Abstract
Background
Despite the increasing interest in sex differences in disease manifestations and responses to treatment, very few data are available on sex differences in seizure types and semiology. The Epilepsy Phenome/Genome Project (EPGP) is a large-scale, multi-institutional, collaborative study that aims to create a comprehensive repository of detailed clinical information and DNA samples from a large cohort of people with epilepsy. We used this well-characterized cohort to explore differences in seizure types as well as focal seizure symptoms between males and females.
Methods
We reviewed the EPGP database and identified individuals with generalized epilepsy of unknown etiology (GE) (n=760; female 446, male 314), non-acquired focal epilepsy (NAFE) (n=476; female 245, male 231), or both (n=64; female 33, male 31). Demographic data along with characterization of seizure type and focal seizure semiologies were examined.
Results
In GE, males reported atonic seizures more frequently than females (6.5% vs. 1.7%; p<0.001). No differences were observed in other generalized seizure types. In NAFE, no sex differences were seen for seizure types with or without alteration of consciousness or progression to secondary generalization. Autonomic (16.4% vs. 26.6%; p=0.005), psychic (26.7% vs. 40.3%; p=0.001), and visual symptoms (10.3% vs. 19.9%; p=0.002) were more frequently reported in females than males. Specifically, of psychic symptoms, more females than males endorsed déjà vu (p=0.001), but not forced thoughts, derealization/depersonalization, jamais vu, or fear. With corrections for multiple comparisons, there were no significant differences in aphasic, motor, somatosensory, gustatory, olfactory, auditory, vertiginous, or ictal headache symptoms between sexes.
Conclusions
Significant differences between the sexes were observed in the reporting of atonic seizures, which was more common in males with GE, and for autonomic, visual, and psychic symptoms associated with NAFE, which were more common in females.
Keywords: Epilepsy, seizures, sex, focal epilepsy, semiology, generalized epilepsy
1. Introduction
Epilepsy affects ~50 million people worldwide and has a lifetime risk of ~3%.[1, 2] The incidence and prevalence of unprovoked seizures is higher in men than women[3-5] and status epilepticus is more frequent in men than women.[6, 7] However, some idiopathic generalized epilepsies are more common in women[4, 8-12], particularly juvenile myoclonic epilepsy[8-11] and absence epilepsy.[4, 8, 12] There are no sex differences for patients with hippocampal sclerosis on MRI.[13] Sex disparities after epilepsy surgery are reported with more favorable outcomes in women[14] as well as men.[15-18]
A few studies have examined sex differences in seizure semiology. A retrospective review of patients with medial temporal lobe epilepsy identified less frequent isolated auras and more frequent secondarily generalized seizures in men, but no other significant semiologic differences between sexes[19]. Others reported an increased incidence of sexual auras[20, 21] and increased frequency of affective, particularly negative affective, ictal symptoms[22] in women. These observations suggest that there may be underlying sex differences in the neurobiology of seizures and epilepsy. Using the prospectively gathered seizure and semiology data from the multi-center Epilepsy Phenome/Genome Project database, we aimed to explore differences in both seizure types and semiology.
2. Materials and Methods
2.1 Subjects
All patients were identified from the Epilepsy Phenome/Genome Project (EPGP). This multi-institutional, collaborative network of 27 academic epilepsy centers throughout the U.S., Australia, New Zealand, and Argentina carried out detailed clinical phenotyping of participants from 2006 to 2013. Enrolled participants in the generalized epilepsy of unknown etiology (GE) or non-acquired focal epilepsy (NAFE) arms had a family history (either sibling or parent) of epilepsy. Participants were identified through a combination of prospective screening of clinic patients, retrospective review of medical records, and education and recruitment of colleagues within the primary EPGP institutions and neighboring institutions.[23] After obtaining informed consent from the subject, all clinical and demographic data were gathered prospectively through semi-structured interviews as well as review of medical records, EEG, and imaging data. Figure 1 depicts the data collection and review processes and the three points at which eligibility was re-assessed following obtaining informed consent. Subjects with GE had to have generalized onset seizures, normal neuroimaging if it was performed, and an EEG showing generalized epileptiform activity with a normal posterior dominant rhythm. If the EEG was normal, there had to be clear clinical history and the data were sent for review and adjudication.[23] For NAFE, subjects had neuroimaging which was either normal or demonstrated mesial temporal sclerosis or focal cortical dysplasia and an unambiguous clinical semiology consistent with focal seizures and/or focal EEG abnormalities. Patients with benign rolandic epilepsy based upon clinical presentation were not required to have neuroimaging.
Figure 1.
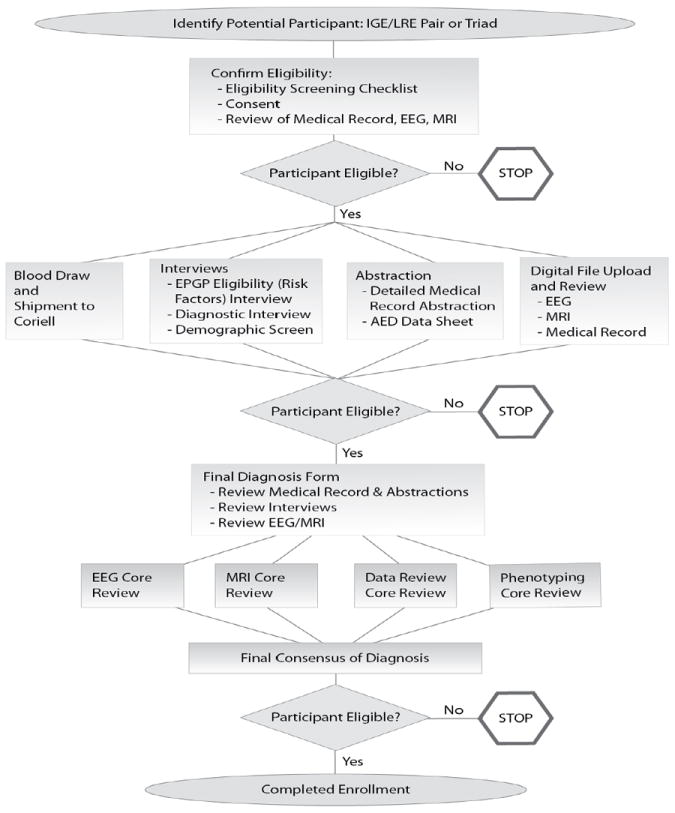
EPGP patient enrollment process. Following the initial eligibility screen, two additional eligibility screens occurred after additional data were gathered and reviewed prior to final enrollment.
2.2 Seizure Classification
Seizures were classified utilizing the International League Against Epilepsy Classification for both generalized and focal (partial) seizure types[24]. Generalized seizures were: absence, atypical absence, tonic, clonic, tonic-clonic, atonic, and myoclonic. Focal seizures were classified utilizing the older terminology of simple partial seizures for focal seizures without dyscognitive features and complex partial seizures for focal seizures with dyscognitive features. Both types of seizures could progress to a secondarily generalized tonic-clonic convulsion. Patients were not restricted to a single seizure type.
2.3 Semiologic Descriptions
Semiology information was gathered through a structured interview (by telephone or in person) and by medical record review.[23] The interview was modified from a previously validated instrument.[25, 26] When necessary, data were reviewed and adjudicated by the Phenotype Core. Ictal semiologies were grouped into the following categories: aphasia, autonomic, motor, psychic, gustatory, olfactory, somatosensory, or visual. These data were gathered at the time of enrollment in the EPGP.
2.4 Data Surveillance and Quality Control
Systematic quality reviews were conducted to identify and correct errors in phenotypic data[23]. All data were stored electronically in a central repository[27]. The following activities are conducted on an ongoing basis: 1) Qualitative and quantitative data monitoring activities by the EPGP Statistician; 2) Automated error checks programmed by the Informatics Core; 3) In-person data review meetings to examine forms and medical records for a subset of participants; 4) Expert reviews by EPGP scientific cores, including EEG, MRI, AED, Phenotype, and Data Review Cores; and 5) Review of final diagnoses by two independent members of the Data Review Core.
2.5 Statistical Analysis
All data were analyzed utilizing SPSS Version 21 for Windows. For continuous variables (i.e. age at enrollment, age at seizure onset, and duration of epilepsy), generalized linear models were employed. For categorical variables (e.g., seizure types, semiology), binary logistic regression was utilized. For all analyses, generalized estimating equations were utilized to adjust the confidence intervals for the non-independence of observations within each family. A Bonferroni correction was used to address multiple comparisons. For generalized seizure types, a corrected alpha level of p≤0.006 was used. For focal seizure symptoms, a corrected alpha level of p≤0.005 was used to address multiple comparisons across the 11 primary symptom classes. For both autonomic and psychic symptoms, additional exploratory analyses of symptoms within those classes was done with an alpha level of p<0.05.
3. Results
Out of a total of 2,751 patients that were consented for enrolment, 545 males and 691 females were analyzed. After obtaining informed consent, there were 813 participants that were ineligible after obtaining and screening their enrolment data. Another 544 participants did not complete the study protocol for data collection and review and were lost to the study; these “inactive” participants were not included in the final data set. An additional 76 males and 82 females were not classifiable due to inadequate clinical data; these patients were excluded from analyses. Table 1 shows the number of males and females enrolled in each of three groups: 1) NAFE, 2) GE, and 3) both GE and NAFE. For each group, the mean age at enrollment, age at onset of epilepsy, and duration of seizures are shown (in years) along with the standard deviations. The mean age at the time of enrollment was younger for males than females for both NAFE (p=0.02) and GE (p<0.001). The duration of epilepsy was shorter for males for both NAFE (p=0.012) and GE (p<0.001). The age at onset was younger for males with GE (p=0.001); there was no significant difference for NAFE. For all subsequent analyses, patients in group 3 (both GE and NAFE) were included in both the NAFE and GE calculations.
Table 1.
Age at enrollment, age at onset of seizures, and epilepsy duration.
| Focal Epilepsy
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age at Enrollment | Age at Onset | Duration of Epilepsy | ||||||||
| Number | Mean | SE | p | Mean | SE | p | Mean | SE | p | |
| Male | 231 | 22.3 | 1.1 | 0.02* | 12.1 | 0.8 | 0.27 | 10.2 | 0.8 | 0.012* |
| Female | 245 | 25.9 | 1.2 | 13.2 | 0.8 | 12.6 | 0.7 | |||
|
|
||||||||||
| Generalized Epilepsy
|
||||||||||
| Age at Enrollment | Age at Onset | Duration of Epilepsy | ||||||||
| Number | Mean | SE | p | Mean | SE | p | Mean | SE | p | |
| Male | 314 | 18.6 | 0.7 | <0.001* | 9.8 | 0.4 | 0.001* | 8.8 | 0.6 | <0.001* |
| Female | 446 | 23.5 | 0.7 | 11.5 | 0.4 | 12 | 0.6 | |||
|
|
||||||||||
| Focal Epilepsy and Generalized Epilepsy
|
||||||||||
| Age at Enrollment | Age at Onset | Duration of Epilepsy | ||||||||
| Number | Mean | SE | p | Mean | SE | p | Mean | SE | p | |
| Male | 31 | 15.8 | 2 | 0.11 | 8.4 | 1 | 0.3 | 7.4 | 1.5 | 0.14 |
| Female | 33 | 20.5 | 2.2 | 10.1 | 1.3 | 10.4 | 1.4 | |||
denotes statistically significant differences.
Table 2 shows the frequency of generalized seizure types for both sexes. Atonic seizures were seen more frequently in males than females (p<0.001); otherwise, no differences were seen.
Table 2.
Sex differences in generalized epilepsy of unknown etiology seizure types
| Male | Female | ||||||
|---|---|---|---|---|---|---|---|
| Yes | No | % Yes | Yes | No | % Yes | Statistical Significance | |
| Absence | 193 | 146 | 56.9% | 292 | 176 | 62.4% | p=0.12 |
|
| |||||||
| Atonic | 22 | 318 | 6.5% | 8 | 466 | 1.7% | p<0.001* |
|
| |||||||
| Atypical Absence | 17 | 318 | 5.1% | 13 | 462 | 2.7% | p=0.08 |
|
| |||||||
| Gen Clonic | 4 | 335 | 1.2% | 3 | 468 | 0.6% | p=0.34 |
|
| |||||||
| Gen Tonic | 9 | 329 | 2.7% | 5 | 463 | 1.1% | p=0.1 |
|
| |||||||
| GTC | 174 | 165 | 51.3% | 238 | 229 | 51.0% | p=0.92 |
|
| |||||||
| Myoclonic | 107 | 233 | 31.5% | 169 | 307 | 35.5% | p=0.25 |
denotes statistically significant after correction for multiple comparisons.
Table 3 shows the frequency of focal seizure types. No differences between the sexes were seen. Table 4 shows the frequencies of the categories of ictal semiologies for both males and females. Motor phenomena were the most commonly reported semiologic element for both males and females followed by psychic features. For males, aphasia and then autonomic symptoms were the next most common features, whereas for females, autonomic symptoms and aphasia were the next most common. Autonomic features (p=0.005), psychic phenomena (p=0.001), and visual phenomena (p=0.002) were more frequent in females compared to males with NAFE. Within autonomic features, visceral/epigastric sensations (p=0.003) were more common in females; no difference was seen for chest tightness, dyspnea, cardiac symptoms, or diaphoresis. Within the category of psychic ictal symptoms, déjà vu (p=0.001) was more common in females; ictal fear, forced thoughts, jamais vu, and derealization/depersonalization had similar frequencies for both males and females.
Table 3.
Sex differences in non-acquired focal epilepsy seizure types
| Male | Female | Statistical Significance | |||||
|---|---|---|---|---|---|---|---|
| Yes | No | % Yes | Yes | No | % Yes | ||
| SPS | 72 | 188 | 27.7% | 92 | 184 | 33.3% | p=0.13 |
|
| |||||||
| SPS to CPS to GTC | 18 | 238 | 7.0% | 25 | 250 | 9.1% | p=0.38 |
|
| |||||||
| SPS to GTC | 36 | 219 | 14.1% | 48 | 227 | 17.5% | p=0.32 |
|
| |||||||
| CPS | 152 | 106 | 58.9% | 173 | 104 | 62.5% | p=0.38 |
|
| |||||||
| CPS to GTC | 74 | 181 | 29.0% | 84 | 189 | 30.8% | p=0.7 |
Table 4.
Sex differences in non-acquired focal epilepsy seizure semiology
| Male | Female | Statistical Significance | |||||
|---|---|---|---|---|---|---|---|
| Yes | No | % Yes | Yes | No | % Yes | ||
| Aphasia | 45 | 217 | 17.2% | 56 | 222 | 20.1% | 0.37 |
|
| |||||||
| Autonomic | 43 | 219 | 16.4% | 74 | 204 | 26.6% | 0.005* |
|
| |||||||
| Dyspnea | 5 | 257 | 1.9% | 16 | 262 | 5.8% | 0.05 |
|
| |||||||
| Chest Tightness | 3 | 259 | 1.1% | 3 | 275 | 1.1% | 0.93 |
|
| |||||||
| Visceral or Epigastric Sensation | 13 | 249 | 5.0% | 33 | 244 | 11.9% | 0.003* |
|
| |||||||
| Cardiac | 4 | 258 | 1.5% | 12 | 265 | 4.3% | 0.07 |
|
| |||||||
| Diaphoresis | 27 | 235 | 10.3% | 34 | 244 | 12.2% | 0.49 |
|
| |||||||
| Motor | 180 | 74 | 70.9% | 187 | 82 | 69.5% | 0.74 |
|
| |||||||
| Psychic | 70 | 192 | 26.7% | 112 | 166 | 40.3% | 0.001* |
|
| |||||||
| Fear | 30 | 230 | 11.5% | 42 | 234 | 15.2% | 0.19 |
|
| |||||||
| Déjà vu | 27 | 233 | 10.4% | 56 | 222 | 20.1% | 0.001* |
|
| |||||||
| Jamais vu | 1 | 258 | 0.4% | 8 | 269 | 2.9% | 0.06 |
|
| |||||||
| Derealization/Depersonalization | 17 | 245 | 6.5% | 26 | 252 | 9.4% | 0.22 |
|
| |||||||
| Forced Thoughts | 1 | 260 | 0.4% | 4 | 274 | 1.4% | 0.23 |
|
| |||||||
| Gustatory | 16 | 245 | 6.1% | 19 | 255 | 6.9% | 0.71 |
|
| |||||||
| Olfactory | 8 | 253 | 3.1% | 16 | 258 | 5.8% | 0.13 |
|
| |||||||
| Somatosensory | 17 | 245 | 6.5% | 32 | 244 | 11.6% | 0.04 |
|
| |||||||
| Visual | 27 | 235 | 10.3% | 55 | 221 | 19.9% | 0.002* |
|
| |||||||
| Auditory | 17 | 245 | 6.5% | 26 | 250 | 9.4% | 0.22 |
|
| |||||||
| Vertigo | 7 | 254 | 2.7% | 14 | 259 | 5.1% | 0.15 |
|
| |||||||
| Ictal Headache | 9 | 253 | 3.4% | 4 | 269 | 1.5% | 0.15 |
denotes statistically significant after correction for multiple comparisons.
The overall reporting of symptoms was higher for females than it was for males in subjects with NAFE; females reported a mean of 4.9 different symptoms for their seizures with males reporting 4 (p=0.002). For generalized seizures, there was no difference between genders.
4. Discussion
This large, prospective sample of patients with rigorous phenotypic classification identified several sex differences with regard to seizure type and semiology. Although no differences in the frequency of focal seizure types were seen between sexes, an increased frequency of autonomic, psychic, and visual features was seen for females compared to males. For generalized seizures, atonic seizures were seen with greater frequency in males than females.
Semiologic differences between sexes have been largely unstudied in the literature. A retrospective study restricted to medial temporal lobe epilepsy identified no differences for psychic auras[19], in contrast to the findings in this series. Our data were not limited to a particular epilepsy localization (e.g. temporal lobe epilepsy) nor restricted to the aura. Although the overall rates of reported psychic symptoms are higher in our study compared to the data reported by Janszky et al (27% males and 40% females versus 24% males and 31% females, respectively), the frequency of psychic symptoms for each sex was not statistically different between the studies for males (p=0.76) nor for females (p=0.16).[19] In our study, more detailed information on the nature of the psychic features of seizures revealed increased reporting of déjà vu for females, but no other differences for specific psychic symptoms.
Similarly, in our study, autonomic features including visceral/epigastric sensations were more frequent in females with NAFE. In contrast to our findings, Janzky et al reported no difference in frequency of reported abdominal auras between males and females. Notably, a higher frequency of abdominal auras was seen in their series compared with ours for both males (58% versus 5.0%, p=0.0001) and females (69% versus 11.9%, p=0.0001). It is unclear what accounts for the marked difference in frequency of this symptom between series. It is possible this is due, in part, to how symptoms were ascertained as well as to the differences in patient populations, as Janzky et al limited enrollment to temporal lobe epilepsy. In addition, the broader range of ictal symptoms explored within the questionnaire for our cohort likely impacts the identified ictal symptoms.
Visual ictal symptoms were more common in females; sex differences for ictal visual symptoms have not been assessed by any previous studies. As was observed in the study by Janszky et al, we found no differences in olfactory, motor, or language symptoms.[19]
In contrast to previously reported studies, we did not observe an increased rate of absence seizures in females.[4, 8, 12] With the exception of atonic seizures, which were seen more frequently in men, no differences were seen amongst generalized seizure types. Although the reason for this finding is not known, it is possible that the increased frequency of atonic seizures may be related to Doose or Doose variants (myoclonic-astatic epilepsy). This syndrome is known to be more frequent in males.[28, 29] Given the EPGP methodology which enrolled patients with a family history of epilepsy, it is possible that there was a relative increase in enrollment of Doose syndrome patients which could lead to more frequent atonic seizures in males.
Differences in the approach to phenotyping may account for some of the discrepancies in results between our study and those reported above. The EPGP inclusion criteria for focal epilepsy required either normal neuroimaging or the findings of mesial temporal sclerosis or focal cortical dysplasia along with EEG findings or clinical semiology consistent with focal seizures. Data were obtained through medical records as well as diagnostic interviews. In contrast, Janzsky et al utilized review of the medical records on admission for pre-surgical evaluation and the video-EEG ictal data for inclusion and characterization.[19] Also, the population in the EPGP GE and NAFE cohorts was enriched for genetic factors; families were required to have at least two affected first degree relatives to be enrolled. The study reported by Janszky et al excluded patients with a family history of epilepsy.[19] These differences in both phenotypic characterization and in patient population may account for some of the differences observed between previous studies and the EPGP data. Lastly, the EPGP population here was relatively young with mean ages well below 30 for both males and females reflecting the inclusion of both adults and children (ranging from one to 82 years of age).
Differences in symptom reporting based upon sex may account for the observed findings. [30] Whether these known reporting differences are psychological, biological, or sociological remains unclear and the impact of one or all of these factors on our results is uncertain. It is possible that sex differences in the frequency of specific seizure symptoms may reflect underlying differences in how males and females engage limbic and other regions for memory and emotional tasks. There is evidence supporting differences between the sexes in the processing of memory and emotion. Differences have been observed using fMRI activations associated with emotional memory[31] and autobiographical memory[32], as well as differences in cerebral blood flow utilizing SPECT in limbic regions in response to procaine infusion.[33] These sex differences in cognitive and emotional processing may lead to differences in activation patterns, and thus semiologies, for seizures.
Given the increasing evidence over the past several decades of the importance of hormones such as estrogen, progesterone, and androgens in neurodevelopment, neuroprotection, memory, and seizures, these differences in focal seizure semiologies may reflect responses to different levels of these important hormones between sexes.[34-36] It is plausible that differences in both brain-derived and systemically-derived estrogens between sexes may lead to differences in responses to the inciting event leading to seizures or the subsequent seizures themselves. It is reasonable to hypothesize that these differences might result in increased involvement of limbic pathways, leading to the experiential phenomena seen with greater frequency in females in this study.
The unique EPGP study population poses some limitations for this study. The EPGP was designed to address the role of genetic variations in the development of epilepsy and treatment resistance by enrolling patients with a family history of epilepsy.[23] Although the study presented here utilized generalized estimating equations to address the non-independence of observations within families, it is possible that the observed sex differences may not be generalizable to a sample of patients without a family history of epilepsy. As patients were recruited primarily from epilepsy centers, they had to have presented for treatment or evaluation leading to a potential bias for patients with less well controlled epilepsy; families who have well controlled epilepsy (i.e. do not need to have regular visits with a neurologist or epileptologist) may be underrepresented in this sample. Similarly, patient populations derived primarily from epilepsy centers may not be representative of a non-tertiary center derived sample. These factors may have led to the higher proportion of females compared to males in the overall study population.
5. Conclusions
The findings presented here show differences in several subjective ictal symptoms between males and females. Although our data cannot directly assess the neurobiological bases of these findings nor can they rule out the possibility of the findings being driven by differences in symptom reporting and recognition, they raise important questions as to differences that exist between the sexes. Nonetheless, these findings may allow for further explorations of processing differences and the ways in which seizures impact these complex systems.
Highlights.
Seizure semiology data from 1,236 patients with epilepsy were analyzed
Males had a higher reported frequency of atonic seizures than females
Autonomic, psychic, and visual symptoms were reported more often in females
Acknowledgments
This work is supported by National Institute of Neurological Diseases and Stroke (NINDS) grant U01 NS053998, as well as planning grants from the Finding a Cure for Epilepsy and Seizures Foundation (FACES) and the Richard Thalheimer Philanthropic Fund.
We would like to acknowledge the recruitment contributions of the EPGP Community Referral Network (CRN). The CRN consists of healthcare professionals not paid by the EPGP grant who refer eligible families to EPGP. A list of individual contributors can be found at www.epgp.org.
Dr. Friedman receives support from the NIH (UL1 TR000038 from the National Center for the Advancement of Translational Science (NCATS), American Epilepsy Society, and NYUfaces, Finding a cure for epilepsy and seizures. Dr. Friedman is an investigator at NYU on studies for UCB Inc/Schwarz Pharma and also receives salary support for work performed on behalf of The Epilepsy Study Consortium, a non-profit organization dedicated to improving the lives of epilepsy patients, and devotes 15% of his time to work done for the Consortium. The Consortium receives payments from a large number of pharmaceutical companies for consulting activities. All payments are made to The Consortium and not to Dr. Friedman directly. Several companies also support the Consortium’s biennial Antiepileptic Drug Trials Symposium.
Footnotes
Conflict of interest
Drs. Carlson, Dugan and Kirsch have no conflicts of interest to disclose.
Since there a so many companies contributing, the amount from each company towards Dr. Friedman’s salary is minimal and is reviewed annually by NYU’s conflict of interest committee.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Linehan C, Kerr M. Epidemiology of epilepsy in developed countries. In: Benbadis SR, Beran RG, Berg AT, Engel J Jr, Galanopoulou AS, Kaplan PW, Koutroumanidis M, Moshe SL, Nordli DR Jr, Serratosa JM, Sisodiya SM, Valeta T, Wilner A, Tatum WO, CP P, editors. Atlas of Epilepsies. London: Springer; 2010. pp. 51–56. [Google Scholar]
- 2.Diagan M, Bhalla D, Ngoungou E, PM P. Epidemiology of epilepsy in resource poor countries. In: Benbadis SR, Beran RG, Berg AT, Engel J Jr, Galanopoulou AS, Kaplan PW, Koutroumanidis M, Moshe SL, Nordli DR Jr, Serratosa JM, Sisodiya SM, Valeta T, Wilner A, Tatum WO, CP P, editors. Atlas of Epilepsies. London: Springer; 2010. pp. 57–63. [Google Scholar]
- 3.Hauser WA. Incidence and Prevalence. In: Engel J, Pedley TA, editors. Epilepsy : a comprehensive textbook. Philadelphia: Lippincott-Raven; 1997. pp. 47–59. [Google Scholar]
- 4.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935-1984. Epilepsia. 1993;34:453–68. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- 5.Kotsopoulos IA, van Merode T, Kessels FG, de Krom MC, Knottnerus JA. Systematic review and meta-analysis of incidence studies of epilepsy and unprovoked seizures. Epilepsia. 2002;43:1402–9. doi: 10.1046/j.1528-1157.2002.t01-1-26901.x. [DOI] [PubMed] [Google Scholar]
- 6.Coeytaux A, Jallon P, Galobardes B, Morabia A. Incidence of status epilepticus in French-speaking Switzerland: (EPISTAR) Neurology. 2000;55:693–7. doi: 10.1212/wnl.55.5.693. [DOI] [PubMed] [Google Scholar]
- 7.Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Incidence of status epilepticus in Rochester, Minnesota, 1965-1984. Neurology. 1998;50:735–41. doi: 10.1212/wnl.50.3.735. [DOI] [PubMed] [Google Scholar]
- 8.Christensen J, Kjeldsen MJ, Andersen H, Friis ML, Sidenius P. Gender differences in epilepsy. Epilepsia. 2005;46:956–60. doi: 10.1111/j.1528-1167.2005.51204.x. [DOI] [PubMed] [Google Scholar]
- 9.Wolf P. Juvenile myoclonic epilepsy. In: Roger J, editor. Epileptic syndromes in infancy, childhood, and adolescence. 2. London: J Libbey; 1992. pp. 213–227. [Google Scholar]
- 10.Kleveland G, Engelsen BA. Juvenile myoclonic epilepsy: clinical characteristics, treatment and prognosis in a Norwegian population of patients. Seizure. 1998;7:31–8. doi: 10.1016/s1059-1311(98)90005-x. [DOI] [PubMed] [Google Scholar]
- 11.Sundqvist A. Juvenile Myoclonic Epilepsy - Events before Diagnosis. Journal of Epilepsy. 1990;3:189–192. [Google Scholar]
- 12.Waaler PE, Blom BH, Skeidsvoll H, Mykletun A. Prevalence, classification, and severity of epilepsy in children in western Norway. Epilepsia. 2000;41:802–10. doi: 10.1111/j.1528-1157.2000.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 13.Briellmann RS, Jackson GD, Mitchell LA, Fitt GJ, Kim SE, Berkovic SF. Occurrence of hippocampal sclerosis: is one hemisphere or gender more vulnerable? Epilepsia. 1999;40:1816–20. doi: 10.1111/j.1528-1157.1999.tb01604.x. [DOI] [PubMed] [Google Scholar]
- 14.Cohen-Gadol AA, Wilhelmi BG, Collignon F, White JB, Britton JW, Cambier DM, Christianson TJ, Marsh WR, Meyer FB, Cascino GD. Long-term outcome of epilepsy surgery among 399 patients with nonlesional seizure foci including mesial temporal lobe sclerosis. J Neurosurg. 2006;104:513–24. doi: 10.3171/jns.2006.104.4.513. [DOI] [PubMed] [Google Scholar]
- 15.Aull-Watschinger S, Pataraia E, Czech T, Baumgartner C. Outcome predictors for surgical treatment of temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2008;49:1308–16. doi: 10.1111/j.1528-1167.2008.01732.x. [DOI] [PubMed] [Google Scholar]
- 16.Burneo JG, Black L, Martin R, Devinsky O, Pacia S, Faught E, Vasquez B, Knowlton RC, Luciano D, Doyle W, Najjar S, Kuzniecky RI. Race/ethnicity, sex, and socioeconomic status as predictors of outcome after surgery for temporal lobe epilepsy. Arch Neurol. 2006;63:1106–10. doi: 10.1001/archneur.63.8.1106. [DOI] [PubMed] [Google Scholar]
- 17.Cappabianca P, Alfieri A, Maiuri F, Mariniello G, Cirillo S, de Divitiis E. Supratentorial cavernous malformations and epilepsy: seizure outcome after lesionectomy on a series of 35 patients. Clin Neurol Neurosurg. 1997;99:179–83. doi: 10.1016/s0303-8467(97)00023-1. [DOI] [PubMed] [Google Scholar]
- 18.Stavem K, Bjornaes H, Langmoen IA. Predictors of seizure outcome after temporal lobectomy for intractable epilepsy. Acta Neurol Scand. 2004;109:244–9. doi: 10.1046/j.1600-0404.2003.00249.x. [DOI] [PubMed] [Google Scholar]
- 19.Janszky J, Schulz R, Janszky I, Ebner A. Medial temporal lobe epilepsy: gender differences. J Neurol Neurosurg Psychiatry. 2004;75:773–5. doi: 10.1136/jnnp.2003.020941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janszky J, Szucs A, Halasz P, Borbely C, Hollo A, Barsi P, Mirnics Z. Orgasmic aura originates from the right hemisphere. Neurology. 2002;58:302–4. doi: 10.1212/wnl.58.2.302. [DOI] [PubMed] [Google Scholar]
- 21.Remillard GM, Andermann F, Testa GF, Gloor P, Aube M, Martin JB, Feindel W, Guberman A, Simpson C. Sexual ictal manifestations predominate in women with temporal lobe epilepsy: a finding suggesting sexual dimorphism in the human brain. Neurology. 1983;33:323–30. doi: 10.1212/wnl.33.3.323. [DOI] [PubMed] [Google Scholar]
- 22.Toth V, Fogarasi A, Karadi K, Kovacs N, Ebner A, Janszky J. Ictal affective symptoms in temporal lobe epilepsy are related to gender and age. Epilepsia. 2010;51:1126–32. doi: 10.1111/j.1528-1167.2009.02396.x. [DOI] [PubMed] [Google Scholar]
- 23.Collaborative E. Abou-Khalil B, Alldredge B, Bautista J, Berkovic S, Bluvstein J, Boro A, Cascino G, Consalvo D, Cristofaro S, Crumrine P, Devinsky O, Dlugos D, Epstein M, Fahlstrom R, Fiol M, Fountain N, Fox K, French J, Freyer Karn C, Friedman D, Geller E, Glauser T, Glynn S, Haut S, Hayward J, Helmers S, Joshi S, Kanner A, Kirsch H, Knowlton R, Kossoff E, Kuperman R, Kuzniecky R, Lowenstein D, McGuire S, Motika P, Nesbitt G, Novotny E, Paolicchi J, Parent J, Park K, Poduri A, Risch N, Sadleir L, Scheffer I, Shellhaas R, Sherr E, Shih JJ, Shinnar S, Singh R, Sirven J, Smith M, Sullivan J, Thio LL, Venkat A, Vining E, von Allmen G, Weisenberg J, Widdess-Walsh P, Winawer M. The epilepsy phenome/genome project. Clin Trials. 2013;10:568–86. doi: 10.1177/1740774513484392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- 25.Ottman R, Hauser WA, Stallone L. Semistructured interview for seizure classification: agreement with physicians’ diagnoses. Epilepsia. 1990;31:110–5. doi: 10.1111/j.1528-1157.1990.tb05368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ottman R, Lee JH, Hauser WA, Hong S, Hesdorffer D, Schupf N, Pedley TA, Scheuer ML. Reliability of seizure classification using a semistructured interview. Neurology. 1993;43:2526–30. doi: 10.1212/wnl.43.12.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nesbitt G, McKenna K, Mays V, Carpenter A, Miller K, Williams M Investigators E. The Epilepsy Phenome/Genome Project (EPGP) informatics platform. Int J Med Inform. 2013;82:248–59. doi: 10.1016/j.ijmedinf.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1989;30:389–99. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 29.Kelley SA, Kossoff EH. Doose syndrome (myoclonic-astatic epilepsy): 40 years of progress. Dev Med Child Neurol. 2010;52:988–93. doi: 10.1111/j.1469-8749.2010.03744.x. [DOI] [PubMed] [Google Scholar]
- 30.Kroenke K, Spitzer RL. Gender differences in the reporting of physical and somatoform symptoms. Psychosom Med. 1998;60:150–5. doi: 10.1097/00006842-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: an FMRI investigation. Learn Mem. 2004;11:261–6. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piefke M, Weiss PH, Markowitsch HJ, Fink GR. Gender differences in the functional neuroanatomy of emotional episodic autobiographical memory. Hum Brain Mapp. 2005;24:313–24. doi: 10.1002/hbm.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adinoff B, Devous MD, Sr, Best SE, Chandler P, Alexander D, Payne K, Harris TS, Williams MJ. Gender differences in limbic responsiveness, by SPECT, following a pharmacologic challenge in healthy subjects. Neuroimage. 2003;18:697–706. doi: 10.1016/s1053-8119(03)00008-9. [DOI] [PubMed] [Google Scholar]
- 34.Shughrue PJ, Merchenthaler I. Estrogen is more than just a “sex hormone”: novel sites for estrogen action in the hippocampus and cerebral cortex. Front Neuroendocrinol. 2000;21:95–101. doi: 10.1006/frne.1999.0190. [DOI] [PubMed] [Google Scholar]
- 35.Frye CA. Effects and mechanisms of progestogens and androgens in ictal activity. Epilepsia. 2010;51(Suppl 3):135–40. doi: 10.1111/j.1528-1167.2010.02628.x. [DOI] [PubMed] [Google Scholar]
- 36.Maki PM. Estrogen effects on the hippocampus and frontal lobes. Int J Fertil Womens Med. 2005;50:67–71. [PubMed] [Google Scholar]


