Abstract
Background: Uterine leiomyomata (fibroids) affect up to 77% of women by menopause and account for $9.4 billion in yearly healthcare costs. Most studies rely on self-reported diagnosis, which may result in misclassification of controls since as many as 50% of cases are asymptomatic and thus undiagnosed. Our objective was to evaluate the performance and accuracy of a fibroid phenotyping algorithm constructed from electronic medical record (EMR) data, limiting to subjects with pelvic imaging.
Methods: Our study population includes women from a clinical population at Vanderbilt University Medical Center (2008–2012). Analyses were restricted to women 18 years and older with at least one fibroid diagnosis confirmed by imaging for cases or at least two separate pelvic imaging procedures without a diagnosis for controls. We randomly reviewed 218 records to evaluate the accuracy of our algorithm and assess the indications for pelvic imaging. Participant characteristics and indications for imaging were compared between cases and controls in unadjusted and adjusted logistic regression analyses.
Results: Our algorithm had a positive predictive value of 96% and negative predictive value of 98%. Increasing age (odds ratio=1.05, 95% confidence interval 1.03–1.08) and Black race (odds ratio=2.15, 95% confidence interval 1.18–3.94) were identified as risk factors for fibroids. The most common indications for imaging in both cases and controls were pain, bleeding, and reproductive factors, and the most common imaging modality was a pelvic ultrasound.
Conclusions: These data suggest that using biorepositories linked to EMR data is a feasible way to identify populations of imaged women that facilitate investigations of fibroid risk factors.
Introduction
Uterine leiomyomata, or fibroids, are benign growths of the uterus and are the most common pelvic tumor in women, accounting for $9.4 billion in healthcare costs each year.1–3 Fibroid risk increases with age until the hormonal changes of menopause, with prevalence estimates ranging from 20 to 77%.4–6 Estimates of fibroid prevalence in the population vary greatly due to poor capture of women with asymptomatic fibroids. Studies have shown that as many as 51% of women are misclassified without clinical confirmation through imaging.7,8 A large proportion of existing fibroid studies are based on study designs that rely on self-reported fibroid diagnosis, with the exception of a few studies that have integrated a standardized fibroid assessment across all enrolled subjects.7,9 In addition to the significant impact of fibroids on a woman's quality of life and the economic burden in the United States, there is poor understanding of fibroid etiology and risk factors that can only be accomplished by better characterization and phenotyping of those with and without fibroids. Overcoming the bias introduced by underreporting of asymptomatic disease is a key challenge in observational fibroid risk research.
The difficulty in implementing a standardized fibroid assessment that conducts pelvic imaging on a large number of subjects is cost. A potential solution to this would be to utilize large clinical databases with linked electronic medical record (EMR) information to identify fibroid cases and controls that have already been imaged. Furthermore, with the development of large networks of biorepositories that link to EMR information, there is an opportunity to conduct studies of fibroids on a large scale to better understand both the epidemiology and etiology of uterine fibroids. Our objective with this study was to evaluate the performance and accuracy of a fibroid phenotyping algorithm constructed from electronic medical record (EMR) data, limiting to subjects with pelvic imaging. We evaluate the algorithm accuracy to identify cases and controls by estimating the positive and negative predictive values (PPV and NPV), as well as sensitivity and specificity. These algorithms are being used to develop a retrospective case-control group for women with and without fibroids. In addition, we characterize our study population with regards to common risk factors, and describe the indications for imaging in the case-control groups.
Materials and Methods
Study population
We utilized the clinical data available from the Synthetic Derivative (SD) EMR database (2008–2014), located at Vanderbilt University, Nashville, Tennessee.10 The SD consists of de-identified clinical data obtained from patients at Vanderbilt University Medical Center, including all clinics that are part of the hospital system. Clinical data from multiple sources are available, including diagnostic and procedure codes, basic demographics, discharge summaries, progress notes, health history, multidisciplinary assessments, laboratory values, imaging reports, medication orders, and pathology reports. The IRB of Vanderbilt University approved this study.
Fibroid diagnosis
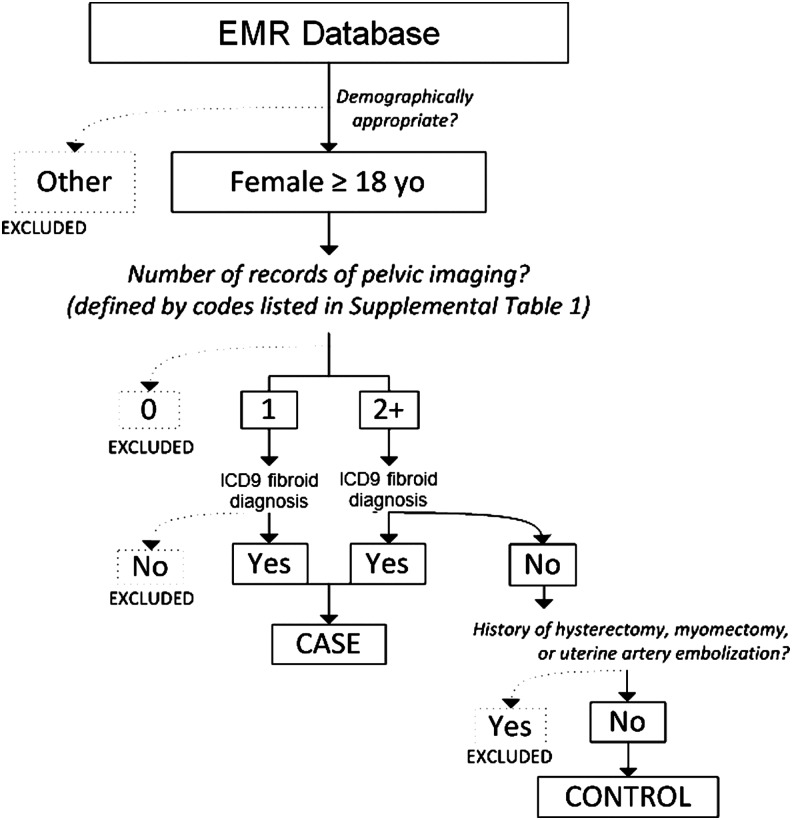
We created a fibroid case and control algorithm for the purposes of developing a retrospective case-control group of women with and without fibroids using EMR data. Our EMR system allows researchers to electronically evaluate clinical records using all available data in a participants' EMR. Our algorithms used a combination of demographic inclusion and exclusion criteria, International Classification of Diseases ninth edition (ICD-9) diagnostic codes, Current Procedural Terminology (CPT) codes, and keyword exclusions from specific notes and reports of a participant in order to identify cases and controls. Keyword searches of EMRs were conducted using natural language processing (NLP) algorithms available for those using our EMR data for research. We found that the NLP approach was able to reliably remove fibroid text mentions in these specific note fields and was able to exclude those subjects that lacked a diagnostic code but had a history of fibroids documented. Our “gold standard” validation of our algorithm was conducted through expert chart review of each participant's notes and reports to confirm that our algorithm accurately diagnosed a subject with or without a fibroid. A detailed summary of our case-control phenotyping algorithm is provided in Fig. 1 and Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/jwh).
Fig. 1.
Summary of study population case inclusion and exclusion criteria. Women aged 18 and older were included in the analysis. A history of pelvic imaging was queried by investigation of Current Procedural Terminology (CPT) codes corresponding to ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) of the pelvis. Those without evidence of pelvic imaging studies were excluded. In records documenting at least one imaging event, those that also carried a fibroid diagnosis were classified as cases. A fibroid diagnosis was defined by International Classification of Diseases (ICD) codes indicating the presence of fibroids or ICD and CPT codes indicating a history of fibroid treatment procedures. Those that did not carry a fibroid diagnosis were included as controls only if a second imaging event on a separate day was also documented. Finally, we determined that since it is unreasonable to assess the fibroid status of women without uteri, we excluded those controls that had a history of hysterectomy. This was accomplished through both the exclusion charts containing certain ICD codes and also with a free text search of records.
Subjects included as a fibroid case or control were women 18 years of age and older. All subjects (both cases and controls) were required to have had imaging procedures performed where they would have been identified as having a fibroid if a fibroid was present. Subjects with procedural codes (one or more for cases and two or more on separate dates for controls) for imaging with ultrasound, magnetic resonance imaging (MRI), or computed tomography (CT) were included (Fig. 1 and Supplementary Table S1). Cases required evidence of a fibroid diagnosis defined by either an ICD-9 code indicating the presence of fibroids or ICD and CPT codes indicating a history of fibroid treatment procedures (e.g., myomectomy or uterine artery embolization). An individual was included as a control if they had two imaging events on separate dates and did not have a fibroid diagnosis or history of fibroid treatment procedures. Excluded from controls were women without an intact uterus (e.g., having had a prior hysterectomy) based on CPT procedural codes and text mentions of hysterectomy (see Supplementary Table S1). Our sampling algorithm to define fibroids cases and controls is informed by a published fibroids algorithm by Hartmann and colleagues using EMRs.3
We randomly selected 218 participants (104 algorithm-defined fibroid cases and 114 controls) for chart review from our larger pool of 44,900 women who met our fibroid case and control algorithm criteria. The 218 subjects were a random draw from the pool of available women in the EMR, regardless of participant characteristics. We evaluated the notes and reports of these subjects to evaluate the quality of the phenotyping algorithm, as well as for abstraction of characteristics for participants. Algorithms were then assessed by calculating PPV, NPV, sensitivity, and specificity as well as evaluating the associations between patient characteristics and indications for imaging with fibroid risk.11–13 Indications for pelvic imaging were determined by manual review of participants EMR. All indications were collected and those reported are the most common indications across records.
Statistical analysis
Analyses were conducted with STATA statistical software version 11.2 (StataCorp LP, College Station). We evaluated known correlates with fibroids risk and tested them for association with fibroid risk in our population using logistic regression. Descriptive statistics of covariate data were expressed as means and standard deviations for continuous covariates and as frequencies and proportions for categorical data. Candidate confounders we modeled included age (years), body mass index (BMI, kg/m2), and indicator variables for race (non-Hispanic white [white, referent], non-Hispanic black [black], other). Although all indications for pelvic imaging were abstracted, here we report only most common indications. Indications for pelvic imaging included pain (pelvic, abdominal, other), bleeding (postmenopausal, dysmenorrhea, menorrhagia, and oligomenorrhea); reproductive (normal pregnancy, pregnancy complications, bleeding during pregnancy, and other); trauma; management of a known issue; malignancy (suspected or known); and other. We evaluated indications for pelvic imaging across cases and controls using logistic regression unadjusted and adjusted for known fibroid candidate confounders. Types of imaging modality included ultrasonography, CT scans, and MRI scans, and were compared between cases and controls using logistic regression adjusted and unadjusted for candidate confounders. We used a two-sided 5% significance level for all statistical inferences for associations between fibroid risk and evaluation of indications and type of modality for pelvic imaging.
Results
Using our phenotyping algorithm, the PPV for cases was 96% and the NPV for controls was 98%, while the sensitivity was 97% and the specificity 98%. The primary reason for case misclassification was due to initial diagnosis or suspicion of fibroid that was later refuted after further evaluation with imaging, and in one circumstance surgery for removal of fibroid proved that it was not a fibroid. Reasons for control misclassification included prior history of fibroid in clinical notes.
Increasing age was associated with increased risk of developing a fibroid (Table 1). The mean age and standard deviation for cases was 44±10 years and 36±14 years for controls. Black race was also associated fibroid risk with an odds ratio (OR)=2.15, and 95% confidence interval (95% CI) of 1.18–3.94. Although we observed increased risk for fibroids among overweight and obese subjects, these associations were not statistically significant (overweight OR=1.68, 95% CI 0.78–3.59; obese OR=1.56, 95% CI 0.77–3.18). Among fibroid cases, the most common type of fibroid was intramural, consistent with published literature, followed by submucous, subserous, and pedunculated fibroids. The majority of cases had only one fibroid, and the average largest diameter dimension was 46 mm±39 mm. The primary surgical treatment for fibroids was hysterectomy (40%) and did not differ by race (data not shown).
Table 1.
Characteristics of Study Population
| 95% CI | ||||||
|---|---|---|---|---|---|---|
| Characteristic | n | Fibroid cases (n=100) %/mean (SD) | Controls (n=112) %/mean (SD) | OR | Lower | Upper |
| Age mean (SD)1 | 212 | 44 (10) | 36 (14) | 1.05 | 1.03 | 1.08 |
| Age categories | ||||||
| <25 | 28 | 2 | 23 | 1.00 | Reference | |
| 25 to 29 | 24 | 3 | 19 | 1.86 | 0.28 | 12.16 |
| 30 to 34 | 29 | 11 | 16 | 7.94 | 1.57 | 40.23 |
| 35–49 | 73 | 49 | 21 | 26.54 | 5.81 | 121.22 |
| 50–64 | 36 | 19 | 15 | 14.53 | 3.00 | 70.54 |
| ≥65 | 22 | 16 | 5 | 34.67 | 6.22 | 193.06 |
| BMI mean (SD) | 204 | 30 (7) | 28 (7) | 1.04 | 0.99 | 1.08 |
| BMI categories | ||||||
| Underweight (<20) | 12 | 3 | 8 | 0.51 | 0.12 | 2.12 |
| Normal weight (20–24.9) | 48 | 19 | 26 | 1.00 | Reference | |
| Overweight (25–29.9) | 63 | 33 | 27 | 1.68 | 0.78 | 3.59 |
| Obese (≥30) | 89 | 45 | 39 | 1.56 | 0.77 | 3.18 |
| Race | ||||||
| White | 138 | 55 | 75 | 1.00 | Reference | |
| Black | 63 | 37 | 23 | 2.15 | 1.18 | 3.94 |
| Other | 10 | 8 | 2 | 6.04 | 1.24 | 29.50 |
| Fibroid type2 | ||||||
| Any submucous | 9 | 16 | - | - | - | - |
| Any intramural | 26 | 47 | - | - | - | - |
| Any subserous | 19 | 35 | - | - | - | - |
| Any pedunculated subserosal | 1 | 2 | - | - | - | - |
| Fibroid number | ||||||
| 1 | 43 | 52 | - | - | - | - |
| >1 | 39 | 48 | - | - | - | - |
| Fibroid largest dimension (mm) | 71 | 46 (39) | - | - | - | - |
| Surgical treatment of fibroid3 | ||||||
| Hysterectomy | 40 | 40 | - | - | - | - |
| Myomectomy | 10 | 10 | - | - | - | - |
| Uterine artery embolization | 4 | 4 | - | - | - | - |
Age is at fibroid diagnosis for cases and second imaging for controls.
Fibroid type values do not sum to total number of fibroids because subjects may have had more than one fibroid type observed.
Surgical treatment percentages do not add to 100 because women have undergone more than one procedure.
95% CI, 95% confidence interval; BMI, body mass index; OR, odds ratio; mean (SD), mean (standard deviation).
Evaluation of the indications for pelvic imaging in both cases and controls (Table 2) showed that on average more cases than controls had experienced bleeding as the primary reason they were evaluated with imaging (39% of cases, 8% for controls). However, there were more controls that were evaluated for reproductive indications occurring during pregnancy (2% of cases and 48% of controls). Pain, trauma, management of a known issue, and malignancy were other common indications, and these did not differ significantly between cases and controls. Since controls required two or more distinct imaging events to be eligible, we evaluated indications across the first and second imaging and did not find significant differences across indications in these two groups among controls (Table 2). Evaluation of indications for imaging were evaluated both unadjusted (Table 2) and adjusted for age, BMI, and race (results not shown) and effect sizes were consistent across both analyses.
Table 2.
Summary of Indications for Pelvic Imaging
| Indication | n | Fibroid cases% (N=90) | Controls %a (N=111) |
|---|---|---|---|
| Pain | 80 | 22 | 28 |
| Bleeding | 51 | 39 | 8 |
| Reproductive | 86 | 2 | 48 |
| Trauma | 5 | 0 | 3 |
| Management of known issue | 20 | 8 | 4 |
| Malignancy | 10 | 2 | 3 |
| Other | 57 | 27 | 17 |
Summarized data are for the first pelvic imaging procedure for controls.
A detailed summary of indications for imaging are provided in Table 3. More controls than cases had indications that included abdominal pain, irregular periods/metrorrhagia, pregnancy complications, and suspected malignancy. More cases than controls had indications that included pelvic pain, menorrhagia, normal pregnancy, and known malignancies.
Table 3.
Detailed Summary of Indications for Pelvic Imaging
| Indication | n | Fibroid cases% (N=90) | Controls %a(N=111) |
|---|---|---|---|
| Pain | |||
| Pelvic | 15 | 70 | 4 |
| Abdominal | 27 | 20 | 82 |
| Other | 6 | 10 | 14 |
| Bleeding | |||
| Postmenopausal | 8 | 21 | 25 |
| Dysmenorrhea | 4 | 11 | 13 |
| Menorrhagia | 19 | 57 | 38 |
| Oligomenorrhea | 3 | 11 | 25 |
| Reproductive | |||
| Normal pregnancy | 28 | 100 | 63 |
| Pregnancy complication | 13 | 0 | 32 |
| Bleeding during pregnancy | 1 | 0 | 2 |
| Other | 1 | 0 | 2 |
| Malignancy | 5 | ||
| Suspected malignancy | 1 | 0 | 33 |
| Known malignancy | 4 | 100 | 67 |
A portion of women were missing indications for pelvic imaging due to incomplete information in their records.
Summarized data are for the first pelvic imaging procedure for controls.
The mean age between the first and last pelvic imaging within cases (if they had more than one imaging event) and controls was 4±5 years for cases and 6±4 years for controls, with a mean of 4±3 and 7±6 imaging events for cases and controls, respectively. The most common type of imaging used to evaluate the pelvis was ultrasound in both cases and controls [88% cases, 59% controls (first image), 65% controls (second image), Table 4]. However, more controls than cases also had imaging performed through CT scans (6% cases, 38% controls).
Table 4.
Imaging Modalities Used in Uterine Fibroid Diagnosis
| Modality | n | Fibroid cases% (N=100)a | Controls % (N=112) |
|---|---|---|---|
| Ultrasound | 214 | 88 | 59 |
| CT Scan | 85 | 6 | 38 |
| MRI | 3 | 0 | 2 |
| Diagnosis as part of surgical proceduredel number | 6 | 7 | 0 |
These women either had a fibroid visualized as part of surgical procedure or had imaging where a mass was seen but not diagnosed until after a follow-up surgical procedure.
CT, computed tomography; MRI, magnetic resonance imaging.
Discussion
We evaluated the utility of EMR data to construct a fibroid case-control cohort of imaged women and demonstrated the accuracy of our phenotyping algorithm for identifying fibroid status. Furthermore, we evaluated the indications for pelvic imaging in both our cases and controls to assess whether indications differed by status. Overall, the most common indications for imaging were pain, bleeding, and pregnancy-related. However, more cases than controls had imaging performed for bleeding, specifically menorrhagia, and more controls than cases had imaging performed related to pregnancy such as routine obstetric ultrasounds or for pregnancy complications. Finally, across cases and controls, ultrasounds were the most common type of imaging modality used, and were more common in cases.
In addition to evaluating the indications for imaging and assessing the accuracy of our phenotyping algorithm, we evaluated a few common fibroid risk factors (age, race, and BMI) to assess whether we had comparable effect sizes within our population. Consistent with prior research, we observed increasing age and Black race to be risk factors for fibroids (age OR=1.05, and Black race OR=2.15).2,4,5,14–17 Our effect sizes for both age and Black race were consistent with prior studies that have observed 2-fold or higher odds of fibroids in Black women compared with White.2,4,5,14–17 Although high BMI has previously associated with fibroid risk, we did not observe a strong association with BMI, although there was evidence that increasing BMI is associated with increasing risk (overweight OR=1.68; obese OR=1.56).18–20 However, the associations between BMI and fibroids have been inconsistent in prior published literature.
The SD is linked to a biorepository of DNA samples (BioVU) that provides a unique resource for conducting genetic epidemiology studies of uterine fibroids. The motivation behind this study was to develop a phenotyping algorithm that could be portable into biorepositories like BioVU that would allow us to pursue large-scale genetic studies of fibroid risk. It would be very difficult and expensive to collect imaging data in a prospective manner in order to properly classify fibroid cases and controls. The major strengths of using populations like BioVU to conduct fibroid genetic analyses are that this information is already available in the EMR, and with the tools described here we show that we can extract that information reliably. Furthermore, the imaging reports available in BioVU essentially provide a “gold standard” diagnosis of fibroid status and “off-the shelf” DNA samples also allow an investigator to immediately conduct studies of fibroids without waiting for sample accrual. BioVU is part of network of biorepositories linked to EMR data called the electronic medical records and genomics (eMERGE) network (http://emerge.mc.vanderbilt.edu), thus phenotyping algorithms can be shared across network sites to develop large-scale genetic studies evaluating EMR-derived traits.
Although using EMR data overcomes a major barrier in fibroid research by helping to eliminate the bias introduced by asymptomatic disease, there are still limitations to consider. Our algorithm may classify some women as controls who will develop fibroids in the future but do not have disease at the time of imaging and being sorted by our algorithm. One strategy to minimize this potential confounder would be to limit the control group to women who are less likely to go on to develop fibroids, such as postmenopausal women. When limiting to the subset of women whom were 55 and older (n=15) among the women evaluated in our study, the algorithm was very reliable with none of the women being misclassified. The majority of these women had pelvic imaging through CT scans. We did not deem it prudent to restrict our control group in our preliminary analyses although the size of the SD and availability of demographic data provide us with ample opportunity to do this in the future. There is also the limitation that when using EMR data it is difficult to assess history of fibroids and/or treatment that occurred outside of the Vanderbilt EMR system. Among cases we can only evaluate women diagnosed and/or treated for fibroids at Vanderbilt; however, for controls we can have the ability to evaluate records through NLP and are able to determine the patient self-reported a fibroid diagnosis and/or treatment of if the diagnosis was reported in their EMR notes and exclude the subjects accordingly. Among controls we also require the women to have had their pelvic imaging at Vanderbilt, therefore, at least at the time of imaging no fibroid was observed and no fibroid was mentioned in their EMR. We also note that our algorithm is only applicable to other clinical populations with similar EMR systems and is meant to be used for retrospective assessment of fibroid status for construction of case-control groups. As a result of the retrospective assessment of fibroid status we cannot apply our algorithm to a prospective design or evaluate accurate fibroid prevalence estimates. Furthermore, our findings show that we capture more symptomatic and severe fibroids due to our data coming from a clinical population. This is due to the fact that pelvic imaging is usually performed for women who present with clinical symptoms, and thus are more likely to have more severe fibroids. Our algorithm is intended to accurately identify case and control individuals particularly for EMR DNA biorepositories that focus on genetic research, where having a more severe condition is a strength in the design. Additionally, in contrast to a prospective cohort where all subjects are imaged, having different indications for imaging across controls results in a pool of subjects that do not represent the general population, as a result evaluation of specific exposures of interest may be confounded by indication for pelvic imagery. A final limitation that cannot be accounted for in this study is the potential physician bias to underreport fibroids in a patient's record, for example, during pregnancy when a fibroid may change in both appearance and/or concentration.
Here we showed that our phenotyping algorithm classifies fibroid case and controls with high accuracy. Despite some differences in indications for pelvic imaging between cases and controls, overall the indications and modalities used to classify subjects were comparable. While the majority of both cases and controls were assessed with ultrasound, more controls were assessed with CT scans. This difference is unlikely to produce an important bias, as MRI is more sensitive for detecting fibroids than ultrasound and so there should not be differential misclassification of controls. Little is known about fibroid pathophysiology or genetic risk factors beyond what has been learned from cell culture studies and tumor biology. The barriers faced by fibroid researchers today include lack of imaging, limited racial diversity in cohorts, and availability of DNA samples. Populations like BioVU that leverage available information regarding the study population, pelvic imaging, and banked DNA samples provide an opportunity to study the molecular causes of uterine fibroids.
Supplementary Material
Acknowledgments
This work was funded by the Building Interdisciplinary Research Careers in Women's Health career development program (2K12HD043483-11) to DRVE, the National Institutes of Health (NIH) grant 1R01HD074711-01 to DRVE, NIH grant 1R03HD078567-01 to DRVE, the Vanderbilt Clinical and Translational Research Scholar Award 5KL2RR024977 to TLE from the National Center for Advancing Translational Sciences, and the Vanderbilt CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences which funded the REDCap database system used for database management for this study, NIH grant 3U01HG006378-03S1; and the BioVU dataset used for the analyses described was obtained from Vanderbilt University Medical Center's BioVU, which is supported by institutional funding and by the Vanderbilt CTSA grant ULTR000445 from NCATS/NIH. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. The material submitted for publication has not been previously reported and is not under consideration for publication elsewhere.
Disclosure Statement
No competing financial interests exist.
References
- 1.Cardozo ER, Clark AD, Banks NK, et al. . The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol 2012;206:211–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Day BD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188:100–107 [DOI] [PubMed] [Google Scholar]
- 3.Hartmann KE, Birnbaum H, Ben-Hamadi R, et al. . Annual costs associated with diagnosis of uterine leiomyomata. Obstet Gynecol 2006;108:930–937 [DOI] [PubMed] [Google Scholar]
- 4.Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol 1990; 94:435–438 [DOI] [PubMed] [Google Scholar]
- 5.Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol 1997;90:967–973 [DOI] [PubMed] [Google Scholar]
- 6.Vollenhoven B. Introduction: the epidemiology of uterine leiomyomas. Baillieres Clin Obstet Gynaecol 1998;12:169–176 [DOI] [PubMed] [Google Scholar]
- 7.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188:100–107 [DOI] [PubMed] [Google Scholar]
- 8.Myers SL, Baird DD, Olshan AF, et al. Self-report versus ultrasound measurement of uterine fibroid status. J Womens Health (Larchmt) 2012;21:285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laughlin SK, Baird DD, Savitz DA, Herring AH, Hartmann KE. Prevalence of uterine leiomyomas in the first trimester of pregnancy: an ultrasound-screening study. Obstet Gynecol 2009;113:630–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulley J, Clayton E, Bernard GR, Roden DM, Masys DR. Principles of human subjects protections applied in an opt-out, de-identified biobank. Clin Transl Sci 2010;3:42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meystre SM, Savova GK, Kipper-Schuler KC, Hurdle JF. Extracting information from textual documents in the electronic health record: a review of recent research. Yearb Med Inform 2008;128–144 [PubMed] [Google Scholar]
- 12.Ritchie MD, Denny JC, Crawford DC, et al. Robust replication of genotype-phenotype associations across multiple diseases in an electronic medical record. Am J Hum Genet 2010;86:560–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilke RA, Berg RL, Peissig P, et al. Use of an electronic medical record for the identification of research subjects with diabetes mellitus. Clin Med Res 2007;5:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laughlin SK, Baird DD, Savitz DA, Herring AH, Hartmann KE. Prevalence of uterine leiomyomas in the first trimester of pregnancy: an ultrasound-screening study. Obstet Gynecol 2009;113:630–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faerstein E, Szklo M, Rosenshein NB. Risk factors for uterine leiomyoma: a practice-based case-control study. II. Atherogenic risk factors and potential sources of uterine irritation. Am J Epidemiol 2001;153:11–19 [DOI] [PubMed] [Google Scholar]
- 16.Faerstein E, Szklo M, Rosenshein N. Risk factors for uterine leiomyoma: a practice-based case-control study. I. African-American heritage, reproductive history, body size, and smoking. Am J Epidemiol 2001;153:1–10 [DOI] [PubMed] [Google Scholar]
- 17.Ojeda VJ. The pathology of hysterectomy specimens. N Z Med J 1979;89:169–171 [PubMed] [Google Scholar]
- 18.Moore AB, Flake GP, Swartz CD, et al. Association of race, age and body mass index with gross pathology of uterine fibroids. J Reprod Med 2008;53:90–96 [PubMed] [Google Scholar]
- 19.Takeda T, Sakata M, Isobe A, et al. Relationship between metabolic syndrome and uterine leiomyomas: a case-control study. Gynecol Obstet Invest 2008;66:14–17 [DOI] [PubMed] [Google Scholar]
- 20.Wise LA, Palmer JR, Spiegelman D, et al. Influence of body size and body fat distribution on risk of uterine leiomyomata in U.S. black women. Epidemiology 2005;16:346–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.