Abstract
The reactivity of cells in vitro was investigated with specimens from various lymphoid organs of seven human fetuses. Thymocytes responded to stimulation by phytohemagglutinin with significant increases in synthesis of DNA, but failed to produce destruction of xenogeneic target cells. In cells from bone marrow, precisely the converse pattern of reactivity to the mitogen was detected. Lymphocytes from spleen and peripheral blood demonstrated both phytohemagglutinin-dependent functions, while hepatic cells did not respond to phytohemagglutinin. Based on the striking dichotomy of phytohemagglutinin-dependent responses in fetal thymocytes and bone-marrow lymphoid cells, we conclude that phytohemagglutinin-dependent cytotoxicity and DNA synthesis are functions of different populations of lymphoid cells during human embryonic development.
Keywords: T and B cells, DNA synthesis, target cells
Full text
PDF
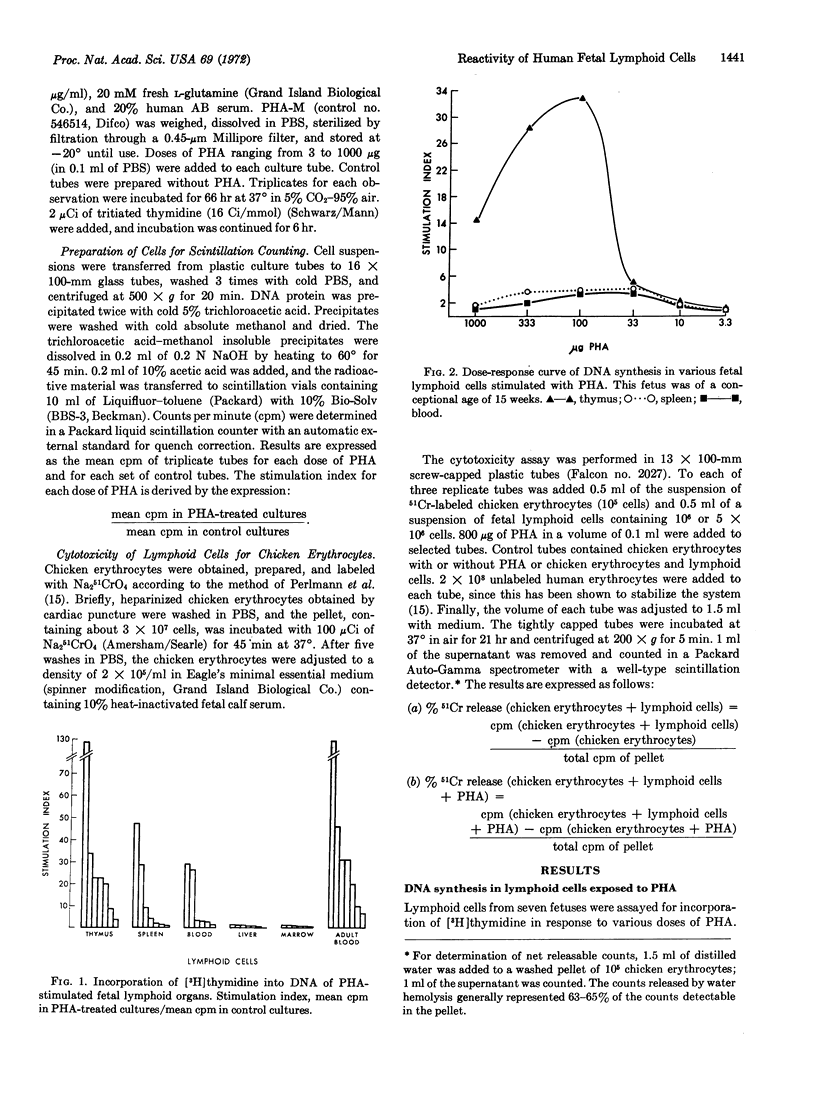
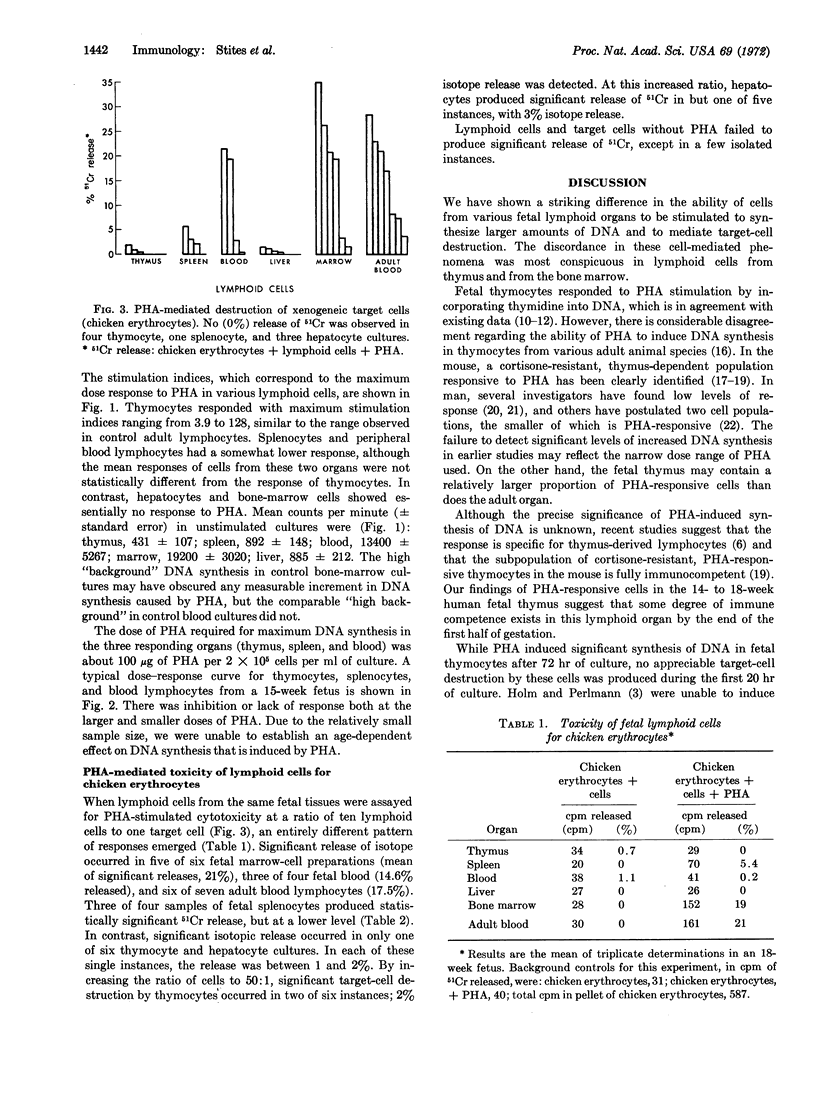


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blomgren H., Svedmyr E. Evidence for thymic dependence of PHA-reactive cells in spleen and lymph nodes and independence in bone marrow. J Immunol. 1971 Mar;106(3):835–841. [PubMed] [Google Scholar]
- Blomgren H., Svedmyr E. In vitro stimulation of mouse thymus cells by PHA and allogeneic cells. Cell Immunol. 1971 Aug;2(4):285–299. doi: 10.1016/0008-8749(71)90063-3. [DOI] [PubMed] [Google Scholar]
- Blomgren H., Takasugi M., Friberg S., Jr Specific cytotoxicity by sensitized mouse thymus cells on tissue culture target cells. Cell Immunol. 1970 Dec;1(6):619–631. doi: 10.1016/0008-8749(70)90027-4. [DOI] [PubMed] [Google Scholar]
- Claman H. N. Human thymus cell cultures-evidence for two functional populations. Proc Soc Exp Biol Med. 1966 Jan;121(1):236–240. doi: 10.3181/00379727-121-30746. [DOI] [PubMed] [Google Scholar]
- Doenhoff M. J., Davies A. J., Leuchars E., Wallis V. The thymus and circulating lymphocytes of mice. Proc R Soc Lond B Biol Sci. 1970 Oct 13;176(1042):69–85. doi: 10.1098/rspb.1970.0035. [DOI] [PubMed] [Google Scholar]
- Forsdyke D. R. Impaired activation of thymus lymphocytes by phytohemagglutinin. J Immunol. 1969 Oct;103(4):818–823. [PubMed] [Google Scholar]
- HOLM G., PERLMANN P., WERNER B. PHYTOHAEMAGGLUTININ-INDUCED CYTOTOXIC ACTION OF NORMAL LYMPHOID CELLS ON CELLS IN TISSUE CULTURE. Nature. 1964 Aug 22;203:841–843. doi: 10.1038/203841a0. [DOI] [PubMed] [Google Scholar]
- Harding B., Pudifin D. J., Gotch F., MacLennan I. C. Cytotoxic lymphocytes from rats depleted of thymus processed cells. Nat New Biol. 1971 Jul 21;232(29):80–82. doi: 10.1038/newbio232080a0. [DOI] [PubMed] [Google Scholar]
- Hardy D. A., Ling N. R., Wallin J., Aviet T. Destruction of lymphoid cells by activated human lymphocytes. Nature. 1970 Aug 15;227(5259):723–725. doi: 10.1038/227723a0. [DOI] [PubMed] [Google Scholar]
- Hartzman R. J., Segall M., Bach M. L., Bach F. H. Histocompatibility matching. VI. Miniaturization of the mixed leukocyte culture test: a preliminary report. Transplantation. 1971 Mar;11(3):268–273. doi: 10.1097/00007890-197103000-00005. [DOI] [PubMed] [Google Scholar]
- Holm G. Lack of cytotoxicity by human thymocytes in vitro. Scand J Haematol. 1967;4(3):230–240. doi: 10.1111/j.1600-0609.1967.tb01624.x. [DOI] [PubMed] [Google Scholar]
- Holm G., Perlmann P. Cytotoxic potential of stimulated human lymphocytes. J Exp Med. 1967 Apr 1;125(4):721–736. doi: 10.1084/jem.125.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm G., Perlmann P. Phytohaemagglutinin-induced cytotoxic action of unsensitized immunologically competent cells on allogeneic and xenogeneic tissue culture cells. Nature. 1965 Aug 21;207(999):818–821. doi: 10.1038/207818a0. [DOI] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- Kay H. E., Doe J., Hockley A. Response of human foetal thymocytes to phytohaemagglutinin (PHA). Immunology. 1970 Mar;18(3):393–396. [PMC free article] [PubMed] [Google Scholar]
- Levine M. A., Claman H. N. Bone marrow and spleen: dissociation of immunologic properties by cortisone. Science. 1970 Mar 13;167(3924):1515–1517. doi: 10.1126/science.167.3924.1515. [DOI] [PubMed] [Google Scholar]
- Lundgren G., Möller G. Non-specific induction of cytotoxicity in normal human lymphocytes in vitro: studies of mechanism and specificity of the reaction. Clin Exp Immunol. 1969 Apr;4(4):435–452. [PMC free article] [PubMed] [Google Scholar]
- MacLennan I. C., Harding B. Failure of certain cytotoxic lymphocytes to respond mitotically to phytohaemagglutinin. Nature. 1970 Sep 19;227(5264):1246–1248. doi: 10.1038/2271246a0. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J. Relationship of in vitro lymphocyte transformation to delayed hypersensitivity in guinea pigs and man. Fed Proc. 1968 Jan-Feb;27(1):21–28. [PubMed] [Google Scholar]
- Papiernik M. Correlation of lymphocyte transformation and morphology in the human fetal thymus. Blood. 1970 Oct;36(4):470–479. [PubMed] [Google Scholar]
- Pegrum G. D., Ready D., Thompson E. The effect of phytohaemagglutinin on human foetal cells grown in culture. Br J Haematol. 1968 Oct;15(4):371–376. doi: 10.1111/j.1365-2141.1968.tb01552.x. [DOI] [PubMed] [Google Scholar]
- Pegrum G. D., Ready D., Thompson E. The in vitro effect of phytohaemagglutinin on separated human bone marrow cells. Br J Haematol. 1968 Oct;15(4):377–380. doi: 10.1111/j.1365-2141.1968.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Perlmann P., Holm G. Cytotoxic effects of lymphoid cells in vitro. Adv Immunol. 1969;11:117–193. doi: 10.1016/s0065-2776(08)60479-4. [DOI] [PubMed] [Google Scholar]
- Perlmann P., Nilsson H., Leon M. A. Inhibition of cytotoxicity of lymphocytes by concanavalin A in vitro. Science. 1970 May 29;168(3935):1112–1115. doi: 10.1126/science.168.3935.1112. [DOI] [PubMed] [Google Scholar]
- Perlmann P., Perlmann H., Holm G. Cytotoxic action of stimulated lymphocytes on allogenic and autologous erythrocytes. Science. 1968 Apr 19;160(3825):306–309. doi: 10.1126/science.160.3825.306. [DOI] [PubMed] [Google Scholar]
- Playfair J. H. Cell cooperation in the immune response. Clin Exp Immunol. 1971 Jun;8(6):839–856. [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D., Rosenthal A. S., Paul W. E. Functional heterogeneity of murine lymphoid cells. I. Responsiveness to and surface binding of concanavalin A and phytohemagglutinin. J Immunol. 1972 Jan;108(1):1–17. [PubMed] [Google Scholar]
- Van Boxel J. A., Stobo J. D., Paul W. E., Green I. Antibody-dependent lymphoid cell-mediated cytotoxicity: no requirement for thymus-derived lymphocytes. Science. 1972 Jan 14;175(4018):194–196. doi: 10.1126/science.175.4018.194. [DOI] [PubMed] [Google Scholar]
- Winkelstein A. Failure of lymphocytes from guinea pig bone marrow to respond to phytohemagglutinin. Proc Soc Exp Biol Med. 1971 Feb;136(2):578–583. doi: 10.3181/00379727-136-35315. [DOI] [PubMed] [Google Scholar]