Abstract
L-3, 4-dihydroxyphenylalanine (L-dopa) is the gold standard for symptomatic treatment of Parkinson's disease (PD), but long-term therapy is associated with the emergence of L-dopa-induced dyskinesia (LID). In the present study, L-dopa and benserazide were loaded by poly (lactic-co-glycolic acid) microspheres (LBM), which can release levodopa and benserazide in a sustained manner in order to continuous stimulate dopaminergic receptors. We investigated the role of striatal DR1/PKA/P-tau signal transduction in the molecular event underlying LID in the 6-OHDA-lesioned rat model of PD. We found that animals rendered dyskinetic by L-dopa treatment, administration of LBM prevented the severity of AIM score, as well as improvement in motor function. Moreover, we also showed L-dopa elicits profound alterations in the activity of three LID molecular markers, namely DR1/PKA/P-tau (ser396). These modifications are totally prevented by LBM treatment, a similar way to achieve continuous dopaminergic delivery (CDD). In conclusion, our experiments provided evidence that intermittent administration of L-dopa, but not continuous delivery, and DR1/PKA/p-tau (ser396) activation played a critical role in the molecular and behavioural induction of LID in 6-OHDA-lesioned rats. In addition, LBM treatment prevented the development of LID by inhibiting the expression of DR1/PKA/p-tau, as well as PPEB mRNA in dyskintic rats.
With a growing elderly population and a reduction in other causes of mortality, the prevalence of Parkinson's disease (PD) is likely to increase1, it has been suggested that the burden of PD is speculated to grow substantially over the next few decades. To our knowledge, L-3,4-dihydroxyphenylalanine (L-dopa) continues to be the chief choice for symptomatic treatment associated with PD even if its long-term use leads to the development of debilitating side effects such as choreic, dystonic, and ballistic movements, collectively referred to as L-dopa-induced dyskinesia (LID)2. The incidence of LID is observed in about 10% of patients in the first year of treatment and in nearly 90% of patients after 9 years of treatment3. Dyskinesia negatively affects patients' quality of life and substantially increases the costs associated with their health care4. Undoubtedly, these involuntary movements represent a serious limitation to the current pharmacotherapy for PD, especially during the advanced stages of the disease2. Current treatment strategies for LID are restricted and include a decrease in L-dopa dosing which has the shortcoming that there is a rebound of parkinsonian symptoms, adjunct therapy with amantadine which has only limited effectiveness or surgical intervention5.
Until now, two major factors appear to underlie the appearance of LID. The first is the primary nigral dopaminergic cell loss6, which determines the degree of drug exposure and time required for the initial onset of involuntary movements, as well as the pulsatile manner in which short-acting dopaminergic agents stimulate striatal postsynaptic dopamine receptors is key to the priming of the basal ganglia for induction of LID7. Based on such theory, numerous studies have shown that continuous dopamine stimulation (CDS) can reduce the responsiveness of dopamine receptor in a direct way, is probably helpful in reducing LID8. Not only restrict in non-human primate researches, in a recent clinical double-blind controlled study reported that continuous delivery of levodopa-carbidopa with an intestinal gel offers a promising option for control of advanced PD with motor complications9.
In addition, accumulating evidence indicates that LID develops in response to activation of sensitized D1 dopamine receptor (DR1) located on the medium spiny neurons (MSNs) of the direct striatonigral pathway10. In rodent and non-human primate models, repeated administration of L-dopa increases the binding of the DR1 antagonist SCH23390 to striatal membranes11, which is associated with increased localization of DR1 at the cell surface. Experimental models of PD further lend support to the hypothesis that L-dopa, through the activation of DR1, triggers profound alterations in the activity of the molecular markers for LID12. Sensitized DR1 transmission may also be caused by increased levels of adenylyl cyclase in striatonigral MSNs. Prolonged administration of L-dopa leads to persistent and intermittent hyper-activation of the cAMP signaling cascade. Activation of cAMP signaling results in increased activity of the cAMP-dependent protein kinase (PKA) and dopamine- and cAMP-dependent phosphoprotein of 32 kDa (DARPP-32)13 Abnormal PKA/DARPP-32 signaling increases the phosphorylation of protein tau and several downstream effector targets14. All of these effects promote the excitability of MSNs and may result in LID occur. However, pharmacological or genetic interventions aimed at reducing abnormal DR1 mediated signal transduction at the level of these various intracellular cascades have been shown to attenuate LID in rodent and non-human primate models.
We recently reported that levodopa/benserazide microspheres (LBM), which release levodopa and benserazide in a sustained manner, could be used to reduce established LID in a rat model of PD15. Previous study showed LBM sustained release within approximately 3 weeks, with the initial burst rate only 18.3% and 30% total drug loading in the first 2 day16. However, whether DR1-mediated transmission and downstream effector targets are involved in the mechanisms by which LBM reduce LID in dyskinetic rats was still unknown. Thus, the present study was mainly to investigate the effect of LBM on DR1, PKA and phosphorylated levels of tau in LID rats. In parallel, we evaluated whether different dose of LBM (20 mg/kg, 40 mg/kg, and 60 mg/kg) affects the occurrence of AIM score and the performance of motor function.
Results
Treatment with LBM prevents the development of LID in 6-OHDA-lesioned rat model of PD
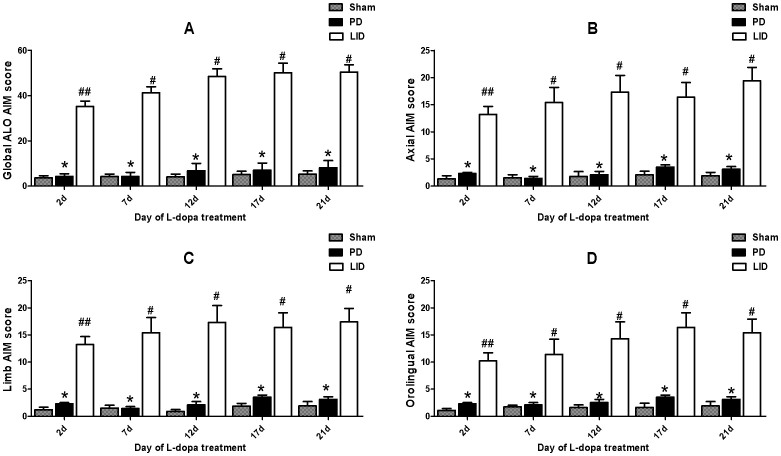
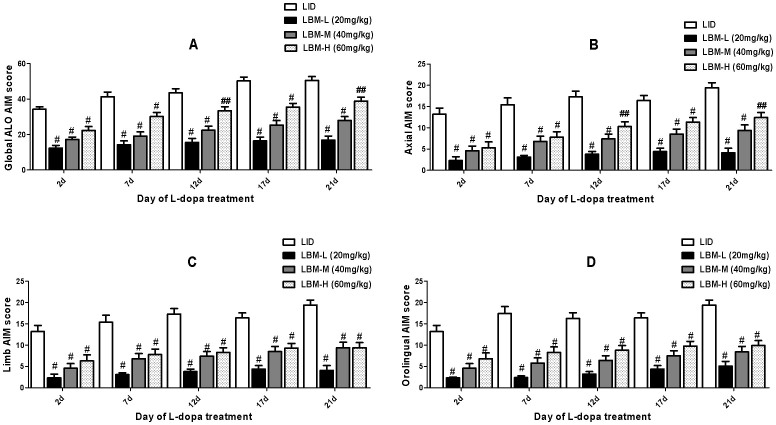
40 SD rats were unilaterally injected with 6-OHDA in the MFB (medial forebrain bundle) except sham group (n = 8). Fig. 1 described the experimental design. 6-OHDA treatment of rats resulted in a parkinsonian syndrome, characterized by bradykinesia, reduced motor activity, reduced attention and a hunched posture. 6-OHDA-lesioned rats treated with L-dopa plus benserazide for 21 days developed a progressive increase in LID (p < 0.0001 for treatment effect, p < 0.0001 for time effect and p < 0.0001 for treatment and time interaction, Fig. 2). Median ALO AIM score increased from 30.2 ± 2.43 on day 2 to 50.5 ± 4.12 on day 21 (n = 8, P < 0.05 vs day 2, Fig. 2A). Meanwhile, the sham and 6-OHDA-lesioned groups received the saline for 21 days did not develop LID features, the median ALO AIM score was range from 0 to 6 (Fig. 2A). This seemed to be the same trend in Axial AIM (Fig. 2B), limb AIM (Fig. 2C) as well as orolingual AIM (Fig. 2D). In contrast, animals treated with LBM did not develop LID over the 21 day treatment period, which differed significantly from the LID groups in all testing sessions (p < 0.001, Fig. 3). In terms of the LBM-L (20 mg/kg, sc) and LBM-M (40 mg/kg, sc) groups, median ALO AIM score increased from 12.3 ± 1.52 on day 2 to 14.9 ± 2.32 on day 21, 16.3 ± 1.32 to 20.9 ± 3.32, respectively, which are obvious reduction compared with the LID group at the same time point (n = 8, P < 0.05 vs LID groups, Fig. 3A). Furthermore, LBM-H (60 mg/kg, sc) groups also demonstrated certain reduction in the ALO AIM score compared with the rats receiving L-dopa plus benserazide, but the rats still showed a mild dyskinesia after 12 days treatment (Fig. 3A). Similarly, evaluation of the individual AIM subtypes showed that LBM significantly decreased both axial (Fig. 3B) and limb AIMs (Fig. 3C) with a similar trend for improvement in orolingual AIMs (Fig. 3D).
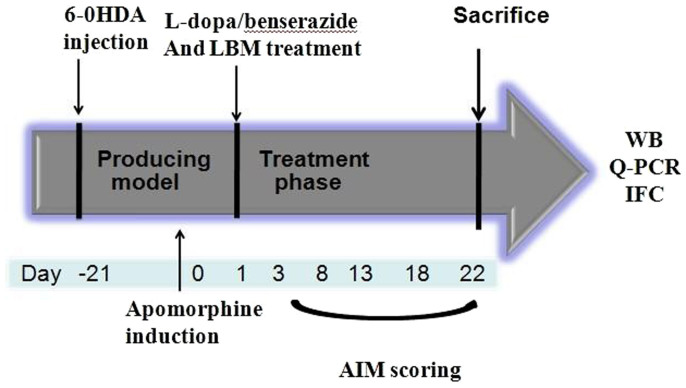
Figure 1. Experimental design of the study.
SD rats (48) were unilaterally injected with 6-OHDA in the medial forebrain bundle (MFB). Contralateral turning behaviours after apomorphine injection were tested 21 days later. Rats in the LID group were administrated twice-daily (9:00 and 15:00) with L-dopa (20 mg/kg, i.p.) plus benserazide (5 mg/kg, i.p.) for 3 weeks; Rats in the LBM group were further divided into three groups, namely LBM low dose group (LBM-L, 20 mg/kg, n = 8), LBM mild moderate dose group (LBM-M, 40 mg/kg, n = 8), and LBM high dose group (LBM-H, 60 mg/kg, n = 8). Rats received LBM subcutaneous (SC) twice one day per week for 3 weeks. AIMs were evaluated during this period, at days 2, 7, 12, 17 and 21. The animals were sacrificed 1 h after the last injection for western blot, Q-PCR and immunofluorescence.
Figure 2. Daily time course of LID scores with L-dopa plus benserazide exposure.
Sham group and 6-OHDA-lesioned rats were daily treated with saline or L-dopa plus benserazide for 21 days and individually tested for ALO AIMs. Scores are shown as the (A) sum of axial, limb and orolingual, (B) axial, (C) limb, and (D) orolingual AIMs on each testing session; # p < 0.01, ## p < 0.05 vs 6-OHDA-lesioned rats; * p > 0.05 vs 6-OHDA-lesioned rats.
Figure 3. Time course of AIMs score development in 6-OHDA-lesioned rats during a 3 weeks treatment with L-dopa + benserazide, LBM-L (20 mg/kg), LBM-M (40 mg/kg), and LBM-H (60 mg/kg).
6-OHDA-lesioned rats treated with LBM did not develop LID over the 21 day treatment period, which differed significantly from the LID groups in all testing sessions. (A) sum of axial, limb, and orolingual, (B) axial, (C) limb, and (D) orolingual AIMs on each testing session were rated following the administration of LBM. Data are presented as mean ± standard error; # p < 0.01, ## p < 0.05 vs LID.
Effects of LBM on cylinder test
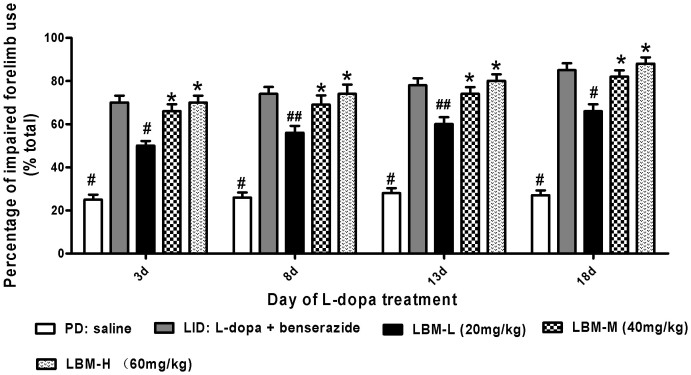
LBM prevents LID without reducing the anti-parkinsonian action (Fig. 4). We attempted to evaluate the effect of LBM on cylinder test in unilaterally lesioned animals, which monitors preferential forelimb usage as the animal spontaneously rears in a cylinder. We observed that 6-OHDA-lesioned rats treated with L-dopa plus benserazide prefer to use the compromised (contralateral) forelimb to touch the inner wall of the cylinder compared with the 6-OHDA-lesioned rats treated with saline (p < 0.05, Fig. 4). If the animals were injected with LBM-L (20 mg/kg) they also demonstrated preferential to touch the wall with contralateral forelimb, but less effect than LBM-M (40 mg/kg) and LBM-H (60 mg/kg) groups as well as 6-OHDA-lesioned rats treated with L-dopa plus benserazide (p < 0.05, Fig. 4). When we continued to measure forelimb preference between LBM-M and LBM-H, we found that it was no significant difference between two groups (p > 0.05, Fig. 4). Namely, LBM-H tested (60 mg/kg) did not produce an additional benefit over the LBM-M (40 mg/kg) administration. Meanwhile, there was no significant difference in total parkinsonian disability between animals treated with L-dopa alone and LBM-M or LBM-H (p > 0.05, Fig. 4). These data suggested that the higher dose of LBM (60 mg/kg) did not result in a greater improvement in cylinder test than LBM-M (40 mg/kg).
Figure 4. Unilaterally 6-OHDA-lesioned rats were tested for the percentage of impaired forelimb use compared with the total numbers.
Animals were injected with either saline, L-dopa + benserazide, LBM-L (20 mg/kg), LBM-M (40 mg/kg) and LBM-H (60 mg/kg). Experiment consisted of four different sessions, namely 3 d, 8 d, 13 d and 18 d in the treatment period. 6-OHDA-lesioned rats treated with L-dopa plus benserazide prefer to use the compromised (contralateral) forelimb to touch the inner wall of the cylinder compared with the 6-OHDA-lesioned rats treated with saline. Rats in the LBM groups also demonstrated preferential to touch the wall with contralateral forelimb. Data are presented as mean ± standard error of the mean; # p < 0.01, ## p < 0.05 vs LID; * p > 0.05 vs LID.
Treatment with LBM prevents activation DR1, PKA and p-tau protein
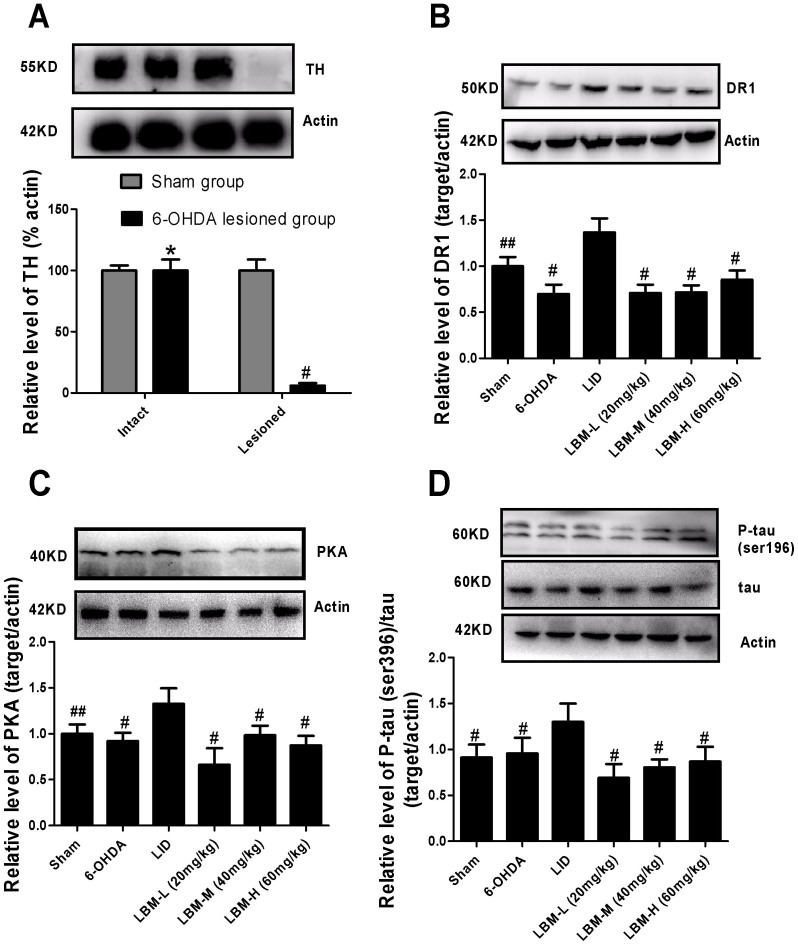
The extent of 6-OHDA-induced nigrostriatal dopamine alteration was verified by western blotting with an antibody raised against Tyrosine hydroxylase (TH). No significant changes were observed in TH levels in the intact hemisphere between sham-operated and 6-OHDA lesioned rats (p > 0.05, Fig. 5A). In contrast, TH protein level was dramatically decreased by more than 90% in the lesioned side of 6-OHDA-injected rats when compared with sham group (p < 0.0001, Fig. 5A). There was mild decrease in the level of DR1 in the lesioned hemispheres of animals treated with vehicle compared with sham group but did not reach statistical difference (p < 0.05, Fig. 5B). However, in 6-OHDA lesioned rats treated with L-dopa, the level of striatal DR1 protein expression in the lesioned hemispheres was significantly increased as compared to the other five groups (p < 0.05, Fig. 5B). This elevation in DR1 expression was apparent prevented in animals treated with LBM (p < 0.05, Fig. 5B). We performed a dose response experiment to measure the protein expression for each of the animals using increasing dose of LBM to verify that the DR1 expression was dependent upon the administration dose of LBM. Meanwhile, we investigated whether LBM subcutaneous altered PKA levels in the striatum of 6-OHDA lesioned rats. Striatal levels of PKA in 6-OHDA-lesioned rats were not different from those seen in sham-operated controls. However, pulsatile L-dopa treatment in 6-OHDA-lesioned animals evoked a robust increment of PKA levels (p < 0.05, Fig. 5C). Remarkably, LBM-L administration totally prevented this effect (p < 0.001 compared with LBM-M and LBM-H, Fig. 5C). When we continued to measure PKA expression between LBM-M and LBM-L, we found there was no significant difference between two groups (p > 0.05, Fig. 5C). Phosphorylated levels of tau (ser396), relative to total tau protein in the striatum of 6-OHDA-lesioned rats were mild increase compared with sham group but without reaching statistical difference (p > 0.05, Fig. 5C). However, pulsatile L-dopa treatment in denervated rats induced a robust increase in the phosphorylated levels of tau compared to other five groups (p < 0.05, Fig. 5D). As the aforementioned results, LBM could totally prevent the increased tau protein phosphorylation at ser396. Further, p-tau expression was dependent upon the administration dose of LBM (Fig. 5D).
Figure 5. LBM prevented increased levels of D1R, PKA, p-tau and total tau after pulsatile L-dopa treatment.
Protein levels were evaluated by western blotting of proteins extracted from the ipsilaterally striatum of the rat brains. They were assessed in extracts from sham or 6-OHDA-lesioned rats treated with vehicle, pulsatile L-dopa (20 mg/kg, bid) or LBM-L (20 mg/kg, sc), LBM-M (40 mg/kg, sc) and LBM-H (60 mg/kg, sc). (A) Extent of the dopaminergic denervation induced by 6-OHDA lesions and sham. Tyrosine hydroxylase (TH) levels expressed relative to actin levels. # p < 0.01 and * p > 0.05 vs sham-intact hemisphere (n = 4); (B) DR1 levels expressed relative to actin levels; (C) PKA levels expressed relative to actin levels; (D) phosphorylated tau levels at ser396 expressed relative to tatal tau levels. The data represent the mean of relative optical density (expressed as a percentage of respective control values and normalized using sham group) ± SD; # p < 0.01 and ## p < 0.05 vs LID group (n = 4).
Effects of LBM on DR1, PKA, PPEB and tau mRNA
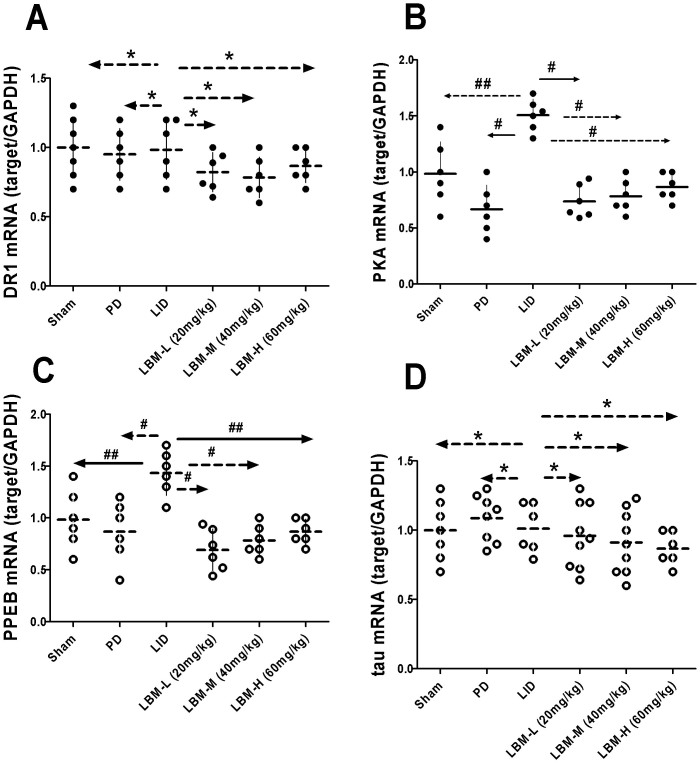
The expression of mRNA encoding DR1, PKA, PPEB and tau mRNA in the ipsilateral striatal was compared between the six different groups of rats (Fig. 6). Six groups did not induce obvious significant difference on the induction of striatal DR1 mRNA (p > 0.05, Fig. 6A), suggesting that it may depend on alterations in the number of functionally available receptors. In 6-OHDA lesioned rats treated with L-dopa, striatal PKA mRNA levels were significant higher in the LID group compared to other five groups (p < 0.05, Fig. 6B). This indicated that intermittent L-dopa treatment was helpful in the expression of PKA mRNA in dyskinetic rats. From the Fig. 6B we found LBM could obvious down-regulate the expression of PKA mRNA levels. Meanwhile, we observed a dose dependent response over a range of LBM-L to LBM-H for inhibited the up-regulation of PKA mRNA to similar levels as in non-dyskinetic rats (Fig. 6B). We also compared striatal PPEB mRNA among the six groups and found treatment with L-dopa (LID group) can apparent rise the PPEB mRNA level (p < 0.05 compared with other five groups). In accordance with previous results, treatment with LBM lowered the expression of PPEB mRNA to similar levels as in 6-OHDA-lesioned rats without treated with L-dopa (p > 0.05, Fig. 6C). Interestingly, dose dependent curve was also observed in the PPEB mRNA level. However, we found that it was no significant difference between LBM-M and LBM-H groups (p > 0.05). Total tau mRNA level did not differ in the six groups (p > 0.05, Fig. 6D), suggesting that treatment with L-dopa or LBM did not impact the total tau gene expression.
Figure 6. mRNA expression of DR1,PKA, PPEB, tau were evaluated by RT-PCR from the ipsilateral sides of the striatum.
mRNA were assessed in extracts from sham group, 6-OHDA-lesioned rats treated with vehicle, pulsatile L-dopa (20 mg/kg, bid) or LBM-L (20 mg/kg), LBM-M (40 mg/kg) and LBM-H (60 mg/kg). (A) DR1 mRNA levels expressed relative to GAPDH mRNA; (B) PKA mRNA levels expressed relative to GAPDH mRNA; (C) PPEB mRNA levels expressed relative to GAPDH mRNA; (D) tau mRNA levels expressed relative to GAPDH mRNA; Results are expressed by RQ from the ABI 7500 and normalized using the sham groups; * p < 0.01, ** p < 0.05 and # p > 0.05 vs LID group (n = 4).
Effects of LBM on p-tau positive neurons in striatum
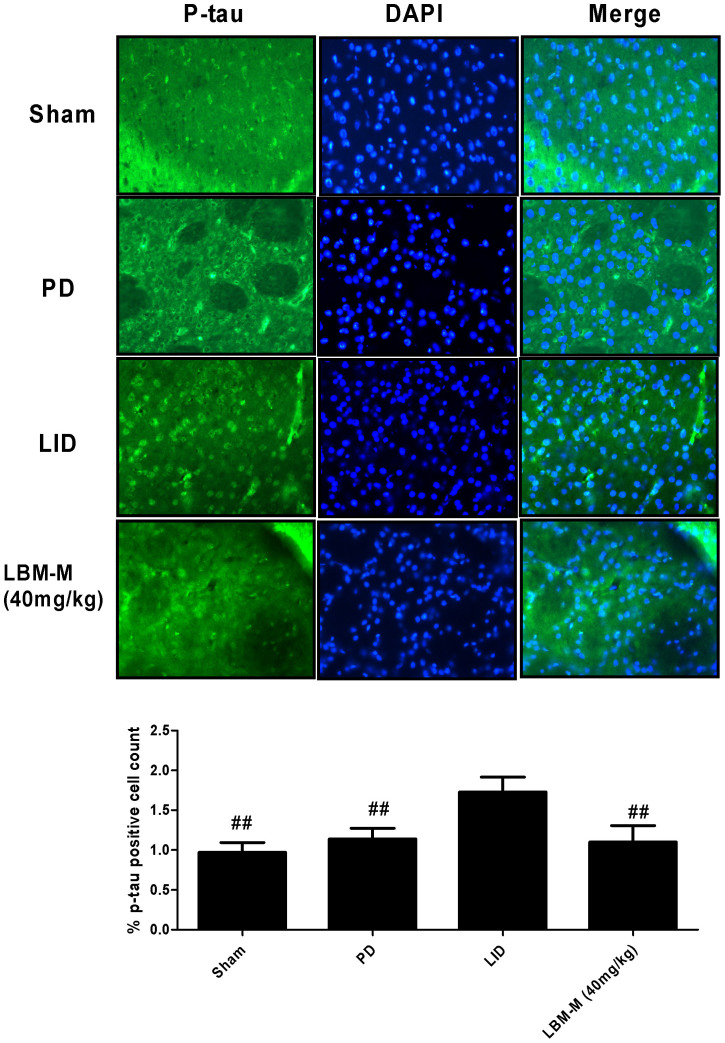
We counted p-tau-positive neurons in the striatum. P-tau-positive neurons (ser396), in the striatum of 6-OHDA-lesioned rats was mild increase compared with sham group but without reaching statistical difference (p > 0.05, Fig. 7). However, pulsatile L-dopa treatment in denervated rats induced a robust increase in the p-tau compared to other three groups (p < 0.05, Fig. 7). As the aforementioned results, LBM-M could totally prevent the increased tau protein phosphorylation at ser396 (p < 0.05 compared with LID group, Fig. 7).
Figure 7. Representative examples of p-tau immunofluorescence studies in sham, 6-OHDA-lesioned rats treated with vehicle, pulsatile L-dopa (20 mg/kg, bid) or LBM-M (40 mg/kg).
In 6-OHDA lesioned rats treated with L-dopa induced approximately increase in p-tau-positive neurons compared to other three group. These L-dopa-induced increases in positive neurons disappear in LBM-M group. Shown is a coronal section of the striatum from the lesioned hemisphere; ## p < 0.05 vs LID rats.
Correlation analysis of DR1, PKA, p-tau expression and dyskinesia levels
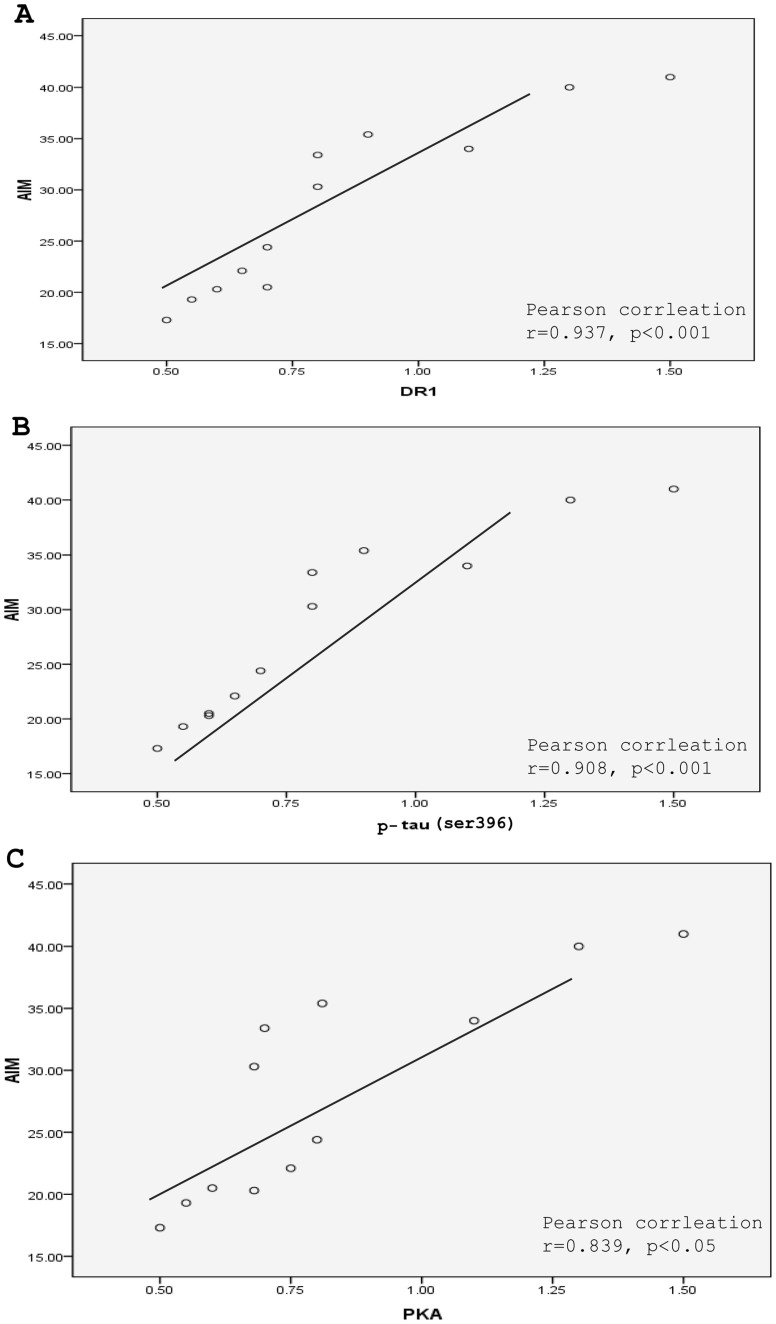
We also compared DR1, PKA and p-tau protein levels from individual dyskinetic and LBM group's rats to their AIMs score (Fig. 8). There was significant positive correlations between the degree of expression up-regulation of DR1 (r = 0.937, p < 0.001, Fig. 8A), p-tau (r = 0.908, p < 0.001, Fig. 8B) and PKA (r = 0.839, p < 0.05, Fig. 8C) and the severity of AIM score exhibited by the rats.
Figure 8. Correlations between AIMs score and DR1 (A), p-tau (ser396) protein (B) and PKA (C) levels from individual dyskinetic and LBM groups rats to their AIMs score.
Correlation coefficients were determined using a two-sided person test.
Discussion
L-dopa treatment is currently the most successful approach to manage motor symptoms in PD. However, the emergence of LID with long-term use is a severe problem for PD patients. Our results from this study showed that treatment with LBM prevented the development of LID in 6-OHDA-lesioned rat model of PD, as well as dose-dependently improvement in motor function by cylinder test. Based on previous study, LBM was encapsulated into poly (lactic-co-glycolic acid) as done previously, which can release levodopa and benserazide in a sustained manner in order to continuous stimulate dopaminergic receptors16. Continuous L-dopa infusion through microsphere, in contrast to intermittent treatment, provides temporally more stable dopamine replacement therapy, relieving AIM score while still producing an anti-parkinsonian effect. The mechanisms behind differences between pulsatile and continuous L-dopa are not entirely clear, but it is supposed that pulsatile L-dopa induces non-physiological activation of dopamine receptors, especially D1 dopamine receptors. Our findings also showed that expression of DR1, PKA and phosphorylation of tau at ser396 were not increased after LBM administration. In conclusion, these data demonstrate that LBM prevents molecular change from occurring when it is given intermittently and lend support to the hypothesis that continuous and stable dopaminergic stimulation is a promising mode of administration to avoid LID.
In the current study, we confirmed a dose range of LBM that is effective in reducing AIM score. LBM-L (20 mg/kg) and LBM-M (40 mg/kg) did not develop LID over the 21 day treatment period, while LBM-H (60 mg/kg) showed a mild dyskinesia after 12 days treatment. In terms of anti-parkinsonian action, LBM-M and LBM-H showed apparent improvement in motor function compared with LBM-L group similar levels as by L-dopa treatment. It has been suggested that LBM-M (40 mg/kg) can obvious prevent AIM score as well as ameliorate motor symptoms in 6-OHDA-lesioned rat models of PD. To our knowledge, changing the delivery profile of an identical drug, even one with a half-life shorter than L-dopa, seems to be able to lower the risk of LID17. In recent years, CDS has largely been replaced by the concept of continuous dopaminergic delivery (CDD). CDD aims to minimize motor fluctuations in PD by delivering the drug in as constant a manner as possible, regardless of serum half-life18. Actually, Levodopa/benserazide microsphere is able to achieve the pharmacokinetics or pharmacodynamics of CDD from our previous studies16.
Several researches lend support to the view that D1-like dopamine receptors are crucially involved in the molecular mechanisms underlying LID19. The pathological augment in the number of DR1 at the plasma membrane is likely to contribute to the increase in DR1 transmission associated to LID and denotes a potential target for therapeutic20. Recently, genetic inactivation of the DR1 in rodents has been used to inspect the involvement of the receptor in LID. DR1 gene knock down blocked the development of dyskinesia and also reduced the associated molecular changes in the lesioned striatum21. In the present study, it was found that DR1 protein level in the membrane of striatum was increased significantly after L-dopa treatment in dyskinetic rats. However, LBM treatment obvious reduced the increase of DR1. More interestingly, this effect is not accompanied by changes in the expression of DR1 mRNA, indicating that it may just alterations in the number of functionally available receptors. Several studies have shown that LID is associated with increased localization of D1Rs at the cell surface, which may be caused by receptor internalization and trafficking22. Previous studies showed that increased responsiveness of the DR1 machinery to L-dopa results in augmented synthesis of cAMP and hyper-activation of PKA23. The abnormal activation of PKA in animal models of LID cause changes in the state of phosphorylation of target effector proteins, which probable have profound repercussion on the excitability of striatal MSNs13. What is more, intrastriatal PKA inhibition significantly attenuates the emergence of AIM score and decrease the expression of ΔFosB, phosphorylation levels of DARPP-32 at threonine34 and activation of ERK1/214. Moreover, one study showed that administration of cannabinoid agonist WIN could ameliorate AIM score in 6-OHDA-treated rats and reversed the concomitant activation of PKA via CB1-mediated mechanism24. Taken together, all these data indicate that PKA plays a pivotal role in molecular priming for dyskinesia by L-dopa and aberrant D1-dependent molecular plasticity in the striatum. Our current results are in line with previous studies showing that intermittent L-dopa evokes a robust increment of PKA protein and mRNA levels. Moreover, LBM overtly blockades of PKA signaling and reduces AIM expression and development.
Tau is one of the key microtubule-associated proteins which involves in the maintenance of dendritic processes that are known to influence synaptic strength and neuronal plasticity25. Normally, tau binds directly to microtubules and facilitates their polymerization, but it is well-established that increasing tau phosphorylation negatively adjusts microtubule-binding and leads to destabilization of the microtubule network, cytoskeletal dysfunction and modification of synaptic plasticity26. Recent studies showed that intermittent L-dopa treatment positively correlates with hyper-phosphorylation of the microtubule-associated protein tau in hemiparkinsonian rats, which was consistent with hypothesis that changes in dopamine levels of the rodent brain were associated with modification of cytoskeletal organization27. Moreover, PKA inhibition had been shown to prevent hyper-phosphorylation of tau, which may contribute to long-lasting synaptic plasticity changes underlying striatal dysfunction and motor impairment14. This indicates that p-tau may play a pivotal role in the expression of LID. In this study, we further confirmed that after repeated administration of L-dopa increased levels of p-tau were observed in dyskinetic rats, which was consistent with our previous study16. Nevertheless, LBM could totally prevent the increased phosphorylation of tau at ser396 while reducing the development of dyskinesia. This suggests that preventing the phosphorylation of tau might be helpful in reducing the emergence of LID in rats. Moreover, it is considered that PPEB is involved in the expression of LID. In the present study, it was found that intermittent L-dopa treatment induced increased levels of PPEB mRNA in the striatum of dyskinetic rats, which may represent increased responsiveness of a direct pathway. However, LBM treatment ameliorated the expression of PPEB mRNA while preventing the expression of LID.
In conclusion, our experiments provide evidence that intermittent administration of L-dopa, but not continuous delivery, and DR1/PKA/p-tau (ser396) activation play a critical role in the molecular and behavioural induction of AIM in 6-OHDA-lesioned rats. In addition, we also found that LBM treatment prevented the development of LID by inhibiting the expression of DR1/PKA/p-tau, as well as PPEB mRNA in dyskintic rats. Based on these results, we speculate the way to achieve CDD by microsphere may have interesting clinical implications for the treatment of LID in PD patients.
Methods
Animals
Forty-eight young adult females Sprague-Dawley (SD) rats weighting 180–220 g were used in this study. Upon their arrival, the animals were housed under a 12 h light:12 h dark cycle, temperature 22.0 ± 2.0°C and relative humidity of 55 ± 10%. The rats had free access to water and were fed with a pelleted complete diet ad libitum. Animals were acclimated for at least one week before the L-dopa/benserazide injections were initiated. All protocols involving the animals were approved by the Institutional Review Board of Xinhua Hospital and were performed according to the guideline of the National Institutes of Health for the care and use of laboratory animals (NIH publication No 80-23). All efforts were made to reduce animal numbers used to the minimum required for valid statistical analysis.
6-hydroxydopamine (6-OHDA) lesioned rat model of PD
Unilateral 6-OHDA lesions were performed according to our standard procedure16. Briefly, 6-OHDA (2 × 16 μg dissolved in 8 μL of 0.9% physiological saline containing 0.2% ascorbic acid; Sigma-Aldrich, St Louis, MO, USA) was stereotaxically injected into the right medial forebrain bundle (MFB) of rats. The coordinates of the right medial forebrain bundle were calculated using the rat brain atlas as follows (in mm relative to the bregma and the dural surface): 1) anterior-posterior (AP), −4.4 mm, medial-lateral (ML), −1.2 mm, dorsal-ventral (DV), −7.8 mm; 2) AP, −3.7 mm, ML, −1.7, DV, −7.8. The tooth bar was set to −2.4 mm. Animals were anesthetized with ketamine (1–2 ml/kg). For the sham-operated rats, two intrastriatal injections of physiological saline containing 0.2% ascorbic acid were given at the same coordinates. Three week post lesion, the lesioned rats were screened behaviorally using an amphetamine-induced (0.5 mg/kg, i.p.) rotation test and all animals exhibited >7 full body turns/min toward the side of the lesioned side were selected for the next experiment.
Drug treatment
The whole process of preparation of levodopa/benserazide microspheres (LBM) was performed according to our previous protocol16. Plasma concentration of L-dopa microspheres reached a high stable level in the first 14 days then reverted to a relatively normal level until nearly 3 weeks had passed. The release profile of benserazide was similar to that of L-dopa microspheres, since they share the same release mechanism in vivo. Our previous research showed that the encapsulation efficiency of L-dopa microspheres was 60.15% ± 4.2%, lower than that of benserazide (62.87% ± 6.9%)16. First, the valid PD rats were divided into two groups: the LID group (n = 8) and the microsphere group (n = 24). Rats in the LID group were administrated twice-daily (9:00 and 15:00) with L-dopa (20 mg/kg, i.p.) plus benserazide (5 mg/kg, i.p.) for 3 weeks to induce a rat model of dyskinesia. Moreover, rats in the microsphere group were further divided into three groups, namely LBM low dose group (LBM-L, L-DOPA 20 mg/kg plus benserazide 5 mg/kg, n = 8), LBM mild moderate dose group (LBM-M, L-DOPA 40 mg/kg plus benserazide 10 mg/kg, n = 8), and LBM high dose group (LBM-H, L-DOPA 60 mg/kg plus benserazide 15 mg/kg, n = 8). Rats in the microsphere group received LBM subcutaneous (SC) one time per week for 3 weeks. Additionally, rats in the PD group (n = 8) and sham group (n = 8) were treated physiological saline for 3 weeks.
AIM ratings
Abnormal involuntary movement (AIM) ratings were performed by an investigator who was unaware of the pharmacological intervention. On test days, rats were individually placed in plastic trays 5 minutes before the drug treatments. Following injections, each rat was assessed for exhibition of axial, limb, and orolingual movements (ALO AIM) as detailed in Lindenbach et al28. For quantification of LID, rats were observed individually every 20 min from 20 to 120 min after the injection of L-dopa or saline as previously described by Cenci et al. (1998)29. For each of these three subtypes, the severity of dyskinesia was scored on a four-point scale, where 0 = absent, 1 = present during less than half of the observation time, 2 = present for more than half of the observation time, 3 = present all the times but suppressible by external stimuli, and 4 = present all the times and not suppressible by external stimuli. For each rat, a total AIMs score was calculated by adding each of the three individual dyskinesia scores. The maximum theoretical score per monitoring session was 72.
Cylinder test
The cylinder test was performed two times a week during the L-dopa treatment period to assess the spontaneous and independent use of each of the rat's forelimb in the context of an instinctive rearing behavior, with the rat standing on its hind legs and leaning on the enclosing walls30. Rats were placed individually in an open ended glass cylinder (22 × 35 cm) without habituation in a dimly lighted room. Wall exploration was expressed in terms of the percentage use of the impaired forelimb (contralateral) compared with the total number of limb use movements.
Western blot
The rats were sacrificed immediately by decapitation. Corpus striatum of rat in the lesioned hemisphere from each animal was obtained. The striatal tissue was homogenized in lysate buffer at 4°C containing Tris at pH 7.4 (50 mM), NaCl (50 mM), 1 mM each of EDTA, EGTA, PMSF, sodium orthovanadate, sodium fluoride, 1% SDS and a protease inhibitor cocktail (Roche Diagnostics, Laval, QC, Canada), and centrifuged at 5000 × g for 5 minutes at 4°C. The supernatant was containing total and membrane-enriched proteins. Briefly, lesioned samples of one animal from each treatment group were loaded onto a single gel 40 μg of protein. The proteins were separated by 8–12% sodium dodecyl polyacrylamide gels, electrophoretically transferred to Polyvinylidene Fluoride (PVDF) membranes. The membrane was incubated with polyclonal rabbit anti-DR1 IgG (diluted 1:1000; Millipore), polyclonal rabbit anti-PKA IgG (diluted 1:1000; Sigma-Aldrich, USA), polyclonal mouse anti-phospho-tau at ser396 IgG2b (diluted 1:1000; Cell Signaling Technology, USA), polyclonal rabbit anti-tau IgG (diluted 1:1000; Sigma-Aldrich, USA) and monoclonal rabbit anti-β-actin IgG (diluted 1:1000; Beyotime Institute of Biotechnology) overnight at 4°C, respectively. The membrane was then incubated with anti-rabbit or anti-mouse Horseradish Peroxidase (HRP) IgGs (diluted 1:1000, Santa Cruz Biotechnology) for 1–2 h at room temperature. Immunoreactive bands were visualized using chemiluminescence (ECL kit; Pierce Biotechnology). Protein bands were scanned with Image-Pro plus 6.0 analyses Software; IDVs were calculated with a computerized image analysis system (Image Lab) and normalized with that of β-actin.
Real-time Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from the brain striatum segments of lesioned hemisphere using TriZol (Invitrogen, USA). cDNA was generated from total RNA samples using the RevertAid First Strand cDNA Synthesis kit (Takara Biotechnology company, Japan). Quantitative PCR was performed using the ABI 7500 Real-Time PCR System (Life Technologies, Carlsbad, CA) and TaqMan universal PCR master mix (Applied Biosystems) according to the supplier's instructions. PCR was carried out with the SYBR Green quantitative RT-PCR system (Takara) and gene-specific primers for 40 cycles according to the manufacturer's protocols. After amplification, melting curve analysis and length verification by gel electrophoresis were carried out to confirm the specificity of PCR products. As a negative control, template RNA was replaced with PCR-grade water. Calculations of threshold cycle and difference were analyzed with ABI 7500 Real-Time PCR System (Life Technologies, Carlsbad, CA). The primer sequences used in this study were as follows:
5-CTATCTCCAGCCCTTTCCAG-3 (forward) and 5-AGTTGTCATCCTCGGTGTCC-3 (reverse) for DR1 mRNA;
5-GGGAACCAGGAGGTTGAGAT-3 (forward) and 5-AACCGCATAAGCAGAAGCAG-3 (reverse) for PKA mRNA;
5-ACGAATCCAACCAGTGTGC-3 (forward) and 5-AACCGCATAAGCAGAAGCAG-3 (reverse) for tau mRNA;
5-GAGAATGAGGTTGCTTTGGAA-3 (forward) and 5-AGACGCTGGTAAGGAGTTGG-3 (reverse) for preproenkephalin B (PPEB) mRNA;
5′-GAGAGGGAAATCGTGCGTGAC-3′ (forward) and 5′-CCATACCCAGGAAGGAAGGCT-3′ (reverse) for GAPDH mRNA.
Immunofluorescence
Whole brain was incubated for 10 min in 5% normal donkey serum in PBS and incubated overnight at 4°C in the primary antibody solution (polyclonal mouse anti-phospho-tau at ser396 IgG2b, same as above). Sections were rinsed in PBS and incubated in FITC-conjugated donkey anti-mouse IgG (1:200). Subsequently, sections were again rinsed in PBS, mounted on slides, coverslipped, and examined with confocal microscopy. Digitized images were analyzed for distribution of immunoreactive cells in the lesioned hemisphere striatum of rats.
Statistical analysis
Data were expressed as the mean ± standard deviation. Behavioral data are non-parametric and were analyzed using a Kruskal Wallis followed by Dunn's test for multiple comparisons in the case of comparing data over multiple days, or a Mann–Whitney U test. All rodent data, conformed to normal distribution were performed using one-way analysis of variance (ANOVA) followed by LSD post-hoc comparisons when appropriate. P-values < 0.05 were considered statistically significant differences. All analyses were carried out using SPSS 16.0.
Author Contributions
C.L.X. and S.F.Z. conceived and participated in its design, searched databases, extracted and assessed studies and helped to draft the manuscript. M.L.Y. and J.Y.C. prepared the Levodopa/benserazide microsphere (LBM) and figures and offer the technical support in the whole process. S.F.Z., J.G. and L.S. carried out the statistical analysis and interpretation of data. W.W.W. offered the help to improve the language of the manuscript. W.E.Y. and Z.G.L. participated in the conceptualization and design of the experiment, data extraction and analysis, and drafted the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Table S1
Acknowledgments
We gratefully acknowledge Professor Zhen-Guo Liu for his help in guiding and revising the manuscript. We also thank Dr. Wen-Wen Wang from Wenzhou Medical University for the language improvement of the paper. The study was supported by the Projects of National Science Foundation of China (No.81171203, 81171204 and 81200871), and Projects of the Shanghai Committee of Science and Technology, China (No.11nm0503300 and 12XD1403800).
References
- Akushevich I., Kravchenko J., Ukraintseva S., Arbeev K. & Yashin A. I. Age patterns of incidence of geriatric disease in the U.S. elderly population: Medicare-based analysis. J Am Geriatr Soc 60, 323–327 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot P., Johnston T. H., Koprich J. B., Fox S. H. & Brotchie J. M. The pharmacology of L-DOPA-induced dyskinesia in Parkinson's disease. Pharmacol Rev 65, 171–222 (2013). [DOI] [PubMed] [Google Scholar]
- Bhidayasiri R. & Truong D. D. Motor complications in Parkinson disease: clinical manifestations and management. J Neurol Sci 266, 204–215 (2008). [DOI] [PubMed] [Google Scholar]
- Dodel R. C., Berger K. & Oertel W. H. Health-related quality of life and healthcare utilisation in patients with Parkinson's disease: impact of motor fluctuations and dyskinesias. Pharmacoeconomics 19, 1013–1038 (2001). [DOI] [PubMed] [Google Scholar]
- Iravani M. M. & Jenner P. Mechanisms underlying the onset and expression of levodopa-induced dyskinesia and their pharmacological manipulation. J Neural Transm 118, 1661–1690 (2011). [DOI] [PubMed] [Google Scholar]
- Jenner P. Molecular mechanisms of L-DOPA-induced dyskinesia. Nat Rev Neurosci 9, 665–677 (2008). [DOI] [PubMed] [Google Scholar]
- Olanow C. W., Obeso J. A. & Stocchi F. Continuous dopamine-receptor treatment of Parkinson's disease: scientific rationale and clinical implications. Lancet Neurol 5, 677–687 (2006). [DOI] [PubMed] [Google Scholar]
- Bezard E. Experimental reappraisal of continuous dopaminergic stimulation against L-dopa-induced dyskinesia. Mov Disord 28, 1021–1022 (2013). [DOI] [PubMed] [Google Scholar]
- Olanow C. W. et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson's disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol 13, 141–149 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthet A. & Bezard E. Dopamine receptors and L-dopa-induced dyskinesia. Parkinsonism Relat Disord 15, S8–12 (2009). [DOI] [PubMed] [Google Scholar]
- Aubert I. et al. Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann Neurol 57, 17–26 (2005). [DOI] [PubMed] [Google Scholar]
- Calabresi P., Di Filippo M., Ghiglieri V., Tambasco N. & Picconi B. Levodopa-induced dyskinesias in patients with Parkinson's disease: filling the bench-to-bedside gap. Lancet Neurol 9, 1106–1117 (2010). [DOI] [PubMed] [Google Scholar]
- Feyder M., Bonito-Oliva A. & Fisone G. L-DOPA-Induced Dyskinesia and Abnormal Signaling in Striatal Medium Spiny Neurons: Focus on Dopamine D1 Receptor-Mediated Transmission. Front Behav Neurosci 5, 71 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel M., Chagniel L., Bureau G. & Cyr M. Striatal inhibition of PKA prevents levodopa-induced behavioural and molecular changes in the hemiparkinsonian rat. Neurobiol Dis 38, 59–67 (2010). [DOI] [PubMed] [Google Scholar]
- Yang X. et al. Levodopa/benserazide microspheres reduced levodopa-induced dyskinesia by downregulating phosphorylated GluR1 expression in 6-OHDA-lesioned rats. Drug Des Devel Ther 6, 341–347 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. et al. Controlled-release levodopa methyl ester/benserazide-loaded nanoparticles ameliorate levodopa-induced dyskinesia in rats. Int J Nanomedicine 7, 2077–2086 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B. A. & Waters C. H. Continuous dopaminergic delivery to minimize motor complications in Parkinson's disease. Expert Rev Neurother 13, 719–729 (2013). [DOI] [PubMed] [Google Scholar]
- Gershanik O. & Jenner P. Moving from continuous dopaminergic stimulation to continuous drug delivery in the treatment of Parkinson's disease. Eur J Neurol 19, 1502–1508 (2012). [DOI] [PubMed] [Google Scholar]
- Calabresi P., Di Filippo M., Ghiglieri V. & Picconi B. Molecular mechanisms underlying levodopa-induced dyskinesia. Mov Disord 23, S570–579 (2008). [DOI] [PubMed] [Google Scholar]
- Fiorentini C., Savoia P., Savoldi D., Barbon A. & Missale C. Persistent activation of the D1R/Shp-2/Erk1/2 pathway in l-DOPA-induced dyskinesia in the 6-hydroxy-dopamine rat model of Parkinson's disease. Neurobiol Dis 54, 339–348 (2013). [DOI] [PubMed] [Google Scholar]
- Picconi B. et al. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat Neurosci 6, 501–506 (2003). [DOI] [PubMed] [Google Scholar]
- Berthet A. et al. Pharmacological analysis demonstrates dramatic alteration of D1 dopamine receptor neuronal distribution in the rat analog of L-DOPA-induced dyskinesia. J Neurosci 29, 4829–4835 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E., Valjent E. & Fisone G. Parkinson's disease: levodopa-induced dyskinesia and signal transduction. FEBS J 275, 1392–1399 (2008). [DOI] [PubMed] [Google Scholar]
- Martinez A., Macheda T., Morgese M. G., Trabace L. & Giuffrida A. The cannabinoid agonist WIN55212-2 decreases L-DOPA-induced PKA activation and dyskinetic behavior in 6-OHDA-treated rats. Neurosci Res 72, 236–242 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt T. et al. Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J Neurosci 23, 6972–6981 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazanetz M. P. & Fischer P. M. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat Rev Drug Discov 6, 464–479 (2007). [DOI] [PubMed] [Google Scholar]
- Lebel M., Patenaude C., Allyson J., Massicotte G. & Cyr M. Dopamine D1 receptor activation induces tau phosphorylation via cdk5 and GSK3 signaling pathways. Neuropharmacology 57, 392–402 (2009). [DOI] [PubMed] [Google Scholar]
- Lindenbach D. et al. Behavioral and cellular modulation of L-DOPA-induced dyskinesia by beta-adrenoceptor blockade in the 6-hydroxydopamine-lesioned rat. J Pharmacol Exp Ther 337, 755–765 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci M. A., Lee C. S. & Björklund A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci 10, 2694–2706 (1998). [PubMed] [Google Scholar]
- Schallert T., Fleming S. M., Leasure J. L., Tillerson J. L. & Bland S. T. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 39, 777–787 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1