Abstract
The replication of influenza virus in chick embryo fibroblast cells is inhibited by α-amanitin added during the first 2 hr of infection at concentrations similar to those required to inhibit cellular DNA-dependent RNA polymerase form II in vivo. Of two periods of increased RNA synthesis observed in cells infected with influenza virus, only the first, occurring from 0 to 2 hr after infection, is sensitive to α-amanitin. During this early period, there is a stimulation of the activity of DNA-dependent RNA polymerase II of nuclei isolated from infected cells. The data suggest that DNA transcription mediated by polymerase II is essential for influenza virus replication.
Keywords: chick embryo fibroblast, nucleus, polymerase II, parainfluenza virus
Full text
PDF
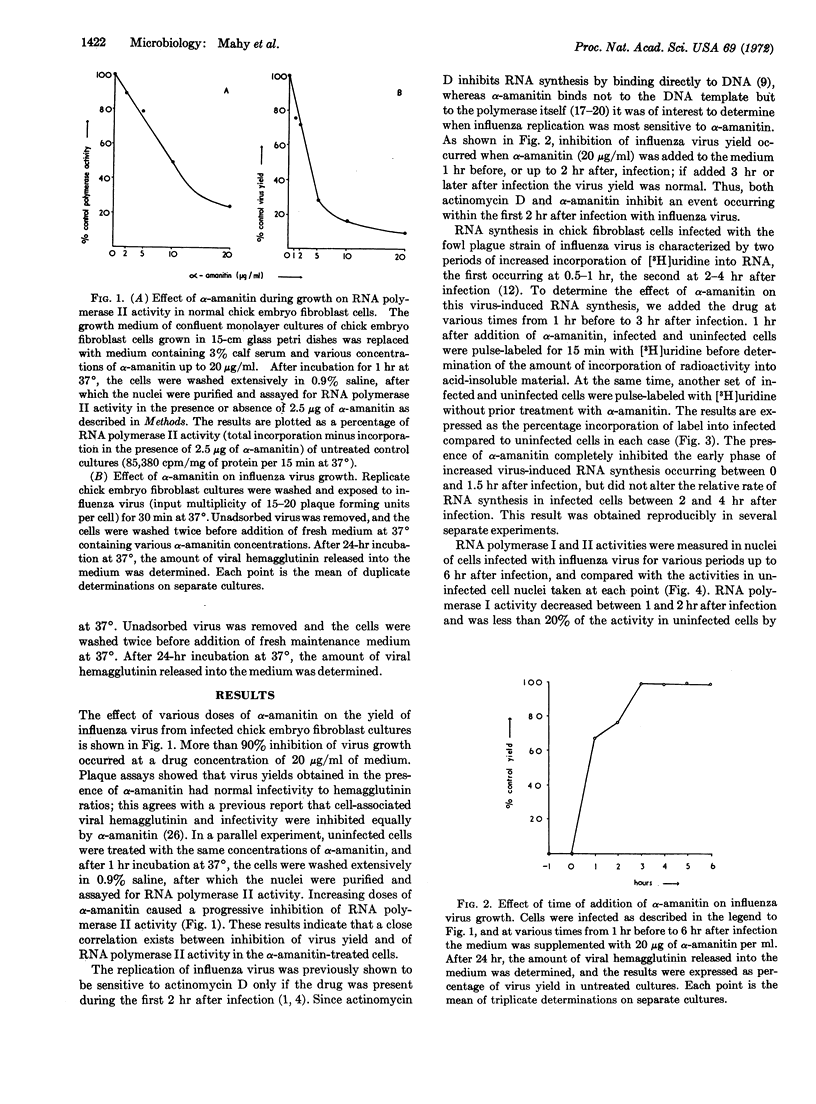
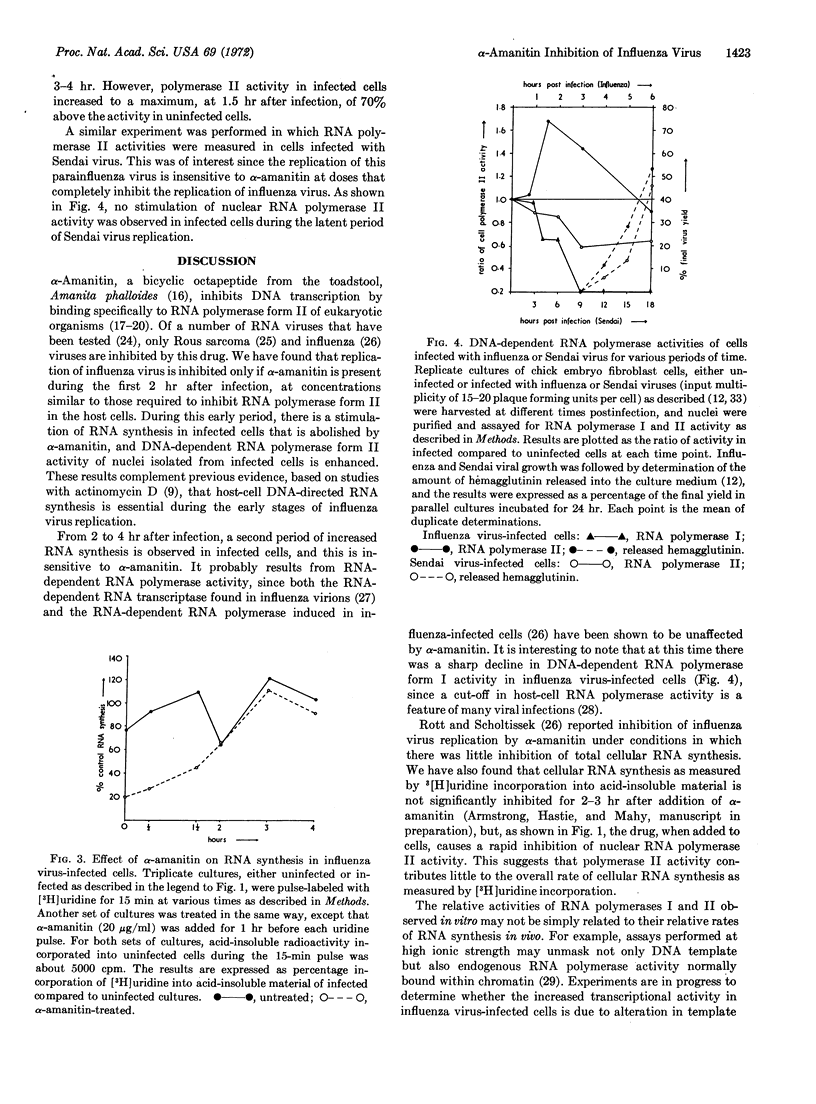

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BADER J. P. THE REQUIREMENT FOR DNA SYNTHESIS IN THE GROWTH OF ROUS SARCOMA AND ROUS-ASSOCIATED VIRUSES. Virology. 1965 Jun;26:253–261. doi: 10.1016/0042-6822(65)90272-2. [DOI] [PubMed] [Google Scholar]
- BARRY R. D., IVES D. R., CRUICKSHANK J. G. Participation of deoxyribonucleic acid in the multiplication of influenza virus. Nature. 1962 Jun 23;194:1139–1140. doi: 10.1038/1941139a0. [DOI] [PubMed] [Google Scholar]
- BARRY R. D. THE EFFECTS OF ACTINOMYCIN D AND ULTRAVIOLET IRRADIATION ON THE PRODUCTION OF FOWL PLAGUE VIRUS. Virology. 1964 Dec;24:563–569. doi: 10.1016/0042-6822(64)90208-9. [DOI] [PubMed] [Google Scholar]
- BUTHALA D. A. CELL CULTURE STUDIES ON ANTIVIRAL AGENTS. I. ACTION OF CYTOSINE ARABINOSIDE AND SOME COMPARISONS WITH 5-IODO-2-DEOXYURIDINE. Proc Soc Exp Biol Med. 1964 Jan;115:69–77. doi: 10.3181/00379727-115-28834. [DOI] [PubMed] [Google Scholar]
- Borland R., Mahy B. W. Deoxyribonucleic acid-dependent ribonucleic acid polymerase activity in cells infected with influenza virus. J Virol. 1968 Jan;2(1):33–39. doi: 10.1128/jvi.2.1.33-39.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth P. H., Cox R. F., Chesterton C. J. Transcription of mammalian chromatin by mammalian DNA-dependent RNA polymerases. Eur J Biochem. 1971 Nov 11;23(2):229–241. doi: 10.1111/j.1432-1033.1971.tb01613.x. [DOI] [PubMed] [Google Scholar]
- CHAUVEAU J., MOULE Y., ROUILLER C. Isolation of pure and unaltered liver nuclei morphology and biochemical composition. Exp Cell Res. 1956 Aug;11(2):317–321. doi: 10.1016/0014-4827(56)90107-0. [DOI] [PubMed] [Google Scholar]
- Chesterton C. J., Butterworth P. H.W. Studies on the origin of the form Ib mammalian DNA-dependent RNA polymerase. FEBS Lett. 1971 Mar 22;13(5):275–278. doi: 10.1016/0014-5793(71)80239-9. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Klenk H. D., Caliguiri L. A., Choppin P. W. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970 Dec;42(4):880–889. doi: 10.1016/0042-6822(70)90337-5. [DOI] [PubMed] [Google Scholar]
- Dimmock N. J. New virus-specific antigens in cells infected with influenza virus. Virology. 1969 Oct;39(2):224–234. doi: 10.1016/0042-6822(69)90042-7. [DOI] [PubMed] [Google Scholar]
- Fiume L., La Placa M., Portolani M. Ricerche sul meccanismo dell'azione citopatogena della alpha-amanitina. Sperimentale. 1966 Jan-Feb;116(1):15–25. [PubMed] [Google Scholar]
- Ghendon Y., Ginsburg V. P., Soloviev G. Y., Markushin S. G. The fate of influenza virus RNA in cells treated with ultraviolet rays. J Gen Virol. 1970 Feb;6(2):249–255. doi: 10.1099/0022-1317-6-2-249. [DOI] [PubMed] [Google Scholar]
- Kedinger C., Gniazdowski M., Mandel J. L., Jr, Gissinger F., Chambon P. Alpha-amanitin: a specific inhibitor of one of two DNA-pendent RNA polymerase activities from calf thymus. Biochem Biophys Res Commun. 1970 Jan 6;38(1):165–171. doi: 10.1016/0006-291x(70)91099-5. [DOI] [PubMed] [Google Scholar]
- Lawson D. E., Wilson P. W., Barker D. C., Kodicek E. Isolation of chick intestinal nuclei. Effect of vitamin D3 on nuclear metabolism. Biochem J. 1969 Nov;115(2):263–268. doi: 10.1042/bj1150263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindell T. J., Weinberg F., Morris P. W., Roeder R. G., Rutter W. J. Specific inhibition of nuclear RNA polymerase II by alpha-amanitin. Science. 1970 Oct 23;170(3956):447–449. doi: 10.1126/science.170.3956.447. [DOI] [PubMed] [Google Scholar]
- Mahy B. W., Bromley P. A. In vitro product of a ribonucleic acid polymerase induced by influenza virus. J Virol. 1970 Sep;6(3):259–268. doi: 10.1128/jvi.6.3.259-268.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahy B. W., Hutchinson J. E., Barry R. D. Ribonucleic acid polymerase induced in cells infected with Sendai virus. J Virol. 1970 Jun;5(6):663–671. doi: 10.1128/jvi.5.6.663-671.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D. P., Rasmussen A. F., Jr Influence of mitomycin C on the replication of influenza viruses. Virology. 1966 Dec;30(4):673–683. doi: 10.1016/0042-6822(66)90172-3. [DOI] [PubMed] [Google Scholar]
- Novello F., Stirpe F. Simultaneous assay of RNA polymerase I and II in nuclei isolated from resting and growing rat liver with the use of alpha-amanitin. FEBS Lett. 1970 May 11;8(1):57–60. doi: 10.1016/0014-5793(70)80225-3. [DOI] [PubMed] [Google Scholar]
- Penhoet E., Miller H., Doyle M., Blatti S. RNA-dependent RNA polymerase activity in influenza virions. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1369–1371. doi: 10.1073/pnas.68.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Multiple forms of DNA-dependent RNA polymerase in eukaryotic organisms. Nature. 1969 Oct 18;224(5216):234–237. doi: 10.1038/224234a0. [DOI] [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Specific nucleolar and nucleoplasmic RNA polymerases. Proc Natl Acad Sci U S A. 1970 Mar;65(3):675–682. doi: 10.1073/pnas.65.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott R., Scholtissek C. Specific inhibition of influenza replication by alpha-amanitin. Nature. 1970 Oct 3;228(5266):56–56. doi: 10.1038/228056a0. [DOI] [PubMed] [Google Scholar]
- SCHOLTISSEK C., ROTT R. [Relation between the synthesis of ribonucleic acid and protein in the propagation of a virus of the influenza group (fowlplague virus)]. Z Naturforsch B. 1961 Oct;16B:663–673. [PubMed] [Google Scholar]
- Schulze I. T. The structure of influenza virus. I. The polypeptides of the virion. Virology. 1970 Dec;42(4):890–904. doi: 10.1016/0042-6822(70)90338-7. [DOI] [PubMed] [Google Scholar]
- WHITE D. O., DAY H. M., BATCHELDER E. J., CHEYNE I. M., WANSBROUGH A. J. DELAY IN THE MULTIPLICATION OF INFLUENZA VIRUS. Virology. 1965 Feb;25:289–302. doi: 10.1016/0042-6822(65)90207-2. [DOI] [PubMed] [Google Scholar]
- Zanetti M., Foa L., Costanzo F., La Placa M. Specific inhibition of Rous sarcoma virus by -amanitin. Arch Gesamte Virusforsch. 1971;34(4):255–260. doi: 10.1007/BF01242970. [DOI] [PubMed] [Google Scholar]
- Zylber E. A., Penman S. Products of RNA polymerases in HeLa cell nuclei. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2861–2865. doi: 10.1073/pnas.68.11.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]



