Abstract
Humpback whales (Megaptera novaeangliae), a mysticete with a cosmopolitan distribution, demonstrate marked behavioural plasticity. Recent studies show evidence of social learning in the transmission of specific population level traits ranging from complex singing to stereotyped prey capturing behaviour. Humpback whales have been observed to employ group foraging techniques, however details on how individuals coordinate behaviour in these groups is challenging to obtain. This study investigates the role of a novel broadband patterned pulsed sound produced by humpback whales engaged in bottom-feeding behaviours, referred to here as a ‘paired burst' sound. Data collected from 56 archival acoustic tag deployments were investigated to determine the functional significance of these signals. Paired burst sound production was associated exclusively with bottom feeding under low-light conditions, predominantly with evidence of associated conspecifics nearby suggesting that the sound likely serves either as a communicative signal to conspecifics, a signal to affect prey behaviour, or possibly both. This study provides additional evidence for individual variation and phenotypic plasticity of foraging behaviours in humpback whales and provides important evidence for the use of acoustic signals among foraging individuals in this species.
Why animals choose to cooperate, with animals from their own or other species, has long been a puzzle to evolutionary biologists1,2,3. More recently, these behaviours are increasingly being viewed as evidence of higher-level cognitive complexity in a wide variety of animal species4. Cooperative behaviours, particularly those associated with foraging, have been described in a wide diversity of taxonomic groups ranging from fish and reptiles5, to higher order mammalian groups including primates, carnivores, elephants, and cetaceans6,7,8,9.
Research on cooperative behaviour and learning in cetaceans has focused extensively on the odontocetes (toothed whales)10. Much less attention has been given to cooperative behaviours and learning in mysticetes, the larger baleen whales11. This may be due to the apparently simple social interactions of many baleen whales and the logistical challenges of observing their behaviours in the open ocean. However, one species of baleen whale, the humpback whale (Megaptera novaeangliae), has a social system with characteristics that are similar to those in complex fission-fusion societies in odontocetes12.
Humpback whales show marked behavioural phenotypic plasticity in acoustic communication and social structuring. Humpback whale song, which varies between populations and changes annually, has provided some of the earliest evidence of behavioural plasticity and acoustic social learning in this species on the breeding grounds13,14,15. The social structure of humpback whales on their feeding grounds varies from solitary individuals to stable short-term associations between both kin and non-kin16,17, and behavioural studies of humpback whales have revealed rapid cultural transmission of acoustic repertoire and social learning contributing to the spread of novel foraging behaviours within a population15,18,19.
Humpback whales also show extensive variation in foraging behaviours, ranging from solitary surface and bottom feeding to highly coordinated and apparently cooperative group feeding behaviours20,21,22. Feeding strategy varies by prey type, and individuals also show distinctive individual variation within specific feeding strategies21,23,24. In Alaska, large groups of surface lunge-feeding humpback whales use tonal acoustic cues to synchronize lunge feeding behaviours22,25,26. These coordinating feeding sounds have not been detected during group feeding in any other humpback whale populations, possibly due to differences in prey type, prey behaviour and habitat characteristics.
This study used digital acoustic and multi-sensor recording tags to describe a novel acoustic signal produced during bottom feeding behaviours by humpback whales in the Northwest Atlantic. Previous studies using tag data have revealed multiple foraging strategies of individuals including diel trends in diving behaviour, likely driven by prey behaviour21,23. A study of tag data collected from associated pairs of humpback whales demonstrated that whales in this population often feed on the seafloor in close coordination, showing tight synchrony in diving and bottom behaviours27. A previously undescribed sound type, the paired burst28, was detected in the acoustic record of tags from the same dataset. The behavioural function of this sound is unknown. It is similar in structure to the humpback ‘megapclick'29, however it is lower in amplitude, has a rhythmic alternating ‘tick-tock' quality, and lacks the variable inter-pulse interval that characterizes the ‘megapclick'. Here we describe the occurrence of paired bursts to assess their potential function, either as a communicative signal to conspecifics, a signal to affect prey behaviour, or both.
Results
Acoustic Analyses
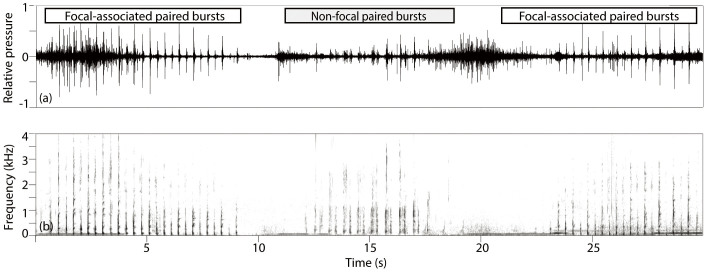
A total of 83 tags were deployed during the study period with 56 tags containing >1 h of acoustic data. Over 430 hours of acoustic data from these 56 tags were analysed for evidence of paired bursts (Supplementary Table S1). Paired bursts were short (<0.25 s) paired broadband pulses with peak energy below 1 kHz (Figure 1) (Supplementary audio clip S1). A paired burst bout consisted of 2 or more paired bursts given in sequence, and bout durations varied widely both between and within individuals. Bouts consisted of 2–120 paired burst pairs in series. Paired bursts were detected on 21 of the 56 tag records, comprising 2358 paired burst bouts. Focal-associated paired burst bouts (signal-to-noise ratio (SNR) > 20 dB) were detected on 15 tag records consisting of 1215 paired burst bouts.
Figure 1. Waveform and spectrogram of paired burst bouts from two whales.
(a) Waveform showing relative amplitude and timing and (b) Spectrogram showing frequency content and timing of paired bursts recorded on tag mn08_189a, ‘Falcon'. The first and last high amplitude bouts were defined as focal-associated, and the middle, lower amplitude bout was defined as non-focal. Spectrogram parameters, 64 kHz sample rate, 4096 Hann window, 50% overlap.
Behavioural context of signal production
Of the 56 tags analysed, 25 tags showed evidence of bottom feeding dives. Paired burst bouts were commonly found on tags with evidence of bottom feeding dives (18/25 tags) and not found on any tags without bottom feeding dives (0/31 tags). All dives containing focal-associated paired burst sound production were flat-bottom dives with rolling behaviours or digging into the substrate, consistent with bottom foraging behaviour23,27 (Figure 2) (Supplementary Table S1). The timing of bottom feeding behaviours by the tagged whale were tightly synchronized with the end time of paired burst bouts, both for focal-associated paired burst bouts (Figure 2), and during louder non-focal associated paired bouts (Figure 3, non-focal paired burst bouts were >15 dB but <20 dB for this example).
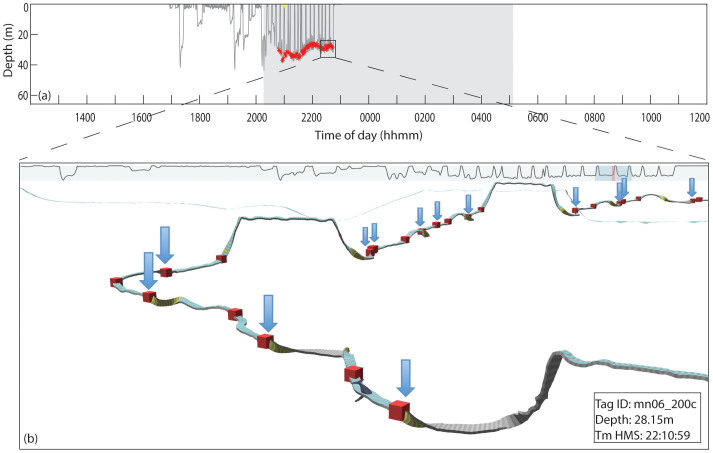
Figure 2. Example of focal paired burst bouts during bottom feeding events.
(a) Dive profile of mn06_200c, ‘Spoon', showing time of paired burst sound production. Red = focal-associated (SNR > 20 dB), Yellow = non-focal (15 dB < SNR < 20 dB). (b) Example of timing of rolls relative to SNR > 20 dB paired burst production. Boxes mark the beginning and end of paired burst bouts. Arrows are placed above the boxes indicating the end time of a focal-associated paired burst bout. Rolls that deviate >45° from vertical orientation of the whale are indicated in the track ribbon by yellow coloration. All bottom feeding rolls were associated with the end of a paired burst bout in this example.
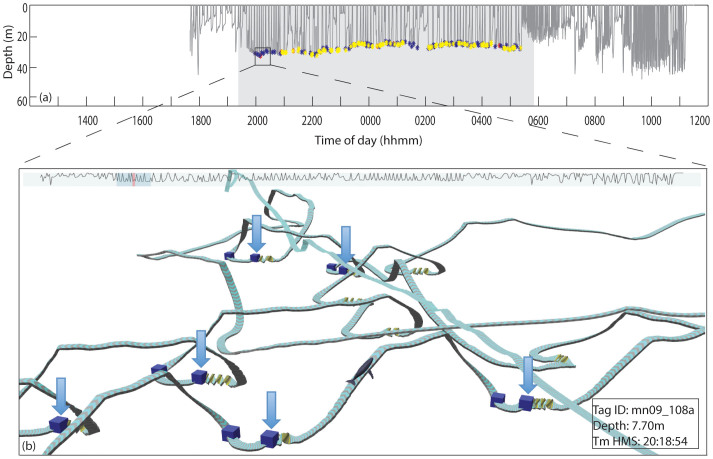
Figure 3. Example of non-focal paired burst bouts during bottom feeding events.
(a) Dive profile of mn09_108a, ‘Division' showing time of paired bursts sound production. Red = focal-associated (SNR > 20 dB), Yellow = non-focal (15 dB < SNR < 20 dB), Blue = non-focal (SNR < 15 dB). (b) Example of timing of rolls relative to SNR < 15 dB paired burst production. Boxes mark the beginning and end of paired burst bouts. Arrows are placed above the boxes indicating the end time of a high SNR paired burst bout. Rolls that deviate >45° from vertical orientation of the whale are indicated in the track ribbon by yellow coloration. Note that multiple bottom feeding rolls in this track had no evidence of any paired burst production.
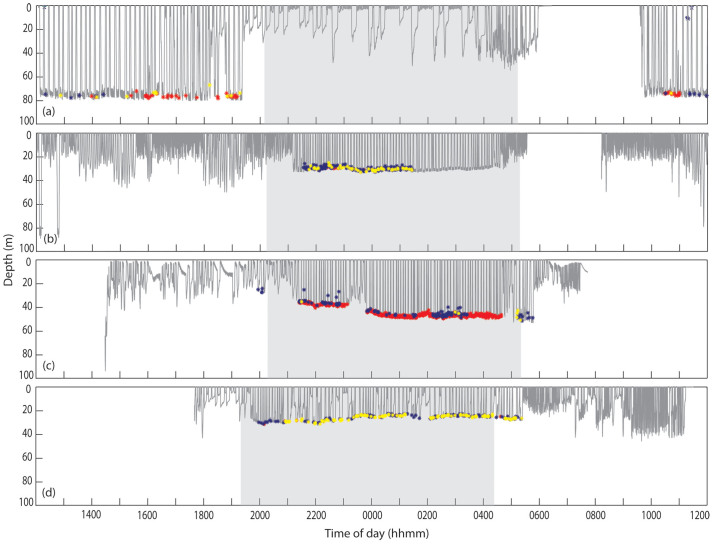
Most of the whales (11/15) with focal-associated paired bursts were tagged on Stellwagen Bank over several years. The remaining four whales were tagged about 50 km east of Chatham, Massachusetts in 2004. Stellwagen Bank is on average only 30–35 m deep and the 2004 tagging area is more than twice the depth at an average of 78 m. Almost all (99%) paired burst bouts from the focal-associated bouts were detected at night for tags attached on Stellwagen Bank (n = 11 tags). The other 1% occurred during dusk hours (Figure 4). In the deeper tagging location east of Chatham in 2004, where bottom light levels would be expected to be less than 1% of the surface light levels30, tagged whales engaged in bottom feeding during all periods of the day but only 68% of paired burst bouts were produced at night and 32% produced during daylight hours (n = 4 tags).
Figure 4. Dive profiles from four tagged individuals.
Asterisks mark the detection of paired bursts. Red = focal-associated (SNR > 20 dB), Yellow = non-focal (15 dB < SNR < 20 dB), Blue = non-focal (SNR < 15 dB). All records were aligned to allow for direct comparison of time of day. Night hours are indicated by grey shading in each plot. (a) July 2004 mn04_189b, ‘Parens'; (b): July 2006 mn06_192a; ‘Division' (c): July 2008 mn08_189a; ‘Falcon'; (d):April 2009 mn09_108a; ‘Division'.
We identified two tag records with repeated sequences of very faint paired burst bouts that increased in received level (RL) on the tag as the whale dived toward the bottom (Figure 5), indicating that the signalling whale either increased signal amplitude as it approached the bottom of its dive, or, more likely, was approaching a conspecific that was producing paired bursts near the bottom.
Figure 5. Example of increasing received signal level of paired bursts for one whale when diving to the seafloor.
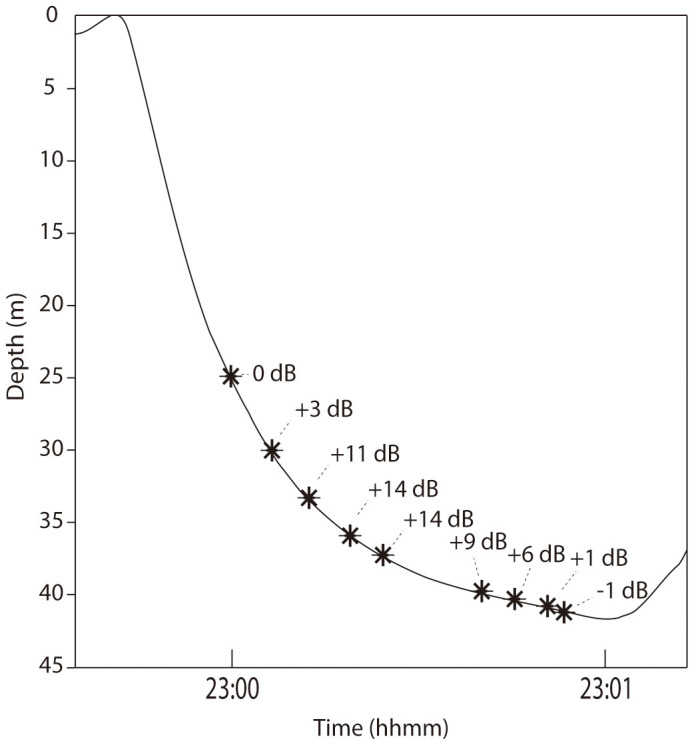
Relative received level of paired burst pulses from a single paired burst bout as mn09_201a, ‘Entropy' descends to the bottom with 0 dB referenced to the first detected paired burst pulse near the surface. Note the received level of the signals increased as the whale dived to the bottom, which was consistent for most paired bursts on this individual's tag record.
Assessment of behavioural associations
For whales with focal-associated paired burst bouts during bottom feeding dives, the majority (12/15) showed evidence of another humpback whale nearby on 100% of their bottom feeding dives containing paired burst bouts (Table 1). The 3 tagged whales that did not have 100% evidence of a nearby conspecific during paired burst dives were closely associated with other humpbacks during their dives based on our conservative criteria ≥73% of the time. All individuals that made bottom feeding dives containing focal-associated paired burst bouts also made very similar bottom foraging dives with no detectable paired bursts. On average, only 60% of bottom feeding dives without paired burst bouts showed evidence of nearby conspecifics, compared to an average value of 97% of bottom feeding dives with paired bursts. Frequently it was bottom feeding dives that occurred during daylight hours that showed evidence of conspecifics nearby without the production of paired bursts. These results suggest that paired burst production is specifically limited to bottom feeding under low-light conditions with conspecifics nearby.
Table 1. Focal-associated paired burst bouts were commonly detected during bottom feeding with a whale nearby. Bottom feeding without paired burst bouts had fewer detections of nearby whales, suggesting that these whales may not have been cooperatively feeding with the tagged whale. Bf = bottom feeding dives; pb = paired burst bouts.
| date | ID | no. of Bf dives in record | % of Bf dives with any pb | % of Bf dives with high SNR pb | % of Bf dives with high SNR pb and evidence of conspecifics |
|---|---|---|---|---|---|
| 7/7/2004 | Pepper | 31 | 32% | 6% | 100% |
| 7/7/2004 | Parens | 60 | 67% | 35% | 100% |
| 7/9/2004 | Deuce | 54 | 67% | 50% | 100% |
| 7/9/2004 | Leonid | 100 | 74% | 24% | 100% |
| 7/11/2006 | Division | 47 | 91% | 38% | 100% |
| 7/14/2006 | Fulcrum | 61 | 74% | 74% | 73% |
| 7/16/2006 | Ivee | 22 | 32% | 18% | 100% |
| 7/19/2006 | Spoon | 13 | 100% | 100% | 92% |
| 7/1/2008 | Perseid | 65 | 5% | 5% | 100% |
| 7/2/2008 | Nile | 88 | 68% | 1% | 100% |
| 7/7/2008 | Falcon | 82 | 94% | 90% | 97% |
| 4/18/2009 | Division | 71 | 96% | 8% | 100% |
| 7/20/2009 | Entropy | 64 | 84% | 81% | 100% |
| 7/25/2009 | Samovar | 92 | 83% | 1% | 100% |
| 6/21/2012 | Touche | 4 | 25% | 25% | 100% |
Discussion
Humpback whales show marked behavioural plasticity, with strong evidence of social learning for both acoustic communication and foraging behaviour13,19. Based on tag data from foraging humpback whales in the Gulf of Maine, paired burst sound production appears to be exclusively associated with bottom feeding and likely plays a role in this foraging strategy. Further analyses indicate that paired bursts were only produced under low-light conditions with conspecifics nearby. Based on these observations, there are several possible functions for these signals.
One potential explanation for these paired burst sounds is that the signals are a non-vocal by-product of kinematic movements of the whales. However, based on our analysis, paired burst bouts, while exclusively produced during bottom feeding, did not occur in all or even most bottom feeding dives. Almost all whales that produced paired bursts engaged in bottom feeding dives both with and without paired burst bout production on the same day and/or between separate tagging dates. This, coupled with the individual variation in the acoustic parameters of the paired bursts production, and the near tonal quality of some individual pulses in some trains, suggest that the paired burst is a deliberate vocal signal produced by humpback whales (Supplementary audio clip S2).
Based on preliminary detection of paired bursts on tags with night data and their similarity to ‘megapclicks'29, we tested the hypothesis that these signals were only produced at night. However, tags from 2004 contained paired burst bouts during bottom feeding during the middle of the day. In these records, all focal paired burst production occurred during bottom foraging events, further strengthening the evidence that this sound is associated strictly with bottom feeding and independent of the time of day. In 2004, the bottom feeding events did take place at significantly greater depth (78 m vs. 30–35 m in other years), where the bottom light levels would be comparable to foraging in shallower depths at night. The visual acuity of humpback whales is unknown but it is possible that during the day in shallow water with good visibility conditions, such as those on Stellwagen Bank, paired burst signals aren't necessary for successful foraging activities. Analysis of additional data on bottom feeding behaviour during daylight hours in other locations will be needed to further test this hypothesis.
Previous studies in a variety of species have investigated the responses of prey species to the detection of both communication and echolocation signals of their predators. In several echolocating species, including bats and odontocetes, prey have been documented to exhibit evasive behavior when detecting these signals, likely reducing predator effectiveness31,32,33,34. Some dolphin species have shown evidence of using sounds to flush hidden prey35. In the Gulf of Maine, bottom feeding behaviours in humpback whales are associated with sand lance (Ammodytes spp.), a species of fish that can burrow into the sediment23. Given the use of paired burst signals at the bottom of foraging dives, it is possible the sound functions to flush sand lance prey out of the substrate into the water column. A previous study has investigated the ability of Ammodytes to detect the ‘megapclick' signal produced by humpback whales in Massachusetts Bay36. This study showed that these fish could hear a low frequency click signal, similar in frequency to the paired burst. This suggests that the bottom-associated prey could detect paired burst signals, though the behavioral response to these signals remains to be tested.
Another necessary condition for paired burst production appears to be the presence of at least one other conspecific in close proximity. In almost all instances of paired burst bouts, calls of nearby humpback whales, either paired bursts or other non-song calls, were detected. In the few cases where no nearby conspecifics were acoustically detected, there was always acoustic evidence of other whales in close proximity in immediately preceding and/or subsequent dives. In bottom feeding dives with no paired burst bouts, acoustic evidence of nearby humpback whales was less common. Given these observations, we propose that these signals likely function as a communicative signal with other foraging humpback whales. These signals may function to coordinate timing of feeding activities under low light conditions or to alert other conspecifics to the location of particularly good patches for feeding, either intentionally or incidentally. In two tag records, the tagged whales showed consistent changes in their depth during the detection of paired burst bouts, mn09_201a (‘Entropy') and mn06_197a (‘Ivee'). In both cases, the SNR of the paired bursts at the surface was low but increased to >15 dB as the whales dove to the bottom. Figure 5 illustrates the increase in received level (RL) of paired burst pulses as mn09_201a dives to the bottom, indicating that the whale either steadily increased the amplitude of paired burst production during diving, or more likely that it was approaching a whale producing the paired burst bouts at the seafloor. We take these two cases as support for our assertion that these signals are cues to conspecifics related to bottom feeding activities. These whales may have been intended recipients, or potential eavesdroppers detecting paired bursts and approaching foraging whales on the bottom. Eavesdropping on echolocation foraging signals has been described in bats and bottlenose dolphins to potentially enhance individual foraging success37,38,39,40.
Since paired burst bouts appear to be intentionally produced acoustic signals, associated solely with bottom feeding, they are likely to function either to manipulate prey behaviour, to communicate with conspecifics, or some combination of these two functions. Given the sum of the evidence presented, we propose that these signals serve to 1) coordinate the timing of behaviours with conspecifics; 2) attract conspecifics to locations with high available prey densities; and/or 3) modify prey behaviour to enhance foraging success on bottom-associated prey. Coordination of behaviour with conspecifics is a possible function of these signals, given previous evidence of very tight coordination of the timing of diving and bottom feeding behaviours in pairs of humpback whales in this population27. An acoustic signal may allow maintenance of tight synchronization of behaviours when visual cues are not available under low light conditions. This hypothesis is consistent with previous reports of acoustic signals used for apparent coordination of surface lunge feeding in humpback whale groups in Alaska22,25, but is the first description of such a coordination sound for any other population of humpback whales and the first signal described in association with bottom feeding. Evidence of individual whales approaching other whales producing paired burst signals on the bottom are suggestive that these signals serve either to directly advertise foraging locations or provide an acoustic cue that can be intercepted by conspecifics. Playback experiments of paired burst sounds could test whether these signals attract humpback whales in the vicinity to explore this potential function. Finally, hearing studies of the primary bottom-associated prey species in this population suggest that the fish likely can detect the paired burst signals36. Field based studies to observe the behavioural response of sand lance to these signals could aid in determining whether these signals may drive prey in a way to improve foraging success of humpback whales. For example, if fish buried in the sediment flush into the water column upon detection of the signal, they may be easier for the whales to capture.
Humpback whales forage across habitats on a wide diversity of prey, ranging from euphausids to larger schooling fish species using a variety of foraging strategies41. Our discovery of a novel acoustic cue used in foraging behaviour on a bottom-associated prey type provides additional evidence of the behavioural plasticity and learning abilities of humpback whales. The variation in behaviour of the diverse prey species targeted by humpback whales has likely created selective pressure for this marked behavioural plasticity. Further research into apparent behavioural mechanisms to coordinate foraging behaviours in humpback whales will likely give us even greater insight into the foraging capabilities of this species. Additional studies, including combinations of video and acoustic tagging of associated pairs of feeding humpback whales, will help to shed light on how these ocean predators are using these rhythmic pulsed signals to facilitate feeding on bottom-associated prey.
Methods
Data were collected from humpback whales in the Gulf of Maine, in the Western North Atlantic between 41.5°N and 43.2°N and 69.3°W and 70.5°W in 2004, 2006–2010, and 2012. Data collection included attachment of non-invasive, suction cup, archival, multi-sensor acoustic tags (Dtags)42 that recorded the sounds in the environment, including those produced by the tagged whale, along with data from multiple sensors that provided data on the depth and body orientation of the tagged whale. Acoustic data were recorded at sampling rates between 16 and 96 kHz, with a flat frequency response of 0.4–8 kHz from all records. Orientation sensors were sampled at 50 Hz. Focal behavioural follows43 during daylight hours collected data on the location, surface behaviour, respiration rates, and social associations of each tagged whale. Tagged whales were identified and categorized by age and sex class using the Whale Center of New England (WCNE) humpback whale catalogue in Gloucester, MA, U.S.A. Details on data collection can be found in Wiley et al (2011)21. All data were collected in accordance with federal and university guidelines, with all protocols approved by the Institution Animal Care and Use Committees at Syracuse University, Duke University, and Penn State University and under a federal permit from the United States National Marine Fisheries Service.
Acoustic Analyses
All tag deployments that remained attached to a whale for >1 h were included in the analysis. Raven Pro 1.5 (Cornell University) was used for all sound analysis measurements. Spectrograms of acoustic data were visually and aurally scanned for all whale calls in the acoustic record. Paired bursts were defined and identified as rhythmic pairs of low frequency pulses (usually <1 kHz peak frequency) given in sequence of two or more pairs. The start and end time of all paired burst sequences (herein termed bouts) were marked for each tag record.
To objectively determine which paired burst bouts may have been produced by the tagged whale or a physically closely associated whale, we assessed the signal-to-noise ratio (SNR) of the received level (RL) of the recorded signals. SNR was calculated for the most intense paired bursts signals within a bout using 0.05 s boxes with a frequency range of 200–1000 Hz around the selected pulse and an identical box around a consecutive period of noise without a pulse present. We used this quantitative SNR measurement to categorize paired burst signals into two categories: signals were assigned to a category labelled ‘focal-associated' with SNR > 20 dB where we had high confidence that the signals were produced either by the tagged whale or a whale immediately adjacent to the tagged whale. All other bouts with SNR < 20 dB were grouped into a second category labelled ‘non-focal'. These bouts likely contained signals that were produced by the tagged whale, close associates, or more distant whales, but since we were unable to confidently distinguish calls at this SNR level, we omitted them from our analyses related to the behaviour of the focal tagged whale. These ‘non-focal' category signals were only used to assess the diel trends of paired burst signal production or as evidence for nearby conspecifics during foraging dives.
Behavioural Context of Signal Production
Only paired burst bouts that met the criteria for the focal-associated category (SNR > 20 dB) were used in analyses regarding behavioural context. Based on the full dataset, we had high confidence that signals with this high SNR were either from the focal animal or a conspecific that was only minimally spatially separated and therefore in a comparable position to the tagged animal in time and space. The depth of paired burst signal production was determined for all bouts that were assigned to the focal-associated category in order to assess where in the dive profile this sound was predominately produced. Trackplot software20 was used to visualize the motor behaviours of the tagged animal in the presence of high SNR (>20 dB) paired burst bouts.
Diel trends in sound production were investigated for all paired burst sounds, both the focal-associated and non-focal categories, comparing sound production in four light regimes: dawn (1 hour prior to sunrise), daylight, dusk (1 hour after sunset), and night. Local sunrise and sunset times were taken from NOAA's marine weather database (http://www.weather.gov/view/states.php?state=MA).
Assessment of behavioural associations
Associations between the tagged whale and conspecifics were determined either through visual observation of surface associations, defined as coordinated movements of whales less than 2 body lengths apart, before and after a dive (during daylight hours), and/or by acoustic evidence of a nearby conspecific from the tag record. For each instance of paired burst bouts >20 dB the presence of clearly audible humpback non-song signals with broadband SNR > 10 dB were taken as evidence of a nearby conspecific. The presence or absence of these signals allowed us to assess possible associations among whales at depth and during the night. Although this process may have resulted in missed detections of some non-focal calls that were of lower intensity and did not allow us to detect silent associates, we wanted to be conservative in our estimation of nearby conspecifics. Therefore these results are likely biased to underestimate the times that whales were associated with conspecifics underwater.
Author Contributions
S.E.P. and D.A.C. designed the experiment and analyzed the data. S.E.P., D.A.C., A.K.S., M.T.W. A.S.F. and D.N.W. collected the data and wrote the paper.
Supplementary Material
Supplementary Audio Clip-S1
Supplementary Audio Clip-S2
Supplementary Table S1
Acknowledgments
Funding was provided by the Stellwagen Bank National Marine Sanctuary, Office of National Marine Sanctuaries, the Office of Naval Research, University of Hawaii Sea Grant College Program and the National Oceanographic Partnership Program. Whale tag data were collected under permit No.775-185 (Northeast Fisheries Science Center) and 605-1904 (Whale Center of New England) issued by the United States National Marine Fisheries Service. Research protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Duke University, the Pennsylvania State University, and Syracuse University. N. D. Merchant and L.E. Matthews provided constructive feedback on early drafts of the manuscript. We thank the officers and crew of the NOAA research vessels Nancy Foster and Auk, and the R/V Stellwagen for their capable assistance during field operations. We also thank the members of our field teams including R. Arsenault, A. Bocconcelli, C. Casey, D. Cholewiak, P. Halpin, E. Hazen, T. Hurst, T. Kirchner, J. Moller, C. Pecarcik, A. Rosner, K. Sardi, J. Smith, J. Tackaberry, M. Thompson, C. Ware, B. Woodward, and J. Winn.
References
- Axelrod R. & Hamilton W. D. The evolution of cooperation. Science 211, 1390–1396 (1981). [DOI] [PubMed] [Google Scholar]
- Schuster R. & Perelberg A. Why cooperate? An economic perspective is not enough. Behav. Process. 66, 261–277 (2004). [DOI] [PubMed] [Google Scholar]
- Enquist M. & Leimar O. The evolution of cooperation in mobile organisms. Anim. Behav. 45, 747–757 (1993). [Google Scholar]
- McNally L., Brown S. P. & Jackson A. L. Cooperation and the evolution of intelligence. P. Roy. Soc. B-Biol. Sci. 279, 3027–3034 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bshary R., Hohner A., Ait-el-Djoudi K. & Fricke H. Interspecific Communicative and Coordinated Hunting between Groupers and Giant Moray Eels in the Red Sea. Plos Biol 4, e431 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesch C. Cooperative hunting in wild chimpanzees. Anim. Behav. 48, 653–667 (1994). [Google Scholar]
- Drea C. M. & Carter A. N. Cooperative problem solving in a social carnivore. Anim. Behav. 78, 967–977 (2009). [Google Scholar]
- Plotnik J. M., Lair R., Suphachoksahakun W. & De Waal F. B. M. Elephants know when they need a helping trunk in a cooperative task. Proc. Natl. Acad. Sci. U.S.A. 108, 5116–5121 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit-Bird K. J. & Au W. W. L. Cooperative prey herding by the pelagic dolphin, Stenella longirostris. J. Acoust. Soc. Am. 125, 125–137 (2009). [DOI] [PubMed] [Google Scholar]
- Marino L. Convergence of complex cognitive abilities in cetaceans and primates. Brain Behav. Evol. 59, 21–32 (2002). [DOI] [PubMed] [Google Scholar]
- Simmonds M. P. Into the brains of whales. Appl. Anim. Behav. Sci. 100, 103–116 (2006). [Google Scholar]
- Clapham P. J. The Humpback Whale. Cetacean Societies: Field studies of dolphins and whales. [Mann, J. et al. (ed)] [173–196] (The University of Chicago Press, 2000).
- Noad M. J., Cato D. H., Bryden M. M., Jenner M. N. & Jenner K. C. S. Cultural revolution in whale songs. Nature 408, 537 (2000). [DOI] [PubMed] [Google Scholar]
- Payne R. S. & McVay S. Songs of humpback whales. Science 173, 585–597 (1971). [DOI] [PubMed] [Google Scholar]
- Eriksen N., Miller L. A., Tougaard J. & Helweg D. A. Cultural change in the songs of humpback whales (Megaptera novaeangliae) from Tonga. Behaviour 142, 305–328 (2005). [Google Scholar]
- Ramp C., Hagen W., Palsbøll P., Bérubé M. & Sears R. Age-related multi-year associations in female humpback whales (Megaptera novaeangliae). Behav. Ecol. Sociobiol. 64, 1563–1576 (2010). [Google Scholar]
- Weinrich M. T., Rosenbaum H., Baker C. S., Blackmer A. L. & Whitehead H. The influence of maternal lineages on social affiliations among humpback whales (Megaptera novaeangliae) on their feeding grounds in the southern Gulf of Maine. J. Hered. 97, 226–234 (2006). [DOI] [PubMed] [Google Scholar]
- Rendell L. & Whitehead H. Culture in whales and dolphins. Behav. Brain. Sci. 24, 309–24– discussion 324–82 (2001). [DOI] [PubMed] [Google Scholar]
- Allen J., Weinrich M., Hoppitt W. & Rendell L. Network-Based diffusion analysis reveals cultural transmission of lobtail feeding in humpback whales. Science 340, 485–488 (2013). [DOI] [PubMed] [Google Scholar]
- Ware C., Friedlaender A. S. & Nowacek D. P. Shallow and deep lunge feeding of humpback whales in fjords of the West Antarctic Peninsula. Mar. Mammal Sci. 27, 587–605 (2011). [Google Scholar]
- Wiley D. et al. Underwater components of humpback whale bubble-net feeding behaviour. Behaviour 148, 575–602 (2011). [Google Scholar]
- D'Vincent C. G., Nilson R. M. & Hanna R. E. Vocalization and coordinated feeding behavior of the humpback whale in southeastern Alaska. Sci. Rep. Whales Res. Inst. 36, 41–47 (1985). [Google Scholar]
- Friedlaender A. S. et al. Diel changes in humpback whale Megaptera novaeangliae feeding behavior in response to sand lance Ammodytes spp. behavior and distribution. Mar. Ecol. Prog. Ser. 395, 91–100 (2009). [Google Scholar]
- Canning C. et al. Population-level lateralized feeding behaviour in North Atlantic humpback whales, Megaptera novaeangliae. Anim. Behav. 82, 901–909 (2011). [Google Scholar]
- Cerchio S. & Dahlheim M. E. Variation in feeding vocalizations of humpback whales Megaptera novaeangliae from southeast Alaska. Bioacoustics 11, 277–295 (2001). [Google Scholar]
- Jurasz C. M. & Jurasz V. P. Feeding modes of the humpback whale, Megaptera novaeangliae, in southeast Alaska. Sci. Rep. Whales Res. Inst. 31, 69–83 (1979). [Google Scholar]
- Ware C. et al. Bottom side-roll feeding by humpback whales (Megaptera novaeangliae) in the southern Gulf of Maine, USA. Mar. Mammal Sci. 30, 494–511 (2014). [Google Scholar]
- Stimpert A. K., Au W. W. L., Parks S. E., Hurst T. & Wiley D. N. Common humpback whale (Megaptera novaeangliae) sound types for passive acoustic monitoring. J. Acoust. Soc. Am. 129, 476–482 (2011). [DOI] [PubMed] [Google Scholar]
- Stimpert A. K., Wiley D. N., Au W. W. L., Johnson M. P. & Arsenault R. ‘Megapclicks': acoustic click trains and buzzes produced during night-time foraging of humpback whales (Megaptera novaeangliae). Biol. Lett. 3, 467–470 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend D. W., Pettigrew N. R. & Thomas A. C. Offshore blooms of the red tide dinoflagellate, Alexandrium sp., in the Gulf of Maine. Cont. Shelf Res. 21, 347–369 (2001). [Google Scholar]
- Roeder K. D. The behaviour of free flying moths in the presence of artificial ultrasonic pulses. Anim. Behav. 10, 300–304 (1962). [Google Scholar]
- Mann D. A., Lu Z. & Popper A. N. A clupeid fish can detect ultrasound. Nature 389, 341 (1997). [Google Scholar]
- Wilson B. & Dill L. M. Pacific herring respond to simulated odontocete echolocation sounds. Can. J. Fish. Aquat. Sci. 59, 542–553 (2002). [Google Scholar]
- Deecke V. B., Slater P. J. B. & Ford J. K. B. Selective habituation shapes acoustic predator recognition in harbour seals. Nature 420, 171–173 (2002). [DOI] [PubMed] [Google Scholar]
- Nowacek D. P. Acoustic ecology of foraging bottlenose dolphins (Tursiops truncatus), habitat-specific use of three sound types. Mar. Mammal Sci. 21, 587–602 (2005). [Google Scholar]
- Strobel S. M. & Mooney T. A. Detection of low-frequency tones and whale predator sounds by the American sand lance Ammodytes americanus. J. Fish Biol. 81, 1646–1664 (2012). [DOI] [PubMed] [Google Scholar]
- Gillam E. H. Eavesdropping by bats on the feeding buzzes of conspecifics. Can. J. Zool. 85, 795–801 (2007). [Google Scholar]
- Gregg J. D., Dudzinski K. M. & Smith H. V. Do dolphins eavesdrop on the echolocation signals of conspecifics? Int. J. Comp. Psych. 20, 65–88 (2007). [Google Scholar]
- Janik V. M. Food-related bray calls in wild bottlenose dolphins (Tursiops truncatus). P. Roy. Soc. B-Biol. Sci. 267, 923–927 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay R. M. Interindividual use of echolocation calls: eavesdropping by bats. Behav. Ecol. Sociobiol. 10, 271–275 (1982). [Google Scholar]
- Goldbogen J. A. et al. Integrative Approaches to the Study of Baleen Whale Diving Behavior, Feeding Performance, and Foraging Ecology. BioScience 63, 90–100 (2013). [Google Scholar]
- Johnson M. P. & Tyack P. L. A digital acoustic recording tag for measuring the response of wild marine mammals to sound. IEEE J. Oceanic Eng. 28, 3–12 (2003). [Google Scholar]
- Altmann J. Observational study of behavior: sampling methods. Behaviour 49, 227–267 (1974). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Audio Clip-S1
Supplementary Audio Clip-S2
Supplementary Table S1