Insight from Kevin Shannon
Adult T cell leukemia/lymphoma (ATLL), caused by human T cell lymphotropic virus 1 (HTLV-1), is an aggressive cancer that is refractory to current therapies. The long latency and low overall penetrance of ATLL in HTLV-1–infected individuals infers the need for cooperating events, which include somatic JAK3, NOTCH1, and FAS mutations. While overexpression of CCR4 is a hallmark of ATLL, it is not clear whether dysregulation of CCR4 function contributes to disease pathogenesis. In this issue, Nakagawa et al. report recurrent somatic mutations in the CCR4 chemokine receptor in ∼25% of ATLL cases, implicating these mutations in ATLL pathogenesis.
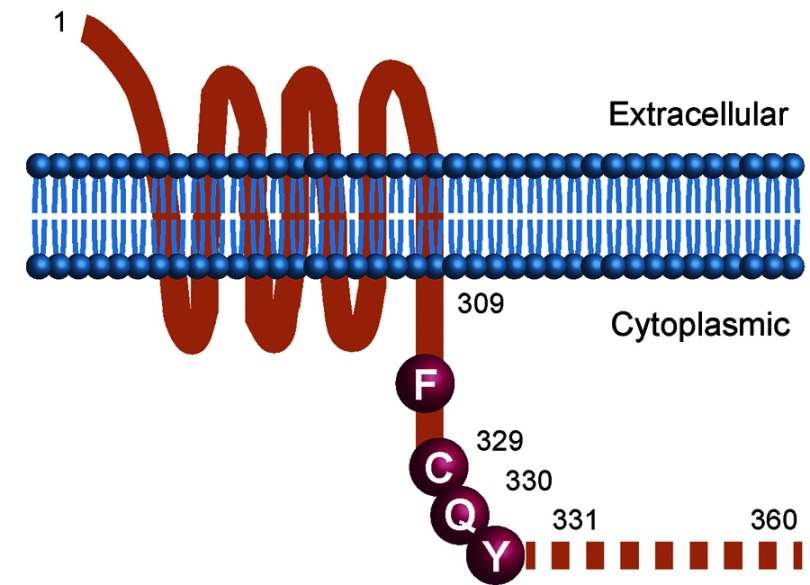
The authors performed RNA transcriptome analysis of two ATLL cases and targeted sequencing of additional ATLL patient samples and cell lines. Remarkably, the CCR4 mutations found in primary ATLL specimens were heterozygous and introduced missense or truncating mutations in a conserved carboxy-terminal domain of CCR4 involved in negative regulation. Together, these findings suggested a dominant gain-of-function mechansim of action. Indeed, elegant functional studies revealed defective internalization of these mutant CCR4 proteins as well as enhanced migration and chemotaxis in response to chemokines. The authors also demonstrated hyperactive PI3 kinase/Akt signaling in ATLL cells expressing CCR4 mutant proteins.
Schematic of CCR4 mutant isoforms in ATLL
So, how do CCR4 mutations promote malignant growth? The authors provide two logical and nonexclusive explanations. First, these mutations might enable ATLL cells to migrate to and colonize niches in tissues such as skin and lymph nodes that are favorable for cancer cell survival and proliferation. If this idea is correct, it is possible that patients with and without CCR4 mutations will exhibit specific patterns of tissue involvement and disease evolution. Second, dysregulated PI3K signaling down-stream of mutant CCR4 might be the key biochemical driver contributing to clonal selection of ATLL cells. Given this underlying biology, it is reasonable to speculate that “seed” and “soil” both contribute to the aberrant growth of ATLL tumors with somatic CCR4 mutations. For example, because CCR4 mutations render PI3K signaling hypersensitive to chemokine stimulation, specific tissue microenvironments likely favor ATLL growth through paracrine mechanisms.
An exciting aspect of these new mechanistic insights is their potential for clinical translation. A “first generation” anti-CCR4 antibody called KW-0761 is showing promise in early phase clinical trials. It will be interesting to determine whether mutant CCR4 is a predictive biomarker of sensitivity to this and other anti-CCR4 agents. Deep genomic analysis of tumors with CCR4 mutations that relapse after an initial response will provide additional insights. PI3 kinase inhibitors—both alone and in combination with other agents—are another potential therapeutic strategy for improving outcomes in this relentless cancer.
References
- Nakagawa, M., et al. 2014. J. Exp. Med. 10.1084/jem.20140987. [DOI] [Google Scholar]