Abstract
The genetic alterations contributing to melanoma pathogenesis are incompletely defined, and few independent prognostic features have been identified beyond the clinicopathological characteristics of the primary tumor. We used transcriptome profiling of 46 primary melanomas, 12 melanoma metastases, and 16 normal skin (N) samples to find genes associated with melanoma development and progression. Results were confirmed using immunohistochemistry and real-time PCR and replicated in an independent set of 330 melanomas using AQUA analysis of tissue microarray (TMA). Transcriptome profiling revealed that transcription factor HMGA2, previously unrecognized in melanoma pathogenesis, is significantly upregulated in primary melanoma and metastases (P-values=1.2 × 10−7 and 9 × 10−5) compared with N. HMGA2 overexpression is associated with BRAF/NRAS mutations (P=0.0002). Cox proportional hazard regression model and log-rank test showed that HMGA2 is independently associated with disease-free survival (hazard ratio (HR)=6.3, 95% confidence interval (CI)= 1.8–22.3, P=0.004), overall survival (OS) (stratified log-rank P=0.008), and distant metastases–free survival (HR=6.4, 95% CI=1.4–29.7, P=0.018) after adjusting for American Joint Committee on Cancer (AJCC) stage and age at diagnosis. Survival analysis in an independent replication TMA of 330 melanomas confirmed the association of HMGA2 expression with OS (P=0.0211). Our study implicates HMGA2 in melanoma progression and demonstrates that HMGA2 overexpression can serve as an independent predictor of survival in melanoma.
INTRODUCTION
The incidence of melanoma in the United States continues to increase; melanoma causes three out of four deaths due to skin cancer (Hayat et al., 2007; Siegel et al., 2012). Early-stage melanoma is frequently curable, in contrast to the poorer prognosis of melanoma with regional lymph node involvement and the dismal outcome of widely metastatic disease. The molecular alterations contributing to the pathogenesis of melanoma are incompletely defined, and few independent prognostic features have been identified beyond the clinical and pathological characteristics of the primary tumor. Melanoma is generally resistant to chemotherapeutic and immunological treatments, and the efficacy of adjuvant therapy of melanoma is modest at best. The contribution of these therapies to overall survival (OS) of melanoma patients is limited. Although the management of melanoma is rapidly changing because of a better understanding of molecular heterogeneity and introduction of novel targeted therapies, new biomarkers can offer opportunities to develop prognostic models and new therapeutic targets.
Clinical trials for melanoma currently concentrate on rational drug development with several classes of drugs, including kinase inhibitors that target BRAF*V600, VEGF/ PDGF receptors, and mutated KIT, as well as drugs that target cell survival signaling pathways involving MEK, PI(3)K, or PTEN/AKT (Gray-Schopfer et al., 2007; Nikolaou et al., 2012; Woodman et al., 2012). Identification of new signaling pathways in melanoma initiation and progression opens new opportunities for targeted melanoma therapy. In 2011, vemurafenib and ipilimumab became the first drugs approved by the Food and Drug Administration for the treatment of metastatic melanoma patients with BRAF V600 mutations and inoperable late-stage melanoma, respectively (Curti and Urba, 2012; Woodman et al., 2012).
Microarray analysis of gene expression has been used to reveal sets of coregulated genes and unpredicted biological relationships in many cancer types, including melanoma (reviewed by Hoek (2007); Schramm et al. (2012)). Studies with different tumor source (cell lines or fresh tumors), microarray platform, and hypotheses considered reported gene expression profiles in melanoma (Bittner et al., 2000; Hoek et al., 2004; Pavey et al., 2004; Bloethner et al., 2005; de Wit et al., 2005; Haqq et al., 2005; Mandruzzato et al., 2006; Winnepenninckx et al., 2006; Jaeger et al., 2007; Kauffmann et al., 2008; Riker et al., 2008; Conway et al., 2009). As demonstrated in a meta-analysis of melanoma gene expression studies, the source of studied material (cell lines or tissue) may affect the list of differentially expressed genes (Hoek, 2007). Partial reporting of gene lists with different significance thresholds also contributes to discordant lists. Although cell lines provide a homogenous and reproducible source of melanoma cells, tissue samples may generate results more relevant to clinical use.
Our study used gene expression profiling of primary melanomas, regional and distant melanoma metastases (MM), and normal skin (N) samples to find novel genes associated with melanoma development and progression. We report genes differentially expressed in primary melanoma and MM, including new and previously reported melanoma genes. Furthermore, we demonstrate that transcription factor HMGA2 is highly overexpressed in melanoma, is strongly associated with regional and distant metastases, and serves as an independent predictor of disease-free survival (DFS) and OS in melanoma.
RESULTS
Sample characteristics for expression microarray and analysis of BRAF/NRAS mutations
Frozen samples were collected from 421 patients out of 7,959 patients treated at the University of Michigan Multidisciplinary Melanoma Clinic. Samples with sufficiently large lesions were selected for the study (n=129); this includes frozen tissue from 67 primary melanomas, 20 MM, and 42 N samples collected from 102 patients. There were 27 paired samples from the same excision (primary melanoma-N), and two patients had two distinct primary melanomas. Clinicopathological characteristics of those samples with microarray data that passed quality control (46 primary melanomas, 12 MM, and 16 N samples) were not significantly different from collected samples (Table 1). Median follow-up was 4 years in primary melanomas.
Table 1.
Clinicopathological characteristics of study
| Characteristics1 | Discovery set (n =74) | Replication set (n =330) | P-value |
|---|---|---|---|
| Normal tissue (n) | 16 | — | |
| Primary melanoma (not metastatic) (n) | 46 | 330 | |
| Melanoma metastases (n) | 12 | — | |
| Mean age, years (range)2 | 59.4 (20–92) | 54.5 (48–87) | 0.0072 |
| Median Breslow thickness, mm (range) | 3 (0.6–19) | 2.45 (0.25–16.3) | 0.5898 |
| Sex, no. (%) | 0.0916 | ||
| Male | 33 (72%) | 183 (56%) | |
| Female | 13 (28%) | 147 (44%) | |
| Site, no. (%) | 0.2810 | ||
| Head and neck | 10 (22%) | 35 (14%) | |
| Trunk | 12 (26%) | 81 (32%) | |
| Legs | 19 (41%) | 79 (32%) | |
| Arms | 10 (22%) | 48 (19.2%) | |
| Others3 | 0 | 7 (3%) | |
| Not available | 0 | 80 | |
| Melanoma type, no. (%) | 0.5897 | ||
| Superficial spreading melanoma | 28 (61%) | 88 (64%) | |
| Nodular melanoma | 8 (17%) | 26 (19%) | |
| Acrolentiginous melanoma | 3 (7%) | 7 (5%) | |
| Lentigo maligna melanoma | 1 (2%) | 2 (2%) | |
| Desmoplastic melanoma | 1 (2%) | 1 (1%) | |
| Nevoid melanoma | 0 | 1 (1%) | |
| MIS | 2 (4%) | 0 | |
| Unclassified | 0 | 0 | |
| Others4 | 3 (7%) | 12 (9%) | |
| Not available | 0 | 193 | |
| AJCC stage at diagnosis | 0.2390 | ||
| 0 | 2 (4%) | 0 | |
| I | 10 (22%) | 191 (69%) | |
| II | 16 (36%) | 11 (4%) | |
| III | 18 (40%) | 60 (22%) | |
| IV | 0 | 14 (5%) | |
| Not available | 0 | 54 | |
| Ulceration, no. (%) | 0.2516 | ||
| Absent | 28 (61%) | 83 (61%) | |
| Present | 18 (39%) | 54 (39%) | |
| Not available | 0 | 193 | |
| Mitotic rate, no. per mm2 | — | ||
| 0 | 13 (28%) | — | |
| 1–6 | 19 (41%) | — | |
| >6 | 13 (31%) | — | |
| Not available | 0 | 330 | |
| Median follow-up, days | 875 | 1,602 | <0.0001 |
Abbreviation: AJCC, American Joint Committee on Cancer.
The characteristics are presented for primary melanomas only.
Age of the patients calculated at the first biopsy.
Oral, male genital, and female vaginal location.
Polypoid, mucosal melanoma, spindle-cell melanoma, Spitz-type melanoma, and amelanotic melanoma.
Primary melanomas (n=46) had 20 BRAF-mutated samples (L597R (n=1), L597S (n=1), V600R (n=1), V600K (n=2), and V600E (n=15)), 6 NRAS-mutated samples (Q61K (n=1), Q61R (n=3), Q61L (n=2)), and 20 samples without mutations in BRAF or NRAS (Figure 1a). Among MM (n=12), eight had mutations in BRAF (V600K (n=1) and V600E (n=7)), two had NRAS mutations (Q61K (n=2)), and two samples had no mutations in BRAF/NRAS. BRAF and NRAS mutations were mutually exclusive in all samples. The observed frequency of BRAF/NRAS mutations in primary melanoma (56%) and MM (83%) is consistent with a previous report (Poynter et al., 2006). Mean mutant to wild-type signal ratio was 1.91 for BRAF and 1.05 for NRAS mutations demonstrating a low level of sample contamination (Supplementary Table S2 online).
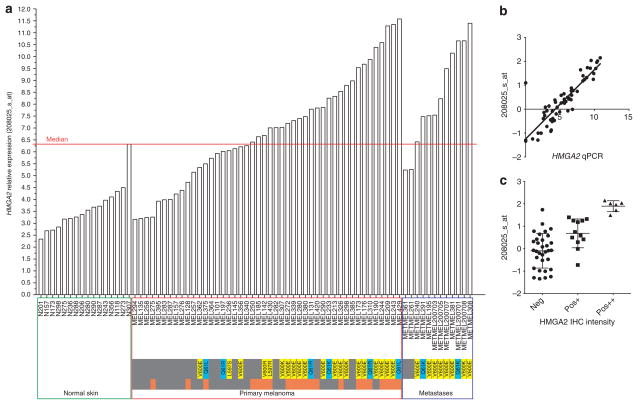
Figure 1. Expression of HMGA2 in normal skin, primary melanoma, and melanoma metastases.
(a) Overexpression of HMGA2 probe 208025_s_at was considered above the median (6.29). Orange–gray bar represents primary melanomas that metastasized later (orange). BRAF mutations are shown in yellow and NRAS mutants are showed in blue bars. (b) Pearson correlation between microarray and quantitative PCR HMGA2 expression data (0.84, P<0.0001). (c) Correlation between HMGA2 expression data from microarray and nuclear immunohistochemistry (IHC) intensity staining against HMGA2 (neg, negative staining; pos+, moderate intensity; pos+ +, strong intensity).
Gene expression differences between primary melanoma, MM, and N
Principal component analysis showed that, although the N and metastases could be distinguished by expression profiles, the expression levels of primary melanomas were heterogenous, and principal components were spread across the data matrix (Supplementary Figure S1 online). We effectively separated N and melanoma using gene expression analysis (Supplementary Figure S2 online). Three melanomas in situ clustered with N.
Differentially expressed genes were identified in four comparisons: primary cutaneous and MM versus N (PCM+MM vs. N); PCM versus N; MM versus N; and MM versus PCM (Supplementary Table S1 online). Compared with N, upregulated gene lists in both PCM and MM were significantly (P-value <1.33E–06) enriched with genes related to nervous system development. The list of genes upregulated in PCM, unlike MM, was significantly (P-value <0.0023) enriched with melanocyte development genes. Upregulated genes in MM related to mitosis, cell division, and cell cycle (P-value <2.53E–09); cell adhesion (P-value <4.36E–07); immune response (P-value <4.29E–06); and cell motility and migration (P-value <0.0125). In MM versus PCM, upregulated genes were associated with transcription and gene expression (P-value <1.71E–04). In all comparisons, downregulated genes were (P-value <4.09E–06) enriched with epidermis development, keratinization, and cell adhesion genes (P-value <3.03E–04).
In PCM versus N, we found upregulated melanoma stem cell markers (ABCB5 and ABCC2), melanoma-associated tumor antigens (MAGEA2, PRAME, ARMC9, and SLC45A2), melanoma progression genes (MIA, GDF15, SPP1, KMO, and S100B), melanocyte development and melanin biosynthesis genes (CITED1, SILV, MLANA, TYR, MITF, PAX3, and TFEC), cell cycle progression genes (S100A1), apoptotic genes (BCL2A1, FAIM3), oncogenic transcription factors (HMGA2, GDF15, HOXD13, and LZTS1), and cell adhesion gene (NRP2). Obviously, the genes related to keratinocyte differentiation (KRT25, KRT71, and KRT85) appeared downregulated in PCM as compared with N. MM versus N comparison showed upregulated melanoma-associated tumor antigens (AKT3 and ARMC9), melanoma progression genes (MIA, SPP1, KMO, and IL8), melanocyte development and melanin biosynthesis genes (CITED1 and PAX3), cell cycle progression genes (BUB1), apoptotic genes (BCL2A1), oncogenic transcription factors (HMGA2, HOXD13, ETV1, and ETV5), and cell adhesion genes (ADAM10 and NRP2). As expected, downregulated genes in MM versus N were involved in keratinocyte differentiation and epidermal development (keratins, sciellin (SCEL), and cystatin (CST6)). Some cell adhesion genes (DSC3, DSG1, and LY6D), tumor suppressors (kallikreins and CLCA2), and cell cycle and apoptosis-regulating genes (FGFR2, BNIPL, and TNS4) were downregulated in MM. Several transcription factors, including ETV1, and a melanoma-associated antigen SPP1 were upregulated in MM as compared with PCM.
To identify new melanoma-associated genes, we focused on the top tenth percentile of upregulated genes with the highest fold change (FC>2.68) in PCM+MM versus N (Supplementary Table S2 online). Within this group, we observed previously characterized melanoma-specific markers (MAGEA3, PRAME, SLC45A2, and SPP1), anti-apoptotic BCL2A1, actin regulator PHACTR1, SPRYD5 involved in cell growth and differentiation, TUBB4 involved in cell migration, and finally the transcription factor HMGA2, which was the only gene associated with melanoma survival as described below.
HMGA2 expression in N, primary melanoma, and MM
Analysis of relative expression of HMGA2 in the microarray demonstrated overexpression (higher than median=6.29) in 57% of primary melanomas and 83% of MM compared with N (Figure 1a). Expression of HMGA2 in N was lower than median in 94% of samples. One N sample (N307) that had higher than median expression of HMGA2 was also clustered with melanoma in the supervised cluster analysis. It is important to note that the HMGA2 expression in N sample N307 was similar to the expression in MEL307 melanoma sample from the same patient.
In the discovery set, we validated the microarray HMGA2 expression using quantitative real-time reverse transcriptase–PCR (RT-PCR) (Figure 1b). Pearson’s correlation coefficient was 0.84 between the expression of HMGA2 microarray probe 208025_s_at and HMGA2 expression from real-time RT-PCR (P<0.0001). To ensure real-time RT-PCR validity, we also ran RT-PCR for SPP1, the well-known melanoma-specific marker (Pearson’s coefficient for 1568574_x_at and SPP1 in RT-PCR was 0.84 (P<0.0001)).
Continuous HMGA2 expression in the microarray was significantly associated with BRAF/NRAS mutations in PCM and MM (odds ratio (OR)=3.4, 95% confidence interval (CI)=1.2–9.3, P=0.017). We confirmed this association in RT-PCR (OR=2.38, 95% CI=1.3–4.2, P=0.003) and validated it using melanoma cell lines with known BRAF/NRAS mutations. Melanoma cell lines A2058 and SK-MEL-31, heterozygous for BRAF*V600E, and A375 cell lines, homozygous for BRAF*V600E, showed overexpressed HMGA2 comparable to melanoma samples. The CHL-1 without BRAF and NRAS mutations had no detectable expression of HMGA2 (Supplementary Figure S3 online).
We also found association of continuous HMGA2 expression with sentinel lymph node status (OR=2.7, 95% CI=1.1–6.5, P=0.034).
Immunohistochemical staining of HMGA2 in N, benign nevi, and melanoma
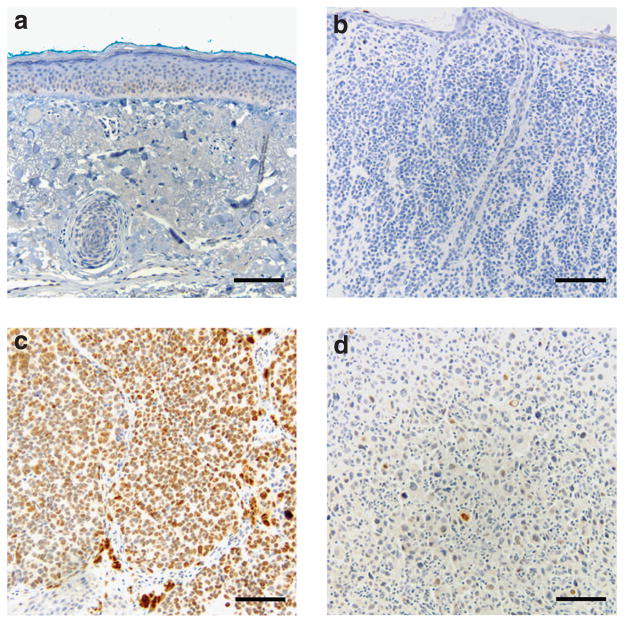
We validated results of HMGA2 expression at the protein level using immunohistochemical staining on 9 N samples, 41 melanoma samples, and 7 MM that were used for the expression microarray (Figures 1c and 2). All N samples demonstrated no nuclear staining. Melanomas with overexpressed HMGA2 had strong nuclear staining. Melanomas with moderate HMGA2 expression were heterogenous, possessing a variable number of cells with moderate nuclear staining. Six out of seven MM had nuclear HMGA2 staining. Twenty benign nevi showed no nuclear staining against HMGA2.
Figure 2. Representative sections with immunohistochemical staining against HMGA2.
(a, b) Normal skin and benign nevus have no nuclear staining, (c, d) whereas both primary melanoma (diffuse) and melanoma metastases (patchy) have strong nuclear staining against HMGA2. Bar=50 μm.
Effect of HMGA2 expression on survival end points in melanoma
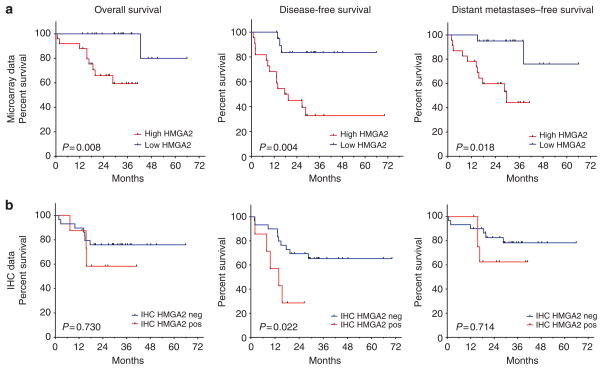
To evaluate the correlation of HMGA2 overexpression with survival of melanoma patients, we categorized expression levels of HMGA2 as high (above the median 6.29) and low (below the median). Multivariate analysis demonstrated a significant association of HMGA2 overexpression (≥6.29) with regional (OR=10.0, 95% CI=1.5–66.6, P=0.0176) and distant metastases (OR=6.8, 95% CI=1.2–39.0, P=0.0318) after adjusting for American Joint Committee on Cancer (AJCC) stage and age at diagnosis. Survival analysis showed that expression of HMGA2 above the median was significantly associated with DFS (hazard ratio (HR)=6.3, 95% CI=1.8–22.3, P=0.004), OS (stratified log-rank P=0.008), and distant metastases–free survival (DMFS) (HR=6.4, 95% CI=1.4–29.7, P=0.018) after adjusting for AJCC stage and age at diagnosis (Figure 3a). Immunohistochemical staining against HMGA2 was also associated with DFS after adjusting for AJCC stage and age at diagnosis (HR=4.8, 95% CI=1.25–18.1, P=0.0221) (Figure 3b). Leave-five-out cross-validation confirmed these results, with a mean HR of 6.76 for DFS (99.9% of resamples with P<0.05) and a mean HR of 7.12 for DMFS across the 1,000 replicates (90.8% of resamples with P<0.05).
Figure 3. Kaplan–Meier curves for overall, disease-free, and distant metastases–free survival associated with HMGA2 expression in 46 primary melanomas.
Cox proportional hazard regression adjusted for American Joint Committee on Cancer stage and age at diagnosis was used for analysis, except for overall survival from microarray data, where stratified log-rank test was used because of sparsity of events. (a) In microarray expression data, HMGA2 overexpression was significantly associated with overall survival (P=0.008), disease-free survival (hazard ratio (HR)=6.29, P=0.004), and distant metastases–free survival (HR=6.41, P=0.018). (b) In immunohistochemistry (IHC) expression data, HMGA2 overexpression was significantly associated with disease-free survival (HR=4.76, P=0.022) and showed the same direction of association for overall and distant metastases-free survival as microarray data, although not statistically significant.
HMGA2 expression and melanoma survival in an independent melanoma replication set
An independent replication tissue microarray (TMA) comprising 580 melanomas (Gould Rothberg et al., 2009a) was used to replicate the association of HMGA2 expression with survival. Only OS was analyzed because data regarding local recurrence and regional metastases were not available. HMGA2 expression was evaluated using AQUA scores. Although 506 passed quality control, survival data were available for only 330 melanomas.
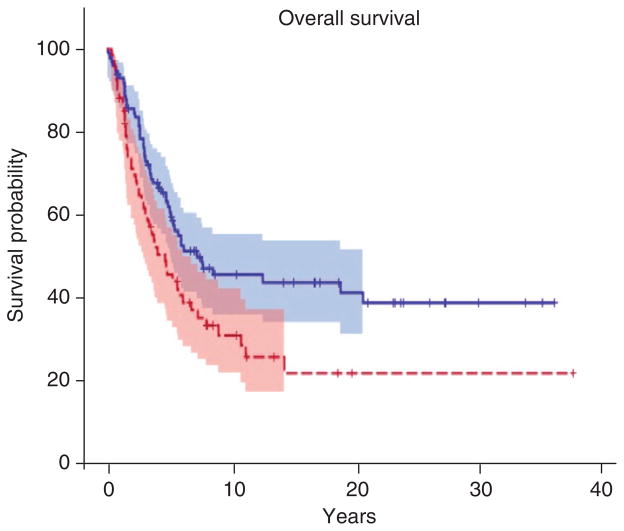
To estimate OS in the replication TMA, we compared survival between melanomas with 75% quartile of HMGA2 expression measured by AQUA to 25% quartile. We found significant association of HMGA2 overexpression with reduced OS of melanoma patients after adjustment for AJCC stage and age at diagnosis (HR=1.72, 95% CI=1.09–2.73, P=0.0211) (Figure 4).
Figure 4. Kaplan–Meier curves for overall survival associated with the expression of HMGA2 protein in an independent set of 330 melanomas.
HMGA2 expression was studied in melanoma tissue microarray (TMA) and analyzed by AQUA. HMGA2 expression was compared between 75% quartile (red) and in 25% quartile (blue). Overall survival was analyzed using stratified multivariate log-rank test including age at diagnosis and American Joint Committee on Cancer stage. HMGA2 overexpression was significantly associated with reduced overall survival of melanoma patients (hazard ratio=1.72, P=0.0211).
DISCUSSION
Our study used fresh frozen tissue from patients with newly diagnosed melanoma to compare gene expression and identify new melanoma-associated genes. Some of the previous melanoma expression studies used tissue samples (Haqq et al., 2005; Talantov et al., 2005; Mandruzzato et al., 2006; Winnepenninckx et al., 2006; Conway et al., 2009), but the majority of studies used cell lines. Although fresh tissue analysis is a strength of our study, relatively small sample size and a large number of melanomas with Breslow depth >2mm limit generalization of our findings. We tried to address this issue by replicating our results in an independent melanoma set.
Gene lists generated by our study include both well-known melanoma genes and novel genes that have not previously been reported in relation to melanoma development. One of the new genes is a transcription factor HMGA2 that was significantly upregulated in both primary melanoma and MM (P-values=1.2 × 10−7 and 9 × 10−5) compared with N. Moreover, HMGA2 overexpression in primary melanoma was significantly associated with survival of melanoma patients, probably explained by the increased metastatic potential of HMGA2-expressing tumors. The oncogene HMGA2 is an embryonic architectural transcription factor that is completely silenced or undetectable in normal adult tissues, but has a significant role in the transformation of many cancer types (Miyazawa et al., 2004; Meyer et al., 2007; Malek et al., 2008). HMGA2 binds to the minor groove of DNA in AT-rich regions using AT-hook sequences, changes DNA conformation, and facilitates binding of other transcription factors (Reeves, 2001). The oncogenic role of HMGA2 has not been previously recognized in the pathogenesis of melanoma. However, HMGA2 has been well documented in other types of cancer, where it can be overexpressed, amplified, or fused with other proteins (Fusco and Fedele, 2007).
A large study examining gene expression in 83 PCMs identified a 254-gene prognostic signature, but HMGA2 was not among these genes (Winnepenninckx et al., 2006). Re-examination of the data shows that HMGA2 was indeed associated with melanoma thickness, supporting our conclusion about biological relevance of HMGA2 in melanoma. Another study of nine melanoma cell lines demonstrated 2.96-fold overexpression of HMGA2 compared with normal human melanocytes (Hoek et al., 2004). Contributing to previously published transcriptome studies of melanoma, our results offer new insights into the pathogenesis of melanoma by highlighting the differential expression and prognostic importance of HMGA2.
Several mechanisms may underlie the oncogenicity of HMGA2, including the activation of transcription factor E2F1 through binding of HMGA2 to pRB (Fedele et al., 2006), direct or indirect induction of cyclin A (Pagano et al., 1992), or negative regulation of nucleotide excision repair genes (Borrmann et al., 2003). The chemokine CXCL1, which is overexpressed in melanoma and is involved in melanoma progression, has been shown to be regulated by HMGA2 (Nirodi et al., 2001). It is noteworthy that both CXCL1 and HMGA2 were significantly overexpressed in melanoma in our study. TGF-beta mediates epithelial–mesenchymal transition by inducing HMGA2 through the SMAD pathway, which may partially explain the association between HMGA2 overexpression and MM in our study (Thuault et al., 2006). HMGA2 also enhances the NF-kB complex formation (Noro et al., 2003).
HMGA2 expression is negatively regulated by the miRNA let-7 family (Peng et al., 2008). Loss of expression of let-7 increases the expression of c-Myc, RAS, CDK4, integrin-β(3), and HMGA2 (Johnson et al., 2005; Park et al., 2007; Muller and Bosserhoff, 2008; Schultz et al., 2008). Clusters of let-7a-1_let-7f-1_let-7d are located in 9p22.3, which is deleted in 81% of cases of cutaneous melanoma (Bastian et al., 1998). The MAPK pathway, activated by BRAF/NRAS mutations, negatively regulates let-7 by inducing LIN28 expression through Myc transcription (Dangi-Garimella et al., 2009). This mechanism may explain the association of HMGA2 overexpression with BRAF/NRAS mutations in our study. A recent study showed that let-7 is downregulated in highly invasive melanoma cell lines (Mueller et al., 2009).
Activated oncogenes in normal cells trigger senescence as a key protective mechanism against cancer (Mooi and Peeper, 2006). For example, INK4/ARF products are essential activators of p53-dependent and p53-independent melanocyte senescence (Bennett and Medrano, 2002). In addition, oncogenic BRAF and NRAS can induce p16 expression and senescence in melanocytes in vitro (Gray-Schopfer et al., 2006). During melanoma progression, c-Myc overexpression continuously suppresses BRAF*V600E- and NRAS*Q61R-dependent senescence programs, independently of p16 and p53 senescence mechanisms (Zhuang et al., 2008).
HMGA2 is also required for normal proliferation and self-renewal of fetal and young adult neural stem cells through repression of the INK4/ARF locus; however, during aging, let7b blocks HMGA2 and contributes to declining neural stem cell function (Tzatsos and Bardeesy, 2008). We speculate that BRAF- and NRAS-activated senescence is overridden by both p16- and p53-independent c-Myc overexpression and by HMGA2 repression of p16- and p53-induced apoptosis and cell senescence. It is noteworthy that HMGA2 is directly regulated by c-Myc (Wood et al., 2000).
Overexpressed HMGA2 correlates with amplification of 12q harboring HMGA2 in gastric cancer, liposarcoma, and carcinoma ex pleomorphic adenoma (Yang et al., 2007; Persson et al., 2009). This correlation was associated with copy number variability in other tumor suppressor genes and oncogenes (MDM2 and CDK4), showing a specific pattern in tumor subtypes.
HMGA2 analysis may have some important clinical applications. Observational noninvasive clinical trials are needed to further estimate the prognostic value of HMGA2 expression in melanoma. Although immunohistochemistry and quantitative PCR are still the methods of choice, recent improvements in next-generation sequencing technology may introduce targeted RNAseq for routine testing of HMGA2 expression in melanoma. The melanoma patients with overexpressed HMGA2 might benefit from more aggressive treatment and closer follow-up. In addition, HMGA2 may be a potential therapeutic target, as HMGA2 expression seems to be non-essential for normal cell survival, and therapeutic gene silencing would be relatively safe. This approach has been successful in ovarian carcinoma, where short-hairpin RNA silencing of HMGA2 inhibited cell proliferation, with G1 cell cycle arrest and increased apoptosis in vitro, as well as tumor growth inhibition in vivo (Malek et al., 2008).
Our study highlights a new aspect of melanoma biology by implicating the well-known oncogene HMGA2 in melanoma progression. We propose that HMGA2 has an important role in melanoma development and serves as an independent prognostic factor. Further studies of HMGA2 in melanoma are warranted to better understand its role in melanoma development and progression.
MATERIALS AND METHODS
Tissue samples
Our report was written to comply with REMARK criteria (McShane et al., 2005; Gould Rothberg et al., 2009b); therefore, we provide relevant information about study design, hypotheses, patient and specimen characteristics, assay methods, and statistical analysis methods. In addition, we conducted a replication study.
Frozen cutaneous melanomas were obtained from the University of Michigan Multidisciplinary Melanoma Clinic where 7,959 patients were treated from 2002 through 2008. The study has been approved by the institutional review board. Samples were selected from 421 frozen primary or metastatic samples including pediatric and non-Caucasian cases consecutively collected during this period. All participating patients received standard-of-care treatment with surgical excision of lesions. OS, DFS, and DMFS data were collected from chart reviews, University of Michigan Cancer Tumor Registry, and by communication with the patient or his/her family. OS was confirmed using the National Death Index.
Detailed description of sample preparation, isolation of RNA, DNA and microarray hybridization, as well as mutational analysis, quantitative RT-PCR, and immunohistochemistry, can be found in Supplementary Methods online.
For independent replication of survival analysis, we obtained well-annotated melanoma TMA slides from Dr David L Rimm (Yale University) comprising 580 primary melanomas and MM. The available de-identified data included clinicopathological features, OS, and demographic characteristics.
Statistical analysis
Microarray expression data were analyzed using Bioconductor for R (Gentleman et al., 2004). Detailed description of statistical analysis of the expression microarray can be found in Supplementary Materials online.
Kaplan–Meier survival curves and log-rank tests were used to analyze OS, DFS (without local recurrence, regional, or distant metastases), and DMFS. Multivariate Cox proportional hazard regression model was adjusted for AJCC stage (stages I/ II vs. stage III) and age at diagnosis. Wald tests based on maximum likelihood estimates of the log-HR parameters in the Cox model were deemed significant at P<0.05. OS was evaluated by the stratified log-rank test adjusted for AJCC stage and age at diagnosis because of sparsity of events. Leave-five-out cross-validation (1,000 random permutations of 46 melanomas) evaluated the consistency and robustness of the survival analysis. We use multivariate logistic regression to estimate the association between HMGA2 expression and metastases, and linear regression for the association between HMGA2 expression and BRAF/NRAS mutations. All analyses were performed in SAS 9.1 (SAS Institute, Cary, NC) using FREQ, LIFETEST, PHREG, and LOGISTIC.
Supplementary Material
Acknowledgments
We thank Dr David L Rimm for melanoma tissue microarray slides provided for the replication study, and Nisha Meireles, Clinical Trials Research Associate, for tissue procurement and data management. We also thank Michelle Vinco and Donita Sanders for help in sample preparation. Dr Leon Raskin had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This work was supported by the University of Michigan Center for Genetics in Health and Medicine, the Lewis and Lillian Becker Fund, the Cooper Fund, the UM Cancer Center Core Grant (5P30CA46592), the Genes, Environment, and Melanoma Study NCI-CA83180, and R01CA112243, and the USC Norris Comprehensive Cancer Center Support Grant P30CA014089.
Abbreviations
- CI
confidence interval
- DFS
disease-free survival
- DMFS
distant metastases–free survival
- HR
hazard ratio
- MM
melanoma metastases
- N
normal skin
- OR
odds ratio
- OS
overall survival
- PCM
primary cutaneous melanoma
- REMARK
reporting recommendations for tumor marker prognostic studies
- RT-PCR
reverse transcriptase–PCR
- TMA
tissue microarray
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
References
- Bastian BC, LeBoit PE, Hamm H, et al. Chromosomal gains and losses in primary cutaneous melanomas detected by comparative genomic hybridization. Cancer Res. 1998;58:2170–5. [PubMed] [Google Scholar]
- Bennett DC, Medrano EE. Molecular regulation of melanocyte senescence. Pigment Cell Res. 2002;15:242–50. doi: 10.1034/j.1600-0749.2002.02036.x. [DOI] [PubMed] [Google Scholar]
- Bittner M, Meltzer P, Chen Y, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–40. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- Bloethner S, Chen B, Hemminki K, et al. Effect of common B-RAF and N-RAS mutations on global gene expression in melanoma cell lines. Carcinogenesis. 2005;26:1224–32. doi: 10.1093/carcin/bgi066. [DOI] [PubMed] [Google Scholar]
- Borrmann L, Schwanbeck R, Heyduk T, et al. High mobility group A2 protein and its derivatives bind a specific region of the promoter of DNA repair gene ERCC1 and modulate its activity. Nucleic Acids Res. 2003;31:6841–51. doi: 10.1093/nar/gkg884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway C, Mitra A, Jewell R, et al. Gene expression profiling of paraffin-embedded primary melanoma using the DASL assay identifies increased osteopontin expression as predictive of reduced relapse-free survival. Clin Cancer Res. 2009;15:6939–46. doi: 10.1158/1078-0432.CCR-09-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curti BD, Urba WJ. Integrating new therapies in the treatment of advanced melanoma. Curr Treat Options Oncol. 2012;13:327–39. doi: 10.1007/s11864-012-0201-9. [DOI] [PubMed] [Google Scholar]
- Dangi-Garimella S, Yun J, Eves EM, et al. Raf kinase inhibitory protein suppresses a metastasis signalling cascade involving LIN28 and let-7. EMBO J. 2009;28:347–58. doi: 10.1038/emboj.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit NJ, Rijntjes J, Diepstra JH, et al. Analysis of differential gene expression in human melanocytic tumour lesions by custom made oligonucleotide arrays. Br J Cancer. 2005;92:2249–61. doi: 10.1038/sj.bjc.6602612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedele M, Visone R, De Martino I, et al. HMGA2 induces pituitary tumorigenesis by enhancing E2F1 activity. Cancer Cell. 2006;9:459–71. doi: 10.1016/j.ccr.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Fusco A, Fedele M. Roles of HMGA proteins in cancer. Nat Rev. 2007;7:899–910. doi: 10.1038/nrc2271. [DOI] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould Rothberg BE, Berger AJ, Molinaro AM, et al. Melanoma prognostic model using tissue microarrays and genetic algorithms. J Clin Oncol. 2009a;27:5772–80. doi: 10.1200/JCO.2009.22.8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould Rothberg BE, Bracken MB, Rimm DL. Tissue biomarkers for prognosis in cutaneous melanoma: a systematic review and metaanalysis. J Natl Cancer Inst. 2009b;101:452–74. doi: 10.1093/jnci/djp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–7. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- Gray-Schopfer VC, Cheong SC, Chong H, et al. Cellular senescence in naevi and immortalisation in melanoma: a role for p16? Br J Cancer. 2006;95:496–505. doi: 10.1038/sj.bjc.6603283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haqq C, Nosrati M, Sudilovsky D, et al. The gene expression signatures of melanoma progression. Proc Natl Acad Sci USA. 2005;102:6092–7. doi: 10.1073/pnas.0501564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat MJ, Howlader N, Reichman ME, et al. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12:20–37. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- Hoek K, Rimm DL, Williams KR, et al. Expression profiling reveals novel pathways in the transformation of melanocytes to melanomas. Cancer Res. 2004;64:5270–82. doi: 10.1158/0008-5472.CAN-04-0731. [DOI] [PubMed] [Google Scholar]
- Hoek KS. DNA microarray analyses of melanoma gene expression: a decade in the mines. Pigment Cell Res. 2007;20:466–84. doi: 10.1111/j.1600-0749.2007.00412.x. [DOI] [PubMed] [Google Scholar]
- Jaeger J, Koczan D, Thiesen HJ, et al. Gene expression signatures for tumor progression, tumor subtype, and tumor thickness in laser-micro-dissected melanoma tissues. Clin Cancer Res. 2007;13:806–15. doi: 10.1158/1078-0432.CCR-06-1820. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Grosshans H, Shingara J, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–47. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Kauffmann A, Rosselli F, Lazar V, et al. High expression of DNA repair pathways is associated with metastasis in melanoma patients. Oncogene. 2008;27:565–73. doi: 10.1038/sj.onc.1210700. [DOI] [PubMed] [Google Scholar]
- Malek A, Bakhidze E, Noske A, et al. HMGA2 gene is a promising target for ovarian cancer silencing therapy. Int J Cancer. 2008;123:348–56. doi: 10.1002/ijc.23491. [DOI] [PubMed] [Google Scholar]
- Mandruzzato S, Callegaro A, Turcatel G, et al. A gene expression signature associated with survival in metastatic melanoma. J Translational Med. 2006;4:50. doi: 10.1186/1479-5876-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97:1180–4. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- Meyer B, Loeschke S, Schultze A, et al. HMGA2 overexpression in non-small cell lung cancer. Mol Carcinog. 2007;46:503–11. doi: 10.1002/mc.20235. [DOI] [PubMed] [Google Scholar]
- Miyazawa J, Mitoro A, Kawashiri S, et al. Expression of mesenchyme-specific gene HMGA2 in squamous cell carcinomas of the oral cavity. Cancer Res. 2004;64:2024–9. doi: 10.1158/0008-5472.can-03-1855. [DOI] [PubMed] [Google Scholar]
- Mooi WJ, Peeper DS. Oncogene-induced cell senescence–halting on the road to cancer. N Engl J Med. 2006;355:1037–46. doi: 10.1056/NEJMra062285. [DOI] [PubMed] [Google Scholar]
- Mueller DW, Rehli M, Bosserhoff AK. miRNA expression profiling in melanocytes and melanoma cell lines reveals miRNAs associated with formation and progression of malignant melanoma. J Invest Dermatol. 2009;129:1740–51. doi: 10.1038/jid.2008.452. [DOI] [PubMed] [Google Scholar]
- Muller DW, Bosserhoff AK. Integrin beta 3 expression is regulated by let-7a miRNA in malignant melanoma. Oncogene. 2008;27:6698–706. doi: 10.1038/onc.2008.282. [DOI] [PubMed] [Google Scholar]
- Nikolaou VA, Stratigos AJ, Flaherty KT, et al. Melanoma: new insights and new therapies. J Invest Dermatol. 2012;132:854–63. doi: 10.1038/jid.2011.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirodi C, NagDas S, Gygi SP, et al. A role for poly(ADP-ribose) polymerase in the transcriptional regulation of the melanoma growth stimulatory activity (CXCL1) gene expression. J Biol Chem. 2001;276:9366–74. doi: 10.1074/jbc.M009897200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noro B, Licheri B, Sgarra R, et al. Molecular dissection of the architectural transcription factor HMGA2. Biochemistry. 2003;42:4569–77. doi: 10.1021/bi026605k. [DOI] [PubMed] [Google Scholar]
- Pagano M, Pepperkok R, Verde F, et al. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992;11:961–71. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SM, Shell S, Radjabi AR, et al. Let-7 prevents early cancer progression by suppressing expression of the embryonic gene HMGA2. Cell Cycle (Georgetown, Tex) 2007;6:2585–90. doi: 10.4161/cc.6.21.4845. [DOI] [PubMed] [Google Scholar]
- Pavey S, Johansson P, Packer L, et al. Microarray expression profiling in melanoma reveals a BRAF mutation signature. Oncogene. 2004;23:4060–7. doi: 10.1038/sj.onc.1207563. [DOI] [PubMed] [Google Scholar]
- Peng Y, Laser J, Shi G, et al. Antiproliferative effects by Let-7 repression of high-mobility group A2 in uterine leiomyoma. Mol Cancer Res. 2008;6:663–73. doi: 10.1158/1541-7786.MCR-07-0370. [DOI] [PubMed] [Google Scholar]
- Persson F, Andren Y, Winnes M, et al. High-resolution genomic profiling of adenomas and carcinomas of the salivary glands reveals amplification, rearrangement, and fusion of HMGA2. Genes Chromosomes Cancer. 2009;48:69–82. doi: 10.1002/gcc.20619. [DOI] [PubMed] [Google Scholar]
- Poynter JN, Elder JT, Fullen DR, et al. BRAF and NRAS mutations in melanoma and melanocytic nevi. Melanoma Res. 2006;16:267–73. doi: 10.1097/01.cmr.0000222600.73179.f3. [DOI] [PubMed] [Google Scholar]
- Reeves R. Molecular biology of HMGA proteins: hubs of nuclear function. Gene. 2001;277:63–81. doi: 10.1016/s0378-1119(01)00689-8. [DOI] [PubMed] [Google Scholar]
- Riker AI, Enkemann SA, Fodstad O, et al. The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Med Genomics. 2008;1:13. doi: 10.1186/1755-8794-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm SJ, Campain AE, Scolyer RA, et al. Review and cross-validation of gene expression signatures and melanoma prognosis. J Invest Dermatol. 2012;132:274–83. doi: 10.1038/jid.2011.305. [DOI] [PubMed] [Google Scholar]
- Schultz J, Lorenz P, Gross G, et al. MicroRNA let-7b targets important cell cycle molecules in malignant melanoma cells and interferes with anchorage-independent growth. Cell Res. 2008;18:549–57. doi: 10.1038/cr.2008.45. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Talantov D, Mazumder A, Yu JX, et al. Novel genes associated with malignant melanoma but not benign melanocytic lesions. Clin Cancer Res. 2005;11:7234–42. doi: 10.1158/1078-0432.CCR-05-0683. [DOI] [PubMed] [Google Scholar]
- Thuault S, Valcourt U, Petersen M, et al. Transforming growth factor-beta employs HMGA2 to elicit epithelial-mesenchymal transition. J Cell Biol. 2006;174:175–83. doi: 10.1083/jcb.200512110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzatsos A, Bardeesy N. Ink4a/Arf regulation by let-7b and Hmga2: a genetic pathway governing stem cell aging. Cell Stem Cell. 2008;3:469–70. doi: 10.1016/j.stem.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Winnepenninckx V, Lazar V, Michiels S, et al. Gene expression profiling of primary cutaneous melanoma and clinical outcome. J Natl Cancer Inst. 2006;98:472–82. doi: 10.1093/jnci/djj103. [DOI] [PubMed] [Google Scholar]
- Wood LJ, Mukherjee M, Dolde CE, et al. HMG-I/Y, a new c-Myc target gene and potential oncogene. Mol Cell Biol. 2000;20:5490–502. doi: 10.1128/mcb.20.15.5490-5502.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman SE, Lazar AJ, Aldape KD, et al. New strategies in melanoma: molecular testing in advanced disease. Clin Cancer Res. 2012;18:1195–200. doi: 10.1158/1078-0432.CCR-11-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Jeung HC, Jeong HJ, et al. Identification of genes with correlated patterns of variations in DNA copy number and gene expression level in gastric cancer. Genomics. 2007;89:451–9. doi: 10.1016/j.ygeno.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Zhuang D, Mannava S, Grachtchouk V, et al. C-MYC overexpression is required for continuous suppression of oncogene-induced senescence in melanoma cells. Oncogene. 2008;27:6623–34. doi: 10.1038/onc.2008.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.