Abstract
A longer lifetime duration of breastfeeding may decrease the risk of breast cancer by reducing breast inflammation and mitigating inflammatory cytokine expression during postlactational involution. However, little is known about how the inflammatory cytokine profile in human breastmilk changes over time. To study temporal trends in breastmilk cytokine expression, we measured 80 human cytokines in the whey fraction of breastmilk samples from 15 mothers at 1, 4, 8, and 12 weeks postpartum. We used mixed models to identify temporal changes in cytokine expression and investigated parity status (multiparous vs. primiparous) as a potential confounder. Nine cytokines (monocyte chemoattractant protein-1, epithelial-derived neutrophil-activating protein-78, hepatocyte growth factor, insulin-like growth factor-binding protein-1, interleukin-16, interleukin-8, macrophage colony-stimulating factor, osteoprotegerin, and tissue inhibitor of metallopeptidase-2) had significantly decreased expression with increasing breastfeeding duration; all nine have known roles in breast involution, inflammation, and cancer and may serve as biomarkers of changing breast microenvironment. No cytokine significantly increased in level over the study period. Total protein concentration significantly decreased over time (p<0.0001), which may mediate the association between length of breastfeeding and inflammatory cytokine expression. Parity status did not confound temporal trends, but levels of several cytokines were significantly higher among multiparous versus primiparous women. Our results suggest that inflammatory cytokine expression during lactation is dynamic, and expressed milk may provide a noninvasive window into the extensive biological changes that occur in the postpartum breast.
Introduction
Longer lifetime duration of breastfeeding is associated with reduced risk of breast cancer later in life,1–4 although little is known about the underlying mechanisms. One possible mechanism may relate to glandular involution. Postlactational breast involution involves extensive breast tissue remodeling and depends on infiltration of inflammatory cells. This inflammatory microenvironment may transiently increase risk of pregnancy-associated breast cancer,1,5,6 but longer breastfeeding duration may reduce breast inflammation and mitigate tissue remodeling,1,7 thereby improving postlactational involution outcomes and decreasing breast cancer risk. However, biomarkers for monitoring breast inflammation during lactation and/or predicting risk have received only limited study.
Animal studies feature prominently in our understanding of postlactational involution. These studies have identified inflammatory cytokines that are differentially expressed during mammary involution and in cancer development.8–11 Although some research has investigated the expression of cytokines and other proteins in human breastmilk,12–23 methods have been diverse, and there is little consensus regarding the cytokine profile of human milk and how it changes over time. In fact, most studies of breastmilk cytokines have focused on exploring cytokines that impact infant nutrition and health,12–16 identifying known cancer-associated cytokines in milk,17–19 or investigating cytokines in conjunction with breast inflammatory diseases and noncancerous maternal conditions.20,21 Few studies have attempted to simultaneously characterize the coordinated expression of multiple cytokines in milk,22,23 and longitudinal analyses examining cytokine expression throughout lactation and involution are rare and have had inconsistent results.12,17,18
The present study investigated temporal trends in the expression of 80 cytokines in the breastmilk of healthy women throughout the first 3 months of lactation. Information on total milk protein concentration was also collected. Our two hypotheses were as follows: (1) global cytokine expression would decrease over the study period, reflecting altered breast microenvironment as breastfeeding progresses, and (2) specific cytokines would be identified as potential biomarkers of the breast microenvironment throughout lactation.
Materials and Methods
Study population
The Mother's Milk Microbiome (3M) study included 15 breastfeeding mothers recruited during their hospitalization in the postpartum unit of a tertiary-care hospital in North Carolina from July through December 2010. Included were English-speaking women with a singleton birth at ≥37 weeks of gestation who intended to breastfeed for at least 3 months and were not experiencing more than mild pain with breastfeeding. Participants were excluded if they lived more than 30 minutes from the recruitment hospital or if their infant had a congenital anomaly that interfered with breastfeeding.
Data collection and follow-up
After providing written informed consent, participants were visited four times in their homes by an International Board Certified Lactation Consultant. At 1 week postpartum, participants provided a breastmilk sample (approximately 2–5 mL from each breast) and completed baseline questionnaires assessing age (continuous), race/ethnicity (white and other), marital status (single, living as married, and married), education level (high school, some college, completed college, and some graduate), body mass index (continuous, based on self-reported prepregnancy weight), and previous breastfeeding history (combined months of previous breastfeeding). Study participants completed three follow-up visits over the course of 3 months (at 4, 8, and 12 weeks postpartum), at which additional milk samples were collected and participants reported their daily breastfeeding frequency and time since last milk expression. Study incentives included four home visits with breastfeeding support and counseling by the study's International Board Certified Lactation Consultant and a gift card ($5–10) for each milk sample donation. The study protocol was approved by the institutional review board of the University of North Carolina at Chapel Hill.
Milk sample characteristics and processing
All breastmilk samples were collected manually using aseptic technique after nipples were cleaned with 70% isopropyl alcohol. Milk was collected in sterile plastic containers, transported on ice to the laboratory, and stored at −80°C until analysis.
Equal parts of the milk from left and right breasts were pooled and processed as a single sample for a total of 56 pooled samples for the 15 participants across the four time points (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/bfm). The whey fraction of the milk was obtained as previously described by Kverka et al.22 In brief, 1–1.5 mL of milk was subjected to two successive centrifugations at 680 g for 10 minutes at 4°C and at 10,000 g for 30 minutes at 4°C, after which the fatty layer and cellular elements were removed. The resulting translucent liquid whey was used for cytokine array analysis. To address the question of cytokine degradation during storage and after freeze–thaw cycles, we measured cytokine expression within one sample that was subjected to different freeze–thaw cycles: (1) fresh milk kept on ice (never frozen), (2) one freeze–thaw cycle (thawed on ice), and (3) two freeze–thaw cycles (thawed on ice). Milk samples were randomized and processed after all samples were received to avoid batch effects.
Cytokine measurement
The RayBio Human Cytokine Antibody Array 5 (80) (RayBiotech, Norcross, GA) was used to measure the expression of 80 cytokines in the whey fraction of the milk samples. We processed the cytokine array glass slides according to the manufacturer's recommendation. In brief, the slides were blocked with blocking buffer at room temperature for 30 minutes and incubated with 100 μL of the whey sample at room temperature for 90 minutes. Slides were washed three times with Wash Buffer I and two times with Wash Buffer II at room temperature for 5 minutes per wash and incubated with biotin-conjugated antibodies overnight at 4°C. The slides were washed and incubated with fluorescent dye-conjugated streptavidin at room temperature for 2 hours. The slides were then washed again with Buffers I and II and dried by centrifugation at 200 g for 3 minutes. Immediately after centrifugation, fluorescent signal was detected on a GenePix® 4000B scanner (Molecular Devices, Sunnyvale, CA) using a cy3 (green) channel (excitation frequency of 532 nm). Each slide was normalized to positive controls on individual slides to obtain relative cytokine expression for comparison across slides.
Total protein quantification
Using the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL), total protein concentration was measured in 45 samples with sufficient remaining whey fraction volume (Supplementary Table S1). In brief, whey samples were diluted 1:10 in phosphate-buffered saline, and 10 μL of diluted sample was incubated with 200 μL of Kit Reagent in 96-well plates for 30 minutes at 37°C. Samples were assayed in triplicate, and total protein concentration was measured using a BioTek (Winooski, VT) EL800 plate reader (excitation frequency of 570 nm).
Statistical analysis
Prior to analysis, all cytokine expression and protein data were logarithm-transformed using base 2. Mixed models were used in a repeated-measures analysis to model changes in cytokine expression and total protein concentration over time. Specifically, PROC MIXED (SAS version 9) was used to model cytokine expression or protein concentration, with week as the fixed factor and subject as the repeated factor using an unstructured covariance matrix. Parity status was dichotomized into multiparous (one or more births prior to the current birth) versus primiparous (no prior births) and evaluated as a potential confounder in multivariable models. Other covariates (age, race/ethnicity, marital status, education, body mass index, and previous breastfeeding history) were collected, but sample size was insufficient to investigate these covariates as confounders. Therefore only parity was included in final models. We also examined daily breastfeeding frequency and time since last milk expression in association with cytokine expression. Statistical significance was defined as p<0.05, although q values were also estimated using an optimized false discovery rate (FDR) approach in all cytokine analyses to adjust p values for multiple testing. Statistical analyses were performed using SAS software, version 9.3 (SAS Institute, Inc., Cary, NC).
Data were analyzed using a two-dimensional cluster generated by Cluster 3.0 and visualized using Java Treeview. Samples were organized by time point (Week 1, 4, 8, and 12), and cytokine values were clustered across all samples.
Results
The 15 women in this study were primarily white, married, and highly educated with an average age of 33.9 years (Table 1). Additionally, the women had a low body mass index (average of 21.7 kg/m2), and all multiparous women had previously breastfed, with an average lifetime duration of 19.1 months.
Table 1.
Demographic Characteristics of Study Participants (n=15)
| Characteristic | Number (%) |
|---|---|
| Age (years) | |
| <35 | 9 (64.3) |
| ≥35 | 5 (35.7) |
| Missing | 1 |
| Race/ethnicity | |
| White | 13 (86.7) |
| Other | 2 (13.3) |
| Marital status | |
| Married | 13 (86.7) |
| Living as married | 2 (13.3) |
| Education level | |
| Some college | 1 (6.7) |
| Completed college | 8 (53.3) |
| Some graduate | 6 (40.0) |
| BMI (prepregnancy weight) (kg/m2) | |
| <25 | 14 (93.3) |
| ≥25 | 1 (6.7) |
| Number of births (including current) | |
| 1 | 6 (40.0) |
| ≥2 | 9 (60.0) |
| Previous breastfeeding among multiparous women | |
| Yes | 9 (100.0) |
| No | 0 |
| NA (primiparous) | 6 |
| Previous breastfeeding duration among multiparous women (months)a | 19.1±10.3 |
Mean±standard deviation.
BMI, body mass index; NA, not applicable.
Breastmilk samples were collected at 1, 4, 8, and 12 weeks postpartum for nearly all women (Supplementary Table S1), and we conducted a series of quality tests to address the question of sample degradation as a result of storage and freeze–thaw cycles. We found that Pearson correlations for overall cytokine expression across the different storage and freeze–thaw conditions ranged from 0.97 to 0.99 (Supplementary Table S2), suggesting that freezing the samples did not substantially affect the results of the cytokine assay. However, two cytokines (epidermal growth factor and GRO) were found to have poor reproducibility (expression values were greater than 0.2 SD from the mean across all freeze–thaw conditions) and were removed from further analysis.
Average cytokine expression and total protein decrease over the first 8 weeks of lactation
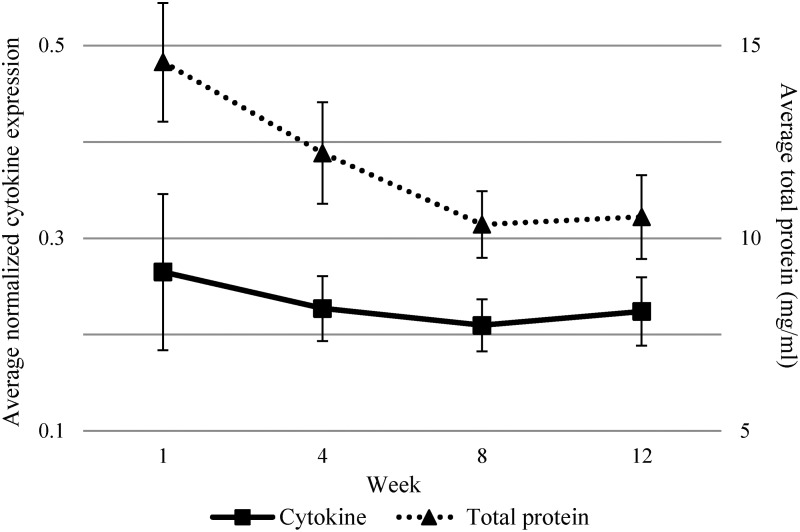
Average cytokine expression decreased over the study period until Week 8, after which expression increased at Week 12 (Fig. 1). Similarly, we found that total protein concentration followed a more pronounced decreasing trend until Week 8 when the trend plateaued (Fig. 1). The trend lines for both cytokine expression and total protein decreased linearly with an inflection point at Week 8, suggesting that model fit could be improved by fitting a linear spline term at Week 8.
FIG. 1.
Average cytokine expression and total protein concentration over the first 12 weeks of lactation (n=15 participants). Seventy-eight cytokines were averaged per participant. Error bars show the 95% confidence interval of the mean.
Three models were considered to evaluate how the inclusion of the spline term and parity status as a potential confounder affected the associations between time and average cytokine expression or total protein. As shown in Table 2, we did not find a significant association between time and average cytokine expression in the crude model, although the model coefficient was negative, suggesting decreased cytokine expression over time. The magnitude of the association nearly doubled with the inclusion of the spline term, although the association remained nonsignificant. Similarly, for total protein concentration (Table 2), the crude model showed that total protein significantly decreased over time (p<0.0001). Adjusting for parity status had no effect on the association between time and total protein or between time and cytokine expression, and thus there was no substantial confounding by parity. The addition of the spline term strengthened the association between time and total protein. Goodness of model fit was assessed by comparing the Akaike information criterion between the crude and spline models, and the Akaike information criterion values were either unchanged (for cytokine models) or improved (for total protein models) with a spline term. Given the shape of the curves in Figure 1, improved flexibility of the spline model, and impact on model fit, the spline term was retained in cytokine and protein models.
Table 2.
Comparing Models: Change in Average Cytokine Expression and Total Protein over Time
| Model, factor | β coefficient | 95% CI | p value |
|---|---|---|---|
| Cytokine expression | |||
| Weeka | −0.016 | −0.046, 0.014 | 0.3 |
| Week+spline | −0.034 | −0.082, 0.013 | 0.2 |
| Week+parity | −0.016 | −0.046, 0.014 | 0.3 |
| Total protein | |||
| Weeka | −0.045 | −0.060, −0.029 | <0.0001 |
| Week+spline | −0.068 | −0.089, −0.048 | <0.0001 |
| Week+parity | −0.045 | −0.061, −0.030 | <0.0001 |
Crude model, with only a continuous variable representing week of sample collection. Cytokine expression was averaged within each sample. The spline term added an inflection point at Week 8. Parity status was defined as multiparous versus primiparous.
CI, confidence interval.
Average cytokine expression is not associated with breastfeeding frequency or time since last milk expression
Daily breastfeeding frequency and time since last milk expression were considered as factors influencing cytokine expression over time. Average daily breastfeeding frequency decreased slightly over the study period (participants averaged 9.8, 9.4, 8.4, and 7.6 daily feedings at Week 1, 4, 8, and 12 postpartum, respectively); however, breastfeeding frequency was not associated with cytokine expression (p=0.8). Time since last milk expression varied widely across the 15 participants and four time points, ranging from 5 to 332 minutes (data not shown). Similar to the trend for average cytokine expression (Fig. 1), average time since last milk expression decreased from 119.4 minutes at Week 1 to 91.3 minutes at Week 8, after which time since last expression increased to 100.5 minutes (Supplementary Fig. S1). However, despite the trend similarities, average time since last milk expression was not significantly associated with cytokine expression (p=0.2).
Individual cytokines show decreased expression over time
The temporal change in the expression of each measured cytokine individually was modeled using mixed models with a spline term. FDR q values of <0.05 were considered to be statistically significant, although unadjusted p values of <0.05 were also noted. Of the 78 cytokines that were measured, the expression of five cytokines (monocyte chemoattractant protein [MCP]-1, osteoprotegerin, tissue inhibitor of metallopeptidase-2 [TIMP-2], insulin-like growth factor-binding protein-1 [IGFBP-1], and epithelial-derived neutrophil-activating protein-78 [ENA-78]) were found to significantly change over the 12-week study period (q<0.05), and four other cytokines (hepatocyte growth factor [HGF], interleukin [IL]-16, IL-8, and macrophage colony-stimulating factor [M-CSF]) had significant changes in expression at α=0.05 (Table 3). Expression values of all significant cytokines decreased over time; no cytokines were found to have significantly (q<0.05 or p<0.05) increased expression over the study period. As shown in Table 3, the magnitude of the temporal trend was stronger for individual cytokines than for average cytokine level.
Table 3.
Individual Cytokines Showing a Significant Decrease over Time or a Significant Association with Parity Status
| Trend, cytokine | β coefficient | 95% CI | p value | q value |
|---|---|---|---|---|
| Decreased over time | ||||
| ENA-78a | −0.157 | −0.260, −0.053 | 0.004 | 0.04 |
| HGF | −0.083 | −0.151, −0.014 | 0.02 | 0.2 |
| IGFBP-1a | −0.110 | −0.176, −0.043 | 0.002 | 0.03 |
| IL-16 | −0.080 | −0.149, −0.010 | 0.03 | 0.2 |
| IL-8 | −0.103 | −0.185, −0.020 | 0.02 | 0.1 |
| MCP-1a | −0.184 | −0.263, −0.105 | <0.0001 | 0.006 |
| M-CSF | −0.070 | −0.139, −0.002 | 0.05 | 0.3 |
| Osteoprotegerina | −0.149 | −0.233, −0.064 | 0.001 | 0.03 |
| TIMP-2a | −0.132 | −0.210, −0.054 | 0.002 | 0.03 |
| Associated with parityb | ||||
| Eotaxin | 0.398 | 0.004, 0.791 | 0.05 | 0.02 |
| Eotaxin-2 | 0.307 | 0.003, 0.610 | 0.05 | 0.02 |
| Eotaxin-3 | 0.398 | 0.045, 0.750 | 0.03 | 0.02 |
| GRO-alpha | 0.447 | 0.148, 0.746 | 0.007 | 0.01 |
| IGFBP-3 | 0.467 | 0.076, 0.858 | 0.02 | 0.02 |
| IGFBP-4 | 0.303 | 0.008, 0.598 | 0.05 | 0.02 |
| IL-15 | 0.378 | 0.004, 0.752 | 0.05 | 0.02 |
| IL-7 | 0.428 | 0.018, 0.837 | 0.04 | 0.02 |
| IP-10 | 0.433 | 0.018, 0.848 | 0.04 | 0.02 |
| TGF-beta1 | 0.375 | 0.010, 0.741 | 0.05 | 0.02 |
| TIMP-1 | 0.352 | 0.128, 0.577 | 0.005 | 0.01 |
| Thrombopoetin | 0.461 | 0.054, 0.869 | 0.03 | 0.02 |
| VEGF | 0.279 | 0.096, 0.462 | 0.006 | 0.01 |
Significant false discovery rate q values (q<0.05); all other p values were significant at p=0.05. Models included a linear spline term at Week 8.
All presented correlations were significant at p=0.05 and q=0.05. Parity status was defined as multiparous (one or more births prior to the current birth) versus primiparous (no prior births).
CI, confidence interval; ENA-78, epithelial-derived neutrophil-activating protein-78; HGF, hepatocyte growth factor; IGFBP, insulin-like growth factor-binding protein; IL, interleukin; IP, interferon gamma-induced protein; MCP-1, monocyte chemoattractant protein-1; M-CSF, macrophage colony-stimulating factor; TIMP-2, tissue inhibitor of metallopeptidase-2; TGF, transforming growth factor; VEGF, vascular endothelial growth factor.
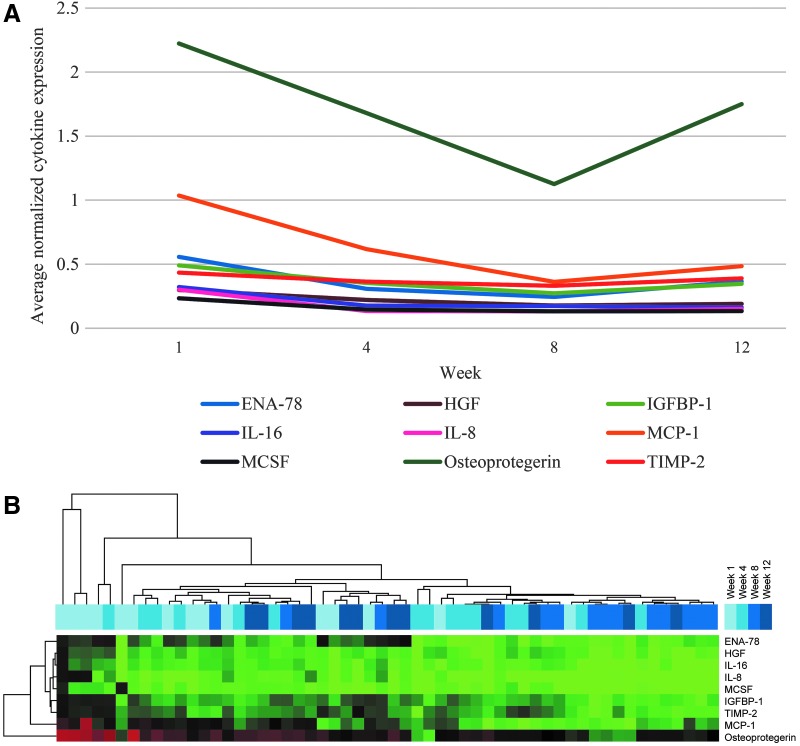
Expression values of all significant cytokines were plotted over time, and two groups of cytokines were identified based on the divergence of trend lines at Week 8 (Fig. 2A). After Week 8, several cytokines (most notably osteoprotegerin, MCP-1, ENA-78, and IGFBP-1), showed increasing expression, whereas HGF, IL-16, IL-8, and M-CSF expression continued to decrease.
FIG. 2.
Expression of significant cytokines over time, averaged across all participants (n=15). (A) Temporal trends of significant cytokines over the study period. All expression values were normalized to positive controls and averaged across all participants. For measures of variability in these temporal cytokine expression trends, see the 95% confidence intervals presented in Table 3 (cytokines decreased with time). (B) Heat map depicting a two-dimensional, supervised cluster of cytokine expression data. Only significant cytokines from Table 3 (cytokines decreased with time) were clustered. Red represents cytokines with high expression, whereas green represents cytokines with low expression relative to the median. ENA-78, epithelial-derived neutrophil-activating protein-78; HGF, hepatocyte growth factor; IGFBP-1, insulin-like growth factor-binding protein-1; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; MCSF, macrophage colony-stimulating factor; TIMP-2, tissue inhibitor of metallopeptidase-2.
Two-dimensional clustering was used to generate a heat map of the expression data for these nine cytokines (Fig. 2B). The samples clustered as expected based on Figure 1, with Week 1 samples clustering together and showing higher cytokine expression and Week 8 samples clustering together with lower expression. It is interesting that samples from Week 12 generally clustered with samples from Weeks 1 and 4, mirroring the trend in Figure 1 and suggesting that cytokine expression may increase at 12 weeks to levels comparable to the first month postpartum.
Total protein is significantly associated with cytokine expression
We also examined the association between cytokine expression and total protein concentration to consider whether our statistical models should adjust for total protein. Cytokines constitute one class of proteins expressed in breastmilk, and Qin et al.17 previously suggested that cytokine analyses in breastmilk should account for total milk protein through statistical adjustment or normalization of expression values to protein concentration. We identified eight cytokines (ENA-78, granulocyte–macrophage colony-stimulating factor, IGFBP-1, IL-16, IL-8, MCP-1, MCP-4, and TIMP-2) that were significantly associated with total protein (Supplementary Table S3), although no q values were significant. These results suggest that some cytokine expression is associated with total protein concentration, as expected given that milk protein levels include the cytokines we measured. However, we chose not to adjust for total protein in this analysis for two reasons.
First, trends in Figure 1 suggest that cytokine expression changes independently of total protein concentration over the first 12 weeks of lactation. Total protein content declined sharply over time and eventually stabilized after Week 8, whereas cytokine expression initially declined but increased after Week 8. Cytokine expression and total protein do not appear to change in parallel, and normalizing cytokine expression values to total protein concentration obscures the interpretation of changes in cytokine levels over time. Second, we considered the biological relationships among cytokine expression, total protein concentration, and duration of lactation (time). Total protein concentration and cytokine expression in breastmilk appear to be independently affected by time, and given that total protein and cytokine expression are correlated, total protein may be an intermediate in the association between time and cytokine expression. Thus, adjusting for total protein would attenuate or obscure the estimated trends in cytokine expression. However, the cytokine expression and total protein data for all participants are provided in Supplementary Table S4.
Multiparous women have higher cytokine expression than primiparous women
Parity status was not identified as a confounder in this analysis; however, we investigated whether cytokines were differentially expressed with respect to parity status. We identified 13 cytokines that were significantly increased among multiparous women (p<0.05), all of which also had q values <0.05 (Table 3). Although temporal trends seem unaffected by parity status, multiparous women appeared to have higher cytokine expression at baseline and persistently at subsequent time points.
Discussion
Our results suggest that average global cytokine expression and total protein concentration decrease over the first 3 months of lactation, with total protein showing a highly significant decreasing trend. We expected to see a similar downward trend for both cytokine expression and total protein content; however, average cytokine expression appeared to increase after Week 8 of lactation.
Our findings confirm and extend earlier work on temporal trends in inflammatory cytokine expression in human milk. Ustundag et al.12 found a similar but significant decrease in cytokine expression in breastmilk samples from 3 weeks and 2 months postpartum compared with colostrum samples from Week 1. Their study did not extend beyond 2 months and thus was not informative regarding trends later in lactation. Qin et al.17 also reported strongly decreased total milk protein concentrations at 2 months compared with baseline (within 10 days postpartum) and observed a nonsignificant increase in total protein content at the time of weaning (an average of 40 weeks postpartum). Similarly, Bauer and Gerss24 observed a significant decline in total protein levels over the first 2 months postpartum in the milk from mothers of term and preterm infants. Our temporal results are consistent with these studies despite differences in sampling time frame, suggesting that cytokine expression and total milk protein content initially decline until 2 months postpartum, after which expression may increase.
Further study is needed to clarify and replicate temporal patterns and link these trends to the onset of weaning. Given that weaning triggers breast involution, one could hypothesize that cytokine expression would increase or level off at weaning. Future longitudinal studies would benefit from regular breastmilk sampling from 3 months postpartum to weaning to better characterize cytokine expression dynamics.
Shifts in lactation biology and breastfeeding behavior over the first 3 months of lactation may influence temporal trends in cytokine expression and total protein concentration. Breastmilk production is thought to switch from endocrine to autocrine control early in lactation,25 after which milk production is regulated locally by the completeness and frequency of breast emptying during feeding.26–28 Given the known roles of cytokines in cell signaling and secretion, it is possible that cytokine profiles would be impacted by the frequency and recentness of milk production. We did not find that breastfeeding frequency or time since last feeding was significantly associated with cytokine expression and were unable to address other breastfeeding behaviors in our study; however, we found substantial variation across participants in reported time since last feeding, and it is possible that a larger sample could better characterize how breastfeeding recentness might impact cytokine expression.
We identified nine cytokines (ENA-78, HGF, IGFBP-1, IL-16, IL-8, MCP-1, M-CSF, osteoprotegerin, and TIMP-2) with significantly varied expression over the study period (p<0.05), and five cytokines (ENA-78, IGFBP-1, MCP-1, osteoprotegerin, and TIMP-2) remained significant after considering FDR q values (q<0.05).
All nine significant cytokines previously have been studied in association with breast cancer, inflammation, or involution: IL-8 and IL-16 expression is associated with several cancers, particularly metastatic breast cancer,29,30 and osteoprotegerin overexpression has been identified as a promising prognostic marker of bone metastasis risk.31,32 IL-8 expression in human milk has been suggested to decrease as breastfeeding progresses, although no previous study had found a significant trend.12,33,34 Regarding involution, HGF signaling is involved in wound healing, and ENA-78 expression is associated with angiogenesis; both cytokines are key players in tissue remodeling during postlactational involution.5,35 MCP-1 is also a known regulator of angiogenesis in addition to promoting macrophage migration and infiltration, and high MCP-1 expression may reflect higher levels of overall breast inflammation.36 M-CSF appears to be essential to successful involution in mice, as mouse knockouts show significantly delayed involution.37 Finally, expression of IGFBP-1 and TIMP-2 has been measured in human breastmilk, and both cytokines have previously been found to decrease in level during lactation,38,39 with the IGFBP-1 level decreasing over the first 3 months postpartum before stabilizing through Month 9 of lactation.38 It is not yet known how expression of these cytokines may be interrelated during lactation, although their known associations with breast involution, inflammation, and breast cancer suggest that these nine cytokines may be strong candidates for breast microenvironment biomarkers. Future experimental work is needed to establish the biological significance of these cytokines at the concentrations found in human breastmilk.
Postlactational biology may vary in primiparous versus parous women, but parity status was not a confounder in our analyses of temporal trends. However, multiparous women more highly expressed 13 cytokines across the time frame studied. To our knowledge, no studies have previously quantified breastmilk cytokine expression by parity status. This difference in cytokine levels with parity is particularly interesting in light of findings that risk of basal-like breast cancer increases with multiparity.2 The regulatory roles of parity-associated cytokines in breastfeeding, postlactational involution, and breast cancer merit further study.
Our findings should be considered in light of some limitations and strengths. Because of our small sample size, we lacked sufficient power to examine potential confounders other than parity. However, we did pursue careful aseptic sample collection at multiple time points, ensuring that our observed time course is free from collection-related microbial contaminants. We identified nine cytokines with potential to serve as biomarkers of the breast microenvironment; however, future mechanistic studies are necessary to clarify the biological significance of these cytokines in the breast microenvironment throughout lactation. Given that breastmilk is produced to provide nutrition to the infant, the cytokines we examined may have primary roles in infant development12,14,16,22 with secondary functions in the breast microenvironment. Additionally, we noted a shift in the cytokine expression trend after Week 8 postpartum, but our study period only extended to Week 12. Future studies collecting additional time points over the first year of breastfeeding are warranted to better understand the trends in cytokine expression associated with longer breastfeeding.
Conclusions
Our study characterizes cytokine expression in the breastmilk of healthy women with repeated sampling during the first 3 months of lactation. We present nine cytokines with potential as biomarkers of the breast microenvironment during breastfeeding and involution, providing a key first step in identifying potential cytokine-related biological mechanisms for how breastfeeding duration may impact future breast cancer risk. Our results suggest that cytokine expression during lactation is dynamic, and expressed milk may provide a noninvasive window into the extensive biological changes that occur in the postpartum breast. Beyond identifying potential biomarkers to understand how breastfeeding alters breast tissue, studies such as ours can also help to inform ideal cutpoints for breastfeeding exposure in epidemiologic studies. Prior studies have had conflicting definitions of breastfeeding exposure, partly because of lack of guidance from biological evidence. Continued study of breastmilk biomarkers has important implications for studying breastfeeding in cancer prevention.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the support of the North Carolina Translational and Clinical Sciences Institute. The M.A.T. Lab was supported by grant U01-ES019472 from the National Institute for Environmental Health Sciences (NIEHS) Breast Cancer and the Environment Research Program. This project benefitted from the NIEHS-funded University of North Carolina (UNC)-based Center for Environmental Health and Susceptibility (grant P30 ES010126) and the National Institute of Diabetes and Digestive and Kidney Diseases–funded UNC Nutrition and Obesity Research Center (grant P30 DK056350). Milk collection was supported by the UNC Medical Alumni Foundation and a National Institutes of Health Clinical and Translational Science Award at UNC (grant 1UL1TR001111). The authors also acknowledge Beth Horton and Xuezheng Sun for their assistance with data management and data analysis for this manuscript.
Disclosure Statement
No competing financial interests exist.
References
- 1.Kobayashi S, Sugiura H, Ando Y, et al. Reproductive history and breast cancer risk. Breast Cancer 2012;19:302–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Millikan RC, Newman B, Tse CK, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat 2008;109:123–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ursin G, Bernstein L, Lord SJ, et al. Reproductive factors and subtypes of breast cancer defined by hormone receptor and histology. Br J Cancer 2005;93:364–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet 2002;360:187–195 [DOI] [PubMed] [Google Scholar]
- 5.Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer 2006;6:281–291 [DOI] [PubMed] [Google Scholar]
- 6.McDaniel SM, Rumer KK, Biroc SL, et al. Remodeling of the mammary microenvironment after lactation promotes breast tumor cell metastasis. Am J Pathol 2006;168:608–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipworth L, Bailey LR, Trichopoulos D. History of breast-feeding in relation to breast cancer risk: A review of the epidemiologic literature. J Natl Cancer Inst 2000;92:302–312 [DOI] [PubMed] [Google Scholar]
- 8.Sohn BH, Moon HB, Kim TY, et al. Interleukin-10 up-regulates tumour-necrosis-factor-alpha-related apoptosis-inducing ligand (TRAIL) gene expression in mammary epithelial cells at the involution stage. Biochem J 2001;360:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson CJ. Immune cell regulators in mouse mammary development and involution. J Anim Sci 2009;87:35–42 [DOI] [PubMed] [Google Scholar]
- 10.Zhao L, Melenhorst JJ, Hennighausen L. Loss of interleukin 6 results in delayed mammary gland involution: A possible role for mitogen-activated protein kinase and not signal transducer and activator of transcription 3. Mol Endocrinol 2002;16:2902–2912 [DOI] [PubMed] [Google Scholar]
- 11.Radisky DC, Hartmann LC. Mammary involution and breast cancer risk: Transgenic models and clinical studies. J Mammary Gland Biol Neoplasia 2009;14:181–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ustundag B, Yilmaz E, Dogan Y, et al. Levels of cytokines (IL-1beta, IL-2, IL-6, IL-8, TNF-alpha) and trace elements (Zn, Cu) in breast milk from mothers of preterm and term infants. Mediators Inflamm 2005;2005:331–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcuzzi A, Vecchi Brumatti L, Caruso L, et al. Presence of IL-9 in paired samples of human colostrum and transitional milk. J Hum Lact 2013;29:26–31 [DOI] [PubMed] [Google Scholar]
- 14.Radillo O, Norcio A, Addobbati R, et al. Presence of CTAK/CCL27, MCP-3/CCL7 and LIF in human colostrum and breast milk. Cytokine 2013;61:26–28 [DOI] [PubMed] [Google Scholar]
- 15.Apaydin K, Ermis B, Arasli M, et al. Cytokines in human milk and late-onset breast milk jaundice. Pediatr Int 2012;54:801–805 [DOI] [PubMed] [Google Scholar]
- 16.Srivastava MD, Srivastava A, Brouhard B, et al. Cytokines in human milk. Res Commun Mol Pathol Pharmacol 1996;93:263–287 [PubMed] [Google Scholar]
- 17.Qin W, Zhang K, Kliethermes B, et al. Differential expression of cancer-associated proteins in breastmilk. Breastfeed Med 2013;8:120–126 [DOI] [PubMed] [Google Scholar]
- 18.Qin W, Zhang K, Kliethermes B, et al. Differential expression of cancer associated proteins in breast milk based on age at first full term pregnancy. BMC Cancer 2012;12:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arcaro KF, Browne EP, Qin W, et al. Differential expression of cancer-related proteins in paired breast milk samples from women with breast cancer. J Hum Lact 2012;28:543–546 [DOI] [PubMed] [Google Scholar]
- 20.Wockel A, Beggel A, Rucke M, et al. Predictors of inflammatory breast diseases during lactation—Results of a cohort study. Am J Reprod Immunol 2010;63:28–37 [DOI] [PubMed] [Google Scholar]
- 21.Erbagci AB, Cekmen MB, Balat O, et al. Persistency of high proinflammatory cytokine levels from colostrum to mature milk in preeclampsia. Clin Biochem 2005;38:712–716 [DOI] [PubMed] [Google Scholar]
- 22.Kverka M, Burianova J, Lodinova-Zadnikova R, et al. Cytokine profiling in human colostrum and milk by protein array. Clin Chem 2007;53:955–962 [DOI] [PubMed] [Google Scholar]
- 23.Nagatomo T, Ohga S, Takada H, et al. Microarray analysis of human milk cells: Persistent high expression of osteopontin during the lactation period. Clin Exp Immunol 2004;138:47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer J, Gerss J. Longitudinal analysis of macronutrients and minerals in human milk produced by mothers of preterm infants. Clin Nutr 2011;30:215–220 [DOI] [PubMed] [Google Scholar]
- 25.Jones E, Spencer S. The physiology of lactation. Paediatr Child Health 2007;17:244–248 [Google Scholar]
- 26.Peaker M, Wilde CJ. Feedback control of milk secretion from milk. J Mammary Gland Biol Neoplasia 1996;1:307–315 [DOI] [PubMed] [Google Scholar]
- 27.Daly SE, Owens RA, Hartmann PE. The short-term synthesis and infant-regulated removal of milk in lactating women. Exp Physiol 1993;78:209–220 [DOI] [PubMed] [Google Scholar]
- 28.Wilde C, Addey C, Bryson J, et al. Autocrine regulation of milk secretion. Biochem Soc Symp 1998;63:81–90 [PubMed] [Google Scholar]
- 29.Todorović-Raković N, Milovanović J. Interleukin-8 in breast cancer progression. J Interferon Cytokine Res 2013;33:563–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richmond J, Tuzova M, Cruikshank W, et al. Regulation of cellular processes by interleukin-16 in homeostasis and cancer. J Cell Physiol 2014;229:139–147 [DOI] [PubMed] [Google Scholar]
- 31.Owen S, Ye L, Sanders AJ, et al. Expression profile of receptor activator of nuclear-kappaB (RANK), RANK ligand (RANKL) and osteoprotegerin (OPG) in breast cancer. Anticancer Res 2013;33:199–206 [PubMed] [Google Scholar]
- 32.Bilgin E, Yasasever V, Soydinc HO, et al. Markers of bone metastases in breast and lung cancers. Asian Pac J Cancer Prev 2012;13:4331–4334 [DOI] [PubMed] [Google Scholar]
- 33.Meki AMA, Saleem TH, Al-Ghazali MHA, et al. Interleukins −6, −8 and −10 and tumor necrosis factor-alpha and its soluble receptor I in human milk at different periods of lactation. Nutr Res 2003;23:845–855 [Google Scholar]
- 34.Michie CA, Tantscher E, Schall T, et al. Physiological secretion of chemokines in human breast milk. Eur Cytokine Netw 1998;9:123–129 [PubMed] [Google Scholar]
- 35.Persson T, Monsef N, Andersson P, et al. Expression of the neutrophil-activating CXC chemokine ENA-78/CXCL5 by human eosinophils. Clin Exp Allergy 2003;33:531–537 [DOI] [PubMed] [Google Scholar]
- 36.Ueno T, Toi M, Saji H, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res 2000;6:3282–3289 [PubMed] [Google Scholar]
- 37.O'Brien J, Martinson H, Durand-Rougely C, et al. Macrophages are crucial for epithelial cell death and adipocyte repopulation during mammary gland involution. Development 2012;139:269–275 [DOI] [PubMed] [Google Scholar]
- 38.Sureshbabu A, Tonner E, Flint DJ. Insulin-like growth factor binding proteins and mammary gland development. Int J Dev Biol 2011;55:781–789 [DOI] [PubMed] [Google Scholar]
- 39.Alexander CM, Selvarajan S, Mudgett J, et al. Stromelysin-1 regulates adipogenesis during mammary gland involution. J Cell Biol 2001;152:693–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.