Abstract
Background
Neovascular age-related macular degeneration (AMD) is associated with rapid vision loss due to choroidal neovascularization (CNV), leakage, and scarring. Steroids have gained attention in their role for the treatment of neovascular AMD for their antiangiogenic and anti-inflammatory properties.
Objectives
This review aims to examine effects of steroids with antiangiogenic properties in the treatment of neovascular AMD.
Search strategy
We searched for trials in CENTRAL, MEDLINE, EMBASE, and LILACS on 2 October 2006.
Selection criteria
We included randomised controlled clinical trials of intra- and peri-ocular steroids in people diagnosed with neovascular AMD.
Data collection and analysis
Review authors extracted the data and assessed trial quality independently. We did not pool data since the included studies evaluated difference comparisons.
Main results
We report the risk of losing three or more lines vision at 12 months - “vision loss”. One trial (139 people randomized) reported that a single dose of intravitreal triamcinolone (n = 75) (4 mg) had no significant effect on the risk of vision loss compared to placebo (n = 76). (Risk ratio vision loss 0.97, 95% confidence interval (CI) 0.74 to 1.26). Eyes treated with triamcinolone were more likely to develop cataracts and experience increased intraocular pressure (IOP) compared to untreated eyes. One trial (128 people randomized) reported the effects of anecortave acetate (3 mg (n = 32), 15 mg (n = 33) or 30 mg (n = 33) single dose with retreatment every six months if indicated) compared to placebo (n = 30). Risk ratio vision loss 0.80 (95% CI 0.45 to 1.45) in the 3 mg group, 0.45 (95% CI 0.21 to 0.97) in the 15 mg group and 0.91 (95% CI 0.52 to 1.58) in the 30 mg group. Side effects were similar in all treatment groups with the anecortave group having a slightly higher incidence of foreign body sensation compared to placebo. There was a high loss to follow-up. The final analysis may have been subject to selection bias as participants who were not selected for retreatment, possibly with worsening disease, were excluded. There was also a possibility of type I error due to multiple statistical comparisons. The sample size was estimated on the basis of a single 2-way comparison but three 2-way comparisons were analysed and presented. One trial reported that anecortave acetate (n = 263) (15 mg administered at beginning of study and six months) gave similar results to photodynamic therapy (n = 267) (risk ratio vision loss 1.08, 95% CI 0.91 to 1.29).
Authors' conclusions
Overall there is weak evidence as to the benefits and harms of steroids with antiangiogenic properties for treating neovascular AMD with only three published trials of variable quality. Intravitreal triamcinolone injection for neovascular AMD does not appear to prevent severe vision loss and is associated with increased IOP and higher risk of cataract formation. Anecortave acetate 15 mg may have a mild benefit in stabilizing vision, but further better quality evidence is needed. The role of steroids in combination with other treatment modalities is yet to be determined.
Medical Subject Headings (MeSH): Angiogenesis Inhibitors [*administration & dosage], Choroidal Neovascularization [complications; *drug therapy], Drug Implants, Macular Degeneration [*drug therapy; etiology], Pregnadienediols [administration & dosage], Randomized Controlled Trials as Topic, Triamcinolone Acetonide [administration & dosage]
MeSH check words: Humans
Background
Introduction
Age-related macular degeneration (AMD) continues to be among the leading causes of blindness in the developed world. It is a degenerative disorder involving the central portion of the retina that is responsible for high-resolution visual acuity. The two major types of AMD are classified based on specific abnormalities of the retinal pigment epithelium (RPE) and retina. The dry, or atrophic, form of AMD typically involves the RPE, choriocapillaris, and photoreceptors in the absence of serous or hemorrhagic leakage. The wet, or neovascular, form includes choroidal neovascularization (CNV), leakage of blood and serum, and fibrovascular scarring. Because of the severity of disease found in the neovascular form, it accounts for the majority of significant vision loss caused by AMD (Ferris 1984). Treatment options for this disease are limited and there are a variety of therapies currently being investigated for neovascular AMD. This review is concerned with the potential use of intravitreal antiangiogenic steroids for the treatment of neovascular AMD. Another Cochrane review is looking at agents that are specific to anti-vascular endothelial growth factors (VEGF) (Krzystolik 2005).
Epidemiology
The World Health Organization (WHO) estimates that at least eight million individuals worldwide are severely visually impaired due to AMD (WHO 1997). It is estimated that in the United States alone 1.6 million people age 50 years and older have evidence of late AMD (Tielsch 2002). Large, population-based studies world-wide have found varying prevalence rates of AMD, though they have consistently shown increased risk with age (Evans 2001). Accordingly, the Beaver Dam Eye Study found an increased incidence of age-related maculopathy lesions with age (Klein 2002). With the growing aging population in the developed world, the number of people at risk of AMD is increasing.
Presentation and diagnosis
Individuals with atrophic AMD typically present with a slow and gradual deterioration in fine discriminate visual acuity, which may eventually lead to central vision loss. Neovascular AMD is associated with a more rapid loss of vision. With CNV and leakage of fluid into the surrounding retina, central blurring or visual distortion occurs. Eventual scarring and extensive leakage can subsequently lead to more significant loss of vision.
Fundus examination of people with AMD reveals characteristic presentations that range from drusen and pigmentary changes in people with the atrophic form, to subretinal fluid or blood in the neovascular form. In neovascular AMD, fluorescein angiography is helpful in detecting subtle exudates associated with CNV. Based on fluorescein studies, CNV can be classified as classic or occult lesions. Classic lesions are characteristically well-defined and have early hyperfluorescence, whereas occult lesions have late leakage or evidence of fibrovascular pigment epithelial detachment.
Treatment options
The Macular Photocoagulation Studies (MPSG 1994) demonstrated a delay in vision loss after laser photocoagulation treatment for extrafoveal and juxtafoveal classic CNV secondary to AMD. Argon thermal laser photocoagulation of CNV is not an option for subfoveal CNV as it causes immediate loss of central vision due to damage to the overlying retina. Recurrence of CNV following thermal laser has been shown to occur within three years (MPSG 1994). A Cochrane review laser photocoagulation was recently published (Virgili 2007).
Photodynamic therapy (PDT) was developed as an alternative non-thermal treatment for subfoveal CNV. In PDT, a photoreactive drug is used and activated with light to induce release of free radicals as the drug fills the proliferative neovascularization. The Treatment of AMD with PDT (TAP) Study Group demonstrated a reduced risk of visual loss for eyes with predominantly classic CNV after PDT treatment (Bressler 2001; TAP 1999). However, most participants receiving PDT need multiple treatments within the first year. Recent fluorescein angiographic guidelines for the evaluation and treatment of subfoveal CNV describe patients who are most likely to benefit from PDT with verteporfin (TAP 1999; VIP 2003). A recently updated systematic review on PDT for AMD is also published in The Cochrane Library (Wormald 2005).
Cochrane reviews have also been published on other interventions such as antiangiogenic therapy with interferon alfa (Reddy 2006), radiotherapy (Sivagnanavel 2004), antioxidants (Evans 2006) and ginkgo biloba (Evans 1999).
There is growing interest in novel medical therapies targeting CNV secondary to AMD. Clinical and basic science research has directed attention to angiogenesis as a key target for the medical treatment of CNV. Several antiangiogenic agents are being investigated in treating neovascular AMD and other pathologic neovascularization of ocular tissues (see Table 1). Intravitreal injection of pegaptanib sodium and ranibizumab have been recently approved by the US Federal Drugs Association (FDA) in the treatment of CNV in patients with AMD. Inflammatory processes have also been shown to play a significant role in the pathogenesis of AMD and development of CNV (Penfold 2001). Given these potential pharmacologic targets for inducing regression of CNV, steroids are being investigated for their potential anti-inflammatory and antiangiogenic effects.
Table 1. Antiangiogenic agents investigated for potential benefit in exudative AMD.
| Agent | Putative mechanism |
|---|---|
| VEGF antagonists i.e. Rhu-fab | Binds and neutralizes/inactivates VEGF |
| Dexamethasone | Down-regulates VEGF expression |
| Triamcinolone acetonide | Effects vascular endothelial cell matrix turnover and down-regulates inflammation |
| Anecortave acetate | Suppression of Plasminogen Activator |
| Interferon-alpha | Blocks angiogenic basic fibroblast growth factor and endothelial cell functions |
| Thalidomide | Inhibits cell migration |
| Growth hormone antagonists | Inhibits endothelial cell proliferation and VEGF release |
Penfold et al reviewed laboratory evidence demonstrating that intravitreal corticosteroids mediate anti-inflammatory responses by inducing the resorption of exudates and down-regulating inflammatory stimuli (Penfold 2001). Angiostatic steroids are a separate class of steroids that mediate specific antiangiogenic activity independent of steroid hormone activity. Angiostatic steroids have been shown to inhibit neovascularization in intraocular tumors in animals (BenEzra 1997; Clark 1999; Penfold 2001). These steroids are believed to inhibit angiogenesis by increasing the synthesis of plasminogen activator inhibitor, which subsequently inhibits plasmin generation, a process essential to the invasion of new vessels (Blei 1993).
The clinical use of steroids for the treatment of neovascular AMD will necessitate an effective and practical method of administering these agents into the eye. Intravitreal injections are used for administering drugs for posterior segment diseases in efforts to minimize unwanted systemic effects and to produce effective concentrations locally. More recently, various intraocular drug delivery systems have been developed for longer-term administration of pharmacologic agents (Kurz 2002). With sustained-release gancyclovir implants being used in the treatment of cytomegalovirus retinitis (Musch 1997), intraocular drug delivery devices for chronic corticosteroid treatment are also being examined (Jaffe 2000). Surgically implanted degradable polymer microspheres are also being tested as a way to administer antiangiogenic agents for the treatment of choroidal and retinal diseases (Carrasquillo 2003; Yasukawa 1999).
Rationale for systematic review
With the support of laboratory evidence and advances in drug delivery systems, intravitreal steroids are currently being examined for the treatment of neovascular AMD in the clinical setting. However, the classes of steroids, dosages, and drug delivery systems being used vary between clinical studies. The diversity in methodology warrants a review of these trials to help draw meaningful conclusions about the effectiveness and relevance of steroids in the future management of neovascular AMD.
Objectives
The primary aim of this review is to investigate the effects of intra-and peri-ocular steroids with antiangiogenic properties in delaying visual loss in neovascular macular degeneration. A secondary aim of this review is to compare the clinical outcomes and side effects of different antiangiogenic steroids. A third aim is to compare the intraocular drug delivery systems used for the administration of steroids in the treatment of neovascular AMD in different clinical trials.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials (RCTs) in this review.
Types of participants
We included trials in which participants were diagnosed with neovascular age-related macular degeneration as defined by study investigators.
Types of interventions
We included trials in which implantation (injection, device, suspension, or micro-sphere) of a steroid with antiangiogenic characteristics was compared to another treatment, placebo, or no treatment.
Types of outcome measures
Primary outcomes
The primary outcome for this review is loss of visual acuity measured at 12 months or more, but no more than 24 months. Depending on the data presented in the trial reports, we also examined the proportion of people with loss of three or more lines of logMAR visual acuity (equivalent to a doubling of the visual angle).
Secondary outcomes
The secondary outcomes for this review are as follows.
Contrast sensitivity measured at 12 months using standardized charts and analyzed using log contrast sensitivity values.
Size and characteristics of lesion as determined by fluorescein angiography.
Retinal thickness and parameters as measured by optical coherence tomography (OCT) at 12 months.
Quality of life measures as assessed by any validated measurement scales
Adverse effects
We categorized adverse effects reported in the included studies as ocular or systemic. We planned to report and describe all adverse effects observed including, but not limited to, those listed below. We enumerated adverse event data from each trial included, recognizing that reliable evidence of rare events was unlikely to emerge from RCTs alone. We did not attempt to summarize observational studies examining rare events associated with steroid use for neovascular macular degeneration.
Ocular: retinal detachment; hemorrhage; infection; cataract; steroid-induced rise in intraocular pressure (IOP) and steroid-induced glaucoma; pupillary abnormalities; rubeosis; pigment epithelial detachment; loss of vision; photopsia; ptosis; anterior chamber inflammation; corneal abrasion; pain; foreign body sensation; chemosis and subconjunctival hemorrhage; lid edema; dry eye; vitreous prolapse; vitreous floaters; intraocular air; tearing.
Systemic: cardiovascular; neurological; respiratory; genitourinary; kidney; joint; fatigue; headache.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Group Trials Register) (The Cochrane Library Issue 3, 2006), MEDLINE (January 1966 to October 2006), EMBASE (January 1980 to October 2006) and the Latin American and Caribbean Literature on Health Sciences (LILACS) (1982 to October 2006). The databases were last searched on 2 October 2006.
Searching other resources
The bibliographies of included trials were searched for details of further relevant trials. We did not conduct a manual search of any conference proceedings for the purpose of this review.
Data collection and analysis
Selection of studies
Two review authors independently scanned the titles and abstracts resulting from electronic searches. Full copies of all potentially or definitely relevant articles were further screened by the two review authors independently. Only those articles meeting the inclusion criteria were assessed for quality. We contacted authors to clarify details as necessary to make a complete assessment of the relevance of each study.
Data extraction and management
Two review authors independently extracted data using a form developed by the Cochrane Eyes and Vision Group. Data entered into RevMan 4.2 by one review author was checked using the double-data entry facility and by a second author.
Assessment of risk of bias in included studies
Two review authors independently assessed the methodological quality of the studies that met the inclusion criteria. The review authors were not masked to author or institution details of included trials during the assessment. We assessed the methodological quality of included studies considering four parameters of quality in our assessment: allocation concealment and method of allocation to treatment; masking of providers and recipients of care; masking of outcome assessment; completeness of follow up. Allocation concealment was independently graded by both review authors as: A - adequate; B - unclear; C - Inadequate. Disagreements between review authors were resolved by discussion. We contacted trial authors for clarification of any of the parameters.
Data synthesis
We planned to combine the data using a random-effects model if we found no substantial heterogeneity with a chi-square test and the I-square statistic. As more studies become available in the future, we will investigate any heterogeneity by examining study characteristics including: participant age, visual acuity, lesion size and composition, drug delivery system, type and dose of steroid used. We will use a fixed-effect model if there are fewer than three studies. Dichotomous outcome measures are summarized as risk ratios. Continuous outcomes were to be summarized as mean difference.
Sensitivity analysis
We planned to conduct a sensitivity analysis to examine the influence of any assumptions made during the review on the conclusions of the review and to examine the impact of exclusion of studies of lower methodological quality (i.e. graded C on allocation concealment), unpublished data and industry funded studies.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
The electronic searches identified 1059 reports of trials. After screening the titles and abstracts, we identified 21 relevant articles. Upon further screening of full-text articles, we found three randomised controlled trials that met the inclusion criteria for this review. Five reports discussed one included trial (AACS 2003) and two reports discussed a second included trial (IVTS 2003). The third included trial (Slakter 2006), designed and analysed as anon-inferiority trial, was discussed in a single report. We excluded 11 studies and listed them in the ‘Characteristics of excluded studies’ table along with reasons for exclusion. Details of the included trials are summarized below and in the ‘Characteristics of included studies’ table.
Types of participants
The Anecortave Acetate Clinical Study (AACS 2003) was a multicentre trial conducted in 18 clinical centres across Europe and the United States. The trial enrolled 128 participants and only one eye per participant was randomized. Participants had known AMD and subfoveal CNV with a classic component, were 50 years or older, and had a best-corrected visual acuity of 20/40 to 20/320. The Intravitreal Triamcinolone Study (IVTS 2003) conducted in a single tertiary care hospital in Sydney, Australia, enrolled participants 60 years or older with known AMD and found to have classic or discrete CNV, best-corrected visual acuity of 20/200 or better, and symptoms lasting no longer than 12 months prior to enrollment. The trial included 139 participants of which 12 had both eyes randomized to different interventions.
Slakter 2006 was conducted in 52 centres across Europe, USA, Canada, Israel and Australia. Participants in this trial were at least 50 years old and had been diagnosed with neovascular AMD, sub-foveal CNV eligible for treatment with PDT and with no history of prior treatment with PDT. The best-corrected visual acuity range for eligibility was 20/240 to 20/400. The study randomized 130 participants.
Type of intervention
AACS 2003 investigated juxtascleral depot injection of an entirely different corticosteroid, acetonide anecortave acetate at three different doses, 3 mg, 15 mg, and 30 mg compared to placebo. A sterile posterior juxtascleral procedure, using a specially designed injecting cannula, was used to administer 0.5 ml of study medication or placebo (vehicle) onto the outer surface of the sclera near the macula.
IVTS 2003 examined the efficacy and safety of a single intravitreal injection of 4 mg triamcinolone acetonide (0.1 ml of Kenacort 40; Bristol-Myers Squibb Pharmaceuticals, Noble Park, Victoria, Australia). A placebo treatment consisting of a subconjunctival injection of isotonic sodium chloride solution was introduced after the 12th participant was enrolled, when it became apparent that participants allocated to no treatment were more likely to drop out of the study.
Slakter 2006 compared PDT with adjunct juxtascleral depot administration of 15 mg anecortave acetate versus PDT alone. Participants randomized to the anecortave acetate 15 mg group received a sham PDT treatment prior to the posterior juxtascleral depot, while the PDT group received a sham posterior juxtascleral depot procedure after the PDT treatment.
Types of outcome measures
AACS 2003 evaluated the mean change from baseline in best-corrected visual acuity as the primary outcome. Other vision outcomes included as percentage of participants with preservation or maintenance of vision (loss of < 3 logMAR lines vision), clinically significant worsening of vision (loss of at least three lines vision), and severe vision loss (at least 30 logMAR letters or six lines). The trial also commented on changes in CNV lesion characteristics. Participants were clinically evaluated at post-injection day one to two, week two and six, month three and six after each posterior juxtascleral administration of treatment. Visual acuity was measured at all study visits except on post-injection day one to two. Fluorescein angiography and fundus photography was performed at week two and six, month three, prior to each additional retreatment and at the exit visit.
IVTS 2003 reported the proportion that developed severe visual loss (3 30 letters on a logMAR chart) at two years as their primary outcome. Other vision outcomes reported include the risk ratio of loss of three or more lines of vision, and loss of six or more lines of vision at 12 months. Participant data was reviewed before treatment, then at three, six, and 12 months when visual acuity was measured. Fluorescein angiography was performedat baseline, and at three and 12 months, while stereocolor photographs of the macula were taken at baseline, and then at six and 12 months. Other outcomes reported include changes in size of CNV and leakage of neovascular membranes.
Slakter 2006 assessed preservation of vision defined as loss of three or fewer logMAR lines of vision. The primary outcome measure was percentage of responders or participants losing fewer than three lines of vision at month 12. The follow up visits were scheduled for months three, six, nine and 12. Digital fluorescein and indocyanine green angiography was done at all study visits and transmitted to a reading center for masked assessment.
Risk of bias in included studies
In AACS 2003, at each month six visit, a masked examining ophthalmologist made the decisionastowhether the participant might benefit from retreatment. Retreatment was performed by an unmasked injecting ophthalmologist using the assigned treatment as originally randomized. If the participant was judged not to benefit from continued participation, then the participant exited the study. The 12-month report of the study did not explicitly report the number of retreatments given to participants of this ongoing study. Based on their reported methods, participants who completed the 12 month follow-up were to have received two treatments at six-month intervals. The authors suggest that masking the evaluation for retreatment meant that both the treatment and placebo groups were equally exposed to the potential bias involved in only retreating those who did not worsen with either intervention. However, the analysis of participants beyond six months follow-up was not by intention-to-treat principle. Further, the authors do not report any predefined explicit criteria based on which the masked ophthalmologist would judge eligibility of participants to continue in the trial, introducing possible selection bias into analyses beyond the first six-month period of follow-up.
Of the 128 participants randomized in AACS 2003, only 76 participants completed their month 12 visit. Of the 52 participants who exited the study, 24 exited secondary to AMD progression. There was no statistical difference in the distribution and explanation for attrition between the treatment groups, and baseline characteristics of the participants exiting before month 12 were not statistically significantly different from baseline characteristics of those not exiting.
IVTS 2003 was a single-center study with 151 eyes in 139 participants. Details of methodological quality are described in the table of included studies. A brief summary of the main issues are discussed here. Overall, the trial was of moderate methodological quality. Randomization was achieved using variable block numbers; a convincing description of allocation concealment was provided. However, 12 (8%) participants had both eyes included in the trial. Except for the first two of such participants, treatment assignment was determined by randomization for one of the eyes and the other eye was allotted to the alternate treatment group. No adjustment was made to allow for the lack of independence between paired eyes. Masking of outcome assessment was achieved by asking the care-giver to assess the visual acuity (primary outcome) without referring to prior notes or examining the participant. Reasonable attempts were also made to mask the participants.
Losses to follow-up differed in the two treatment groups with 4% lost to follow-up due to death in the treatment group and 7.9% lost to follow-up in the placebo group. Reasons for losses to follow-up in the placebo group included death in two participants, withdrawal from the trial by four participants of whom one received treatment outside of the trial settings. While the unit of analysis in the review was participants, the unit of analysis for the study was not clear from the published reports. No separate analysis was done for addressing the fact that 12 participants had both eyes included in the trial.
Slakter 2006 was designed as a non-inferiority trial comparing posterior juxtascleral depot of anecortave acetate 15 mg and PDT. Since there is no placebo group in this study, the non-inferiority study design relies on historical data demonstrating superiority of PDT to placebo to provide an indirect comparison between a novel intervention to placebo. The authors used the TAP study as their reference trial and specified a non-inferiority margin a priori. Demographic and lesion characteristics between the two groups were similar suggesting adequate randomization. Sham procedures were also performed for both treatment groups to ensure masking. Data from 18.6% in the anecortave acetate group and 17.6% in the PDT group were not available for 12-month analyses. Reasons included adverse events, disease progression, loss to follow-up, packaging error, participant decision and transportation issues.
Effects of interventions
We did not conduct a meta-analysis since the included studies evaluated different comparisons.
Anecortave acetate versus placebo
Visual acuity
AACS 2003 compared three different doses of anecortave acetate with placebo and reported visual acuity as both a dichotomous and continuous outcome. Data from this trial at 12 months included last observation carried forward for participants who were determined not to benefit with further treatment at six months by a masked investigator.
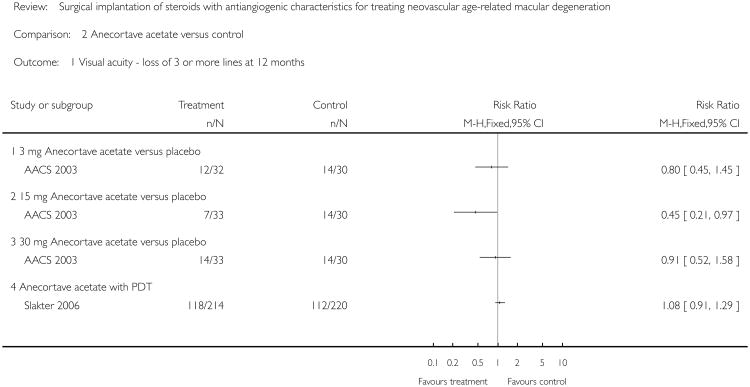
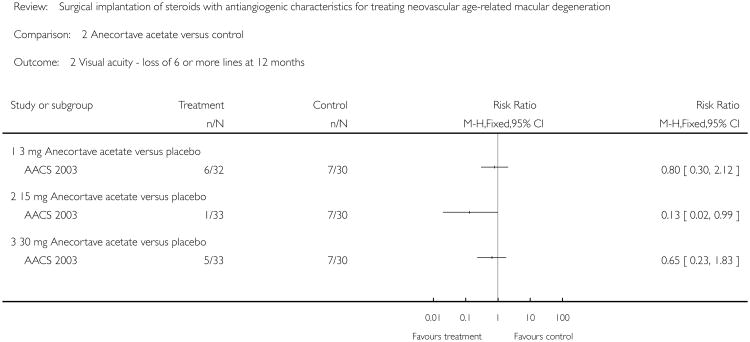
The risk ratio of loss of three or more lines at 12 months was 0.8 (95% CI = 0.45 to 1.45) in the 3 mg group, 0.45 (95% CI = 0.21 to 0.97) in the 15 mg group and 0.91 (95% CI = 0.52 to 1.58) in the 30 mg anecortave acetate group all compared with placebo. The risk ratio of loss of six or more lines at 12 months was 0.8 (95% CI = 0.30 to 2.12) in the 3 mg group, 0.13 (95% CI = 0.02 to 0.99) in the 15 mg group and 0.65 (95% CI = 0.23 to 1.83) in the 30 mg anecortave acetate group all compared with placebo. The 95% confidence interval for the 15 mg group alone excludes the null value suggesting treatment benefit in decreasing the risk of moderate and severe vision loss. A pooled analysis of all three treatment groups, 3 mg, 15 mg, and 30 mg, resulted in a risk ratio of 0.72 (95% CI = 0.24 to 1.33).
Triamcinolone acetonide versus placebo
Visual acuity
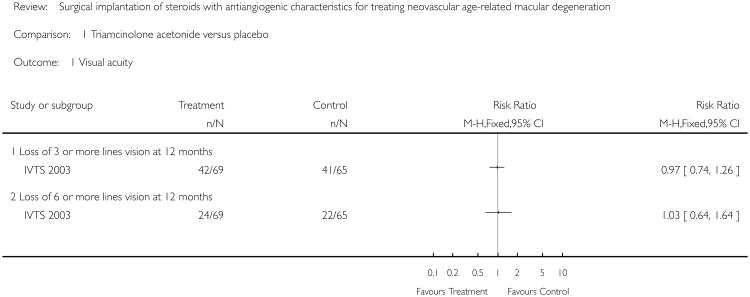
Analysis for visual acuity showed no evidence of beneficial effect with treatment. The risk ratio of loss of three or more lines at 12 months was 0.97 (95% CI = 0.74 to 1.26) and the risk ratio of loss of six or more lines vision at 12 months was 1.03 (95% CI = 0.64 to 1.64). Participants who were lost to follow-up, who died and who refused treatment in the placebo group were not included in the analysis (six of 70 or 8.5% of participants at baseline). In the treatment group, however, all participants randomised were analysed despite loss of two participants to follow-up due to death, leading to confusion about the unit of analysis adopted in the trial.
Lesion characteristics
No change in lesion size or growth was observed at 12 months, but at three months there were more eyes in the treated group (35 out of 74) compared to the placebo group (26 out of 74) that had small and medium neovascular lesions. However, in about 30% of the participants in each group, lesion characteristics could not be assessed. Data on mean lesion size allowing us to calculate weighted averages were not reported.
Anecortave acetate 15 mg versus PDT
Visual acuity
The risk ratio of loss of three or more lines of vision at 12 months follow-up (figure not shown) was 1.08 (95% CI = 0.91 to 1.29) in an available case analysis and 1.08 (95% CI = 0.91 to 1.27) in an intention-to-treat analysis conducted using the last observation carried forward method. However, Slakter 2006 was designed to be a non-inferiority trial. The investigators reported a primary outcome of preservation of visual acuity in terms of loss of fewer than three lines of vision at 12 months to compare anecortave acetate 15 mg versus PDT. The risk ratio of loss of fewer than three lines at 12 months was 1.08 (95% CI = 0.91 to 1.29) only slightly favouring PDT over anecortave acetate.
In the subgroup analysis performed within the anecortave acetate group, there was a higher proportion of responders in the group treated with anecortave acetate 15 mg within the six-month treatment window and for whom reflux was controlled. Overall however, there was no statistical difference between the two groups, which in the context of the non-inferiority trial, suggests that anecortave acetate is comparable to PDT for treatment of subfoveal CNV.
Adverse effects
In the AACS trial, the most common adverse events included vision loss defined as loss of four or more lines of vision and cataract progression. This occurred in all four treatment groups. The only ocular adverse events that occurred more frequently in the anecortave acetate groups were “vision abnormalities” and foreign body sensation. There is no mention of any dose-dependent response for any of these ocular events.
The IVTS analyzed adverse events in 142 eyes at 12 months, 100 eyes at two years and in 35 eyes at three years (IVTS 2003). Procedure related events such as transient discomfort or blurring were reported in both groups. Elevated IOP, however, was noted in 31 participants in the treatment group and only in three participants in the placebo group at 12 months. One additional participant in each group developed increased IOP over the three year follow-up period.
There was significant progression of posterior subcapsular cataracts in eyes treated with intravitreal triamcinolone. After 12 months, five participants had progression of cataract by two or more Age-related Eye Disease Study Grades in the treatment group and only one participant in the placebo group (IVTS 2003). In two years, eight participants in the treatment group compared to none in the placebo group developed progression of posterior subcapsular cataract. Other adverse events reported included arteritic anterior ischemic optic neuropathy in one participant in the treatment group and massive subretinal or break-through vitreous hemorrhage in three eyes in the placebo group.
Slakter 2006 evaluated safety data on participants who received at least one dose of anecortave acetate or PDT. As in AACS, the most frequently reported adverse event was vision loss as defined as a drop of four or more lines of vision. There was little difference between the participants treated with anecortave and PDT, with 31.9% and 30.4% having a decrease in vision respectively. Other adverse events were mostly unrelated to therapy except for one participant treated with anecortave acetate 15 mg who discontinued participation due to retinal artery occlusion, which is possibly related to the study drug.
Discussion
Though randomized clinical trials have shown that laser treatment, PDT and pegaptanib sodium are effective in preserving vision in specific groups of participants with AMD, other pharmacological therapies are still being developed. Steroids have gained attention in their role for the treatment of CNV for their antiangiogenic and anti-inflammatory properties. However, side effect profiles and differing drug administration techniques have necessitated clinical investigation to provide guidance in the use and design of steroids for AMD. This review suggests that even within this class of drugs, there are diverse therapeutic and safety profiles for individual agents.
Triamcinolone acetonide
Intravitreal injection of triamcinolone acetonide has been used in practice for the treatment of various retinal diseases. The only randomized clinical trial looking at the long-term effects of the drug on CNV secondary to AMD is included in this review. This trial suggests that a single dose of intravitreal triamcinolone (4 mg) has little to no effect on the risk of vision loss and lesion progression after one year. This contradicts conclusions from prior, smaller clinical studies.
Danis et al conducted a small (27 participants), randomized clinical trial comparing a single injection of intravitreal triamcinolone (4 mg) to observation alone (Danis 2000). The study suggests possible short-term benefit in visual acuity; at six months, mean acuity of the treatment group was 0.04 logMAR units better than baseline compared to a decrease by 0.39 units in the observation group. Changes in lesion characteristics also differed between the groups at six months (69% of the treated participants versus 29% of controls had improved or stable lesions). The study was not included in this review because it only reports short-term (six month) data and long-term follow-up was not available.
Numerous uncontrolled clinical trials have also been done and have suggested that there may be some benefit with intravitreal triamcinolone. A study by Challa et al showed that 18 months after triamcinolone treatment (4 mg) only 30% (6/20) of eyes with an initial visual acuity of 6/60 or better developed severe vision loss (Challa 1998). This is in comparison to the 35% of treated eyes that developed severe vision loss after one year in the trial reviewed here. In another case series by Ranson et al only one out of 11 (9%) eyes treated with intravitreal triamcinolone (4 mg) developed severe vision loss after one year (Ranson 2002). However, given the lack of prospective controls and the small size of the previous trials, it is difficult to draw meaningful conclusions regarding the relative benefit of treatment versus observation alone.
The absence of evidence of benefit from a single dose of 4 mg intravitreal triamcinolone as reported by IVTS investigators (IVTS 2003), suggest that the effects of intraocular steroid treatment is transient. Jonas et al (Jonas 2004) report an increase in visual acuity in three out of six participants after a second injection of triamcinolone. In another interventional case series reported by Jonas et al (Jonas 2003) a higher dose of 25 mg intravitreal triamcinolone was used and suggested a potential benefit in eyes treated with this dose. Higher doses of intravitreal triamcinolone bring up concerns for increased incidence of adverse effects, but Jonas et al claim that the adverse events noted in their study were comparable to those previously reported in studies using 4 mg triamcinolone.
The most common adverse events associated with intravitreal triamcinolone include increased IOP and cataract progression. In the clinical trial reviewed here, 41% of eyes treated with 4 mg triamcinolone developed an increase in IOP, whereas this occurred in only 25% of treated participants in the report by Danis et al. In the IVTS (IVTS 2003), there was a higher rate of progression of posterior subcapsular cataracts in the treatment group compared to placebo. Danis et al also found an increased frequency in cataract progression in the treatment group (Danis 2000). Other serious side effects previously reported including endophthalmitis, rheg-matogenous retinal detachment, or proliferative vitreoretinopa-thy, were not reported in the study reviewed here. Gillies et al (IVTS 2003), however, reported a hemiretinal vein occlusion that was possibly related to treatment. There have been case reports of non-infectious endophthalmitis presenting as a dense vitreous haze after injection with intravitreal triamcinolone. These reports suggest that in the absence of eye pain, it may be appropriate to observe participants who develop this pseudo-endophthalmitis since it may resolve without specific treatment with antimicrobials.
Anecortave acetate
Unlike triamcinolone acetonide, anecortave acetate is a synthetic cortisol derivative with specific modifications to its structures, thought to have less of the usual side effect profile of glucocorticoids. A study sponsored by the manufacturers of the drug looked at the effects of various doses of anecortave acetate on visual acuity in participants with neovascular AMD. The anecortave acetate study (AACS) showed little difference in IOP or cataract progression in participants receiving the juxtascleral injection of anecortave acetate compared to placebo. A pooled analysis of the three dosages was not statistically significant. Though 15 mg of anecortave acetate may have some benefit in stabilizing vision and preventing vision loss in participants with AMD, results from the AACS should be taken in the context of the trial's small sample size, a notable attrition rate, and the absence of a classic dose-response with treatment. While the sample size calculation was based on a single two-way comparison, 3 two-way comparisons were produced. Many statisticians would have advocated for a larger number of eyes in the control arm if three comparisons were to be made. Thus, the sample size was inadequate. The study results should also be regarded cautiously, since the limits of the 95% CI were close to no difference and were not adjusted for multiple comparison. Reasons for participants exiting the study were reported in detail and were partly based on physician assessment of participants for possible retreatment at month six. This is a potentially significant source of selection bias since the final analysis excluded patients who were not selected for retreatment and who likely had worsening disease despite intervention (either with placebo or anecortave acetate).
Prior randomized clinical trials provide evidence that participants with subfoveal CNV, such as those included in AACS, may benefit from PDT. Slakter et al (Slakter 2006) compares anecortave acetate to PDT and shows no significant difference between the two treatment groups. Through a non-inferiority study design, the authors suggest that anecortave acetate 15 mg may be a safe alternative to PDT in treating AMD-related subfoveal CNV.
Though the above studies evaluated the efficacy of steroid treatment alone, future studies investigating the combination therapy of PDT and triamcinolone acetonide are being done in lieu of a possible synergistic effect of the two treatment modalities.
Authors' Conclusions
Implications for practice
Intravitreal triamcinolone injections for neovascular age-related macular degeneration (AMD) does not appear to have clear benefits in preventing severe vision loss and preserving visual acuity, contrary to prior reports. The adverse events reported in this study and others suggest that intravitreal triamcinolone is not a benign therapy and there is only uncontrolled evidence supporting its efficacy in treating choroidal neovascularization (CNV) secondary to AMD.
Anecortave acetate 15 mg may have a slight benefit in treating subfoveal CNV related to AMD. However, the data presented in the AACS trial is of low quality given the high attrition rate, inadequate sample size, lack of adjustment for multiple comparisons, and potential bias in the non-randomized retreatment schedule. The drug appears to be relatively well tolerated using the posterior juxtascleral depot procedure, and Slakter et al suggest it is at least a safe alternative to PDT, although it must be kept in mind that Slakter et al is a non-inferiority study design. Given its safety profile, there may be potential application of anecortave acetate in other neovascular or inflammatory ocular diseases.
Implications for research
Given the small size and quality of trials reported, there is little evidence to support the use of steroids in the treatment of neovascular AMD. In designing future clinical trials, it would be important to assess long-term outcomes since AMD is a chronic disease and to evaluate other relevant outcome measures in patients with AMD (i.e. contrast sensitivity, quality of life data). Cost is another practical consideration in the development of novel treatments that will provide long-term benefit to patients with AMD. As more pharmaceutical agents and different methods of administration are becoming available for the treatment of CNV, clinical evidence will be critical in guiding future treatments. However, future studies are warranted to examine the potential role of anecortave acetate in combination therapy with other treatment modalities in treating neovascular AMD. An interesting new development is the use of steroid microcannular delivery to the suprachoiroidal space beneath the macula (Olsen 2006).
Plain Language Summary.
Steroids with antiangiogenic properties for treating neovascular age-related macular degeneration
Neovascular age-related macular degeneration (AMD) is associated with rapid loss of vision due to abnormal growth of blood vessels in the macula. Corticosteroids that reduce this growth of blood vessels have been tested for treatment of such vision loss. This review includes three trials evaluating two different types of steroids, triamcinolone acetonide and anecortave acetate, for the treatment of neovascular AMD. One trial found no evidence of benefit with intravitreal triamcinolone 4 mg. One trial found posterior juxtascleral depot of anecortave acetate 15 mg may be effective in preventing severe vision loss. However, further research is necessary to confirm the efficacy of anecortave acetate due to the small sample size and high attrition of the reviewed study.
Acknowledgments
The Trials Search Co-ordinator for Cochrane Eyes and Vision Group prepared and executed the electronic searches for this review. We thank Jennifer Evans and Anupa Shah for their assistance with the development of the protocol for this review. We acknowledge Elizabeth Ssemanda's assistance in responding to peer review comments and co-ordination of the review. We acknowledge Barbara Hawkins, Roberta Scherer, and Gianni Virgili for their comments on this review.
Sources Of Support
Internal sources
Brown University, USA.
Johns Hopkins University, USA.
External sources
Contract N-01-EY-2-1003, National Eye Institute, National Institutes of Health, USA.
Characteristics of Studies
Characteristics of included studies [ordered by study ID]
AACS 2003.
| Methods | Method of randomization: Not clearly described - “randomization schedule generated by the biostatistics department of Alcon Research”. Method of allocation concealment: Study medication and supplies for posterior juxtascleral administration were placed in sealed, opaque, sequentially numbered boxes identified by participant number only. Masking of participants and care-givers: Adequate. Masking of outcome assessment: Adequate. Losses to follow-up: At 6 months, 6.25%, 24.2%, 3% and 13.3% participants were lost to follow-up in the 3 mg, 15 mg, 30 mg Anecortave acetate and placebo groups respectively. At 1 year, however, 37.5%, 48.5%, 36.4% and 40% participants were not analysed in the 3 mg, 15 mg, 30 mg Anecortave acetate and placebo groups respectively. Intention-to-treat analysis: The 6 month analysis was by intention-to-treat with the last observation carried forward to impute missing values. However, the 12 month analysis was not. Reported sample size calculation: Assuming a standard deviation of 0.285 logMAR lines in visual acuity, the study reported a 80% power to detect a difference between treatment means of approximately 2 logMAR lines in a 2-tailed t-test with a 0.05 level of significance with 30 patients per group Unusual study design: The patients were examined by a masked ophthalmologist at 6 months who determined whether the patient was likely to benefit with further treatment. Patients judged as unlikely to benefit from further treatment were excluded from further follow-up. |
|
| Participants | # randomized in treatment arm: 3 mg (n = 32), 15 mg (n =33), and 30 mg (n=33) # randomized in control arm: n=30 Inclusion criteria:
Exclusion criteria:
Age: Mean age was 78.1, 75.8, 75.7 and 78.3 years in 3 mg, 15 mg, 30 mg Anecortave acetate and placebo groups, respectively. Gender: 46.9%, 54.5%, 54.5% and 60% were females in 3 mg, 15 mg, 30 mg Anecortave acetate and placebo groups, respectively Equivalence of baseline characteristics: All groups were similar at baseline with respect to age, race, composition of lesions, logMAR visual acuity, sizes of choroidal neovascularization and classic component of the lesions. |
|
| Interventions | Treatment: Juxtascleral depot injection of three doses of acetonide anecortave acetate: 30 mg, 15 mg, and 3 mg. At each month 6 visit, a masked ophthalmologist decided if the patient might benefit from retreatment. Retreatment was performed by an unmasked ophthalmologist using the assigned treatment as originally randomized. Control: Placebo injection in the same fashion. |
|
| Outcomes | Primary outcome: Mean change in logMAR visual acuity from baseline. Secondary outcomes: Percentage of all participants exhibiting stabilized vision (< 3 lines lost). Percentage of participants with clinically significant worsening of vision defined as loss of at least 3 lines vision. Percentage of participants with severe vision loss (at least 6 logMAR lines lost). Percentage of participants that demonstrated reduced lesion growth (percent change from baseline). |
|
| Notes | Country: 18 centers across United States and Europe. Time period of study: April 1999 to May 2001. |
|
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Yes | A-Adequate |
IVTS 2003.
| Methods | Method of randomization: List of computer generated pseudorandom numbers with variable block size was used. For 12 participants with bilateral eye disease, one eye was randomized and the other eye was allocated to the alternate treatment group. Method of allocation concealment: Opaque, sealed, numbered envelopes were kept locked in a cabinet and issued in sequence by a designated member of the clinical staff. Neither the member nor the surgeon enrolling the participants was involved in the trial in any other way. Masking of participants and care-givers: Adequate. Masking of outcome assessment: Adequate. Losses to follow-up: 2 in the triamcinolone group and 6 in placebo group at the end of 1 year. Intention-to-treat analysis: Authors state it is an intention-to-treat analysis but participants lost to follow-up (due to death or withdrawal from the study) were not analysed. Reported sample size calculation: A sample size of 130 participants was calculated to be required for detecting a reduction in risk of severe visual loss (less than or equal to 30 letters on a logMAR chart) from 55% to 25% over 2 years with 90% power at a 0.05 level of significance. Unusual study design: 12 participants had both eyes randomized and analysis was not controlled for correlation. |
|
| Participants | # randomized in treatment arm: 75 # randomized in control arm: 76 Inclusion criteria:
Exclusion criteria:
Age: Mean age was 76 years in triamcinolone group and 77 years in the placebo group. Gender: 57% in triamcinolone and 64% in placebo group were females. Equivalence of baseline characteristics: Participants were similar at baseline with respect to age, gender, smoking history and number of letters read. |
|
| Interventions | Treatment: Triamcinolone acetonide, 4 mg (40 mg/mL: 0.1 mL of Kenacort 40; Bristol-Myers Squibb Pharmaceuticals) injected into the vitreous 5 minutes after subconjunctival injection of 2% lidocaine and digital massage to reduce intraocular pressure. A small amount of 1% chloramphenicol ointment was instilled after the procedure. Control: Placebo was not administered to the first 12 participants enrolled in the trial. Placebo consisted of a subconjunctival injection of isotonic sodium chloride solution. |
|
| Outcomes | Primary outcome: Rate of development of severe visual loss (greater than or equal to 30 letters on a logMAR chart). Secondary outcomes:
|
|
| Notes | Country: Sydney, Australia. Time period of study: Not explicitly reported. |
|
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Yes | A-Adequate |
Slakter 2006.
| Methods | Method of randomization: Not explicitly reported (“randomized”). Method of allocation concealment: Not clearly reported. Masking of participants and care-givers: Participants were masked (covered infusion-tubing, similar set of instructions as indicated for PDT. Physicians administering the medication were not. Masking of outcome assessment: Yes, outcome assessors were masked. Losses to follow-up: Only 3 participants in the PDT group and none in the anecortave acetate group were lost to follow-up. Data from 18.6% in anecortave acetate group and 17.6% in PDT group were not available for 12-month analyses. Intention-to-treat analysis: The trial was designed as a non-inferiority study and the authors reported a per-protocol analysis as well as an intention-to-treat with the last observation carried forward. Reported sample size calculation: Yes, an a priori sample size calculation was reported. 261 per group to provide 90% coverage probability to achieve non-inferiority margin of 7% points. |
|
| Participants | # randomized in treatment arm: 263 #randomized in control arm: 267 Inclusion criteria:
Exclusion criteria:
Age: Average age was 76.6 years (51-96 years), group-wise data not provided. Gender: Overall, 52% were females, no group-wise data available Equivalence of baseline characteristics: Age, gender, mean baseline visual acuity were similar at baseline |
|
| Interventions | Treatment: Posterior juxtascleral depot administration of 15 mg anecortave acetate every 6 months, a sham PDT every 3 months if there was leakage on fluorescein angiogram. Control: PDT, administered every 3 months and influenced by investigator's decision based on fluorescein angiographic evidence of leakage, and sham anecortave acetate at baseline and at 6 months. |
|
| Outcomes | Primary outcome: Visual acuity, measured as loss of fewer than 3 lines of vision at month 12. Visual acuity was measured at 2 meters using the ETDRS and TAP study methodology. Visual acuity was also reported as logMAR scores. Secondary outcomes: Safety outcomes/adverse events. |
|
| Notes | Country: USA, Canada, Europe, Israel, Australia. Time of study: Not reported. |
|
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
AMD: age-related macular degeneration
BCVA: best corrected visual acuity
CNV: choroidal neovascularization
ETDRS: early treatment diabetic retinopathy study
PDT: photodynamic therapy
TAP: treatment of age-related macular degeneration with photodynamic therapy
Characteristics of excluded studies [ordered by study ID].
| Arevalo 2005 | Non-randomised study |
| Argurto 2005 | Only 6-month follow-up |
| Bakri 2006 | Review |
| Challa 1998 | Case series |
| Danis 2000 | Primary outcome not at 12-months follow-up |
| Jonas 2003 | Case series |
| Jonas 2004 | Case series |
| Penfold 1995 | Case series |
| Ranson 2002 | Case series |
| Spaide 2003 | Case series |
| Van De Moere 2005 | Historical controls |
Data and Analyses
Comparison 1. Triamcinolone acetonide versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Visual acuity | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Loss of 3 or more lines vision at 12 months | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable | |
| 1.2 Loss of 6 or more lines vision at 12 months | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
Comparison 2. Anecortave acetate versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Visual acuity - loss of 3 or more lines at 12 months | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 1.1 3 mg Anecortave acetate versus placebo | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable | |
| 1.2 15 mg Anecortave acetate versus placebo | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable | |
| 1.3 30 mg Anecortave acetate versus placebo | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable | |
| 1.4 Anecortave acetate with PDT | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable | |
| 2 Visual acuity - loss of 6 or more lines at 12 months | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 2.1 3 mg Anecortave acetate versus placebo | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable | |
| 2.2 15 mg Anecortave acetate versus placebo | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable | |
| 2.3 30 mg Anecortave acetate versus placebo | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
Analysis 1.1. Comparison 1 Triamcinolone acetonide versus placebo, Outcome 1 Visual acuity.
|
Analysis 2.1. Comparison 2 Anecortave acetate versus control, Outcome 1 Visual acuity - loss of 3 or more lines at 12 months.
|
Analysis 2.2. Comparison 2 Anecortave acetate versus control, Outcome 2 Visual acuity - loss of 6 or more lines at 12 months.
|
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Macular Degeneration
#2 MeSH descriptor Retinal Degeneration
#3 MeSH descriptor Retinal Neovascularization
#4 MeSH descriptor Choroidal Neovascularization
#5 MeSH descriptor Macula Lutea
#6 macula* near lutea*
#7 ((macul* OR retina* OR choroid*:TI) AND (degener* OR neovasc*:TI))
#8 ((macul* OR retina* OR choroid*:AB) AND (degener* OR neovasc*:AB))
#9 maculopath*
#10 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9)
#11 MeSH descriptor Angiogenesis Inhibitors
#12 angiogen* or antiangiogen* or neovasculari* or vasculari*
#13 macugen or pegaptanib or lucentis or rhufab or ranibizumab
#14 (#11 OR #12 OR #13)
#15 (#10 AND #14)
Appendix 2. MEDLINE on OVID search strategy
exp clinical trial/[publication type]
(randomized or randomised).ab,ti.
placebo.ab,ti.
dt.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1-7
exp animals/
exp humans/
9 not (9 and 10)
8 not 11
exp macular degeneration/
exp retinal degeneration/
exp retinal neovascularization/
exp choroidal neovascularization/
exp macula lutea/
maculopath$.tw.
((macul$ or retina$ or choroid$) adj3 degener$).tw.
((macul$ or retina$ or choroid$) adj3 neovasc$).tw.
(macula$ adj2 lutea).tw.
or/13-21
exp angiogenesis inhibitors/
(angiogen$ or antiangiogen$ or neovasculari$ or vasculari$).tw.
(macugen or pegaptanib or lucentis or rhufab or ranibizumab).tw.
or/23-25
22 and 26
27 and 12
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville (Glanville 2006).
Appendix 3. EMBASE on OVID search strategy
exp randomized controlled trial/
exp randomization/
exp double blind procedure/
exp single blind procedure/
random$.tw.
or/1-5
(animal or animal experiment).sh.
human.sh.
7 and 8
7 not 9
6 not 10
exp clinical trial/
(clin$ adj3 trial$).tw.
((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
exp placebo/
placebo$.tw.
random$.tw.
exp experimental design/
exp crossover procedure/
exp control group/
exp latin square design/
or/12-21
22 not 10
23 not 11
exp comparative study/
exp evaluation/
exp prospective study/
(control$ or prospectiv$ or volunteer$).tw.
or/25-28
29 not 10
30 not (11 or 23)
11 or 24 or 31
exp retina macula degeneration/
exp retinal degeneration/
exp retinal neovascularization/
exp subretinal neovascularization/
exp retina maculopathy/
exp retina macula lutea/
maculopath$.tw.
((macul$ or retina$ or choroid$) adj3 degener$).tw.
((macul$ or retina$ or choroid$) adj3 neovasc$).tw.
(macula$ adj2 lutea).tw.
or/33-42
exp angiogenesis inhibitors/
exp angiogenic factor/
(angiogen$ or antiangiogen$ or neovasculari$ or vasculari$).tw.
(macugen or pegaptanib or lucentis or rhufab or ranibizumab).tw.
or/44-47
43 and 48
49 and 32
Appendix 4. LILACS search terms
macul$ or retina$ or choroid$ and degener$ or neovasc$
Footnotes
Indicates the major publication for the study
Contributions Of Authors: Conceiving the review: AG
Designing the review: AG, AT
Coordinating the review: SSV
- Designing search strategies: Cochrane Eyes and Vision Group editorial base
- Undertaking electronic searches: Cochrane Eyes and Vision Group editorial base
- Undertaking manual searches: AT, SSV
- Screening search results: AG, AT, SSV
- Organizing retrieval of papers: AT, SSV
- Screening retrieved papers against inclusion criteria: AT, SSV
- Appraising quality of papers: AT, SSV
- Extracting data from papers: AT, SSV
- Writing to authors of papers for additional information: AG, AT, SSV
- Entering data into RevMan: SSV
- Analysis of data: AT, SSV
- Interpretation of data: AT, SSV
Writing the review: AG, AT, SSV
Securing funding for the review: AG, SSV
Performing previous work that was the foundation of the current study: AG
Declarations of Interest: AG was a paid consultant for Neurotech USA on a research study of the use of intravitreal vehicles in retinitis pigmentosa until 2002. AG has not been involved with any company that has conducted trials on surgical implantation of steroids with anti-angiogenic characteristics for treating neovascular macular degeneration.
AT and SSV have no known conflicts of interest.
References to studies included in this review
- Augustin AJ, D'Amico DJ, Mieler WF, Schneebaum C, Beasley C. Safety of posterior juxtascleral depot administration of the angiostatic cortisene anecortave acetate for treatment of subfoveal choroidal neovascularization in patients with age-related macular degeneration. Graefe's Archive for Clinical and Experimental Ophthalmolog y. 2005;243(1):9–12. doi: 10.1007/s00417-004-0961-4. [DOI] [PubMed] [Google Scholar]
- Regillo CD, D'Amico DJ, Mieler WF, Beasley CH, Schneebaum C. Safety of anecortave acetate administered as posterior juxtascleral injection in patients with subfoveal choroidal neovascularization. American Academy of Ophthalmology. 2002;282 [Google Scholar]
- Schmidt-Erfurth U, Michels S, Michels R, Aue A. Anecortave acetate for the treatment of subfoveal choroidal neovascularization secondary to age-related macular degeneration. European Journal of Ophthalmology. 2005;15(4):482–5. doi: 10.1177/112067210501500411. [DOI] [PubMed] [Google Scholar]
- The Anecortave Acetate Clinical Study Group. Anecortave acetate as monotherapy for the treatment of subfoveal lesions in patients with exudative age-related macular degeneration (AMD) - Interim (month 6) analysis of clinical safety and efficacy. Retina. 2003;23:14–23. doi: 10.1097/00006982-200302000-00003. [DOI] [PubMed] [Google Scholar]
- *.The Anecortave Acetate Clinical Study Group. Anecortave acetate as monotherapy for treatment of subfoveal neovascularization in age-related macular degeneration - twelve-month clinical outcomes. Ophthalmology. 2003;110(12):2372–83. doi: 10.1016/j.ophtha.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Gillies MC, Simpson JM, Billson FA, Luo W, Penfold P, Chua W, et al. Safety of an intravitreal injection of triamcinolone. Results from a randomized clinical trial. Archives of Ophthalmology. 2004;122(3):336–40. doi: 10.1001/archopht.122.3.336. [DOI] [PubMed] [Google Scholar]
- *.Gillies MC, Simpson JM, Luo W, Penfold P, Hunyor ABL, Chua W, et al. A randomized clinical trial of a single dose of intravitreal triamcinolone acetonide for neovascular age-related macular degeneration. One-year results. Archives of Ophthalmology. 2003;121(5):667–73. doi: 10.1001/archopht.121.5.667. [DOI] [PubMed] [Google Scholar]
- Slakter JS, Bochow T, D'Amico DJ, Marks B, Jerdan J, Sullivan EK, et al. Anecortave acetate (15 milligrams) versus photodynamic therapy for treatment of subfoveal neovascularization in age-related macular degeneration. Ophthalmology. 2006;113(1):3–13. doi: 10.1016/j.ophtha.2005.10.019. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Arevalo JF, Mendoza AJ, Fernandez CF. Indocyanine green-mediated photothrombosis with and without intravitreal triamcinolone acetonide for subfoveal choroidal neovascularization in age-related macular degeneration: a pilot study. Retina. 2005;25(6):719–26. doi: 10.1097/00006982-200509000-00006. [DOI] [PubMed] [Google Scholar]
- Agurto Rivera R, Diaz Rubio J, Torres Bernal L, Macky TA, Colina Luquez J, Papa Oliva G, et al. Intravitreal triamcinolone with transpupillary therapy for subfoveal choroidal neovascularization in age related macular degeneration. A randomized controlled pilot study [ISRCTN74123635] BMC Ophthalmology. 2005;5:27. doi: 10.1186/1471-2415-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakri SJ, Kaiser PK. Anercortave Acetate. Expert Opinion on Investigational Drugs. 2006;15(2):163–9. doi: 10.1517/13543784.15.2.163. [DOI] [PubMed] [Google Scholar]
- Challa JK, Gillies MC, Penfold PL, Gyory JF, Hunyor AB, Billson FA. Exudative macular degeneration and intravitreal triamcinolone: 18 month follow up. Australia New Zealand Journal of Ophthalmology. 1998;26(4):277–81. doi: 10.1111/j.1442-9071.1998.tb01330.x. [DOI] [PubMed] [Google Scholar]
- Danis RP, Ciulla TA, Pratt LM, Anliker W. Intravitreal triamcinolone acetonide in exudative age-related macular degeneration. Retina. 2000;20(3):244–50. [PubMed] [Google Scholar]
- Jonas JB, Kreissig I, Hugger P, Sauder G, Panda-Jonas S, Degenring R. Intravitreal triamcinolone acetonide for exudative age related macular degeneration. British Journal of Ophthalmology. 2003;87(4):462–8. doi: 10.1136/bjo.87.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas JB, Akkoyun I, Budde WM, Kreissig I, Degenring RF. Intravitreal reinjection of triamcinolone for exudative age-related macular degeneration. Archives of Ophthalmology. 2004;122(2):218–22. doi: 10.1001/archopht.122.2.218. [DOI] [PubMed] [Google Scholar]
- Penfold PL, Gyory JF, Hunyor AB, Billson FA. Exudative macular degeneration and intravitreal triamcinolone. A pilot study. Australia New Zealand Journal of Ophthalmology. 1995;23(4):293–8. doi: 10.1111/j.1442-9071.1995.tb00179.x. [DOI] [PubMed] [Google Scholar]
- Ranson NT, Danis RP, Ciulla TA, Pratt L. Intravitreal triamcinolone in subfoveal recurrence of choroidal neovascularisation after laser treatment in macular degeneration. British Journal of Ophthalmology. 2002;86(5):527–9. doi: 10.1136/bjo.86.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaide RF, Sorenson J, Maranan L. Combined photodynamic therapy with verteporfin and intravitreal triamcinolone acetonide for choroidal neovascularization. Ophthalmology. 2003;110(8):1517–25. doi: 10.1016/S0161-6420(03)00544-X. [DOI] [PubMed] [Google Scholar]
- Van De Moere A, Sandhu SS, Kak R, Mitchell KW, Talks SJ. Effect of posterior juxtascleral triamcinolone acetonide on choroidal neovascular growth after photodynamic therapy with verteporfin. Ophthalmology. 2005;112(11):1896–1903. doi: 10.1016/j.ophtha.2005.06.018. [DOI] [PubMed] [Google Scholar]
Additional references
- BenEzra D, Griffin BW, Maftzir G, Sharif NA, Clark AF. Topical formulations of novel angiostatic steroids inhibit rabbit corneal neovascularization. Investigative Ophthalmology & Visual Science. 1997;38(10):1954–62. [PubMed] [Google Scholar]
- Blei F, Wilson EL, Mignatti P, Rifkin DB. Mechanism of action of angiostatic steroids: suppression of plasminogen activator activity via stimulation of plasminogen activator inhibitor synthesis. Journal of Cellular Physiology. 1993;155(3):568–78. doi: 10.1002/jcp.1041550315. [DOI] [PubMed] [Google Scholar]
- Bressler NM Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporforin: two-year results of 2 randomized clinical trials - TAP report 2. Archives of Ophthalmology. 2001;119(2):198–207. [PubMed] [Google Scholar]
- Carrasquillo KG, Ricker JA, Rigas IK, Miller JW, Gragoudas ES, Adamis AP. Controlled delivery of the anti-VEGF aptamer EYE001 with poly (lactic-co-glycolic) acid microspheres. Investigative Ophthalmology & Visual Science. 2003;44(1):290–9. doi: 10.1167/iovs.01-1156. [DOI] [PubMed] [Google Scholar]
- Clark AF, Mellon J, Li XY, Ma D, Leher H, Apte R, et al. Inhibition of intraocular tumor growth by topical application of the angiostatic steroid anecortave acetate. Investigative Ophthalmology & Visual Science. 1999;40(9):2158–62. [PubMed] [Google Scholar]
- Evans JR. Ginkgo Biloba extract for age-related macular degeneration. Cochrane Database of Systematic Reviews. 1999;(3) doi: 10.1002/14651858.CD001775. Art. No.: CD001775. [DOI] [PubMed] [Google Scholar]
- Evans JR. Risk factors for age-related macular degeneration. Progress in Retinal and Eye Research. 2001;20(2):227–53. doi: 10.1016/s1350-9462(00)00023-9. [DOI] [PubMed] [Google Scholar]
- Evans JR. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database of Systematic Reviews. 2006;(2) doi: 10.1002/14651858.CD000254.pub2. Art. No.: CD000254. [DOI] [PubMed] [Google Scholar]
- Ferris FL, 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Archives of Ophthalmology. 1984;102(11):1640–2. doi: 10.1001/archopht.1984.01040031330019. [DOI] [PubMed] [Google Scholar]
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association. 2006;94(2):130–6. [PMC free article] [PubMed] [Google Scholar]
- Jaffe GF, Yang CH, Guo H, Denny JP, Lima C, Ashton P. Safety and pharmacokinetics of an intraocular fluocinolone acetonide sustained delivery device. Investigative Ophthalmology & Visual Science. 2000;41(11):3569–75. [PubMed] [Google Scholar]
- Klein R, Klein BE, Tomany SC, Meuer SM, Huang G. Ten-year incidence and progression of age-related maculopathy: The Beaver Dam Eye Study. Ophthalmology. 2002;109(10):1767–79. doi: 10.1016/s0161-6420(02)01146-6. [DOI] [PubMed] [Google Scholar]
- Krzystolik MG, Woodcome HA, Reddy U. Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for neovascular age-related macular degeneration. Cochrane Database of Systematic Reviews. 2005;(1) doi: 10.1002/14651858.CD005139. Art. No.: CD005139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz D, Cuilla TA. Novel approaches for retinal drug delivery. Ophthalmology Clinics of North America. 2002;15(3):405–10. doi: 10.1016/s0896-1549(02)00034-2. [DOI] [PubMed] [Google Scholar]
- Macular Photocoagulation Study Group. Laser photocoagulation for juxtafoveal choroidal neovascularization: Five-year results from randomized clinical trials. Archives of Ophthalmology. 1994;112(4):500–9. [PubMed] [Google Scholar]
- Musch DC, Martin DF, Gordon JF, Davis MD, Kupperman BD The Ganciclovir Implant Study Group. Treatment of cytomegalovirus retinitis with a sustained-release ganciclovir implant. The New England Journal of Medicine. 1997;337(2):83–90. doi: 10.1056/NEJM199707103370203. [DOI] [PubMed] [Google Scholar]
- Olsen TW, Feng X, Wabner K, Contson S, Sierra DH, Folden DV, Smith ME, Cameron JD. Cannulation of the suprachoroidal space: A novel drug delivery methodology to the posterior segment. American Journal of Ophthalmology. 2006;142(5):777–87. doi: 10.1016/j.ajo.2006.05.045. [DOI] [PubMed] [Google Scholar]
- Penfold PL, Madigan MC, Gillies MC, Provis JM. Immunological and etiological aspects of macular degeneration. Progress in Retinal and Eye Research. 2001;20(3):385–414. doi: 10.1016/s1350-9462(00)00025-2. [DOI] [PubMed] [Google Scholar]
- Reddy U, Krzystolik M. Antiangiogenic therapy with interferon alfa for neovascular age-related macular degeneration. Cochrane Database of Systematic Reviews. 2006;(1) doi: 10.1002/14651858.CD005138.pub2. Art. No.: CD005138. [DOI] [PubMed] [Google Scholar]
- Sivagnanavel V, Evans JR, Ockrim Z, Chong V. Radiotherapy for neovascular age-related macular degeneration. Cochrane Database of Systematic Reviews. 2004;(3) doi: 10.1002/14651858.CD004004.pub2. Art. No.: CD004004. [DOI] [PubMed] [Google Scholar]
- TAP Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporforin: One-year results of 2 randomized clinical trials -TAP Report 1. Archives of Ophthalmology. 1999;117(10):1329–45. [PubMed] [Google Scholar]
- Tielsch JM, Friedman DS, Congdon N, Kempen J. Vision Problems in the U S Prevalence of adult vision impairment and age-related eye disease in America. Prevent Blindness America; 2002. [Google Scholar]
- TAP and VIP Study Groups. Photodynamic therapy of subfoveal choroidal neovascularization with verteporfin: Fluorescein angiographic guidelines for evaluation and treatment - TAP and VIP report No 2. Archives of Ophthalmology. 2003;121(9):1253–68. doi: 10.1001/archopht.121.9.1253. [DOI] [PubMed] [Google Scholar]
- Virgili G, Bini A. Laser photocoagulation for neovascular age-related macular degeneration. Cochrane Database of Systematic Reviews. 2007;(3) doi: 10.1002/14651858.CD004763.pub2. Art. No.: CD004763. [DOI] [PubMed] [Google Scholar]
- WHO. [accessed April 2003];Fact sheet. 1997 www.who.int/inf-fs/en/fact144.html.
- Wormald R, Evans J, Smeeth L, Henshaw K. Photodynamic therapy for neovascular age-related macular degeneration. Cochrane Database of Systematic Reviews. 2005;(4) doi: 10.1002/14651858.CD002030.pub2. Art. No.: CD002030. [DOI] [PubMed] [Google Scholar]
- Yasukawa T, Kimura H, Tabata Y, Miyamoto H, Honda Y, Ikada Y, et al. Targeted delivery of anti-angiogenic agent TNP-470 using water soluble polymer in the treatment of choroidal neovascularization. Investigative Ophthalmology & Visual Science. 1999;40(11):2690–6. [PubMed] [Google Scholar]


