Modulation of orexin and its effects on sleep/wakefulness affect amyloid-β pathology in the brain of mouse models for Alzheimer’s disease.
Abstract
Age-related aggregation of amyloid-β (Aβ) is an upstream pathological event in Alzheimer’s disease (AD) pathogenesis, and it disrupts the sleep–wake cycle. The amount of sleep declines with aging and to a greater extent in AD. Poor sleep quality and insufficient amounts of sleep have been noted in humans with preclinical evidence of AD. However, how the amount and quality of sleep affects Aβ aggregation is not yet well understood. Orexins (hypocretins) initiate and maintain wakefulness, and loss of orexin-producing neurons causes narcolepsy. We tried to determine whether orexin release or secondary changes in sleep via orexin modulation affect Aβ pathology. Amyloid precursor protein (APP)/Presenilin 1 (PS1) transgenic mice, in which the orexin gene is knocked out, showed a marked decrease in the amount of Aβ pathology in the brain with an increase in sleep time. Focal overexpression of orexin in the hippocampus in APP/PS1 mice did not alter the total amount of sleep/wakefulness and the amount of Aβ pathology. In contrast, sleep deprivation or increasing wakefulness by rescue of orexinergic neurons in APP/PS1 mice lacking orexin increased the amount of Aβ pathology in the brain. Collectively, modulation of orexin and its effects on sleep appear to modulate Aβ pathology in the brain.
Age-related aggregation of amyloid-β (Aβ) is an upstream pathological event in Alzheimer’s disease (AD) pathogenesis (Holtzman et al., 2011; Sperling et al., 2011). As the accumulation and aggregation of Aβ in the brain is known to develop ∼10–15 yr before the initial symptoms of AD, understanding the factors that lead to Aβ aggregation are likely to be important in delaying the onset of this pathology and delaying/preventing AD (Holtzman et al., 2011; Sperling et al., 2011). Diverse lines of in vitro and in vivo studies have shown that synaptic activity and specifically synaptic vesicle release is coupled with presynaptic Aβ release (Kamenetz et al., 2003; Cirrito et al., 2008; Bero et al., 2011; Roh et al., 2012). In addition, sleep plays a role in the regulation of synaptic weight in the brain such that sleep appears to downscale the slow wave activity of the brain caused by accumulated load of synaptic potentiation during wakefulness (Vyazovskiy et al., 2009). Poor sleep quality and insufficient amounts of sleep have been noted in humans with preclinical evidence of AD (Roh et al., 2012; Ju and Holtzman, 2013; Ju et al., 2014). These observations suggest that understanding how integrated synaptic and network activity as measured by the sleep–wake cycle regulates Aβ may provide novel insights into AD pathogenesis. However, how the amount and quality of sleep affect Aβ aggregation is not yet well understood (Ju et al., 2014). Orexins (hypocretins) initiate and maintain wakefulness, and loss of orexin-producing neurons causes narcolepsy (de Lecea et al., 1998; Chemelli et al., 1999).
Levels of soluble Aβ in the extracellular interstitial fluid (ISF) of the hippocampus in mice are dynamically and positively associated with minutes awake per hour and negatively associated with time asleep (Kang et al., 2009). In addition, the sleep–wake cycle also affects the Aβ pathology in the brain. A study in mice showed that intracerebral administration of orexin can acutely increase both wakefulness and Aβ levels and systemic treatment with an orexin receptor antagonist decreased Aβ deposition in amyloid precursor protein (APP) transgenic mouse models (Kang et al., 2009). Pharmacological experiments suggest that orexin and the sleep–wake cycle appear to be related to regulation of Aβ levels. We sought to determine for the first time whether genetic manipulation of orexin has similar effects as pharmacological manipulation and, importantly, whether orexin is influencing Aβ levels and Aβ pathology directly via orexin signaling or indirectly via its effects on the sleep–wake cycle.
RESULTS AND DISCUSSION
Marked reduction of Aβ pathology in the APP/Presenilin 1 (PS1)/Orexin knockout (OR−/−) mice
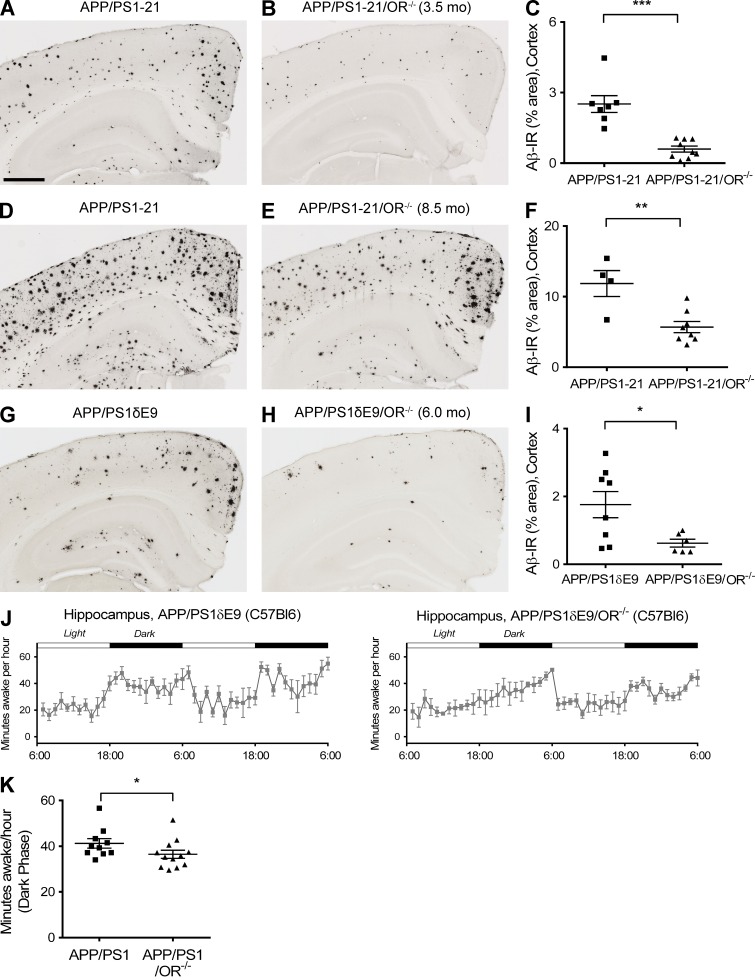
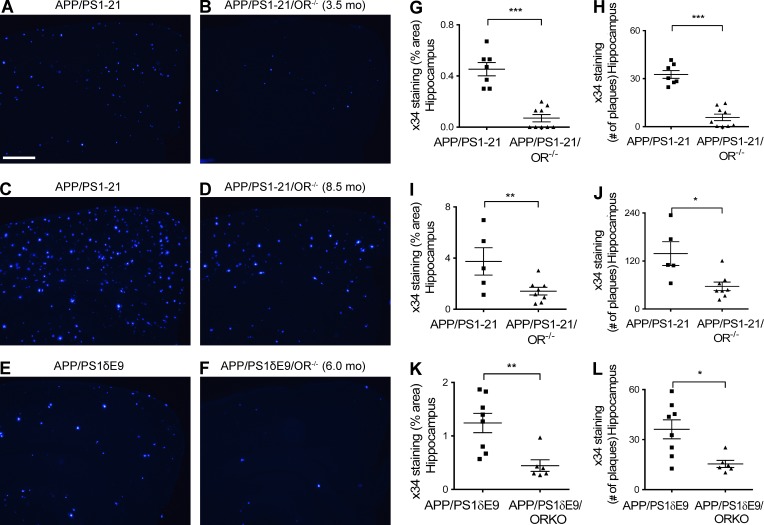
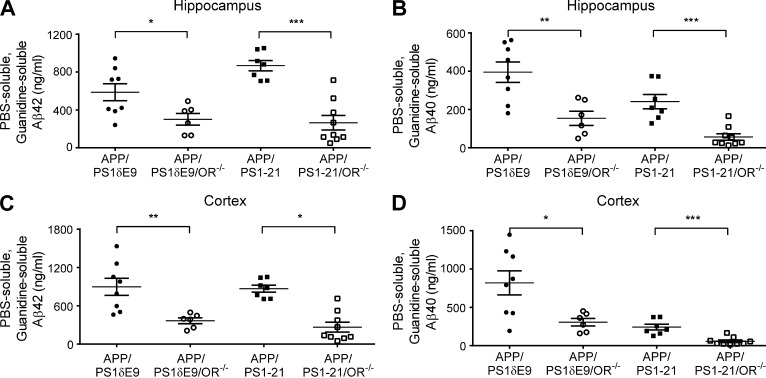
To more specifically address the role of orexin in regulating Aβ levels and pathology, we used APP/PS1-21 transgenic mice (Radde et al., 2006), which begin to develop Aβ deposition at ∼2 mo of age, and bred them to OR−/− mice (de Lecea et al., 1998; Chemelli et al., 1999). When orexin was knocked out in APP/PS1-21 mice (APP/PS1-21/OR−/− mice), there was a marked reduction of Aβ plaque pathology at 3.5 mo as well as at 8.5 mo of age compared with APP/PS1-21 mice (Fig. 1, A–F). Similar patterns of reduction in Aβ deposition were also seen when orexin was knocked out in another mouse model that develops Aβ deposition, APP/PS1δE9 mice (Jankowsky et al., 2004), which begin to develop Aβ deposition at 4 mo of age. APP/PS1δE9/OR−/− mice had a marked reduction in Aβ deposition at 6 mo of age compared with APP/PS1δE9 mice expressing orexin (Fig. 1, G–I). APP/PS1δE9/OR−/− mice demonstrated significantly decreased wakefulness by 11.5% during the 12-h dark phase at 3 mo of age compared with mice expressing orexin, before the onset of Aβ pathology in the brain (Fig. 1, J and K). The difference in wakefulness was more prominent during the early phases of the dark period (6 p.m. to 12 a.m. with an 18.3% difference). Fibrillar Aβ deposition assessed by X-34 staining was also markedly reduced in the brains of APP/PS1-21/OR−/− mice and APP/PS1δE9/OR−/− mice compared with mice expressing orexin (Fig. 2). We also assessed Aβ levels biochemically. The total amount of both Aβ40 and Aβ42 solubilized by 5M guanidine was also significantly reduced in the hippocampus as well as in the cortex in APP/PS1-21/OR−/− mice compared with APP/PS1-21 mice. Similar patterns of pathological Aβ reduction were also noted in APP/PS1δE9/OR−/− mice compared with APP/PS1δE9 mice (Fig. 3).
Figure 1.
Marked reduction of Aβ pathology in the APP/PS1/OR−/− mice compared with APP/PS1 mice. (A–I) The amount of Aβ pathology was noted at 3.5 (A–C) and 8.5 mo (D–F) in APP/PS1-21 mouse line and at 6 mo in APP/PS1δE9 mouse line (G–I). (J and K) Amount of wakefulness at 3 mo of age before the onset of Aβ pathology in the brain was compared between APP/PS1δE9 and APP/PS1δE9/OR−/− mice. Each mouse was investigated independently one time. Data are presented as mean ± SEM (n = 4–12 in each group, two-tailed Student’s t test in C, F, and I and Mann-Whitney test in J). *, P < 0.05; **, P < 0.01; and ***, P < 0.001. Bar, 500 µm.
Figure 2.
Marked reduction of amyloid pathology in the APP/PS1/OR−/− mice compared with APP/PS1 mice. (A–L) Amyloid pathology measured by Aβ immunoreactivity and number of amyloid plaques after X-34 staining is noted at 3.5 (A, B, G, and H) and 8.5 mo (C, D, I, and J) in the APP/PS1-21 mouse line and at 6 mo in the APP/PS1δE9 mouse line (E, F, K, and L). Each mouse was investigated independently one time. Data are presented as mean ± SEM (n = 5–9 in each group, two-tailed Student’s t test). *, P < 0.05; **, P < 0.01; and ***, P < 0.001. Bar, 100 µm.
Figure 3.
Strong reduction in Aβ species in APP/PS1 mice lacking orexin. (A–D) The amount of PBS-soluble and guanidine-soluble forms of Aβ40 and Aβ42 were compared in APP/PS1δE9/OR−/− and APP/PS1δE9 mice (left two columns) and in APP/PS1-21/OR−/− and APP/PS1-21 mice (right two columns). Results were obtained from the hippocampus (A and B) and from the cortex (C and D) of each group of mice. Each mouse was investigated independently one time. All samples were measured in triplicate. Data are presented as mean ± SEM (n = 5–9 in each group, two-tailed Student’s t test). *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
Reversal of Aβ pathology by modulation of sleep rather than focal overexpression of orexin
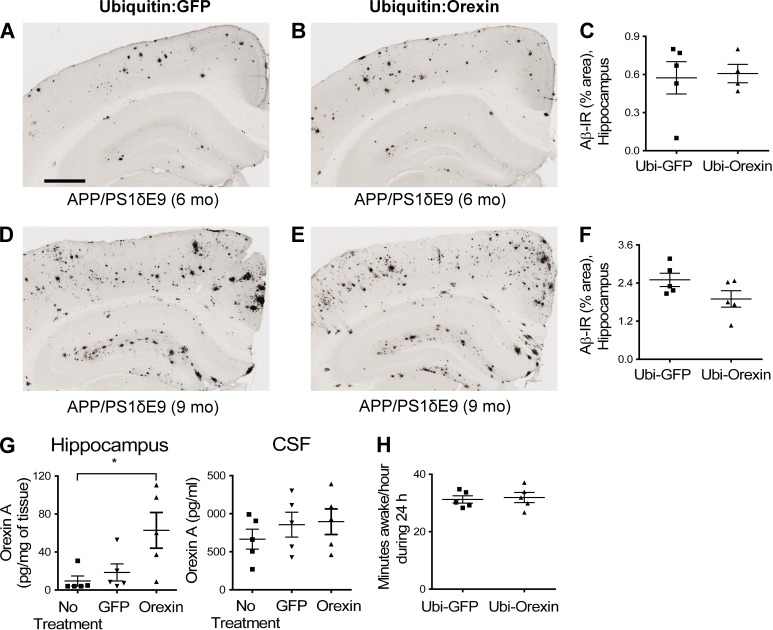
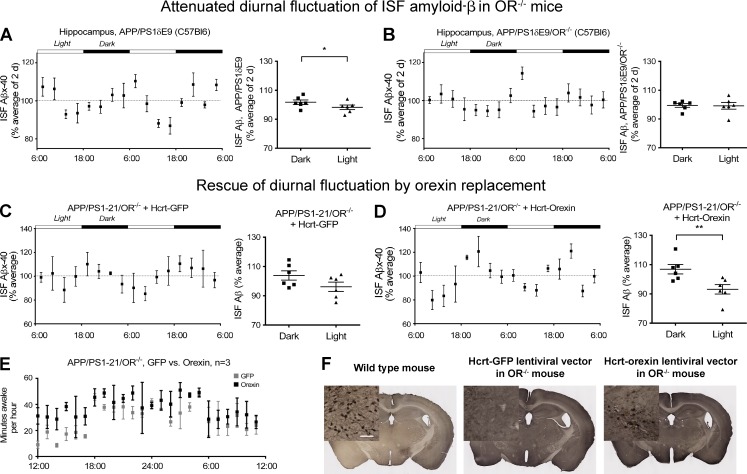
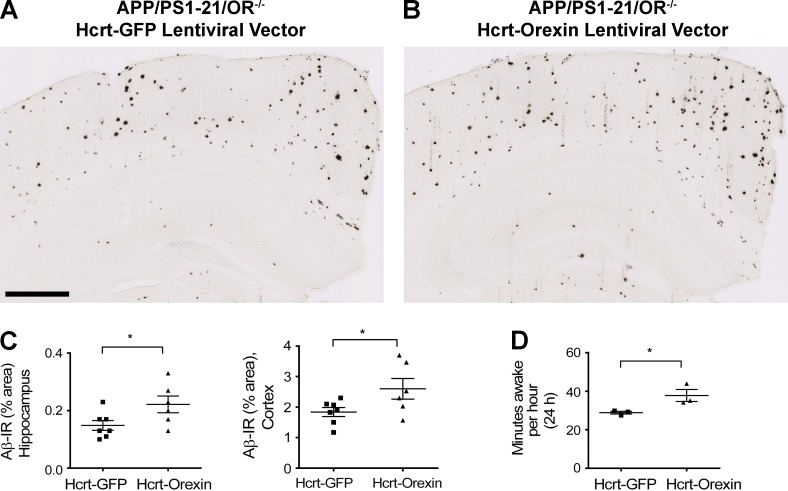
Orexin receptors are present on hippocampal neurons, so we focally overexpressed orexin by stereotaxic injection of a lentiviral vector expressing orexin into the hippocampus of APP/PS1δE9 mice to determine whether local orexin signaling was directly leading to the effects observed on Aβ deposition. This treatment resulted in no changes in levels of Aβ deposition in the brain nor changes in sleep time despite focal increases in the level of orexin in the hippocampus (Fig. 4). To further investigate whether the reduction in Aβ pathology in orexin knockout mice is mainly caused by the effects of orexin signaling on sleep, expression of orexin specifically in orexinergic neurons was instituted in 1.5-mo-old APP/PS1-21/OR−/− mice. We used an orexin lentiviral vector in which orexin is driven by the hypocretin/orexin promoter. After injection of this vector bilaterally in the hypothalamus, expression of orexin in orexinergic neurons was restored (Fig. 5 F). 6 wk after injection with the hypocretin promoter–driven orexin lentiviral vector, APP/PS1-21/OR−/− mice had a significantly increased amount of wakefulness as well as increased amount of Aβ deposition in the hippocampus and cortex compared with mice injected with a control vector (Figs. 5 E and 6). Collectively, results from focal and generalized orexin expression in different brain regions using different promoters strongly support the idea that the reduction in Aβ pathology noted in APP/PS1/OR−/− mouse models is caused by secondary changes in sleep time induced by hypothalamic expression of orexin rather than by alteration in orexin signaling in hippocampus and other brain regions.
Figure 4.
No changes in Aβ deposition and amount of wakefulness by focal overexpression of orexin. (A–F) Amount of Aβ pathology was compared after focal injection of ubiquitin-driven orexin lentiviral vector versus ubiquitin-driven GFP lentiviral vector in the hippocampus from 3 to 6 mo (A–C) or 5 to 9 mo (D–F) in APP/PS1δE9 mice. (G) Levels of orexin in the hippocampus and CSF were compared after focal injection of orexin or GFP lentiviral vector driven by ubiquitin promoter. (H) The amount of wakefulness in APP/PS1δE9 mice was compared after focal injection of ubiquitin-driven orexin lentiviral vector versus ubiquitin-driven GFP lentiviral vector. Each mouse was investigated independently one time. Data are presented as mean ± SEM (n = 4–5 in each group, two-tailed Student’s t test in C, F, and H; one-way ANOVA, followed by Tukey’s post hoc test in G). *, P < 0.05. Bar, 500 µm.
Figure 5.
Restoration of diurnal fluctuation of ISF Aβ40 via rescue of orexin expression in APP/PS1/OR−/− mice. (A and B) Diurnal fluctuation of ISF Aβ40 in young APP/PS1δE9 mice was compared with the diurnal fluctuation in the same mice when orexin was knocked out (APP/PS1δE9/OR−/− mice). (C–E) Diurnal fluctuation of ISF Aβ40 in the hippocampus and minutes awake per hour of APP/PS1-21/OR−/− mice treated with hypocretin/orexin promoter–driven lentiviral vector overexpressing orexin in the hypothalamus bilaterally were compared with mice treated with the same lentiviral vector expressing GFP. (F) Expression of orexin by immunostaining in orexinergic neurons in the hypothalamus of APP/PS1-21/OR−/− mice treated with hypocretin/orexin promoter–driven lentiviral vector expressing GFP or orexin (middle and right) compared with wild-type mice (left). Each mouse was investigated independently one time. All ISF Aβ measurements were performed in duplicate. Data are presented as mean ± SEM (n = 6 in each group, two-tailed Student’s t test in A–D and Mann–Whitney test in E). *, P < 0.05; and **, P < 0.01. Bar, 100 µm.
Figure 6.
Increase in Aβ deposition and wakefulness by rescue of orexin expression in APP/PS1/OR−/− mice via expression of orexin with lentiviral vector driven by hypocretin/orexin promoter in the bilateral hypothalamus. (A–C) Amount of Aβ pathology in the hippocampus and cortex of the APP/PS1-21/OR−/− mice after bilateral injection of hypocretin promoter–driven orexin lentiviral vector in the hypothalamus of the APP/PS1/OR−/− mice was compared with results from injection of the same lentiviral vector overexpressing GFP. (D) The amount of wakefulness as measured by polysomnography was compared between the same groups of mice. Each mouse was investigated independently one time. Data are presented as mean ± SEM (n = 6–7 in each group, two-tailed Student’s t test in C; n = 3 in each group, Mann–Whitney test in D). *, P < 0.05. Bar, 500 µm.
We also analyzed the functional effect of APP/PS1/OR−/− mice in which orexin expression was rescued in orexinergic neurons by measuring the diurnal fluctuation of Aβ in the extracellular space using in vivo microdialysis. In APP/PS1-21/OR−/− mice injected with a lentiviral vector in which the hypocretin/orexin promoter is driving orexin expression bilaterally in the hypothalamus, restoration of diurnal fluctuation of ISF Aβx-40 was found, suggesting possible functional rescue of orexinergic neurons in the hypothalamus bilaterally (Fig. 5, A–D). There was, however, no difference in the amplitude of circadian rhythms assessed by cosinor analysis between the groups (Fig. 5, C and D). This is likely caused by the number of mice used in this experiment.
Sleep deprivation induced Aβ pathology in OR−/− mice
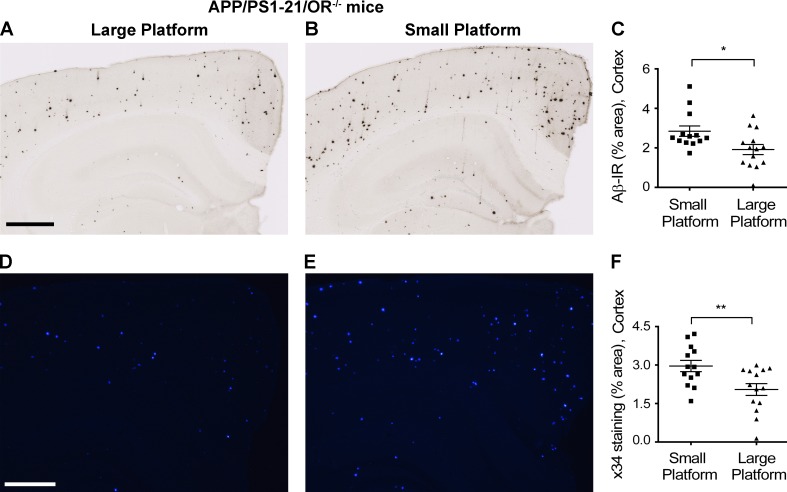
Finally, we wanted to determine whether sleep deprivation increased Aβ deposition in the absence of orexin, as previously observed in the presence of orexin (Kang et al., 2009). We sleep-deprived APP/PS1-21/OR−/− mice using the platform above water method as previously described (Kang et al., 2009). Interestingly, relative to mice that were placed on a large platform for 20 h per day for 3 wk, where they can maintain a normal sleep–wake cycle, mice placed on a small platform that experienced sleep deprivation had a significant increase in Aβ plaque pathology (Fig. 7). Collectively, these findings strongly support the idea that the amount of sleep, as modulated by hypothalamic but not local orexin signaling, is crucial in regulating Aβ metabolism and AD pathogenesis.
Figure 7.
Increased Aβ deposition with chronic sleep deprivation in APP/PS1-21/OR−/− mice. (A–F) The amount of Aβ plaques stained with HJ3.4B (A–C) and fibrillar Aβ stained with X-34 (D–F) were compared between APP/PS1-21/OR−/− mice exposed to a large platform and a small platform. Sleep deprivation experiments were performed using a small and large platform in a cage with water on the bottom, where a mouse cannot sleep on the small platform because of its size, whereas they can maintain a normal sleep–wake cycle on the larger platform. Mice exposed to small platforms (n = 3) and to large platforms (n = 3) were analyzed together within a set of experiments. The results are the sum of five repeats in different mice. Each mouse was investigated independently. Data are presented as mean ± SEM (n = 13–14 in each group, two-tailed Student’s t test). *, P < 0.05; and **, P < 0.01. Bars: (A) 500 µm; (D) 100 µm.
Increasing evidence suggests that neuronal activity in many brain regions is physiologically regulated by the sleep–wake cycle (Bero et al., 2011). It has been noted in animals that decreases in sleep time and increases in amount of Aβ levels and deposition have a reciprocal relationship (Kang et al., 2009; Roh et al., 2012; Ju et al., 2014). Intriguingly, recent results from humans also indicate that the quality or amount of sleep is inversely associated with the amount of Aβ measured in the cerebrospinal fluid (CSF; Ju and Holtzman, 2013; Ooms et al., 2014) and that the amount of sleep inversely correlates with fibrillar forms of Aβ quantified by amyloid PET scan (Spira et al., 2013). Treatment with active vaccination with Aβ42 prevented development of Aβ pathology and abnormalities of the sleep–wake cycle in APP/PS1δE9 mice (Roh et al., 2012). This demonstrated that Aβ aggregation and accumulation caused a sleep abnormality. In this study, the opposite is also true, i.e., an increase in sleep time caused by orexin deficiency resulted in a decrease in the amount of Aβ pathology that developed in the brain. Collectively, these findings suggest that increasing certain types of sleep should be considered as a therapeutic target to decrease Aβ-associated AD pathogenesis. Possible side effects of sleep induction such as excessive daytime sleepiness or cataplexy driven by knocking out or decreasing orexin function need to be considered in the process of designing new therapeutic interventions targeting sleep.
It is notable that a 12% increase in the sleep time during the dark phase was associated with >50% reduction in the development of Aβ pathology in the brain. This matches well with the previous findings that an ∼15% decrease in the amount of soluble forms of Aβ in the extracellular space resulted in an up to 50% reduction of Aβ plaque growth and formation in the brain (Yan et al., 2009). It suggests that a small increase in NREM sleep or small improvement in sleep quality and the resulting decrease in Aβ release into the extracellular space could be beneficial for the delay or attenuation of emergence of Aβ-associated pathologies in AD. Although our data support a mechanism whereby decreased neuronal activity during sleep results in a decrease in ISF Aβ, we cannot yet rule out the contribution of increased Aβ clearance from the ISF as proposed by Xie et al. (2013). It will be important in future experiments to determine whether different methods of sleep or orexin modulation will trigger new methods of intervention targeting Aβ pathophysiology in the brain. Given the association between neuronal activity and release of tau in the brain, further studies to determine whether the sleep–wake cycle also influences this key molecule in the pathogenesis of AD and other neurodegenerative diseases will be critical (Yamada et al., 2014). It is important to note that other systems involved in the sleep–wake cycle are also likely to affect the pathophysiology of AD. Even though we concluded that not orexin itself but rather the secondary changes in the sleep–wake cycle affect AD pathophysiology in brains of mice, modulation of other molecules that increase or decrease wakefulness such as noradrenergic and GABAergic modulation are also likely to influence AD pathophysiology (Matsuki et al., 2009; Carter et. al, 2010).
The location of Aβ deposition in the human brain overlaps very closely with a brain network called the “default mode” network, which is made up of brain regions that appear to have the highest measures of aerobic glycolysis when individuals are at rest (Raichle et al., 2001; Vlassenko et al., 2010). In addition to the global reduction in neuronal activity resulting from an increase in sleep time as shown here, focal alteration of brain activity, connectivity, or even focal sleep modulation through pharmacologic or other methods may also merit consideration as potential therapies in AD and other neurodegenerative disorders (Nir et al., 2011; Vyazovskiy et al., 2011). Such manipulation may be a powerful way to modulate Aβ levels and pathology by taking advantage of normal brain physiology.
MATERIALS AND METHODS
Mice.
All experiments were approved by the Animal Studies Committee at Washington University in St. Louis. Female APPswe/PS1δE9 mice on a B6 background (The Jackson Laboratory) were crossed with OR−/− mice on a C57BL/6 background to produce APPswe/PS1δE9 hemizygous/OR+/− mice. Then, APPswe/PS1δE9; OR+/− mice were crossed with OR−/− mice to obtain APPswe/PS1δE9 hemizygous OR−/− mice (APPswe/PS1δE9/OR−/−). APP/PS1-21/OR−/− mice were obtained using the same methods beginning from crossing the APP/PS1-21 mice (C57BL/6) with OR−/− mice (C57BL/6). APP/PS1-21 mice (C57BL/6) were provided by M. Jucker at the University of Tubingen (Tubingen, Germany).
ELISA.
Microdialysis samples were analyzed for Aβx-40, Aβx-42, or Aβ1-x with sandwich ELISAs. In brief, Aβx-40, Aβx-42, and Aβ1-x were captured with monoclonal antibodies targeted against amino acids 35–40 (HJ2; Cirrito et al., 2003), 37–42 (HJ7.4; Roh et al., 2012), and 13–28 (m266; Cirrito et al., 2003) of Aβ, respectively. For Aβx-40 and Aβx-42 assays, a biotinylated central domain monoclonal antibody (HJ5.1; Koenigsknecht-Talboo et al., 2008) followed by streptavidin–poly-HRP40 (Fitzgerald) was used for detection. For Aβ1-x assays, a biotinylated N-terminal domain monoclonal antibody (3D6; Koenigsknecht-Talboo et al., 2008) followed by streptavidin–poly-HRP20 (Fitzgerald) was used. The antibodies m266 and 3D6 were gifts from Eli Lilly. All assays were developed with Super Slow ELISA TMB (Sigma-Aldrich) and read on a Bio-Tek Synergy 2 plate reader at 650 nm. Hippocampal tissue lysates were analyzed for Aβ1-40 and Aβ1-42 using a denaturing, sandwich ELISA specific for human Aβ1-40 and Aβ1-42 after solubilizing the tissue in 5M guanidine as previously described (Cirrito et al., 2003).
Sleep–wake monitoring.
Polysomnographic sleep–wake cycle analysis of mice was performed as described previously (Bero et al., 2011; Roh et al., 2012). In brief, electroencephalogram (EEG) and electromyogram (EMG) electrodes were implanted simultaneously with a microdialysis guide cannula. For EEG recording, two stainless steel screws attached to wire electrodes were placed over the right frontal bone (bregma +1.0 mm, 1.5 mm lateral to midline) and the right parietal bone (bregma −3.0 mm, 2.5 mm lateral to midline). Two wire electrodes were directly inserted into the neck musculature for EMG recording. The ground electrode was placed on the skull over the cerebellum. Insulated leads from the EEG and EMG electrodes were soldered to a mini-connector. After surgery, mice were housed in 12-h light/12-h dark for 2 wk before recording began. To monitor the sleep–wake cycle, we transferred the mice to recording cages maintained in 12-h light/12-h dark conditions (light phase began at 6 a.m.), and we connected the mini-connector to flexible recording cables. Mice were habituated to the recording cages for 3 d. At the end of the habituation period, EEG and EMG recording began simultaneously with collection of microdialysis samples. EEG and EMG signals were displayed on a monitor and stored in a computer for analysis of sleep states. EEG and EMG recordings were assessed with a P511K AC preamplifier (Grass-Telefactor Instruments), digitized with a DigiData 1440A Data Acquisition System (Molecular Devices), and recorded digitally with pCLAMP 10.2 (Molecular Devices). EEG and EMG signals were filtered (EEG: high pass 1 Hz, low pass 30 Hz; EMG: high pass 10 Hz, low pass 100 Hz) and used to identify vigilance states. EEG and EMG recordings were scored semiautomatically with sleep-scoring software (SleepSign; Kissei Comtec Co. Ltd.) and binned into 10-s epochs as wakefulness, REM sleep, and NREM sleep on the basis of standard criteria of rodent sleep. Semiautomatic sleep scoring was visually inspected and corrected when appropriate. The automatic analysis and visual inspection was performed in a blinded state to the genotype and age of mice. Chronic sleep deprivation was performed as previously described (Kang et al., 2009).
Plaque deposition analyses.
After mice were perfused with PBS transcardially, brains were removed, fixed in 4% paraformaldehyde for 24 h (4°C), cryoprotected with 30% sucrose in PBS (4°C), frozen in powdered dry ice, and cut on a freezing sliding microtome. Serial coronal sections (50 µm thick) were collected from the genu of the corpus callosum to caudal hippocampus. Sections (each separated by 300 µm) were stained with biotinylated HJ3.4 (Aβ 1–13) antibody to visualize Aβ-immunopositive plaques or X-34 dye to visualize fibrillar amyloid plaques (Roh et al., 2012). Immunostained sections and X-34–stained sections were imaged with a NanoZoomer slide scanner (Hamamatsu Photonics). Quantitative analysis of percent area covered by HJ3.4- or X-34–positive staining was performed using a neurostereological method as described previously (Kim et al., 2009). In brief, images of immunostained sections were exported with NDP viewer software (Hamamatsu Photonics), converted to 8-bit grayscale with ACDSeePro 2 software (ACD Systems), thresholded to highlight Aβ-specific staining, and analyzed with ImageJ software (National Institutes of Health). Images of X-34–stained sections were converted to 16-bit grayscale, thresholded to highlight X-34–specific staining, and analyzed with ImageJ software. A mouse brain atlas was used to identify hippocampal sections relative to bregma (−1.7, −2.0, −2.3 mm) for quantitative analysis of immuno- and X-34–positive staining as described previously (Roh et al., 2012).
Overexpression of orexin by lentiviral vector and measurement of orexin.
Lentiviral vectors were prepared in the Hope Center Viral Vectors Core. For focal overexpression of orexin, orexin or GFP driven by an ubiquitin promoter was overexpressed via lentivirus in the hippocampus bilaterally in APPswe/PS1δE9 mice (B6C3) starting at 5 mo of age, and the amount of sleep and Aβ pathology from both groups were compared at 9 mo. To investigate the effect of focal overexpression of orexin before Aβ plaque pathology was formed in the hippocampus, the same lentiviral injection experiments were performed in APPswe/PS1δE9 mice (B6C3) with viral injection at 3 mo and pathological assessment at 6 mo. For rescue of orexinergic neurons, orexin or GFP driven by an hypocretin/orexin promoter was expressed via lentivirus in the bilateral hypothalamus in APPswe/PS1-21/Orexin knockout mice (C57BL/6) starting at 1.5 mo of age, and the amount of sleep and Aβ pathology from both groups were compared at 3 mo. The mouse hypocretin/orexin promoter was provided by L. de Lecea. Brains were sectioned on a freezing microtome at 50-µm thickness. Floating brain sections at a 1:6 series were processed for anti–orexin-A and anti–glial fibrillary acidic protein (GFAP) immunohistochemistry as follows: tissue was washed in Tris-buffered saline (TBS), then quenched in 3% hydrogen peroxide solution for 10 min, washed again in TBS, and then incubated in 0.25% Triton-X solution plus 5% normal goat serum for 30 min. Finally, the slides were incubated in primary antibody plus 5% normal goat serum, anti–orexin-A at 1:10,000 overnight (rabbit anti–mouse Orexin-A; Phoenix Pharmaceuticals, Inc.) or anti-GFAP at 1:1,000 (polyclonal chicken anti-GFAP; EMD Millipore). The next day, sections were incubated in a solution containing goat anti–rabbit IgG biotinylated secondary antibody for orexin or donkey anti–chicken secondary antibody for GFAP at 1:1,000 dilution, and signal was then amplified using the Vectastain ABC kit at 1:400 (Vector Laboratories) followed by visualization with DAB-nickel. Sections were carefully mounted onto glass microscope slides, air-dried, and then dehydrated in ascending ethanols and coverslipped with Cytoseal. CSF and brain tissue lysate samples were analyzed for orexin-A (human, rat, mouse, porcine, bovine, and ovine) levels using a commercially available fluorescent enzymatic immunoassay (EIA) kit (Phoenix Pharmaceuticals). All samples were assayed in duplicate, and the mean of the two values was reported.
In vivo microdialysis.
In vivo microdialysis to assess Aβ and lactate in the brain ISF of awake, freely behaving mice was performed as described previously (Cirrito et al., 2003; Roh et al., 2012). In brief, guide cannulae (BR style; Bioanalytical Systems) were stereotaxically implanted into hippocampus (bregma −3.1 mm, 2.5 mm lateral to midline, and 1.2 mm below the dura at a 12° angle). Probe placement in the regions of interest was confirmed by cresyl violet staining. Microdialysis probes (2 mm; 38-kD molecular size cutoff; BR style; Bioanalytical Systems) were connected to a syringe pump (Stoelting Co.), and artificial CSF, pH 7.35, containing 1.3 mM CaCl2, 1.2 mM MgSO4, 3 mM KCl, 0.4 mM KH2PO4, 25 mM NaHCO3, and 122 mM NaCl was continuously perfused through the microdialysis probe. For measurement of Aβx-40, a flow rate of 0.5 µl/min was used. Guide cannulae were implanted 2 wk before the beginning of microdialysis. After insertion of the microdialysis probe, mice were habituated to a 12-h light/dark cycle for three more days. On the fourth day, samples were collected and stored for analyses.
Statistical analysis.
Statistical significance was determined by two-tailed Student’s t test if the datasets fulfilled the normality test (Kolmogorov–Smirnov test). When the dataset did not meet the assumptions of a parametric test, Mann–Whitney rank sum test was performed. One-way ANOVA followed by Tukey’s post hoc test for multiple comparisons was performed if the datasets fulfilled the equal variance test (Levene’s test) and normality test (Kolmogorov–Smirnov test). All statistical analyses were performed with Prism version 4.0 for Windows (GraphPad Software) and SPSS 15.0 for Windows (SPSS Inc.). Values were accepted as significant if P < 0.05.
Acknowledgments
We thank Eli Lilly and Co. for providing m266 and biotinylated 3D6 anti-Aβ antibodies. We thank M. Lim, E.S. Musiek, E.D. Herzog, and S. Maloney for their advice and help for sleep studies in animals and R. Perez in the Hope Center Animal Surgery Core for animal surgeries.
This work was supported by the American Academy of Neurology Clinical Research Training Fellowship, the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2013R1A1A1012925), NRF MRC grant funded by the Korean government (MSIP; 2008-0062286), the Korea Institute of Science and Technology Institutional Program (2E24242-13-110), grants (2014-0783, 2014-7203, and 2014-9077) from the Asan Institute for Life Sciences (to J.H. Roh), an Ellison Medical Foundation Senior Scholar Award (to D.M. Holtzman), P01NS074969 (to D.M. Holtzman), R01NS090934 (to D.M. Holtzman), P30NS057105 (to D.M. Holtzman), the JPB Foundation, and the Cure Alzheimer’s Fund (to D.M. Holtzman).
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- Aβ
- amyloid-β
- AD
- Alzheimer’s disease
- APP
- amyloid precursor protein
- CSF
- cerebrospinal fluid
- EEG
- electroencephalogram
- EMG
- electromyogram
- GFAP
- glial fibrillary acidic protein
- ISF
- interstitial fluid
References
- Bero A.W., Yan P., Roh J.H., Cirrito J.R., Stewart F.R., Raichle M.E., Lee J.M., and Holtzman D.M.. 2011. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat. Neurosci. 14:750–756 10.1038/nn.2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M.E., Yizhar O., Chikahisa S., Nguyen H., Adamantidis A., Nishino S., Deisseroth K., and de Lecea L.. 2010. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 13:1526–1533 10.1038/nn.2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli R.M., Willie J.T., Sinton C.M., Elmquist J.K., Scammell T., Lee C., Richardson J.A., Williams S.C., Xiong Y., Kisanuki Y., et al. 1999. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 98:437–451 10.1016/S0092-8674(00)81973-X [DOI] [PubMed] [Google Scholar]
- Cirrito J.R., May P.C., O’Dell M.A., Taylor J.W., Parsadanian M., Cramer J.W., Audia J.E., Nissen J.S., Bales K.R., Paul S.M., et al. 2003. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-β metabolism and half-life. J. Neurosci. 23:8844–8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito J.R., Kang J.E., Lee J., Stewart F.R., Verges D.K., Silverio L.M., Bu G., Mennerick S., and Holtzman D.M.. 2008. Endocytosis is required for synaptic activity-dependent release of amyloid-β in vivo. Neuron. 58:42–51 10.1016/j.neuron.2008.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L., Kilduff T.S., Peyron C., Gao X., Foye P.E., Danielson P.E., Fukuhara C., Battenberg E.L., Gautvik V.T., Bartlett F.S. II, et al. 1998. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. USA. 95:322–327 10.1073/pnas.95.1.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman D.M., Goate A., Kelly J., and Sperling R.. 2011. Mapping the road forward in Alzheimer’s disease. Sci. Transl. Med. 3:114ps48 10.1126/scitranslmed.3003529 [DOI] [PubMed] [Google Scholar]
- Jankowsky J.L., Fadale D.J., Anderson J., Xu G.M., Gonzales V., Jenkins N.A., Copeland N.G., Lee M.K., Younkin L.H., Wagner S.L., et al. 2004. Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: evidence for augmentation of a 42-specific γ secretase. Hum. Mol. Genet. 13:159–170 10.1093/hmg/ddh019 [DOI] [PubMed] [Google Scholar]
- Ju Y.E., and Holtzman D.M.. 2013. Sleep evaluation by actigraphy for patients with Alzheimer disease—reply. JAMA Neurol. 70:1074–1075 10.1001/jamaneurol.2013.3490 [DOI] [PubMed] [Google Scholar]
- Ju Y.E., Lucey B.P., and Holtzman D.M.. 2014. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat Rev Neurol. 10:115–119 10.1038/nrneurol.2013.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenetz F., Tomita T., Hsieh H., Seabrook G., Borchelt D., Iwatsubo T., Sisodia S., and Malinow R.. 2003. APP processing and synaptic function. Neuron. 37:925–937 10.1016/S0896-6273(03)00124-7 [DOI] [PubMed] [Google Scholar]
- Kang J.E., Lim M.M., Bateman R.J., Lee J.J., Smyth L.P., Cirrito J.R., Fujiki N., Nishino S., and Holtzman D.M.. 2009. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science. 326:1005–1007 10.1126/science.1180962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Castellano J.M., Jiang H., Basak J.M., Parsadanian M., Pham V., Mason S.M., Paul S.M., and Holtzman D.M.. 2009. Overexpression of low-density lipoprotein receptor in the brain markedly inhibits amyloid deposition and increases extracellular A β clearance. Neuron. 64:632–644 10.1016/j.neuron.2009.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsknecht-Talboo J., Meyer-Luehmann M., Parsadanian M., Garcia-Alloza M., Finn M.B., Hyman B.T., Bacskai B.J., and Holtzman D.M.. 2008. Rapid microglial response around amyloid pathology after systemic anti-Aβ antibody administration in PDAPP mice. J. Neurosci. 28:14156–14164 10.1523/JNEUROSCI.4147-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuki T., Nomiyama M., Takahira H., Hirashima N., Kunita S., Takahashi S., Yagami K., Kilduff T.S., Bettler B., Yanagisawa M., and Sakurai T.. 2009. Selective loss of GABAB receptors in orexin-producing neurons results in disrupted sleep/wakefulness architecture. Proc. Natl. Acad. Sci. USA. 106:4459–4464 10.1073/pnas.0811126106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y., Staba R.J., Andrillon T., Vyazovskiy V.V., Cirelli C., Fried I., and Tononi G.. 2011. Regional slow waves and spindles in human sleep. Neuron. 70:153–169 10.1016/j.neuron.2011.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms S., Overeem S., Besse K., Rikkert M.O., Verbeek M., and Claassen J.A.. 2014. Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol. 71:971–977 10.1001/jamaneurol.2014.1173 [DOI] [PubMed] [Google Scholar]
- Radde R., Bolmont T., Kaeser S.A., Coomaraswamy J., Lindau D., Stoltze L., Calhoun M.E., Jäggi F., Wolburg H., Gengler S., et al. 2006. Aβ42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 7:940–946 10.1038/sj.embor.7400784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., and Shulman G.L.. 2001. A default mode of brain function. Proc. Natl. Acad. Sci. USA. 98:676–682 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh J.H., Huang Y., Bero A.W., Kasten T., Stewart F.R., Bateman R.J., and Holtzman D.M.. 2012. Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer’s disease pathology. Sci. Transl. Med. 4:150ra122 10.1126/scitranslmed.3004291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M., Iwatsubo T., Jack C.R. Jr, Kaye J., Montine T.J., et al. 2011. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7:280–292 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira A.P., Gamaldo A.A., An Y., Wu M.N., Simonsick E.M., Bilgel M., Zhou Y., Wong D.F., Ferrucci L., and Resnick S.M.. 2013. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 70:1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassenko A.G., Vaishnavi S.N., Couture L., Sacco D., Shannon B.J., Mach R.H., Morris J.C., Raichle M.E., and Mintun M.A.. 2010. Spatial correlation between brain aerobic glycolysis and amyloid-β (Aβ) deposition. Proc. Natl. Acad. Sci. USA. 107:17763–17767 10.1073/pnas.1010461107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy V.V., Olcese U., Lazimy Y.M., Faraguna U., Esser S.K., Williams J.C., Cirelli C., and Tononi G.. 2009. Cortical firing and sleep homeostasis. Neuron. 63:865–878 10.1016/j.neuron.2009.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy V.V., Olcese U., Hanlon E.C., Nir Y., Cirelli C., and Tononi G.. 2011. Local sleep in awake rats. Nature. 472:443–447 10.1038/nature10009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L., Kang H., Xu Q., Chen M.J., Liao Y., Thiyagarajan M., O’Donnell J., Christensen D.J., Nicholson C., Iliff J.J., et al. 2013. Sleep drives metabolite clearance from the adult brain. Science. 342:373–377 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Holth J.K., Liao F., Stewart F.R., Mahan T.E., Jiang H., Cirrito J.R., Patel T.K., Hochgräfe K., Mandelkow E.M., and Holtzman D.M.. 2014. Neuronal activity regulates extracellular tau in vivo. J. Exp. Med. 211:387–393 10.1084/jem.20131685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan P., Bero A.W., Cirrito J.R., Xiao Q., Hu X., Wang Y., Gonzales E., Holtzman D.M., and Lee J.M.. 2009. Characterizing the appearance and growth of amyloid plaques in APP/PS1 mice. J. Neurosci. 29:10706–10714 10.1523/JNEUROSCI.2637-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]