Abstract
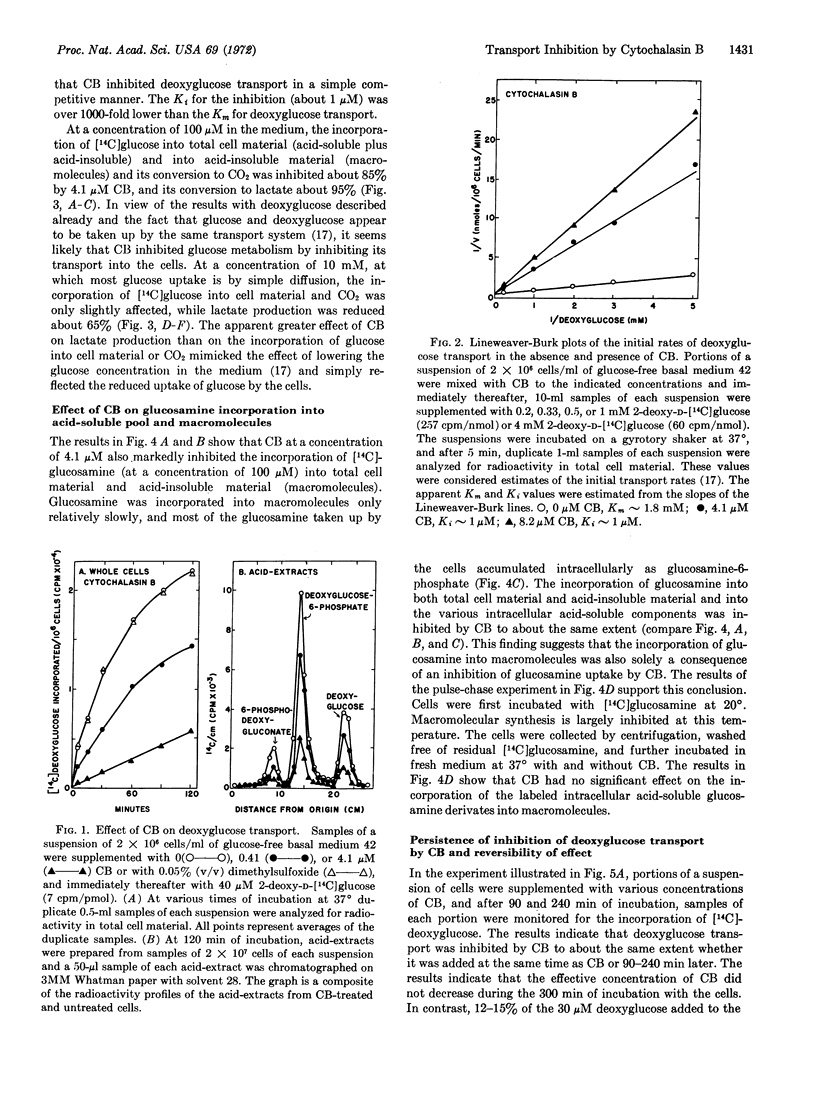
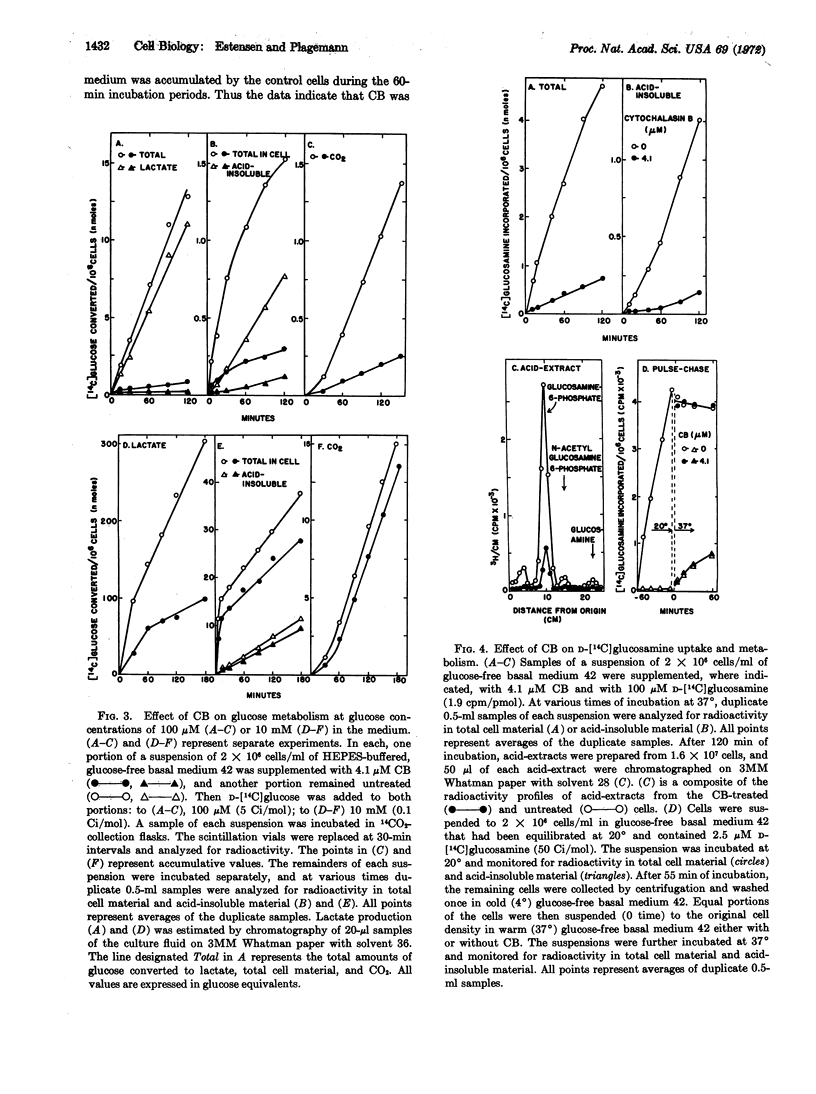
Cytochalasin B has been shown to potently inhibit the transport of glucose, deoxyglucose, and glucosamine by Novikoff hepatoma cells in suspension culture without affecting their intracellular phosphorylation and metabolism. Deoxyglucose transport is inhibited by cytochalasin B in a simple competitive manner. Although this inhibition is not sufficient to explain the biological action of the drug on cytokinesis, it does explain earlier observations on inhibition by cytochalasin B of the incorporation of glucose and glucosamine, and probably of other extracellular precursors, into macromolecules by various types of cells.
Keywords: deoxyglucose, Novikoff rat hepatoma cells, cell membrane
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter S. B. Effects of cytochalasins on mammalian cells. Nature. 1967 Jan 21;213(5073):261–264. doi: 10.1038/213261a0. [DOI] [PubMed] [Google Scholar]
- Davis A. T., Estensen R., Quie P. G. Cytochalasin B. 3. Inhibition of human polymorphonuclear leukocyte phagocytosis. Proc Soc Exp Biol Med. 1971 May;137(1):161–164. doi: 10.3181/00379727-137-35535. [DOI] [PubMed] [Google Scholar]
- Estensen R. D. Cytochalasin B. I. Effect on cytokinesis of Novikoff hepatoma cells. Proc Soc Exp Biol Med. 1971 Apr;136(4):1256–1260. doi: 10.3181/00379727-136-35470. [DOI] [PubMed] [Google Scholar]
- Estensen R. D., Rosenberg M., Sheridan J. D., Wessells N. K., Spooner B. S., Ash J. F., Ludueña M. A., Wrenn J. T. Cytochalasin B: microfilaments and "contractile" processes. Science. 1971 Jul 23;173(3994):356–359. doi: 10.1126/science.173.3994.356. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G. Effects of phenethyl alcohol on transport reactions, nucleotide pools and macromolecular synthesis in Novikoff rat hepatoma cells growing in suspension culture. J Cell Physiol. 1970 Jun;75(3):315–327. doi: 10.1002/jcp.1040750308. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G. Nucleoside transport by Novikoff rat hepatoma cells glowing in suspension culture. Specificity and mechanism of transport reactions and relationship to nucleoside incorporation into nucleic acids. Biochim Biophys Acta. 1971 Jun 1;233(3):688–701. doi: 10.1016/0005-2736(71)90168-4. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G. Nucleotide pools of Novikoff rat hepatoma cells growing in suspension culture. I. Kinetics of incorporation of nucleosides into nucleotide pools and pool sizes during growth cycle. J Cell Physiol. 1971 Apr;77(2):213–240. doi: 10.1002/jcp.1040770212. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Roth M. F. Permeation as the rate-limiting step in the phosphorylation of uridine and choline and their incorporation into macromolecules by Novikoff hepatoma cells. Competitive inhibition by phenethyl alcohol, persantin, and adenosine. Biochemistry. 1969 Dec;8(12):4782–4789. doi: 10.1021/bi00840a020. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Swim H. E. Replication of mengovirus. I. Effect on synthesis of macromolecules by host cell. J Bacteriol. 1966 Jun;91(6):2317–2326. doi: 10.1128/jb.91.6.2317-2326.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger J. W., Holtzer H. Cytochalasin B: effects on cell morphology, cell adhesion, and mucopolysaccharide synthesis (cultured cells-contractile microfilaments-glycoproteins-embryonic cells-sorting-out). Proc Natl Acad Sci U S A. 1972 Jan;69(1):253–257. doi: 10.1073/pnas.69.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield J. G. Cytochalasin B and release of growth hormone. Nat New Biol. 1971 Sep 15;234(50):215–216. doi: 10.1038/newbio234215a0. [DOI] [PubMed] [Google Scholar]
- Schroeder T. E. The contractile ring. I. Fine structure of dividing mammalian (HeLa) cells and the effects of cytochalasin B. Z Zellforsch Mikrosk Anat. 1970;109(4):431–449. [PubMed] [Google Scholar]
- Spooner B. S., Wessells N. K. Effects of cytochalasin B upon microfilaments involved in morphogenesis of salivary epithelium. Proc Natl Acad Sci U S A. 1970 Jun;66(2):360–361. doi: 10.1073/pnas.66.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R., Rosenberg M., Estensen R. Endocytosis in Chang liver cells. Quantitation by sucrose- 3 H uptake and inhibition by cytochalasin B. J Cell Biol. 1971 Sep;50(3):804–817. doi: 10.1083/jcb.50.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward G. A., Plagemann P. G. Fluctuations of DNA-dependent RNA polymerase and synthesis of macromolecules during the growth cycle of Novikoff rat hepatoma cells in suspension culture. J Cell Physiol. 1969 Jun;73(3):213–231. doi: 10.1002/jcp.1040730307. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Wolff J. Cytochalasin B inhibits thyroid secretion. Biochem Biophys Res Commun. 1971 Jul 16;44(2):422–425. doi: 10.1016/0006-291x(71)90617-6. [DOI] [PubMed] [Google Scholar]
- Wrenn J. T., Wessells N. K. Cytochalasin B: effects upon microfilaments involved in morphogenesis of estrogen-induced glands of oviduct. Proc Natl Acad Sci U S A. 1970 Jul;66(3):904–908. doi: 10.1073/pnas.66.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]


