | High risk | Low risk | Unclear risk | Low risk |
| Walensky et al48 | Low risk | Unclear risk | Low risk | Unclear risk | Low risk |
Table 2.
Characteristics of included studies—randomised controlled trials
| Study | Study method | Participants | Country | Intervention arm | Control arm | Target period (if applicable) | Outcome measures |
|---|---|---|---|---|---|---|---|
| Anaya et al49 | Randomised controlled trail | Age: 18–65 years Sex: male and female Setting: adult clinic waiting room patients within the Department of Veteran Affairs |
USA |
|
Conventional VCT: 83 participants | Testing rates | |
| Coates et al59 60 | Cluster randomised controlled trial | Age: 16–32 years Sex: male and female Setting: area of HIV prevalence >1% |
Tanzania, Zimbabwe, Thailand, and South Africa | Community-based rapid VCT: 63 000 participants | Conventional VCT: 52 900 participants | 36 months | HIV incidence |
| Lugada et al51 | Cluster randomised controlled trial | Age: 15–49 years Sex: male and female Setting: five SE districts in Uganda* HIV prevalence: 5.6% |
Uganda | Home-based rapid VCT with ART programme: 4798 participants | Conventional clinic-based ART programme: 2386 participants | 2 years | Uptake of testing; HIV prevalence |
| Malonza et al50 | Randomised controlled trail | Age: 18–44 years Sex: female Setting: antenatal clinic attendees HIV prevalence: 15–35% |
Kenya | Rapid VCT in health facility: 627 participants | Conventional HIV testing (ELISA) test: 622 participants | 1 year | Wait period for tests; receipt of test results; uptake into antiretroviral treatment programmes |
| Read et al58 | Randomised controlled trial | Age: Sex: male Setting: sexual health service |
Australia | Rapid VCT 200 participants |
Conventional VCT 200 participants |
18 months | Incidence of HIV testing, including testing outside study clinic |
| Spielberg et al52 | Cluster randomised trial | Age: 14 years and older Sex: male and female Setting: needle exchange and bathhouse |
USA |
|
|
221 days | Uptake of HIV testing; receipt of test results |
| Sweat et al20 57 | Cluster randomised controlled trial | Age: 16–32 years Sex: male and female Setting: area of HIV prevalence >1% |
Tanzania, Zimbabwe, Thailand | Community-based rapid VCT: 28 240 participants | Conventional VCT: 28 916 participants | 3 years | HIV prevalence; uptake of HIV testing, repeat testing |
| Walensky et al48 | Prospective randomised controlled trial | Age: mean=37.1 years Sex: male and female Setting: emergency department patients in a large Boston Hospital |
USA | Counsellor facilitated rapid VCT in emergency department: 2446 participants | Convention HIV testing in emergency department 2409 participants | 18 months | Overall testing: offer, acceptance |
*Jinja, Kamuli, Iganga, Mayuge and Mukuno. ART, antiretroviral therapy; VCT, voluntary counselling and testing.
Table 3.
GRADE evidence profile and summary of findings for use of rapid approaches for improving health outcomes
| Outcomes | Effects of rapid testing approaches on HIV outcomes | Relative effect (95% CI) | Anticipated absolute effect with control | Risk difference with intervention | Number of participants (studies) | Quality of the evidence (GRADE) |
|---|---|---|---|---|---|---|
|
Uptake of testing Follow-up: 12–36 months |
Three RCTs included in the analysis provided consistent point estimates showing uptake of testing was significantly better among participants randomised to rapid testing approaches | RR=2.95 (1.69 to 5.16) | 145 more per 1000 | 282 cases more per 1000 (100–602) | 80 400 (4 studies) 18 350* |
⊕⊕⊕⊝ Moderate† |
| Receipt of results Follow-up: 12–24 months | Two RCTs reported rapid approaches resulted in higher receipt of HIV test results. However due to the heterogeneity-variations in population characteristics, the pooled estimates were not statistically significant | RR=2.14 (1.04 to 4.24) |
213 more per 1000 | 243 cases per 1000 (17–691) | 18 426 (3 studies) 4680* |
⊕⊕⊕⊝ Moderate† |
|
Combined effect of repeat testing Follow-up: 36 months |
One large Cluster RCT found a very large effect for this outcome with participants randomised to rapid testing approaches twice more likely to have repeat HIV tests | RR=2.28 (0.35 to 15.07) | 97 more per 1000 | 124 cases per 1000 (63 fewer–1000 more) | 10 706 (1 study) | ⊕⊕⊕⊝ Moderate† |
|
HIV incidence Follow-up: 36 months |
HIV incidence did decrease in intervention clusters compared with control clusters, but this effect was not statistically significant | RR=0.89 (0.63 to 1.24) | 81 more per 1000 | 9 cases per 1000 (30 fewer–19 more) | 115 300 7189* (1 study) |
⊕⊕⊝ Low‡, § |
GRADE Working Group grades of evidence.
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
*Adjusted sample size after applying the intracluster correlation coefficient.
†Outcome of HIV incidence was downgraded because allocation concealment was unclear, blinding of intervention not possible and inability to determine blinding of researchers.
‡Outcome of HIV incidence was downgraded because allocation concealment was unclear, blinding of intervention not possible and inability to determine blinding of researchers and imprecision of estimates.
§Number of participants included in the analysis is not available from the abstracts.
RCT, randomised controlled trial; RR, relative risk.
One cluster RCT of 16–32-year-olds also referred to as Project Accept in South Africa, Tanzania, Zimbabwe and Thailand examined community-mobilisation/outreach, mobile rapid testing and post-test counselling compared with conventional VCT on uptake of tests, retesting and incidence of HIV.20 Another RCT looked at incidence of testing between rapid VCT versus convention testing in men from sexually transmitted disease (STD) clinics in Australia.58 An RCT looked at rapid VCT versus conventional testing in women in an antenatal clinic in Kenya and looked at receipt of results and uptake of treatment.50 An RCT in US emergency departments (ED) looked at a HIV counsellor-facilitated rapid VCT versus rapid testing by ED staff (laboratory technicians) on testing uptake.48 A cluster RCT looked at rapid VCT in home setting compared with conventional testing in Uganda.51 Another RCT in the USA looked at the effect of nurse-facilitated rapid VCT versus conventional testing on HIV-testing rates in veterans’ affairs hospital.49 The final RCT conducted among high-risk populations at a needle exchange programme and bathhouses in the USA examined four alternative testing approaches and assessed the effect on uptake of testing.52
We found data on the following outcomes: uptake of testing (n=5 studies), receipt of HIV test results (n=3 studies), HIV incidence (n=1 study), repeat testing (n=1 study) and stigma (n=1 study).
Observational studies included four cohort studies. A description and appraisal of the studies and main outcomes is provided in tables 4 and 5.29 53–56
Table 4.
Newcastle-Ottawa Quality Assessment Scale—Observational Study Star Template
| Study | Selection | Comparability | Outcome/exposure |
|---|---|---|---|
| Appiah et al56 | ***/**** | */** | **/*** |
| Huebner et al55 | ***/**** | */** | **/*** |
| Liang et al54 | ***/**** | */** | **/*** |
| Shrestha et al29 | ***/**** | */** | **/*** |
| White et al53 | ****/**** | */** | **/*** |
Selection—maximum of 4 stars (representativeness of exposed cohort; selection of non-exposed cohort; and exposure).
Comparability—maximum of 2 stars (comparability between cohorts).
Outcome/ Exposure—maximum of 3 stars (adequacy of outcome, follow-up duration).
Adapted from Wells et al.47 Available at http://www.evidencebasedpublichealth.de.
Table 5.
Characteristics of included studies—observational studies
| Authors | Study method | Participants | Country | Intervention arm | Control arm | Target period (if applicable) | Outcome measures |
|---|---|---|---|---|---|---|---|
| Appiah et al56 | Cross-sectional observational study | Tuberculosis clinic patients and VCT clients | Ghana | Tuberculosis clinic-based testing | VCT clinic-based testing | 1–6 months | Uptake of testing and results |
| Huebner et al55 | Controlled before and after studies | Bathhouse patrons | USA | Rapid VCT | Conventional VCT | 11–13 months | Uptake of testing; receipt of test results |
| Liang et al54 | Prospective cohort study | Men who have sex with men, injection drug users and commercial sex workers | USA | Rapid HIV testing | Traditional serum-based HIV testing | 9 months | Uptake of testing; HIV prevalence; post-test counselling |
| Shrestha et al29 | Cohort study | High-risk members of the population11 | USA | Outreach-based HIV testing | Clinic-based HIV testing | 23 months | Cost per notification |
| White et al24 | Retrospective cohort study | Emergency department patients | USA | Point-of-care HIV testing | Laboratory-based HIV testing | 6 months | HIV-testing rates |
VCT, voluntary counselling and testing.
Unit of analysis issues
Our analysis included three cluster-randomised trials and we adjusted these clusters using the methods described in section 8.11.2.3 of the Cochrane Handbook.45 We used the ICC derived from a Zambian HIV study.46 Adjustments were not made for the individually randomised studies.
Risk of bias
All RCTs reported adequate randomisation. No study, however, provided explicit statements about allocation concealment. The risk due to blinding study participants was unclear across studies likewise was the risk due to incomplete outcome data. We particularly do not think the attrition rate across studies was significant to introduce a risk of bias in our analysis (table 1).
The five observational studies showed moderately representative cohorts, comparability between cohorts and adequacy of selected outcomes (see table 4).
Uptake of HIV testing
All randomised trials compared rapid VCT with conventional testing and reported uptake of HIV testing as an individual outcome. We report the findings from Anaya et al separately because this was conducted in a community healthcare facility unlike the other three CB studies.20 48 51 We excluded one study from the meta-analysis because randomisation was done after the participants had already accepted voluntary HIV testing.50
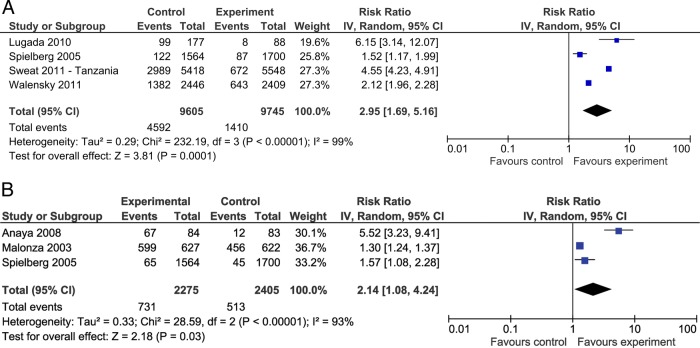
As shown in figure 2A, these studies show a threefold increase in uptake of HIV testing in the rapid VCT arm (RR=2.95, 95% CI 1.69 to 5.16). Heterogeneity between the studies was high I2=99%. When the results from the Sweat study, a large, tri-country pragmatic CB trial, are considered, there is significant heterogeneity in the site estimates but there is a consistency in the increased likelihood of the participants to accept HIV testing with rapid VCT.20 57 The heterogeneity suggests different modes of implementation and varied populations between these countries. This is further highlighted by the failure to measure some outcomes such as repeat testing rates in Thailand. The HIV-testing programmes were not limited to specific participants and there was a higher degree of contamination within geographically accessible cluster sites.
Figure 2.
Forest plot of rapid HIV voluntary counselling and testing versus conventional care (A) on uptake of HIV testing and (B) on receipt of HIV results
Results from two observational studies also showed that participants were more likely to get tested for HIV using a rapid approach.54 55
Receipt of HIV test results
Results for the receipt of HIV tests from three randomised trials reporting this outcome showed an almost twofold increased likelihood among participants randomised to the rapid approach study arms to receive test results (RR=2.14, 95% CI 1.08 to 4.24). Heterogeneity between the studies was high (I2=93%; p=<0.0001; figure 2).49 50
These estimates were supported by evidence from one of the observational studies conducted among bathhouse patrons in a large US city with a significant proportion being men who had sex with men.55 The results showed a significant difference in the proportion of men who received results of HIV tests: among HIV-positive men there was a 34.6% risk difference, and among HIV-negative men a 26% risk difference, when rapid VCT was compared with conventional testing.
Repeat HIV testing and test incidence rate
The international cluster RCT20 sought to indirectly measure personal awareness, entry of participants into a HIV care and treatment programme, and maturation of prevention initiatives. This study included three countries: Tanzania, Zimbabwe and Thailand that recruited 10, 8 and 14 community clusters, respectively. Because each country reported data separately, and because there appeared to be differences in methods, we treated each country as a separate study in this meta-analysis. Thailand was not included in our repeat testing meta-analysis because data was not available for this outcome. The analysis showed an RR=2.28, 95% CI 0.35 to 15.07 suggesting increased HIV repeat testing among those in the intervention arm (RR=2.28, 95% CI 0.35 to 15.07; figure 3).20 Sweat et al also reported a consistent increase in repeat HIV testing in Thailand and Zimbabwe reaching 28% of all testing done in the CB VCT sites.
Figure 3.
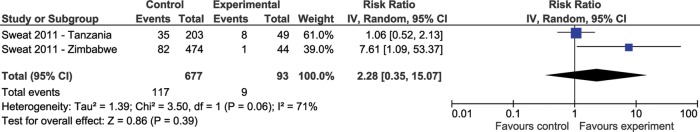
Forest plot of effect of rapid voluntary counselling and testing testing approaches versus conventional care on repeat testing.
The Australia RCT of men known to the public sexual health service reported a test incidence rate ratio 1.15, 95% CI 0.96 to 1.38.58 Men randomised to the conventional testing reported the wait for the test result was too long (p<0.001) and reported anxiety because of the wait (p<0.002) while men in the rapid VCT reported convenience in obtaining results (p<0.001). Other RCTs did not report on repeat testing preferences.
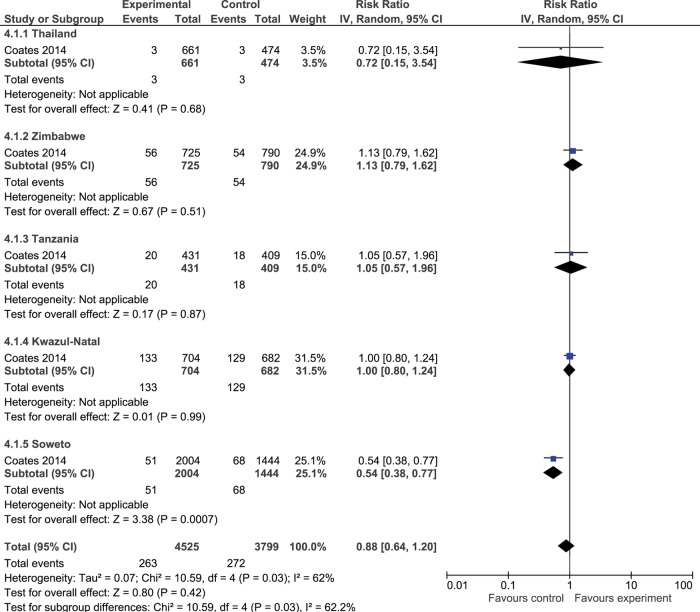
HIV incidence
HIV incidence data by Coates et al59 60 over a 36-month period in five countries showed an 11% reduction in estimated incidence in intervention vs control communities (RR=0.89, 95% CI=0.63 to 1.24; see figure 4).
Figure 4.
Forest plot of effect of rapid voluntary counselling and testing versus convention testing on HIV incidence.
Treatment programme uptake
Malonza et al50 reported that all of the women in the study were offered free antiretroviral drugs irrespective of study arm and the study found OR=1.7, 95% CI 0.8 to 3.7 for the uptake of perinatal HIV-1 interventions between rapid VCT versus conventional VCT.
HIV-related stigma
HIV-related stigma was assessed only in Project Accept and showed that stigma was low at baseline with little room for further decrease.59
Heterogeneity and sensitivity analysis
Our analysis included studies conducted in a range of countries, contexts, settings and populations. The studies also involved different variants of rapid VCT. Heterogeneity was statistically significant for all outcomes with more than one study.
Age: Sweat et al reported a reduction in HIV incidence of 1.5% among 18–24-year-olds and a 25% reduction in HIV incidence among participants aged 25–32 years (RR=0.75; 95% CI 0.54 to 1.04, p=0.08).60 In Uganda, Lugada et al51 reports that persons aged 15–24 years were least likely to get tested.
Sex/gender: We were only able to report subgroup analysis on gender in one trial because data was not disaggregated in the other studies.20 57 The Sweat study reported a greater reduction in HIV incidence in men than women in the intervention arm. An 11.6% reduction in HIV incidence among women was reported (RR=0.89; 95% CI 0.73 to 1.07, p=0.17) and 19.3% in men (RR=0.81; 95% CI 0.57 to 1.15, p=0.19). In addition, women older than 24 years in the intervention arm had a 30.2% reduction in HIV incidence versus conventional testing (95% CI 0.54 to 0.90, p=0.009).20 57 60
In another RCT, females were significantly more likely to accept HIV testing than men, adjusted OR (1.18, 95% CI 1.07 to 1.30).51
Education: Subgroup analyses by Lugada et al51 and Malonza et al50 also showed that irrespective of level of education, participants were more likely to accept HIV testing if it employed a rapid VCT.
Applicability and quality of evidence
This review aimed to be relevant for people at high risk for exposure to HIV. The GRADE summary of findings table with patient important outcome and certainty estimates can be found in table 3.44 61–63 The evidence for uptake of HIV testing, receipt of results and repeat testing were considered moderate quality because of randomisation and allocation concerns. The evidence for HIV incidence is considered low quality due to concerns of risk of bias and the imprecision of the estimates. We did not downgrade for indirectness because the included studies were conducted in community and health facility settings making the estimates applicable across a wide range of settings.
Discussion
While HIV awareness is improving, many communities and individuals still face barriers to HIV testing and viral load suppression. Our systematic review studied a complex intervention with three critical components designed to improve voluntary counselling and uptake of testing (engagement), reduce travel and improve receipt of test results (convenience), and to facilitate provision of results with appropriate information on treatment and counselling (long-term intervention). Rapid VCT was studied in health facility and CB interventions and in diverse settings where there is a high risk for HIV exposure; such as bathhouses, STD clinics, inner city ED, tuberculosis (TB) programmes and antenatal programmes in endemic regions. Rapid VCT showed large increase in update of testing and receipt of test results. Observational studies have also shown that VCT is associated with a reduction in the HIV disease burden.39
In the studies analysed, no harms were identified despite hypothetical concerns of test inaccuracy, lack of privacy and abuse to healthcare workers in non-hospital environments. A recent systematic review of observational studies focusing on home-based rapid VCT also failed to identify harms.27
Until recently, some organisations have argued that HIV testing should continue using the conventional clinic or hospital testing approach.11 This is changing and our findings clearly suggest high-risk populations benefit from rapid VCT compared with conventional testing, especially in terms of uptake and receipt of results.35 64 CB VCT, which also uses a facilitated rapid approach with community engagement, has received considerable WHO and research attention.39 65 Our systematic review corroborates these and other CB findings.26
Our review however specifically focused on populations at high risk of exposure to HIV, with the hypothesis that use of rapid VCT will increase HIV testing and receipt of testing rates and increase access to HIV-related treatment and services. Evidence from our study showed consistency of effect across settings, evidence for improved uptake in men, no uptake difference with low education status. These findings were corroborated by the evidence from prospective observational studies.54 55 Sweat et al20 and Coates et al60 reported CB rapid VCT was associated with improved behaviour change and prevention. The results from our study are applicable across a variety of settings; for example, among at-risk youth, women and hard to reach men in Zimbabwe and Thailand, repeat testing rates were comparable with those found in facilitated testing in high-risk men who have sex with men population in the USA.55 Lugada et al51 demonstrated that men used rapid-testing approaches but that this usage was slightly less than women. This is an important finding because men are usually harder to reach for HIV testing and treatment programmes.14 66 The findings of our review are also similar to a recent meta-analysis that shows increased receipt of HIV testing with rapid HIV VCT in medical facilitities.67
Rapid VCT has emerged as a complex intervention that can be used in community settings and health facilities in low-income and high-income countries. Previous systematic reviews have not included rapid VCT studies conducted in health facilities, thus leaving rapid VCT approach primarily directed at CB initiatives; for example, the WHO HIV guidelines highlights CB VCT, but not rapid VCT for health facilities.
Finally, our study highlights the importance of three key components within a counselling and testing strategy. Complex interventions include components with varying degrees of interaction.68 We suggest ongoing research is needed to improve HIV testing and viral load suppression: and this should include recognition of interacting components within the intervention, the number and difficulty of behaviours required by those delivering or receiving the intervention, the number of organisational levels targeted by the intervention, the number and variability of outcomes, and the degree of flexibility or tailoring of the intervention.69 Understanding this variability is also important for economic analysis.
Implication for policy and practice
Our study has shown the benefit of rapid VCT on uptake of HIV testing and receipt of results.70 This testing approach was effective in health facilities as well as community settings. CB VCT has received explicit attention in the recent WHO HIV testing and treatment guidelines and WHO consolidated guidelines for key populations.39 42 Our work supports CB VCT, but also finds that persons at high risk of exposure to HIV who use health facilities benefit from rapid VCT. This finding is not yet reflected in the WHO Consolidated Guidelines for key populations.42 We also found some emerging evidence for increased HIV awareness in most care settings.71
Implementing rapid VCT, with testing components tailored for high-risk communities, could improve health equity through earlier HIV diagnosis with possible retention in viral suppression programmes, reduced transmission and longer lifespans.7–9 In high-income countries, our results have particular importance for Aboriginal population, persons who inject drugs, prison populations, and certain migrant and minority populations. Additionally, routine use of rapid VCT may avoid human rights violations among marginalised populations where testing may occur without informed consent and were existing stigma may create barriers to testing and treatment. Given the significant heterogeneity in the trials, we suggest more research to study the components of the rapid VCT and identify what works, for whom and in what settings.
Strengths and limitations
We used a rigorous and transparent systematic review method, with an a priori protocol. The equity-focus allowed us to explicitly report how we assessed effects in populations at high risk of exposure to HIV; for example, by using explicit data collection methods to assess robustness of effect across SES, sex and level of education.40 In addition, our analysis included studies from various geographical areas covering four continents (Africa, Asia, Australia and North America) and diverse high-risk populations and testing settings (community and hospital), unlike previous reviews.26 Three of eight studies in our analysis were cluster RCTs, a design that is good for evaluating health service and CB interventions where the intervention is delivered at a group level.20 51 72
Despite the strengths of our analysis, there are a number of limitations. First, the components of the rapid VCT intervention varied across studies yet our data did not allow for quantitative comparisons of the components. Second, the studies were conducted in a variety of community and health facility settings and these settings also contributed to variations in implementation and convention testing. Third, while our analysis included studies from four continents, there were no studies from Europe and only eight RCTs in total. Fourth, we identified limited allocation concealment in the CB studies as well as healthcare facility-based studies. Finally, the studies that were included did not report on the impact on HIV-related stigma.
Conclusion
There still exist a significant proportion of HIV-infected people who are unaware of their status, lack access to HIV services such as VCT and are at risk of transmitting the virus within their communities. Our review focused on people at high risk of exposure to HIV to study the effect of rapid VCT compared with conventional testing. We studied a complex intervention with three critical components to improve initial counselling and update, optimise receipt of results and to facilitate trust in provision of results, counselling and treatment. Rapid VCT showed large increases in update of testing and in receipt of test results. Results were applicable to health facilities or CB interventions and in diverse settings such as bathhouses, prisons, home-based testing, ED, antenatal programmes, TB programmes and primary care clinics. No significant harms were identified in this testing approach. Evidence from our studies showed consistency of effect, evidence for favourable uptake in men in Africa and an 11% decrease in HIV incidence after 3 years of CB testing; however, precision of this estimate was low. These findings contribute new evidence for HIV-testing components that are relevant for HIV guidelines, supporting CB rapid VCT and highlighting a role for more health facility-based rapid VCT for populations at high risk of exposure. More research is needed to examine the relative effectiveness of the three components within rapid VCT and to study the association of rapid VCT and uptake of HIV treatment and long-term viral suppression.
Acknowledgments
The authors would like to acknowledge the Canadian Institutes of Health Research for funding this systematic review and synthesis. They would also like to acknowledge Joy Oliver of the Cochrane Collaboration HIV Review Group for her expert librarian services and Kamila Premji for her helpful comments on an earlier version of this manuscript.
Footnotes
Contributors: KP conceived the study and received funding with VW, TR. KP and VW were involved in the development and oversight of the statistical analysis plan and in the writing of the review. OM analysed the data and prepared the initial draft and revised the paper. GPD designed the data extraction tool, reviewed the studies for inclusion in the analysis and review of the draft. MT provided clinical HIV expertise and revised the draft paper. TR developed the search strategy used for identifying the relevant studies. GW reviewed and provided additional expertise for the complex intervention and statistical analysis plan. All authors approved the final version.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Joint United Nations Panel on HIV/AIDS (UNAIDS) Global Report—UNAIDS report on the global AIDS epidemic 2012. Geneva, 2012. [Google Scholar]
- 2.Joint United Nations Panel on HIV/AIDS (UNAIDS)—UNAIDS Terminology Guidelines—revised version Geneva, 2011. [Google Scholar]
- 3.Joint United Nations Panel on HIV/AIDS (UNAIDS). AIDS epidemic update. Geneva, 2007. [Google Scholar]
- 4.Miro JM, Manzardo C, Mussini C et al. . Survival outcomes and effect of early vs. deferred cART among HIV-infected patients diagnosed at the time of an AIDS-defining event: a cohort analysis. PLoS ONE 2011;6:e26009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegfried N, Uthman OA, Rutherford GW. Optimal time for initiation of antiretroviral therapy in asymptomatic HIV-infected treatment-naive adults (review). Cochrane Database Syst Rev 2010;(3):CD008272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulissa Z, Jerene D, Lindtjørn B. Patients present earlier and survival has improved, but pre-ART attrition is high in a six-year HIV cohort data from Ethiopia. PLoS ONE 2010;5:e13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gianella S, von Wyl V, Fischer M et al. . Effect of early antiretroviral therapy during primary HIV-1 infection on cell-associated HIV-1 DNA and plasma HIV-1 RNA. Antivir Ther 2011;16:535–45. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MS, Chen YQ, McCauley M et al. . Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bärnighausen T, Salomon JA, Sangrujee N. HIV treatment as prevention: issues in economic evaluation. PLoS Med 2012;9:e1001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC). Vital signs: HIV infection, testing, and risk behaviors among youths—United States. Morb Mortal Wkly Rep 2012;61:971–6. [PubMed] [Google Scholar]
- 11.Chou R, Cantor AG, Zakher B et al. . Screening for HIV in pregnant women: systematic review to update the 2005 U.S. Preventive Services Task Force recommendation. Ann Intern Med 2012;157:719–28. [DOI] [PubMed] [Google Scholar]
- 12.Niccolai LM, Verevochkin SV, Toussova OV et al. . Estimates of HIV incidence among drug users in St. Petersburg, Russia: continued growth of a rapidly expanding epidemic. Eur J Public Health 2011;21:613–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coovadia HM. Access to voluntary counseling and testing for HIV in developing countries. Ann N Y Acad Sci 2000;918:57–63. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO). Global HIV/AIDS response—Progress Report 2011. Geneva. [Google Scholar]
- 15.MacArthur GJ, Minozzi S, Martin N et al. . Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ 2012;345:e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood E, Montaner JSG, Li K et al. . Burden of HIV infection among aboriginal injection drug users in Vancouver, British Columbia. Am J Public Health 2008;98:515–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruneau J, Daniel M, Abrahamowicz M et al. . Trends in human immunodeficiency virus incidence and risk behavior among injection drug users in Montreal, Canada: a 16-year longitudinal study. Am J Epidemiol 2011;173:1049–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiessing L, Likatavicius G, Hedrich D et al. . Trends in HIV and hepatitis C virus infections among injecting drug users in Europe, 2005 to 2010. Euro Surveill 2011;16:1–5. [PubMed] [Google Scholar]
- 19.Prentice T. Alarming rates of HIV/AIDS for Canada's Aboriginal women. Can Womens Health Netw 2005;8 http://www.cwhn.ca/node/39483 [Google Scholar]
- 20.Sweat M, Morin S, Celentano D et al. . Community-based intervention to increase HIV testing and case detection in people aged 16–32 years in Tanzania, Zimbabwe, and Thailand (NIMH Project Accept, HPTN 043): a randomised study. Lancet Infect Dis 2011;11:525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obermeyer CM, Osborn M. The utilization of testing and counseling for HIV: a review of the social and behavioral evidence. Am J Public Health 2007;97:1762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spielberg F, Kurth A, Gorbach PM et al. . Moving from apprehension to action: HIV counseling and testing preferences in three at-risk populations. AIDS Educ Prev 2001;13:524–40. [DOI] [PubMed] [Google Scholar]
- 23.de la Fuente L, Delgado J, Hoyos J et al. . Increasing early diagnosis of HIV through rapid testing in a street outreach program in Spain. AIDS Patient Care STDs 2009;23:625–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White DA, Scribner AN, Schulden JD et al. . Results of a rapid HIV screening and diagnostic testing program in an urban emergency department. Ann Emerg Med 2009;54:56–64. [DOI] [PubMed] [Google Scholar]
- 25.Gordon MS, Kinlock TW, Rich JD. Rapid HIV testing for individuals on probation/parole: outcomes of an intervention trial. AIDS Behav 2013;17:2022–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bateganya M, Abdulwadud OA, Kiene S. Home-based HIV voluntary counselling and testing (VCT) for improving uptake of HIV testing. Cochrane Database Syst Rev 2010;(7):CD006493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabapathy K, Van den Bergh R, Fidler S et al. . Uptake of home-based voluntary HIV testing in sub-Saharan Africa: a systematic review and meta-analysis. PLoS Med 2012;9:e1001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plate DK. Evaluation and implementation of rapid HIV tests: the experience in 11 African Countries. AIDS Res Hum Retroviruses 2007;23:1491–8. [DOI] [PubMed] [Google Scholar]
- 29.Shrestha RK, Clark HA, Sansom SL et al. . Cost-effectiveness of finding new HIV diagnoses using rapid HIV testing in community-based organizations. Public Health Rep 2008;123(Suppl):94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders GD, Anaya HD, Asch S et al. . Cost-effectiveness of strategies to improve HIV testing and receipt of results: economic analysis of a randomized controlled trial. J Gen Intern Med 2010;25:556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farnham PG, Hutchinson AB, Sansom SL et al. . Comparing the costs of HIV screening strategies and technologies in health-care settings. Public Health Rep 2008;123(Suppl):51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutchinson AB, Branson BM, Kim A et al. . A meta-analysis of the effectiveness of alternative HIV counseling and testing methods to increase knowledge of HIV status. AIDS 2006;20:1597–604. [DOI] [PubMed] [Google Scholar]
- 33.King AM, Osterwalder JJ, Vernazza PL. A randomised prospective study to evaluate a rapid HIV-antibody assay in the management of cases of percutaneous exposure amongst health care workers. Swiss Med Wkly 2001;131:10–13. [DOI] [PubMed] [Google Scholar]
- 34.Branson BM, Handsfield HH, Lampe MA et al. . Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006;55:1–17. [PubMed] [Google Scholar]
- 35.McCollum ED, Preidis GA, Maliwichi M et al. . Clinical versus rapid molecular HIV diagnosis in hospitalized African infants: a randomized controlled trial simulating point-of-care infant testing. J Acquir Immune Defic Syndr 2014;66:e23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corbett EL, Makamure B, Cheung YB et al. . HIV incidence during a cluster-randomized trial of two strategies providing voluntary counselling and testing at the workplace, Zimbabwe. AIDS 2007;21:483–9. [DOI] [PubMed] [Google Scholar]
- 37.Hensen B, Baggaley R, Wong VJ et al. . Universal voluntary HIV testing in antenatal care settings: a review of the contribution of provider-initiated testing & counselling. Trop Med Int Health 2012;17:59–70. [DOI] [PubMed] [Google Scholar]
- 38.Thornton AC, Delpech V, Kall MM et al. . HIV testing in community settings in resource-rich countries: a systematic review of the evidence. HIV Med 2012;13:416–26. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva, 2013. [PubMed] [Google Scholar]
- 40.Welch V, Petticrew M, Tugwell P et al. . PRISMA-Equity 2012 extension: reporting guidelines for systematic reviews with a focus on health equity. PLoS Med 2012;9:e1001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pottie K, Dahal G, Logie C et al. . Rapid testing for improving uptake of HIV/AIDS services in people with HIV infection. Cochrane Database Syst Rev 2011;(11):CD003507. [Google Scholar]
- 42.World Health Organization (WHO). Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. Geneva, Switzerland: 2014(in press). [PubMed] [Google Scholar]
- 43.Higgins JP, Altman DG, Gøtzsche PC et al. . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guyatt GH, Oxman AD, Vist G et al. . GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol 2011;64:407–15. [DOI] [PubMed] [Google Scholar]
- 45.Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions 4.2.6. 4th edn Chichester, UK: The Cochrane Collaboration, 2006. [Google Scholar]
- 46.Fylkesnes K, Sandøy IF, Jürgensen M et al. . Strong effects of home-based voluntary HIV counselling and testing on acceptance and equity: a cluster randomised trial in Zambia. Soc Sci Med 2013;86:9–16. [DOI] [PubMed] [Google Scholar]
- 47.Wells GA, Shea B, Connell DO, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta- analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 48.Walensky RP, Reichmann WM, Arbelaez C et al. . Counselor- versus provider-based HIV screening in the emergency department: results from the universal screening for HIV infection in the emergency room (USHER) randomized controlled trial. Ann Emerg Med 2011;58:S126–32.e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anaya HD, Hoang T, Golden JF et al. . Improving HIV screening and receipt of results by nurse-initiated streamlined counseling and rapid testing. J Gen Intern Med 2008;23:800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malonza IM, Richardson BA, Kreiss JK et al. . The effect of rapid HIV-1 testing on uptake of perinatal HIV-1 interventions: a randomized clinical trial. AIDS 2003;17:113–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lugada E, Levin J, Abang B et al. . Comparison of home and clinic-based HIV testing among household members of persons taking antiretroviral therapy in Uganda: results from a randomized trial. J Acquir Immune Defic Syndr 2010;55:245–52. [DOI] [PubMed] [Google Scholar]
- 52.Spielberg F, Branson BM, Goldbaum GM et al. . Choosing HIV counseling and testing strategies for outreach settings. Epidemiol Soc Sci 2005;38:348–55. [PubMed] [Google Scholar]
- 53.White DA, Tran T, Dideum PJ et al. . Physician-initiated rapid HIV testing in an urban emergency department: comparison of testing using a point-of-care versus a laboratory model. Ann Emerg Med 2011;58:S53–9. [DOI] [PubMed] [Google Scholar]
- 54.Liang TS, Erbelding E, Jacob CA et al. . Rapid HIV testing of clients of a mobile STD/HIV clinic. AIDS Patient Care STDs 2005;19:253–7. [DOI] [PubMed] [Google Scholar]
- 55.Huebner DM, Binson D, Dilworth SE et al. . Rapid vs. standard HIV testing in bathhouses: what is gained and lost? AIDS Behav 2010;14:688–96. [DOI] [PubMed] [Google Scholar]
- 56.Appiah LT, Havers F, Gibson J et al. . Efficacy and acceptability of rapid, point-of-care HIV testing in two clinical settings in Ghana. AIDS Patient Care STDs 2009;23:365–9. [DOI] [PubMed] [Google Scholar]
- 57.Sweat M, Morin S, Celentano D et al. . Increases in HIV testing and case detection from NIMH Project Accept (HPTN 043) among 16–32 year olds: a randomized community-based intervention in Tanzania, Zimbabwe, and Thailand. Lancet Infect Dis 2012;11:525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Read TRH, Hocking JS, Bradshaw CS et al. . Provision of rapid HIV tests within a health service and frequency of HIV testing among men who have sex with men: randomised controlled trial. BMJ 2013;347:f5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coates TJ, Kulich M, Celentano DCD et al. . Effect of community-based voluntary counselling and testing on HIV incidence and social and behavioural outcomes (NIMH Project Accept; HPTN 043): a cluster-randomised trial. Lancet Glob Health 2014;2:e267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coates T, Eshleman SH, Chariyalertsak S et al. ; the H 043 PAST. Community-level reductions in estimated HIV incidence: HIV Prevention Trials Network 043, Project Accept. Conference on Retroviruses and Opportunistic Infections Atlanta, 2013. http://www.retroconference.org/2013b/Abstracts/47715.htm (accessed 18 Apr 2013). [Google Scholar]
- 61.Guyatt GH, Oxman AD, Kunz R et al. . Going from evidence to recommendations. BMJ 2008;336:1049–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guyatt G, Oxman AD, Akl EA et al. . GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94. [DOI] [PubMed] [Google Scholar]
- 63.Guyatt GH, Oxman AD, Kunz R et al. . GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol 2011;64:1294–302. [DOI] [PubMed] [Google Scholar]
- 64.Vermund SH, Wilson CM. Barriers to HIV testing—where next. Lancet 2002;360:1186–7. [DOI] [PubMed] [Google Scholar]
- 65.Suthar AB, Ford N, Bachanas PJ et al. . Towards universal voluntary HIV testing and counselling: a systematic review and meta-analysis of community-based approaches. PLoS Med 2013;10:e1001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cherutich P, Kaiser R, Galbraith J et al. . Lack of knowledge of HIV status a major barrier to HIV prevention, care and treatment efforts in Kenya: results from a nationally representative study. PloS ONE 2012;7:e36797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y, Guo J, Lu W. Effects of rapid versus standard HIV voluntary counselling and testing on reception rate: a meta-analysis. Int J STD AIDS 2014. [DOI] [PubMed] [Google Scholar]
- 68.Noyes J, Gough D, Lewin S et al. . A research and development agenda for systematic reviews that ask complex questions about complex interventions. J Clin Epidemiol 2013;66:1262–70. [DOI] [PubMed] [Google Scholar]
- 69.Craig P, Dieppe P, Macintyre S et al. . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Cock KM, Bunnell R, Mermin J. Unfinished business—expanding HIV testing in developing countries. N Engl J Med 2006;354:440–2. [DOI] [PubMed] [Google Scholar]
- 71.World Health Organization (WHO). Delivering HIV test results and messages for re-testing and counselling in adults 2010. http://whqlibdoc.who.int/publications/2010/9789241599115_eng.pdf
- 72.Banerfee A, Duflo E. Poor economics: a radical thinking of the way to fight global poverty. New York, NY: Public Affairs, 2012. [Google Scholar]