Abstract
The distribution of food resources in space and time is likely to be an important factor governing the type of foraging strategy used by ants. However, no previous systematic attempt has been made to determine whether spatiotemporal resource distribution is in fact correlated with foraging strategy across the ants. In this analysis, I present data compiled from the literature on the foraging strategy and food resource use of 402 species of ants from across the phylogenetic tree. By categorizing the distribution of resources reported in these studies in terms of size relative to colony size, spatial distribution relative to colony foraging range, frequency of occurrence in time relative to worker life span, and depletability (i.e., whether the colony can cause a change in resource frequency), I demonstrate that different foraging strategies are indeed associated with specific spatiotemporal resource attributes. The general patterns I describe here can therefore be used as a framework to inform predictions in future studies of ant foraging behavior. No differences were found between resources collected via short-term recruitment strategies (group recruitment, short-term trails, and volatile recruitment), whereas different resource distributions were associated with solitary foraging, trunk trails, long-term trail networks, group raiding, and raiding. In many cases, ant species use a combination of different foraging strategies to collect diverse resources. It is useful to consider these foraging strategies not as separate options but as modular parts of the total foraging effort of a colony.
Keywords: Review, trunk trails, group recruitment, networks, collective behavior, honeydew, phylogeny, evolution, pheromone, framework
Introduction
Myrmecologists have long recognized that the distribution of food resources in time and space are important determinants of the foraging strategy used by ants (Oster & Wilson 1978, Hölldobler & Lumsden 1980, Traniello 1989, Hölldobler & Wilson 1990). In particular, we expect that ants will use different recruitment strategies depending on whether resources are patchily distributed, dispersed, temporally stable or ephemeral (Dornhaus & Powell 2009, Gordon 2012), and make predictions about foraging based on these expectations (e.g., Traniello & Levings 1986, Sundstrom 1993). We intuitively expect, for instance, that solitary foraging will occur in species that collect small, randomly dispersed, and quickly replenished resources (Plowes & al. 2013), whereas we expect raiding and nomadism to occur in species that utilize spatiotemporally unpredictable and depletable resources (Kronauer 2009). Yet, despite these expectations, no systematic attempt has been made to determine whether spatiotemporal resource distribution is in fact correlated with foraging strategy across the ants. Here for the first time, I have compiled data on the reported diet and foraging behavior for a large number of species across the ant phylogenetic tree. With these data, I explore whether resource type and foraging strategy are indeed correlated, and determine the spatiotemporal signature of resources associated with each foraging type.
Data set
Is foraging strategy correlated with resource distribution across the ants? In order to find out, I compiled as many references as possible that describe either the diet or foraging strategy of ants. In both the Web of Science and Google Scholar databases, I systematically searched for every extant genus of ants listed in the Bolton world catalog (Bolton & al. 2007), as well as for newly described genera and common older synonyms of each genus (listed on AntWeb, (AntWeb 2002) and the Hymenoptera Name Server (Johnson 2007)). To find papers that did not include genus names in the title, abstract, or key words, I also systematically searched for the terms “ant” and “forage”, “foraging”, “trail”, “raid”, and various other terms in both databases. Lastly, to find older references lacking abstracts and key words, I looked at cited references in more recent works and searched for prolific authors by name.
Because this analysis deals with the foraging strategies of free-living ants, I excluded all papers on myrmecophytic ants (Fonseca & Ganade 1996), which feed exclusively on a plant species they occupy (but included cases in which the plant provides only housing and ants forage beyond the plant), parasitic ants, and slave-making ants. Overall, I found 1252 papers that met these criteria and reported on some aspect of ant foraging behavior. Table S1 (Appendix, as digital supplementary material to this article, at the journal’s web pages) provided in the Digital Supplementary Material includes the diets, foraging strategies, and 498 associated citations for 402 species of ants. Although this data set is large, it represents only approximately 4% of the described ant species in the world (AntWeb 2002, Bolton & al. 2007). I provide these data in the hope that other myrmecologists will use and contribute to the list, and that researchers will be motivated to consistently report natural history observations such as diet and foraging in their future publications.
Foraging strategies of ants
In Box 1 and Figure 1, I categorize the variety of foraging strategies used by ants, including solitary foraging, recruitment of groups, and chemical mass recruitment. I use the term “foraging strategy” herein to refer to colony-level patterns of foraging behavior. Because literature sources vary in both the terminology used and the ways in which authors classified foraging behavior, I was careful to match the description of the actual behavior in the paper against my own categories in Box 1. For instance, in order for an observed behavior to be listed as “group recruitment” in this review, the authors must have observed a scout leading a group of workers to a previously discovered food source, and preferably should have tested whether the recruits could proceed when the scout was removed. In some cases uncertainties or additional information is noted alongside the citation in the Supplementary Information tables.
Box 1.
Foraging strategies of ants.
No recruitment
Solitary foraging
Workers leave the nest, search for food, and return alone. No information about the location of resources is communicated, although the return of successful workers may stimulate other foragers to leave the nest.
Recruitment of groups
Social carrying
A scout ant discovers a resource and returns to the nest, where she physically picks up a nestmate and carries her to the resource. This recruitment behavior was originally described for nest moving, but can occur during foraging (Möglich & Hölldobler 1974, Liefke & al. 2001). Only one worker is recruited at a time.
Tandem running
Upon discovering a food source, the scout ant returns to the nest and recruits a follower. The scout leads the follower to the food source in a tandem pair, such that the antennae of the follower frequently touch the gaster of the leader (Wilson 1959). Only one worker is recruited at a time, proceeding at a slower pace than a single ant can run, and pausing if the pair loses contact. Both mechanical and chemical signals may be used during tandem running (Liefke & al. 2001).
Group recruitment
A scout ant discovers the food source and usually lays a chemical trail back to the nest. However, the chemical signal alone does not cause workers to follow it. For recruitment to occur the scout must engage in a recruitment motor display inside the nest, activating recruits that then must follow the leading scout along the trail back to the food (Hölldobler 1971). Small numbers of workers are recruited, typically between two and a few dozen.
Group raids
A scout discovers a food source and lays a chemical trail back to the nest, where she then leads a group of recruits in a manner similar to group recruitment. However, instead of just a few ants, a large proportion of the forager population can be recruited. Group raids can look similar to true raiding columns in the field, but the difference is that a scout is necessary to discover the food source beforehand and lead the column (Mill 1984).
Chemical mass recruitment (Wilson 1962)
Short-term trails
A scout discovers the food and lays a chemical trail back to the nest. Other foragers begin following the trail, and if they are successful in finding food, they add pheromone to reinforce the trail as they return. The greater the amount of pheromone on the trail, the more likely individuals are to follow it. Unsuccessful foragers do not add to the trail and recruitment ceases once the resource has been depleted and the pheromone dissipates (Wilson 1962). Dissipation can occur rapidly once the trail is no longer being enhanced (Jeanson & al. 2003). Mechanical recruitment by the scout in the nest may or may not occur, but is not necessary for workers to begin following the trail (Wilson 1962).
A similar method of recruitment, termed “leader-independent trail communication” is described by Liefke & al. (2001). This method differs from short-term trail recruitment only in that mechanical recruitment by the scout within the nest is necessary to induce trail-following by workers. Because most literature sources do not differentiate between these two types of foraging, I have categorized them both as short-term trail recruitment for analysis.
Volatile alarm recruitment
A worker discovering a food source releases a volatile chemical signal that attracts nearby workers. Volatile alarm recruitment draws recruits from the population of nearby foragers, rather than from the pool of potential foragers inside the nest.
Trunk trails, foraging columns, and fans
Trunk trails are long-lasting trails that persist for months or years, radiating outward from the nest in a dendritic pattern. At the end of the trail, ants search individually. Trunk trails are typically cleared of debris or obstacles, and sometimes can be partially underground (Fowler 1985). As they proceed outward from the nest, trunk trails can branch, and the location of the terminal branches are more variable over time (Hölldobler 1976, Plowes & al. 2013). Colonies can have one or multiple trunk trails, but may not use them all at the same time (Plowes & al. 2013). Trunk trails are frequently defended as part of the colony territory (Hölldobler & Lumsden 1980).
Foraging columns also radiate outward from the nest, but are shorter-lived than trunk trails, can lack cleared paths, and last hours or days. Ants follow the foraging column trail to its end, then fan out and engage in individual search (Plowes & al. 2013). Foraging columns may proceed in different directions over time, and areas searched by columns can overlap with areas searched by neighboring colonies.
Foraging fans or plumes are similar to column foraging, but instead of first following a trail workers fan out directly from the nest entrance in a particular direction. Trunk trails, foraging columns, and fans are similar in that they radiate outward from a central point and cover a large search area, and may occur in both polydomous and monodomous colonies.
The exact form and duration of trunk trails and columns can vary considerably between ant genera (e.g., Messor and Pogonomyrmex), and future research may enable us to further refine these categories. However, due to lack of detail in many reports and the interchangeable use of “trunk trail” and “column” in the literature, I have grouped these three foraging strategies for some of the analyses.
Long-term trail networks
Long-term trail networks persist for months or years, but lack the dendritic pattern of trunk trails. Instead, they take the form of a network, linking polydomous nests and temporally persistent food sources. Long-term trail networks can be relatively simple (e.g., van Wilgenburg & Elgar 2007) or extremely large and complex (e.g., Way & Khoo 1991) and are a hallmark of many highly polydomous or unicolonial ant species. Despite these differences, many previous authors have used the terms “trunk trail” and “column” to describe long-term trail networks (see text).
Column and swarm raids
Raids are similar to trunk trails, foraging columns, and fans, in that they radiate outward from a central point in a particular direction and cover a large search area. However, raids last for a short period of time, rarely cover the same area twice, and are typically performed by nomadic species that frequently move in order to cover new foraging areas. Column raids are dendritic in form, branching into narrower columns as groups of workers search for food (e.g., Eciton hamatum (Fabricius, 1782)). Swarm raids (Schneirla 1934) begin with a dense carpet of ants sweeping into an area, forming a column behind that connects back to the bivouac (e.g., Eciton burchellii (Westwood, 1842)). Swarm and column raids are not discrete categories, with many army ant species exhibiting intermediate forms of raiding (Kronauer 2009).
Fig. 1.
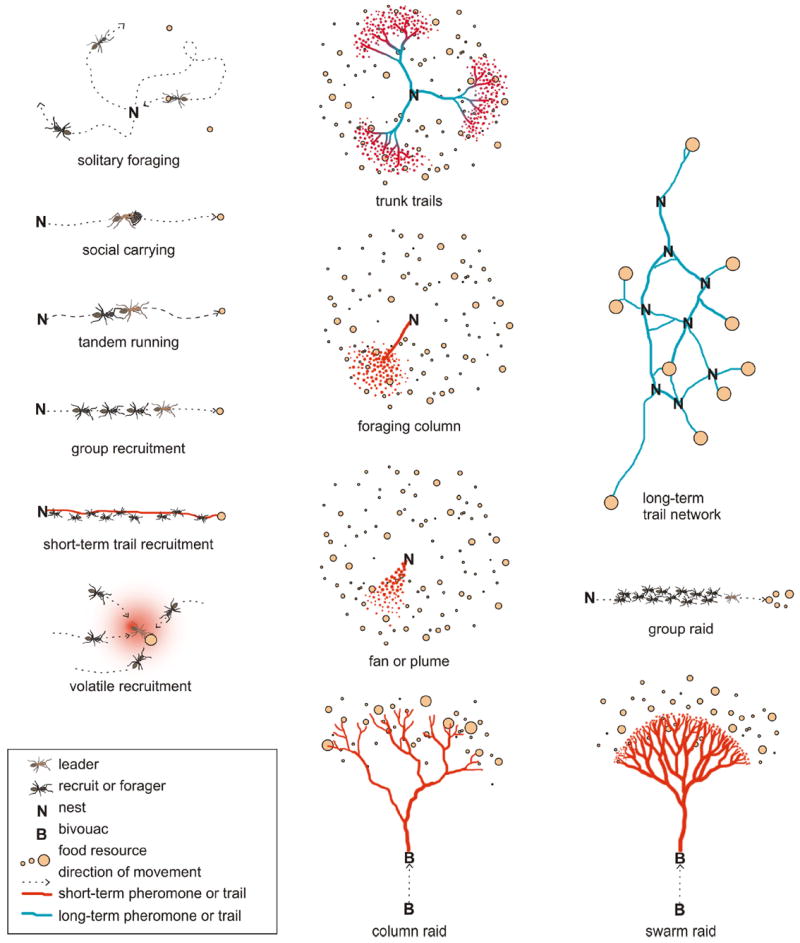
Foraging strategies of ants.
The categories of foraging I list in Box 1 are similar to those used by previous authors (Hölldobler & Wilson 1990, Dornhaus & Powell 2009), with one important exception. Previous authors have grouped all long-term trail systems together as “trunk trails”, defined as “long-term routes which are marked with persistent trail” (Levings & Traniello 1981). Just as the properties of networks vary depending upon whether they are centralized or distributed in structure (Barrat & al. 2004), the properties of longterm trails vary depending upon whether they are dendritic in form like the trunk trails of Pheidole xerophila Wheeler, 1908 (Fig. 2) or distributed like the long-term trail networks of Dolichoderus mapped by Way & Khoo (1991). I have classified long-term trails that branch out in a dendritic manner to cover an area as “trunk trails”, and distributed trail networks that connect many points as “long-term trail networks” (Fig. 1). These are not discrete categories and some ant species exhibit long-term trails that are intermediate in structure or that vary from dendritic to network form depending on resource distribution (e.g., Zakharov 1980). In order to categorize cases of long-term trail foraging, I either placed species in the category they were most similar to using the available maps and description, or listed them as having long-term trails of uncertain type. Although long-term trail networks are more commonly associated with polydomous nest structures, they can also occur with monodomous nesting habits, just as trunk trails can co-occur with both monodomy and polydomy (Acosta & al. 1995).
Fig. 2.

Long trunk trails of the seed harvesting ant Pheidole xerophila Wheeler, 1908. Trunk trails radiating out from the nest on the small hill in the background are traced out on the ground with fluorescent orange flagging. This colony harvests seeds in the highly patchy environment at the edge of the Willcox playa in Arizona (USA), crossing areas of salt flats to reach patches of grass. Photo by M. Lanan.
The terms “trunk trails” and “columns” have also frequently been used interchangeably in the literature, and a great deal of variation within each category may also occur between species. Many papers do not provide enough information to accurately distinguish between trunk trails and columns or between different varieties of trunk trails. For this reason, I have grouped all dendritic long-term trails together for several analyses (described below).
Food resources collected by ants
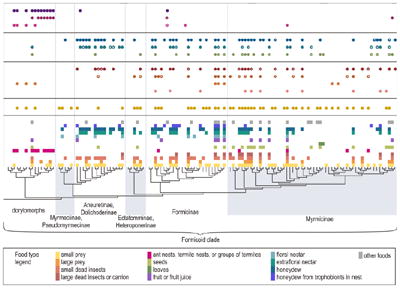
In order of the number of ant species reported to use them, the most common food resources collected by ants are small prey (202), honeydew (110), small and large dead insects (106), seeds (86), extrafloral nectar (EFN) (50), large prey (33), honeydew from trophobionts (defined here as honeydew- secreting insects housed within the nest) (29), leaves as fungal substrate (28), ant or termite colonies (20), fruit or fruit juice (14), vertebrate carrion or very large invertebrate carrion (13), floral nectar (13), elaiosomes (10), groups of small prey, typically termites (9), vertebrate droppings (6), other sugary plant secretions such as sap (6), detritus as fungal substrate (5), assorted plant material (2), flower parts (2), mushrooms (2), pollen (1), and starchy roots (1) (Tab. S1). I excluded baits and human garbage from the list of food resources. This list reflects not only the frequency at which ants collect different food resources, but the biases of researchers towards studying certain groups of ants. For instance, a disproportionate amount of research has focused on the seed-harvesting ants, whereas relatively few studies have reported the diets of non-myrmecophytic ants in the Pseudomyrmecinae. Some resources such as elaiosomes (lipid-rich bodies attached to seeds of certain plants) are also likely to be underrepresented in this list because researchers studying ant-mediated seed dispersal often did not determine the identity of the ants collecting them to species.
These data on food type and foraging strategy are placed in a phylogenetic context in Figure 3. Many of the foods collected by ants can be grouped into two broad categories, carbohydrate foods and protein foods. Common sources of protein include prey and dead arthropods, and use of these foods occurs across the ant phylogenetic tree (yellow, light orange, orange, and brown rectangles in Fig. 3). Carbohydrates are mainly collected in liquid form, including floral nectar, extrafloral nectar, and honeydew. Use of these sugary liquids (light blue, teal, blue, and dark blue rectangles in Fig. 3) is concentrated in the Formicoid clade, particularly the Dolichoderinae, Formicinae, and some of the Myrmicinae. Associations between specific food types and foraging strategies, as well as other phylogenetic patterns are discussed in more detail below.
Fig. 3.
The distribution of food types and foraging strategies across the phylogeny of the ants. The genus-level phylogeny is drawn to reflect the current understanding of the ant phylogenetic tree based on recently published molecular studies (Brady & al. 2006, Moreau & al. 2006, Lapolla & al. 2010, Mehdiabadi & Schultz 2010, Ward & al. 2010, Schmidt 2013). Use of each food type within a genus is indicated with colored rectangles, while occurrence of foraging strategies within a genus are indicated with solid circles. Possible, but uncertain occurrence of a foraging strategy is indicated with open circles. References for the diet and foraging data are provided in Table S1. In addition, Figure S1 in the Supplemental Information presents a more detailed, larger version of this phylogeny with taxa names.
Is foraging strategy correlated with resource distribution in space and time?
To answer this question I first identified four components of spatiotemporal resource distribution (Tab. 1). “Size of the resource unit relative to colony size” ranges from small items that a single ant can retrieve, to large items that require most of the forager workforce to collect. “Spatial distribution of the resource relative to colony foraging range” varies from dispersed to extremely patchy or clumped. “Frequency of resource occurrence in time relative to worker life span” ranges from items that are continuously replenished and found on every foraging bout, to those that are infrequently encountered during foraging bouts. The fourth component, “depletability”, refers to the ability of a colony to cause a change in the frequency of resource occurrence. Although an individual item is always depleted once it has been collected, I considered only whether foraging by the colony could cause a change in the frequency at which the next similar item might be encountered. Thus this measure is relative to colony foraging effort, and a resource that is non-depletable for one species may be depletable for another.
Tab. 1.
Categories used to classify the spatiotemporal characteristics of food resources in this review. Numbers for each category correspond to the axes of the graphs in Figure 4. Classifications for each data point and associated citations and justifications are listed in Table S2.
| Size of resource unit relative to colony size | |
| 1 | The resource occurs as a small unit that one ant can retrieve without help. |
| 2 | The resource is medium-sized, and several ants are necessary to either subdue, process, protect, or transport it. |
| 3 | The resource is large, and a significant portion of the forager workforce is needed to subdue, process, protect, or transport it. |
| Spatial distribution of the resource (patchy or dispersed) relative to colony foraging range | |
| 1 | After the resource is depleted, it could next occur anywhere within the foraging range with equal likelihood (dispersed, or random distribution). |
| 2 | After the resource is depleted, it will most likely be found next in the same general area, and does not occur throughout the foraging range with equal likelihood (patchy). |
| 3 | After the resource is depleted, it will most likely reoccur in the exact same location (very predictable patch). |
| Frequency of resource occurrence in time relative to worker lifespan | |
| 1 | The resource is quickly and continuously replenished or extremely common, and can definitely be encountered during a worker’s next foraging bout. |
| 2 | The resource is moderately common, and a new, similar item is likely to be found again in future foraging bouts within the current foraging range, within the lifespan of a forager. However, the next foraging bout may or may not be successful. |
| 3 | The resource is uncommon, and a new, similar item is unlikely to be found again within the current foraging range, within the lifespan of a forager. |
| Depletability – whether the colony can cause a change in the frequency of resource occurrence | |
| 1 | Foraging by the colony will not cause a decrease in the frequency at which the resource will be encountered in future foraging bouts. |
| 2 | Concerted foraging effort by the colony can temporarily cause a decrease in the frequency at which the resource occurs. |
| 3 | Concerted foraging effort by the colony can cause the resource to be depleted in the entire foraging range for a while. |
Using the categories listed in Table 1, I classified each reported instance of foraging that I found in the literature. Enough information was provided in the literature to include data for 149 ant species in this analysis (data, references, and detailed justifications for every data point are provided in Tab. S2). I considered the spatiotemporal availability of each resource only during the season in which it is present. For instance, ripe Opuntia (prickly pear) fruit occur during approximately two months of the year in the Sonoran Desert, USA, during which time the fruits are a medium-sized, patchy, moderately common, non-depletable resource collected by Aphaenogaster cockerelli André, 1893 (Tab. S2).
For each case, I compared the description in the paper against my own three levels for each category in Table 1. For each data point that I was able to categorize, I recorded my justification and relevant citation in Table S2. Although some bias is unavoidable in an analysis of this type, I attempted to base my categorizations only on the information in the paper and on very basic assumptions (e.g., if an item is retrieved by a solitary forager, it must be a small unit that one ant can retrieve without help, and if ants repeatedly visit the same clump of aphids over time, the standing crop of honeydew is likely being replenished by those aphids). Some data points are based on actual measurements of resource distribution, while others are based simply on statements by the authors (e.g., they describe food distribution in the habitat as “patchy” but do not provide actual data). Notes accompanying the data in Table S2 describe whether each point comes from a qualitative or quantitative source. In many cases, I was able to assign a category for only two or three of the four categories based on the available information. Multiple instances of foraging were included in the data set for some species, when multiple foraging strategies or foods were used. For instance, Camponotus socius Roger, 1863 uses a long-term trail network to visit aggregations of honeydew-secreting insects, but uses group recruitment to retrieve dead insects on the ground (Hölldobler 1971). If two food types were collected using the same foraging strategy by the same species, those data were averaged. Some references provided enough information to include only some of the reported food types or foraging strategies in the analysis.
To determine whether different foraging strategies are associated with unique spatiotemporal resource distributions, I plotted the data using the four aspects of spatiotemporal resource availability listed in Table 1 as axes of resource space. Because most data points were available for only a subset of the four spatiotemporal categories, I calculated the mean and standard deviation in order to represent the data as spheres in resource space. Figure 4 shows these data in four dimensions (with the first axis, size, represented with color) for eight foraging strategies for which there were enough data, as well as for polydomy and nomadism In addition, instances for which at least two axes were categorized are plotted as black dots on the walls of the graphs. A small amount of scatter was added to points that overlapped in order to make them more apparent in the figure.
Fig. 4.
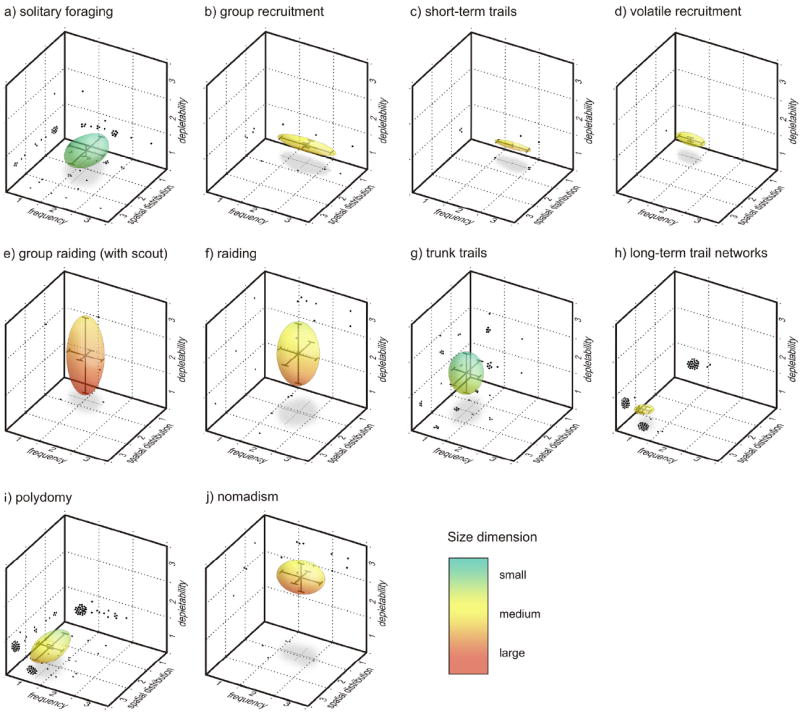
The spatiotemporal distribution of food resources collected by a) solitary foraging, b) group recruitment, c) short-term trails, d) volatile recruitment, e) group raiding, f) raiding, g) trunk trails, h) long-term trail networks, i) polydomy, and j) nomadism. Axes of the graphs are explained in Table 1, with the fourth axis, size, represented by color. Spheres represent the mean and standard deviation of the data for each axis. Small black points on the walls of the graph represent individual data points for which data on two dimensions was available. Data sources are listed in Table S2.
Several general patterns are apparent from this analysis. For instance, strategies for recruitment of small groups including group recruitment, short-term trails, and volatile recruitment appear to have similar spatiotemporal resource distributions, as do group raiding, raiding, and nomadism. Solitary foraging and trunk trails are somewhat similar to one another, as are trail networks and polydomy (Fig. 4). Using this analysis we can identify common features most associated with certain foraging strategies, such as small resources with trunk trails, clumped resources with trail networks and polydomy, and more depletable resources associated with group raiding, raiding, and nomadism. Although it would be possible to determine which spatiotemporal distributions differ from one another significantly using this data set, I did not feel that a statistical analysis would be appropriate based on the subjective nature of the data itself. Rather, the purpose of this analysis is to present the general patterns of spatiotemporal resource distribution as a framework on which we can base hypotheses for future investigations.
In addition to being associated with different spatiotemporal resource distributions, the different foraging strategies were also associated with different types of resources (Fig. 5). Data used in the analysis shown in Figure 5 include every report in which a specific foraging strategy was used to collect a specific resource (All data in Tab. S2, plus some cases from Tab. S1). The types of resources collected using group recruitment, short-term trails, and volatile recruitment (Fig. 5 b - d) do not differ from each other significantly (Pearson χ2 18,48 = 19.27, p = 0.38), whereas solitary foraging, group raiding, raiding, trunk trails, and long-term trail networks were all associated with unique types of resources (Fig. 5 a, e - i, p < 0.05 for all pairs). Below, I discuss the relationship between spatiotemporal resource distribution, food type, and different foraging strategies in greater detail.
Fig. 5.
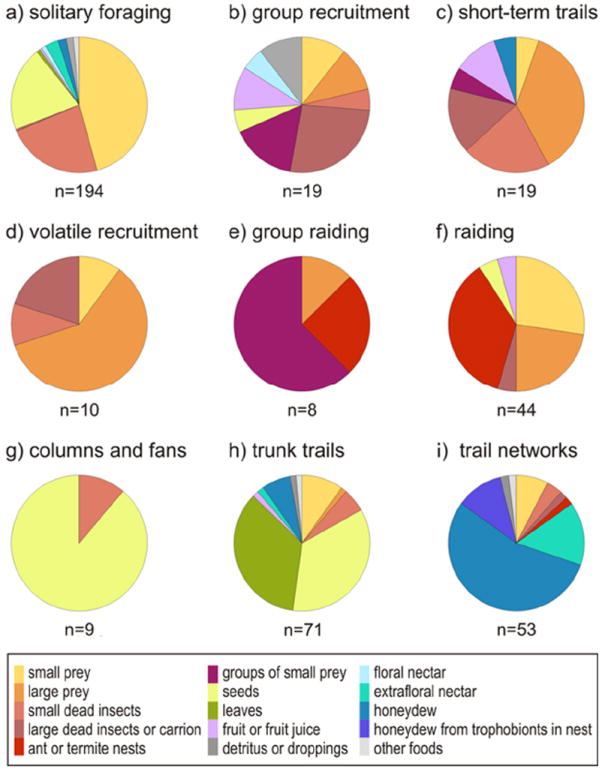
The types of food collected by a) solitary foraging, b) group recruitment, c) short-term trails, d) volatile recruitment, e) group raiding, f) raiding, g) columns and fans, h) trunk trails, and i) long-term trail networks. Only data for which a particular food type was described as being gathered with a particular foraging strategy were included. Prey are defined as large if they require more than one ant to retrieve.
Solitary foraging
Solitary foragers typically collect small, fairly common resources that are distributed unpredictably in space and that are not depleted by colony foraging effort (Fig. 4). Solitary foraging occurs across the ant phylogenetic tree, with examples occurring in nearly every group of ants (Fig. 3), and it frequently co-occurs with other foraging strategies within species (Tab. S1). Small arthropod prey (prey that can be captured and retrieved by a single ant) are the most common resource collected by solitary foragers (Fig. 5), often by species that live and hunt in forest leaf litter (Tab. S2). Small prey can be either dispersed or clumped in distribution, for instance dispersed throughout the leaf litter (Raimundo & al. 2009) or clustered in humid patches (Durou & al. 2001). In most cases, small prey are also quickly replenished in time relative to the lifespan of a worker, because prey are abundant in the area relative to the abundance of ants, because prey quickly reproduce, or because prey are mobile and continuously dispersing into the colony foraging area. Ants collect a wide variety of small prey, including larval and adult insects, earthworms, isopods, Collembola, millipedes, centipedes, spiders, and diplurans. Some ants that specialize in a particular type of small prey may have related morphology, such as species in the genus Leptogenys that capture oniscoid isopods with their unusually curved mandibles (Dejean & Evraerts 1997). Morphological adaptations of such specialized hunters are reviewed by Dornhaus & Powell (2009).
Small dead insects are the second most common resource collected by solitary foragers (Fig. 6a). Similar to small prey, dead insects are typically a dispersed resource that can be encountered anywhere in the foraging range of a colony, and are quickly replenished. Some ant species rely almost entirely on dead insects as a food source. The best-known examples are found in the North African desert ant genus Cataglyphis, which rely almost entirely on dead insects that have succumbed to the heat and have blown into the featureless salt-pan habitat of the ants (Seidl & Wehner 2006). Due to their solitary foraging habits and simple habitat, Cataglyphis have become a model system for studying animal navigation (reviewed in Wehner 2009). The desert genera Melophorus and Ocymyrmex are also solitarily foraging specialists on dead insects (Marsh 1984, Muser & al. 2005). Most generalist ant species will also take dead insects when they encounter them.
Fig. 6.

a) A solitary forager of Dorymyrmex bicolor Wheeler, 1906 retrieves a dead insect from a dry riverbed near Tucson, Arizona (USA). b) Multiple Crematogaster sp. workers tend a group of aphids on a grass stem at Reddington Pass, Arizona (USA). Photos by M. Lanan.
Seeds are the third most common resource collected by solitary foragers, and are also typically small, fairly common, and either distributed or patchy in space. However, seeds are also one of the most commonly collected resources by species that use trunk trail and column foraging (Fig. 5). Colony size appears to be the main determining factor in whether species use solitary foraging or trunk trail foraging to collect seeds (Beckers & al. 1989). Large colonies are more likely to temporarily deplete patches, and trunk trail or column foraging enables the colony to shift foraging effort elsewhere in response to depletion (Goss & Deneubourg 1989, Brener & Sierra 1993). However, recent work by Flanagan & al. (2012) has shown that large Pogonomyrmex colonies that use trunk trails are no more efficient at exploiting clumped resources than are small solitarily foraging colonies, when foraging range and forager number are accounted for.
Short-term recruitment strategies
Social carrying, tandem running, group recruitment, short-term trails, and volatile recruitment are all strategies that mobilize groups of workers for a short period of time. These strategies vary in the size of the mobilization, from one additional worker for social carrying and tandem running to hundreds or thousands of workers for short-term trails (Hölldobler & Wilson 1990). They also vary in the speed of recruitment, from relatively slow tandem running to rapid volatile recruitment. However, these strategies are all used to collect similar types of resources with similar spatiotemporal distributions (Figs. 4, 5). Short-term recruitment strategies are typically used to retrieve medium sized resources that require more than one ant to handle and that are not predictable in space. These resources often occur relatively frequently and are non-depletable, although short-term recruitment can also be used to retrieve uncommon items like vertebrate carrion. The main difference between resources collected by solitary foraging and those collected via short-term recruitment strategies is the size of the food item (Fig. 4), as recruitment is necessary only for items too large for one ant to handle.
Large arthropod prey and dead insects or carrion are the most common resources collected via short-term recruitment strategies, followed by groups of small prey such as aggregations of foraging termites (Fig. 5). Like solitary foraging, short-term recruitment strategies occur in ant genera across the phylogenetic tree (Fig. 3) and frequently co-occur with other foraging strategies within species (Tab. S1). Group recruitment is particularly common in the poneroids, Formicinae, and Myrmicinae. Although short-term trail recruitment does occur in the poneroids, it is most common in the Dolichoderinae, Formicinae, and Myrmicinae.
The mechanics of short-term trail recruitment have been extensively studied in the laboratory and with modeling approaches. Lasius niger (Linnaeus, 1758) has been the species of choice for many studies of short-term trail recruitment. Work on this ant includes studies of the interaction between the use of pheromones and route memory in navigation (Evison & al. 2008, Czaczkes & al. 2011, Grüter & al. 2011, Czaczkes & al. 2013a), the effect of numerous factors on pheromone deposition and recruitment including individual variation (Mailleux & al. 2005), crowding (Czaczkes & al. 2013b), substrate (Detrain & al. 2001), food type, quality, or quantity (Beckers & al. 1993, Mailleux & al. 2000, Detrain & al. 2010), and colony nutritional needs (Portha & al. 2002, 2004, Mailleux & al. 2006, 2010, 2011), the effects of bottlenecks (Dussutour & al. 2005), u-turning (Beckers & al. 1992, Devigne & Detrain 2006), and crowding (Dussutour & al. 2006) on the temporal organization of trail traffic, and use of multiple signals in regulating trail recruitment, including home range marking (Devigne & al. 2004) and negative feedback (Grüter & al. 2012). It is ironic then, that very little information about the behavior and natural history of this ant in the field has been reported in the literature.
Why do some ant species rely on one short-term recruitment strategy rather than another? In particular, the resources collected via group recruitment and short-term trail recruitment are spatiotemporally similar, and in many cases both strategies occur within the same phylogenetic clade (Fig. 3). It is possible that the two strategies are functionally equivalent, or that there are advantages and disadvantages to each that are not apparent in this analysis. Teasing apart the proximate and ultimate causes of variation among species in short-term recruitment strategy may be a rich area for future research.
Trunk trails and columns
Trunk trails and columns, which direct the foraging effort of a colony to a patch within its foraging range, are most commonly used to collect resources that are small, fairly common, and either patchy or dispersed in space (Fig. 4). Unlike resources collected via solitary foraging or short-term recruitment strategies, resources collected by trunk trails can be temporarily depletable. For instance, leaf fragments collected by fungus-gardening ants can be clumped or dispersed depending on the distribution of plants and the selectiveness of the colony (Tab. S2). A patch of leaves on a particular plant might be temporarily depleted by a large leaf-cutting ant colony, replenished only when the plant grows new leaves.
Similarly, harvester ants gathering seeds on the ground are collecting a small resource that can sometimes be temporarily depleted by larger colonies. In some cases, for instance when seeds have fallen from an isolated fruiting plant or when seed-producing plants are clumped, the resource can be patchily distributed within the foraging range of the colony (Wilby & Shachak 2000). However, in many cases seed harvesters are collecting dispersed seeds that are continuously turned out of the soil seed bank (Gordon 1993). A patch of soil that is temporarily depleted through concerted foraging effort will be replenished as seeds are turned out of the ground by abiotic factors like wind and rain, or as new seeds fall.
Foraging columns and fans rotate or move from day to day, focusing colony foraging effort in different areas in response to changing resource abundance (Plowes & al. 2013). Although trunk trails are more permanent structures, colonies may not use the same trunk trail from day to day (Gordon 1991), and the location of trunk trails may change over a longer time scale (Silva & al. 2013). This movement allows time for depleted resources to become replenished before foraging effort again focuses in that area.
Two of the most studied ant genera, Atta and Pogonomyrmex, use trunk trail foraging. Research on Atta has included studies of forager size polymorphism (Stradling 1978, Wetterer 1999, Clark 2006, Evison & Ratnieks 2007) including the relationship between load size and body size (Rudolph & Loudon 1986, Wetterer 1990a, b, 1991, Vanbreda & Stradling 1994, Wetterer 1994, Burd 1995, 2000b, 2001, Burd & Howard 2005, Lima & al. 2006, Helanterä & Ratnieks 2008), locomotion and the energetics of load carrying (Lighton & al. 1987, Burd 1996, 2000a, Roschard & Roces 2002, Lewis & al. 2008, Moll & al. 2010, 2012, 2013), and body size and foraging distance (Shutler & Mullie 1991). In addition, much research has focused on fungal substrate selection and preference (Cherrett 1968, 1972, Berish 1986, Nicholsorians & Schultz 1989, Nicholsorians 1992, Folgarait & al. 1996, Vasconcelos & Cherrett 1996, Vasconcelos 1997, Meyer & al. 2006, Mundim & al. 2009, Saverschek & al. 2010, Silva & Vasconcelos 2011, Vitorio & al. 2011, Neto & al. 2012) and the response of the trunk trail system to resource distribution (Fowler & Robinson 1979, Shepherd 1982, 1985, Kost & al. 2005, Sousa-Souto & al. 2008). Studies have also examined the role of head-on encounters along the trail in information flow (Burd & Aranwela 2003, Farji-Brener & al. 2010) and the effect of branching, trail widths (Bruce & Burd 2012, Farji-Brener & al. 2012), speed, and crowding (Burd & al. 2002, Dussutour & al. 2007, 2009a, Farji-Brener & al. 2011) on traffic flow.
Pogonomyrmex has also become perhaps the best-understood model system for detailed mechanistic investigations of foraging behavior (e.g., Hölldobler 1976, Gordon 1983, 1986, 1991, 2002, Gordon & al. 2005, Schafer & al. 2006, Greene & Gordon 2007a, b, Gordon & al. 2008). Work on Pogonomyrmex has also included studies of size matching and load carrying (Golley 1964, Traniello & Beshers 1991, Ferster & Traniello 1995), as well as food preference (Fewell & Harrison 1991, Crist & Macmahon 1992, Johnson & al. 1994) and interspecific and intraspecific competition (Gordon 1988, Gordon & Kulig 1996, Barton & al. 2002).
A small number of predatory ants also use trunk trails to forage for small prey. Arboreal colonies of Gnamptogenys moelleri (Forel, 1912) use trunk trails to lead workers to foraging areas and help returning workers find the nest (Gobin & al. 1998), and colonies of Pachycondyla (Brachyponera) sennaarensis (Mayr, 1862) use underground tunnels as trunk trails that radiate outward from the nest and deliver foragers to different hunting areas (Dejean & Lachaud 1994). Honeydew is also sometimes collected using trunk trails that radiate outward from the nest (Quinet & al. 1997).
Long-term trail networks
Just as the interstate highway system links cities in the United States, long-term trail networks guide ant traffic between fixed locations within ant colony territories. These networks typically link multiple nests and spatiotemporally stable food sources such as aggregations of honeydewsecreting insects. They may be simple in structure, linking only several locations, or large and highly complex (e.g., Greenslade & Halliday 1983). Most studied examples are above-ground, but many ants also use underground tunnel networks to forage (e.g., Zakharov & Tompson 1998, Kenne & Dejean 1999, Buczkowski 2012). The most common resource collected via long-term trail networks is honeydew from either free-living aggregations of hemipteran insects or from trophobionts housed within the nest itself. Considered as a unit, an aggregation of honeydewproducing insects is a medium-sized resource that usually requires at least several ants to collect liquid, tend the insects, and defend the patch (Fig. 6b). Honeydew is continuously produced by phloem-feeding insects, and is thus quickly replenished (Ness & al. 2009). Hemipteran aggregations do not typically move, and may persist in the same location for months (Henderson & Jeanne 1992), even occurring on the same trees year after year (Csata & al. 2012). Trophobionts are even more spatially and temporally stable, as colonies will move groups or build structures in order to accommodate their foraging needs (Malsch & al. 2001, Rohe & Maschwitz 2003).
Although extrafloral nectaries (glands on non-floral plant parts that secrete sugary liquids) can be small and dispersed in vegetation, certain plants produce clusters of them that are more similar to honeydew in spatiotemporal availability. For instance, the Sonoran Desert barrel cactus Ferocactus wislizeni (Britton & Rose, 1922) secretes nectar continuously from a large cluster of nectaries at the crown of the plant over the course of many years (Morris & al. 2005). The ants that collect this resource maintain large, territorial, polydomous colonies linked by a long-term trail network, and will even build shelters covering the nectaries on the plants (Lanan & Bronstein 2013). Both honeydew and extrafloral nectar are resources that ants obtain via mutualism with insect or plant partners, and thus long-term trail networks and polydomy can be viewed as colonylevel ant traits associated with mutualism. Ant participation in mutualisms has recently been analyzed in a phylogenetic context by Oliver & al. (2008).
Compared with trunk trail foraging, relatively little research has focused on the use of long-term trail networks in ants. However, many if not most species that use longterm trail networks are territorial. A number of studies have used these ants to investigate intraspecific and interspecific competition (e.g., Ettershank & Ettershank 1982, Savolainen 1990, Tanner & Adler 2009, Tanaka & al. 2012, Ribeiro & al. 2013) and ant mosaics (Adams 1994, Dejean & al. 1994, Armbrecht & al. 2001). The best-known examples of territorial, polydomous ants that use long-term trail networks are found in the arboreal genus Oecophylla (e.g., Hölldobler & Wilson 1977, Dejean & Beugnon 1991, Way & Khoo 1991) and the moundbuilding Formica (e.g., Cherix 1980, McIver & al. 1997, Sorvari & Hakkarainen 2004). Multiple pheromones may be used by trail network foraging ants, with both longlasting trail marking signals and short-term recruitment signals (Dussutour & al. 2009b).
By distributing the work force among multiple nests, polydomous long-term trail network foraging species can improve foraging efficiency via dispersed central place foraging (McIver 1991, Holway & Case 2000, Buczkowski & Bennett 2006). The dispersed central place forager Camponotus gigas (Latreille, 1802) exhibits division of labor among foragers, with one specialized caste carrying liquid food between nests (Pfeiffer & Linsenmair 1998). Similarly, the largest workers of Camponotus modoc Wheeler, 1910 are also tasked with transporting liquid foods on the between-nest trails (Tilles & Wood 1986). In network foraging ants, nests are often built or moved to be closer to food sites (Shattuck & McMillan 1998, Holway & Case 2000). Outstations that house only workers may be built near food as well (McIver & Steen 1994, Lanan & al. 2011). Surprisingly, with the exception of Buhl & al. (2009), no studies have directly investigated the shape and efficiency of long-term trail networks in response to resource distribution.
Many of the most problematic invasive ant species are polydomous and use long-term trail networks, including Linepithema humile (Mayr, 1868), Pheidole megacephala (Fabricius, 1793), and Monomorium pharaonis (Linnaeus, 1758). The ability of these ants to exploit liquid food sources like honeydew is thought to be an important factor in their invasive success (Helms 2013). “Crazy ants“ in the genera Paratrechina, Nylanderia, and Anoplolepis also include many invasive or potentially invasive species, and deserve greater attention to their foraging biology. These ants are also highly polydomous and can use long-term trails and multiple pheromone signals (Lizon A l’Allemand & Witte 2010). However, they are most notable for their habit of running in large numbers at high tempo (Lohr 1992, Abbott 2005, Kenne & al. 2005, Kuate & al. 2008, Anonymous 2010). For the invasive yellow crazy ant Anoplolepis gracilipes (Smith, 1857), densities of up to 7000 ants per m2 have been reported to engage in apparently random search on the ground (Abbott 2005). Crazy ant foraging trails are frequently described as “loose” (Meyers 2008, Anonymous 2010) with Anoplolepis custodiens (Smith, 1858) exhibiting traffic of up to 1180 ants per minute (Way 1953). Curiously, crazy ant trails may take a form that is difficult to classify as either trunk trails or trail networks (see map in Way 1953), although data on trail structure are lacking for most species. More research is needed to understand whether crazy ant foraging can be described as longterm trail networks and polydomy combined with particularly dense solitary foraging and volatile recruitment (Kenne & al. 2005) or whether it should be considered as a unique foraging strategy.
Raiding and group raids
Raiding species generally collect spatially unpredictable, depletable resources that can be either common or relatively rare (Fig. 4), and social insect nests are the most commonly reported resource collected by raids (Fig. 5). Raiding typically, although not always, co-occurs with nomadism (Tab. S1). An ant nest is usually a medium or large resource relative to the raiding colony, and a large number of workers may be needed to overpower colony defenses and transport captured brood and adults (Swartz 1998). Following a raid, ant nests in an area can be depleted (Berghoff & al. 2003), presumably until they have a chance to recover and produce more brood. If the raided nests do not survive, the space is entirely depleted until new colonies can become established. Small and large prey are also frequently collected by raiding ant species (Fig. 5). Raids can cause temporary depletion of prey populations in an area (Schöning & al. 2010, Kaspari & al. 2011), however, some raid foraging ants may collect prey in a sustainable manner (Berghoff & al. 2002). The depletability of resources collected by raiding species is hypothesized to be the major determining factor in the evolution of nomadism (Franks & Fletcher 1983, Kronauer 2009), but more work is needed to determine how commonly resource depletion co-occurs with nomadism.
True raiding occurs mostly within the clade of new and old world army ants (the AenEcDo clade in Kronauer 2009, the dorylomorphs in Fig. 3), although several genera within the poneroid clade also use raiding, as do and two species of Pheidologeton in the Myrmicinae, studied by Moffett (1988a, b). Group raiding is a second behavior that often appears similar to true raiding and also functions to bring a large number of ants to an area at once. The difference, however, is that a scout is necessary to discover the food source and lead the recruits during group raiding. This behavior is often used to attack groups of termites, and occurs in the poneroids, the dorylomorph clade (especially in Cerapachys; e.g., Hölldobler 1982, Ravary & al. 2006), Formicinae, and Myrmicinae. Pheidole titanis Wheeler, 1903 uses group raiding to attack termites (Feener 1988), and slave-raiding Polyergus use group raids to collect brood and pupae from nearby nests (Mori & al. 2001).
Although the overwhelming majority of raiding and group raiding ant species are predatory, two species collect unusual types of spatially unpredictable, depletable resources. The nomadic formicine Euprenolepis procera (Emery, 1900) uses columns that may be similar to either raids or group raids to collect its sole food source, mushrooms, in tropical forest (Witte & Maschwitz 2008). Dorylus orientalis Westwood, 1835, although it belongs to the genus of Old World driver ants, apparently feeds exclusively on plant roots and can be a major pest of crops such as potatoes and peanuts (Niu & al. 2010). More research on these two unusual ant species may be helpful for understanding the evolution of raiding and nomadism.
A number of studies have investigated various aspects of army ant behavior, recently reviewed by Kronauer (2009). Due to the inaccessibility of subterranean army ants, much more is currently known about epigaeic army ant behavior. Due to differences in resource distributions below ground, subterranean army ants may move less frequently than epigaeic army ants and may combine raiding with longer-term trunk trails (Berghoff & al. 2002).
Multiple foraging strategies for multiple food types
Many ant species use multiple foraging strategies, often in order to collect different types of resources (Tab. S1). Solitary foraging is a necessary component of many other foraging strategies, serving as the first step in all short-term recruitment strategies. Workers also engage in solitary search at the end point of trunk trails, columns, and fans. Many of the species reported to use long-term trail networks also have a subset of foragers that leave the nests or trails solitarily to search for live prey or dead insects (e.g., Greaves & Hughes 1974, Mabelis 1979, Traniello 1980, 1983, McIver 1991), and some network foragers employ group or short term trail recruitment when retrieving larger items (Dejean & al. 2012). In addition, many groups of ants have independently developed collective transport strategies that can be employed with group, trail and raid foraging strategies (Czaczkes & Ratnieks 2013).
It may be useful to consider the foraging strategies of ants not as separate options, but as modular units that can be combined in response to distinct resource distributions. For instance, the Sonoran Desert ant Forelius pruinosus (Roger, 1863) makes long-term trail networks linking polydomous nests containing trophobionts and plants with honeydew-secreting insects, but can also use short-term trails to carrion and foraging columns or fans leading out from the network to collect dead insects scattered on the ground (Fig. 7a; M. Lanan, unpubl.). An unusual combination of foraging strategies also occurs in Pheidologeton diversus (Jerdon, 1851), which uses a combination of trunk trails, short-term trails, and raids to collect a large variety of foods (Fig. 7b, Moffett 1988b). Future studies of ant foraging will likely reveal many more examples of combined foraging strategies in ants.
Fig. 7.
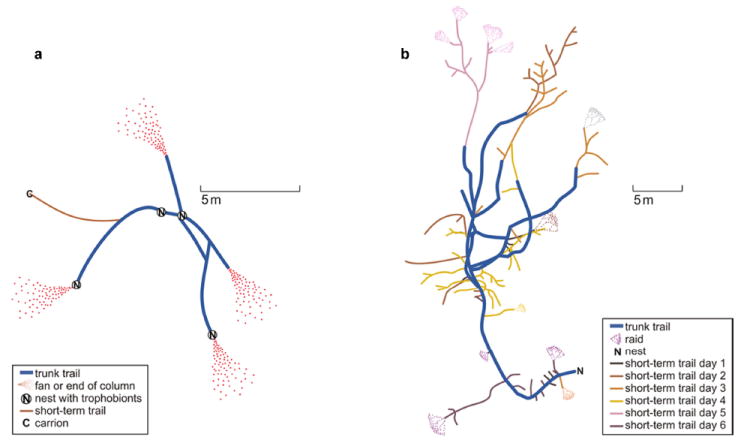
Two examples of unusual combinations of foraging strategies used by ants. a) Forelius pruinosus (Roger, 1863) uses a long-term trail network linking multiple nests where workers tend root coccids, and workers also search for dead insects along fans and columns that radiate outward from the network. A short-term trail is also used to collect vertebrate carrion (a dead baby bird) (M. Lanan, unpubl.). b) Pheidologeton diversus (Jerdon, 1851) uses a combination of trunk trails, short-term trails, and swarm raids (redrawn from Moffett 1988b; reproduced with permission from publisher).
The mechanism enabling ants to use such flexible combined foraging strategies is likely to be the use of multiple physical and pheromone signals. For instance, the weaver ant Oecophylla longinoda (Latreille, 1802) uses complex set of pheromones and recruitment behaviors to produce a large variety of colony-level behaviors (Hölldobler & Wilson 1978), and Monomorium pharaonis (Linnaeus, 1758) uses three different pheromones during recruitment including a short-term attractant, a short-term repellent, and a long-term trail marking signal (Robinson & al. 2008). A relatively unexplored question is whether the same set of behavioral rules and communication signals can produce different patterns of foraging behavior in response to different spatiotemporal resource distributions, or whether there are fundamental differences among the behavioral and chemical repertoire of species that use different foraging strategies. One intriguing example comes from the army ant Neivamyrmex nigrescens (Cresson, 1872). In this species, Topoff & Mirenda (1980) demonstrated that by supplementing food and thus changing the resource from depletable to replenished, one could partially stop migration.
Conclusions and future directions
In this analysis, I have shown that different ant foraging strategies are indeed likely to be associated with different spatiotemporal resource distributions. While solitary foraging, trunk trails, long-term trail networks, and raiding are all associated with unique resource types, short-term recruitment strategies (group recruitment, short-term trails, and volatile recruitment) are used to collect similar resources. The general patterns presented here on foraging strategies and spatiotemporal resource distribution can serve as a framework on which to base hypotheses and inform predictions in future studies.
I have also shown that in addition to differing in spatial structure, trunk trails and long-term trail networks are used to collect very different types of resources. It is therefore important to distinguish between these two foraging types in future studies. Long-term trail networks are a comparatively understudied foraging strategy with great potential for explorations of collective behavior and the properties of dispersed networks.
Understanding the evolutionary history of the ants is a major challenge for myrmecologists (Ward 2009), one that can be informed by a deeper understanding of ant natural history. In particular, the data I present here support the contention by previous authors (e.g., Wilson & Hölldobler 2005, Moreau & al. 2006) that the use of honeydew and other liquid foods is particularly widespread among ants in the formicoid clade (Fig. 3). The question of whether the diversification of the formicoids co-occurred with the rise of the angiosperms and the increasing availability of honeydew remains to be resolved (Brady & al. 2006, Moreau & al. 2006, Moreau 2009, Ward 2009). Future work will analyze the phylogenetic patterns of food use and foraging strategy presented here in greater detail.
Ant foraging behavior has served as inspiration for a large body of theoretical work in mathematics and computer science on the behavior of complex systems (Dorigo & al. 2000), with important applications for understanding the collective behavior of systems ranging from groups of neurons to human societies (Camazine & al. 2001). By describing the variety of different foraging strategies used by ants in the field and exploring how spatiotemporal resource distribution affects foraging, we can better inform this work. In particular, more research is needed to understand how ants use simple behavioral rules and communication to produce a wide diversity of foraging patterns in response to resource distribution, and how ant colonies integrate multiple foraging patterns as modular parts of the total colony foraging strategy.
Supplementary Material
Acknowledgments
I wish to thank Brian Hallmark, Kim Franklin, Katie Burke, Terry McGlynn, Andrew Waser, Peter Waser, Mary Jane Epps, Judith Bronstein, Nicholas Gotelli, and two anonymous reviewers for their feedback on the manuscript. I also thank Diana Wheeler, Sean O’Donnell, Pedro Rodrigues, and Gordon Snelling for stimulating discussions that helped shape the ideas presented here. This work was supported by the University of Arizona Postdoctoral Excellence in Research and Teaching grant NIH 5K12GM000708-13.
References
- Abbott KL. Supercolonies of the invasive yellow crazy ant, Anoplolepis gracilipes, on an oceanic island: Forager activity patterns, density and biomass. Insectes Sociaux. 2005;52:266–273. [Google Scholar]
- Acosta FJ, Lopez F, Serrano JM. Dispersed versus central-place foraging – intracolonial and intercolonial competition in the strategy of trunk trail arrangement of a harvester ant. The American Naturalist. 1995;145:389–411. [Google Scholar]
- Adams ES. Territory defense by the ant Azteca trigona – maintenance of an arboreal ant mosaic. Oecologia. 1994;97:202–208. doi: 10.1007/BF00323150. [DOI] [PubMed] [Google Scholar]
- Anonymous. Tawny (rasberry) Crazy Ant Nylanderia fulva. 2010 < http://urbanentomology.tamu.edu/ants/rasberry.html>, retrieved on 1 May 2013.
- AntWeb. AntWeb. 2002 < http://www.antweb.org>, retrieved 2013.
- Armbrecht I, Jimenez E, Alvarez G, Ulloa-Chacon P, Armbrecht H. An ant mosaic in the Colombian rain forest of Choco (Hymenoptera: Formicidae) Sociobiology. 2001;37:491–509. [Google Scholar]
- Barrat A, Barthelemy M, Pastor-Satorras R, Vespignani A. The architecture of complex weighted networks. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3747–3752. doi: 10.1073/pnas.0400087101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton KE, Sanders NJ, Gordon DM. The effects of proximity and colony age on interspecific interference competition between the desert ants Pogonomyrmex barbatus and Aphaenogaster cockerelli. The American Midland Naturalist. 2002;148:376–382. [Google Scholar]
- Beckers R, Deneubourg JL, Goss S. Trails and Uturns in the selection of a path by the ant Lasius niger. Journal of Theoretical Biology. 1992;159:397–415. [Google Scholar]
- Beckers R, Deneubourg JL, Goss S. Modulation of trail laying in the ant Lasius niger (Hymenoptera: Formicidae) and its role in the collective selection of a food source. Journal of Insect Behavior. 1993;6:751–759. [Google Scholar]
- Beckers R, Goss S, Deneubourg JL, Pasteels JM. Colony size, communication, and ant foraging strategy. Psyche. 1989;96:239–256. [Google Scholar]
- Berghoff SM, Maschwitz U, Linsenmair KE. Influence of the hypogaeic army ant Dorylus (Dichthadia) laevigatus on tropical arthropod communities. Oecologia. 2003;135:149–157. doi: 10.1007/s00442-002-1173-4. [DOI] [PubMed] [Google Scholar]
- Berghoff SM, Weissflog A, Linsenmair KE, Hashim R, Maschwitz U. Foraging of a hypogaeic army ant: a long neglected majority. Insectes Sociaux. 2002;49:133–141. [Google Scholar]
- Berish CW. Leaf-cutting ants (Atta cephalotes) select nitrogen-rich forage. The American Midland Naturalist. 1986;115:268–276. [Google Scholar]
- Bolton B, Alpert GD, Ward PS, Naskrecki P. Bolton’s catalogue of ants of the world: 1758 - 2005. Harvard University Press; Cambridge, MA: 2007. CD-ROM. [Google Scholar]
- Brady SG, Schultz TR, Fisher BL, Ward PS. Evaluating alternative hypotheses for the early evolution and diversification of ants. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18172–18177. doi: 10.1073/pnas.0605858103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener AGF, Sierra C. Distribution of attacked plants along trails in leaf-cutting ants (Hymenoptera, Formicidae) – consequences in territorial strategies. Revista de Biología Tropical. 1993;41:891–896. [Google Scholar]
- Bruce AI, Burd M. Allometric scaling of foraging rate with trail dimensions in leaf-cutting ants. Proceedings of the Royal Society B-Biological Sciences. 2012;279:2442–2447. doi: 10.1098/rspb.2011.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczkowski G. Colony spatial structure in polydomous ants: complimentary approaches reveal different patterns. Insectes Sociaux. 2012;59:241–250. [Google Scholar]
- Buczkowski G, Bennett GW. Dispersed central-place foraging in the polydomous odorous house ant, Tapinoma sessile as revealed by a protein marker. Insectes Sociaux. 2006;53:282–290. [Google Scholar]
- Buhl J, Hicks K, Miller E, Persey S, Alinvi O, Sumpter D. Shape and efficiency of wood ant foraging networks. Behavioral Ecology and Sociobiology. 2009;63:451–460. [Google Scholar]
- Burd M. Variable load size ant size matching in leaf-cutting ants, Atta colombica (Hymenoptera: Formicidae) Journal of Insect Behavior. 1995;8:715–722. [Google Scholar]
- Burd M. Foraging performance by Atta colombica, a leafcutting ant. The American Naturalist. 1996;148:597–612. [Google Scholar]
- Burd M. Body size effects on locomotion and load carriage in the highly polymorphic leaf-cutting ants Atta colombica and Atta cephalotes. Behavioral Ecology. 2000a;11:125–131. [Google Scholar]
- Burd M. Foraging behaviour of Atta cephalotes (leafcutting ants): an examination of two predictions for load selection. Animal Behaviour. 2000b;60:781–788. doi: 10.1006/anbe.2000.1537. [DOI] [PubMed] [Google Scholar]
- Burd M. Leaf tissue transport as a function of loading ratio in the leaf-cutting ant Atta cephalotes. Ecological Entomology. 2001;26:551–556. [Google Scholar]
- Burd M, Aranwela N. Head-on encounter rates and walking speed of foragers in leaf-cutting ant traffic. Insectes Sociaux. 2003;50:3–8. [Google Scholar]
- Burd M, Archer D, Aranwela N, Stradling DJ. Traffic dynamics of the leaf-cutting ant, Atta cephalotes. The American Naturalist. 2002;159:283–293. doi: 10.1086/338541. [DOI] [PubMed] [Google Scholar]
- Burd M, Howard JJ. Global optimization from suboptimal parts: foraging sensu lato by leaf-cutting ants. Behavioral Ecology and Sociobiology. 2005;59:234–242. [Google Scholar]
- Camazine S, Deneubourg JL, Franks N, Sneyd J, Theraulaz G, Bonabeau E. Self-organization in biological systems. Princeton University Press; Princeton, NJ: 2001. p. 538. [Google Scholar]
- Cherix D. A preliminary note about structure, phenology and diet of a super-colony of Formica lugubris ZETT. Insectes Sociaux. 1980;27:226–236. [Google Scholar]
- Cherrett JM. Foraging behaviour of Atta cephalotes L. (Hymenoptera: Formicidae) 1. Foraging pattern and plant species attacked in tropical rain forest. Journal of Animal Ecology. 1968;37:387–403. [Google Scholar]
- Cherrett JM. Some factors involved in selection of vegetable substrate by Atta cephalotes (L.) (Hymenoptera: Formicidae) in tropical rain-forest. Journal of Animal Ecology. 1972;41:647–660. [Google Scholar]
- Clark E. Dynamic matching of forager size to resources in the continuously polymorphic leaf-cutter ant, Atta colombica (Hymenoptera: Formicidae) Ecological Entomology. 2006;31:629–635. [Google Scholar]
- Crist TO, Macmahon JA. Harvester ant foraging and shrub steppe seeds – interactions of seed resources and seed use. Ecology. 1992;73:1768–1779. [Google Scholar]
- Csata E, Marko B, Eros K, Gal C, Szasz-Len AM, Czekes Z. Outstations as stable meeting points for workers from different nests in a polydomous system of Formica exsecta Nyl. (Hymenoptera: Formicidae) Polish Journal of Ecology. 2012;60:177–186. [Google Scholar]
- Czaczkes TJ, Grüter C, Ellis L, Wood E, Ratnieks FLW. Ant foraging on complex trails: route learning and the role of trail pheromones in Lasius niger. Journal of Experimental Biology. 2013a;216:188–197. doi: 10.1242/jeb.076570. [DOI] [PubMed] [Google Scholar]
- Czaczkes TJ, Grüter C, Jones SM, Ratnieks FLW. Synergy between social and private information increases foraging efficiency in ants. Biology Letters. 2011;7:521–524. doi: 10.1098/rsbl.2011.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaczkes TJ, Grüter C, Ratnieks FLW. Negative feedback in ants: crowding results in less trail pheromone deposition. Journal of the Royal Society Interface. 2013b;10 doi: 10.1098/rsif.2012.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaczkes TJ, Ratnieks FLW. Cooperative transport in ants (Hymenoptera: Formicidae) and elsewhere. Myrmecological News. 2013;18:1–11. [Google Scholar]
- Dejean A, Akoa A, Djieto-Lordon C, Lenoir A. Mosaic ant territories in an African secondary rain forest (Hymenoptera: Formicidae) Sociobiology. 1994;23:275–292. [Google Scholar]
- Dejean A, Beugnon G. Persistent intercolonial trunkroute- marking in the African weaver ant Oecophylla longinoda LATREILLE (Hymenoptera: Formicidae) – Tom-Thumb versus Ariadne orienting strategies. Ethology. 1991;88:89–98. [Google Scholar]
- Dejean A, Delabie JHC, Corbara B, Azemar F, Groc S, Orivel J, Leponce M. The ecology and feeding habits of the arboreal trap-jawed ant Daceton armigerum. Public Library of Science One. 2012;7:8. doi: 10.1371/journal.pone.0037683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejean A, Evraerts C. Predatory behavior in the genus Leptogenys: A comparative study. Journal of Insect Behavior. 1997;10:177–191. [Google Scholar]
- Dejean A, Lachaud JP. Ecology and behavior of the seed-eating ponerine ant Brachyponera senaarensis (MAYR) Insectes Sociaux. 1994;41:191–210. [Google Scholar]
- Detrain C, Natan C, Deneubourg JL. The influence of the physical environment on the self-organised foraging patterns of ants. Naturwissenschaften. 2001;88:171–174. doi: 10.1007/s001140100217. [DOI] [PubMed] [Google Scholar]
- Detrain C, Verheggen FJ, Diez L, Wathelet B, Haubruge E. Aphid-ant mutualism: how honeydew sugars influence the behaviour of ant scouts. Physiological Entomology. 2010;35:168–174. [Google Scholar]
- Devigne C, Detrain C. How does food distance influence foraging in the ant Lasius niger: the importance of homerange marking. Insectes Sociaux. 2006;53:46–55. [Google Scholar]
- Devigne C, Renon AJ, Detrain C. Out of sight but not out of mind: modulation of recruitment according to home range marking in ants. Animal Behaviour. 2004;67:1023–1029. [Google Scholar]
- Dorigo M, Bonabeau E, Theraulaz G. Ant algorithms and stigmergy. Future Generation Computer Systems-the International Journal of Grid Computing and Escience. 2000;16:851–871. [Google Scholar]
- Dornhaus A, Powell S. Foraging and defence strategies in ants. In: Lach L, Parr CL, Abbott KL, editors. Ant ecology. Oxford University Press; Oxford, UK: 2009 [2010]. pp. 210–230. [Google Scholar]
- Durou S, Lauga J, Dejean A. Intensive food searching in humid patches: Adaptation of a myrmicine ant to environmental constraints. Behaviour. 2001;138:251–259. [Google Scholar]
- Dussutour A, Beshers S, Deneubourg JL, Fourcassie V. Crowding increases foraging efficiency in the leafcutting ant Atta colombica. Insectes Sociaux. 2007;54:158–165. [Google Scholar]
- Dussutour A, Beshers S, Deneubourg JL, Fourcassie V. Priority rules govern the organization of traffic on foraging trails under crowding conditions in the leaf-cutting ant Atta colombica. Journal of Experimental Biology. 2009a;212:499–505. doi: 10.1242/jeb.022988. [DOI] [PubMed] [Google Scholar]
- Dussutour A, Deneubourg JL, Fourcassie V. Temporal organization of bi-directional traffic in the ant Lasius niger (L.) Journal of Experimental Biology. 2005;208:2903–2912. doi: 10.1242/jeb.01711. [DOI] [PubMed] [Google Scholar]
- Dussutour A, Nicolis SC, Deneubourg JL, Fourcassie V. Collective decisions in ants when foraging under crowded conditions. Behavioral Ecology and Sociobiology. 2006;61:17–30. [Google Scholar]
- Dussutour A, Nicolis SC, Shephard G, Beekman M, Sumpter DJT. The role of multiple pheromones in food recruitment by ants. Journal of Experimental Biology. 2009b;212:2337–2348. doi: 10.1242/jeb.029827. [DOI] [PubMed] [Google Scholar]
- Ettershank G, Ettershank JA. Ritualized fighting in the meat ant Iridomyrmex purpureus (Smith) (Hymenoptera: Formicidae) Journal of the Australian Entomological Society. 1982;21:97–102. [Google Scholar]
- Evison SEF, Petchey OL, Beckerman AP, Ratnieks FLW. Combined use of pheromone trails and visual landmarks by the common garden ant Lasius niger. Behavioral Ecology and Sociobiology. 2008;63:261–267. [Google Scholar]
- Evison SEF, Ratnieks FLW. New role for majors in Atta leafcutter ants. Ecological Entomology. 2007;32:451–454. [Google Scholar]
- Farji-Brener AG, Amador-Vargas S, Chinchilla F, Escobar S, Cabrera S, Herrera MI, Sandoval C. Information transfer in head-on encounters between leaf-cutting ant workers: food, trail condition or orientation cues? Animal Behaviour. 2010;79:343–349. [Google Scholar]
- Farji-Brener AG, Chinchilla FA, Rifkin S, Cuervo AMS, Triana E, Quiroga V, Giraldo P. The “truck-driver” effect in leaf-cutting ants: how individual load influences the walking speed of nest-mates. Physiological Entomology. 2011;36:128–134. [Google Scholar]
- Farji-Brener AG, Morueta-Holme N, Chinchilla F, Willink B, Ocampo N, Bruner G. Leaf-cutting ants as road engineers: the width of trails at branching points in Atta cephalotes. Insectes Sociaux. 2012;59:389–394. [Google Scholar]
- Feener DH. Effects of parasites on foraging and defense behavior of a termitophagous ant, Pheidole titanis Wheeler (Hymenoptera: Formicidae) Behavioral Ecology and Sociobiology. 1988;22:421–427. [Google Scholar]
- Ferster B, Traniello JFA. Polymorphism and foraging behavior in Pogonomyrmex badius (Hymenoptera: Formicidae) – worker size, foraging distance, and load size associations. Environmental Entomology. 1995;24:673–678. [Google Scholar]
- Fewell JH, Harrison JF. Flexible seed selection by individual harvester ants, Pogonomyrmex occidentalis. Behavioral Ecology and Sociobiology. 1991;28:377–384. [Google Scholar]
- Flanagan T, Letendre K, Burnside W, Fricke G, Moses ME. Quantifying the effect of colony size and food distribution on harvester ant foraging. Public Library of Science One. 2012;7:e39427. doi: 10.1371/journal.pone.0039427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folgarait PJ, Dyer LA, Marquis RJ, Braker HE. Leaf-cutting ant preferences for five native tropical plantation tree species growing under different light conditions. Entomologia Experimentalis et Applicata. 1996;80:521–530. [Google Scholar]
- Fonseca CR, Ganade G. Asymmetries, compartments and null interactions in an Amazonian ant-plant community. Journal of Animal Ecology. 1996;65:339–347. [Google Scholar]
- Fowler HG. Leaf-cutting ants of the genera Atta and Acromyrmex of Paraguay (Hymenoptera: Formicidae) Deutsche Entomologische Zeitschrift. 1985;32:19–34. [Google Scholar]
- Fowler HG, Robinson SW. Foraging by Atta sexdens (Formicidae: Attini) - seasonal patterns, caste and efficiency. Ecological Entomology. 1979;4:239–247. [Google Scholar]
- Franks NR, Fletcher CR. Spatial patterns in army ant foraging and migration – Eciton burchelli on Barro Colorado Island, Panama. Behavioral Ecology and Sociobiology. 1983;12:261–270. [Google Scholar]
- Gobin B, Peeters C, Billen J, Morgan ED. Interspecific trail following and commensalism between the ponerine ant Gnamptogenys menadensis and the formicine ant Polyrhachis rufipes. Journal of Insect Behavior. 1998;11:361–369. [Google Scholar]
- Golley FB. Bioenergetics of the southern harvester ant, Pogonomyrmex badius. Ecology. 1964;45:217–225. [Google Scholar]
- Gordon DM. The relation of recruitment rate to activity rhythms in the harvester ant, Pogonomyrmex barbatus (F. Smith) (Hymenoptera: Formicidae) Journal of the Kansas Entomological Society. 1983;56:277–285. [Google Scholar]
- Gordon DM. Thy dynamics of the daily round of the harvester ant colony (Pogonomyrmex barbatus) Animal Behaviour. 1986;34:1402–1419. [Google Scholar]
- Gordon DM. Nest-plugging: interference competition in desert ants (Novomessor cockerelli and Pogonomyrmex barbatus) Oecologia. 1988;75:114–118. doi: 10.1007/BF00378823. [DOI] [PubMed] [Google Scholar]
- Gordon DM. Behavioral flexibility and the foraging ecology of seed-eating ants. The American Naturalist. 1991;138:379–411. [Google Scholar]
- Gordon DM. The spatial scale of seed collection by harvester ants. Oecologia. 1993;95:479–487. doi: 10.1007/BF00317431. [DOI] [PubMed] [Google Scholar]
- Gordon DM. The regulation of foraging activity in red harvester ant colonies. The American Naturalist. 2002;159:509–518. doi: 10.1086/339461. [DOI] [PubMed] [Google Scholar]
- Gordon DM. The dynamics of foraging trails in the tropical arboreal ant Cephalotes goniodontus. Public Library of Science One. 2012;7:7. doi: 10.1371/journal.pone.0050472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DM, Chu J, Lillie A, Tissot M, Pinter N. Variation in the transition from inside to outside work in the red harvester ant Pogonomyrmex barbatus. Insectes Sociaux. 2005;52:212–217. [Google Scholar]
- Gordon DM, Holmes S, Nacu S. The short-term regulation of foraging in harvester ants. Behavioral Ecology. 2008;19:217–222. doi: 10.1093/beheco/arq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DM, Kulig AW. Founding, foraging, and fighting: Colony size and the spatial distribution of harvester ant nests. Ecology. 1996;77:2393–2409. [Google Scholar]
- Goss S, Deneubourg JL. The self-organizing clock pattern of Messor pergandei (Formicidae: Myrmicinae) Insectes Sociaux. 1989;36:339–346. [Google Scholar]
- Greaves T, Hughes RD. The population biology of the meat ant. Journal of the Australian Entomological Society. 1974;13:329–351. [Google Scholar]
- Greene MJ, Gordon DM. How patrollers set foraging direction in harvester ants. The American Naturalist. 2007a;170:943–948. doi: 10.1086/522843. [DOI] [PubMed] [Google Scholar]
- Greene MJ, Gordon DM. Interaction rate informs harvester ant task decisions. Behavioral Ecology. 2007b;18:451–455. [Google Scholar]
- Greenslade PJM, Halliday RB. Colony dispersion and relationships of meat ants Iridomyrmex purpureus and allies in arid locality in south Australia. Insectes Sociaux. 1983;30:82–99. [Google Scholar]
- Grüter C, Czaczkes TJ, Ratnieks FLW. Decision making in ant foragers (Lasius niger) facing conflicting private and social information. Behavioral Ecology and Sociobiology. 2011;65:141–148. [Google Scholar]
- Grüter C, Schürch R, Czaczkes TJ, Taylor K, Durance T, Jones SM, Ratnieks FLW. Negative feedback enables fast and flexible collective decision-making in ants. Public Library of Science One. 2012;7:11. doi: 10.1371/journal.pone.0044501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helanterä H, Ratnieks FLW. Geometry explains the benefits of division of labour in a leafcutter ant. Proceedings of the Royal Society B-Biological Sciences. 2008;275:1255–1260. doi: 10.1098/rspb.2008.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms KR. Mutualisms between ants (Hymenoptera: Formicidae) and honeydew-producing insects: Are they important in ant invasions? Myrmecological News. 2013;18:61–71. [Google Scholar]
- Henderson G, Jeanne RL. Population biology and foraging ecology of prairie ants in southern Wisconsin (Hymenoptera: Formicidae) Journal of the Kansas Entomological Society. 1992;65:16–29. [Google Scholar]
- Hölldobler B. Recruitment behavior in Camponotus socius (Hymenoptera: Formicidae) Zeitschrift für vergleichende Physiologie. 1971;75:123–142. [Google Scholar]
- Hölldobler B. Recruitment behavior, home range orientation and territoriality in harvester ants, Pogonomyrmex. Behavioral Ecology and Sociobiology. 1976;1:3–44. [Google Scholar]
- Hölldobler B. Communication, raiding behavior and prey storage in Cerapachys (Hymenoptera: Formicidae) Psyche. 1982;89:3–24. [Google Scholar]
- Hölldobler B, Lumsden CJ. Territorial strategies in ants. Science. 1980;210:732–739. doi: 10.1126/science.210.4471.732. [DOI] [PubMed] [Google Scholar]
- Hölldobler B, Wilson EO. Colony specific territorial pheromone in African weaver ant Oecophylla longinoda (LATREILLE) Proceedings of the National Academy of Sciences of the United States of America. 1977;74:2072–2075. doi: 10.1073/pnas.74.5.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölldobler B, Wilson EO. Multiple recruitment systems of African weaver ant Oecophylla longinoda (Latreille) (Hymenoptera: Formicidae) Behavioral Ecology and Sociobiology. 1978;3:19–60. [Google Scholar]
- Hölldobler B, Wilson EO. The ants. Belknap Press of Harvard University Press; Cambridge, MA: 1990. p. 732. [Google Scholar]
- Holway DA, Case TJ. Mechanisms of dispersed central-place foraging in polydomous colonies of the Argentine ant. Animal Behaviour. 2000;59:433–441. doi: 10.1006/anbe.1999.1329. [DOI] [PubMed] [Google Scholar]
- Jeanson R, Ratnieks FLW, Deneubourg JL. Pheromone trail decay rates on different substrates in the Pharaoh’s ant, Monomorium pharaonis. Physiological Entomology. 2003;28:192–198. [Google Scholar]
- Johnson NF. The Hymenoptera Name Server. 2007 < http://hns.osu.edu>, retrieved 2013.
- Johnson RA, Rissing SW, Killeen PR. Differential learning and memory by cooccurring ant species. Insectes Sociaux. 1994;41:165–177. [Google Scholar]
- Kaspari M, Powell S, Lattke J, O’donnell S. Predation and patchiness in the tropical litter: do swarm-raiding army ants skim the cream or drain the bottle? Journal of Animal Ecology. 2011;80:818–823. doi: 10.1111/j.1365-2656.2011.01826.x. [DOI] [PubMed] [Google Scholar]
- Kenne M, Dejean A. Spatial distribution, size and density of nests of Myrmicaria opaciventris Emery (Formicidae: Myrmicinae) Insectes Sociaux. 1999;46:179–185. [Google Scholar]
- Kenne M, Mony R, Tindo M, Njaleu LCK, Orivel J, Dejean A. The predatory behaviour of a tramp ant species in its native range. Comptes Rendus Biologies. 2005;328:1025–1030. doi: 10.1016/j.crvi.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Kost C, de Oliveira EG, Knoch TA, Wirth R. Spatio-temporal permanence and plasticity of foraging trails in young and mature leaf-cutting ant colonies (Atta spp.) Journal of Tropical Ecology. 2005;21:677–688. [Google Scholar]
- Kronauer DJC. Recent advances in army ant biology (Hymenoptera: Formicidae) Myrmecological News. 2009;12:51–65. [Google Scholar]
- Kuate AF, Tindo M, Hanna R, Kenne M, Goergen G. Foraging activity and diet of the ant, Anoplolepis tenella Santschi (Hymenoptera: Formicidae), in southern Cameroon. African Entomology. 2008;16:107–114. [Google Scholar]
- Lanan MC, Bronstein JL. An ant’s-eye view of an ant-plant protection mutualism. Oecologia. 2013;172:779–790. doi: 10.1007/s00442-012-2528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanan MC, Dornhaus A, Bronstein JL. The function of polydomy: the ant Crematogaster torosa preferentially forms new nests near food sources and fortifies outstations. Behavioral Ecology and Sociobiology. 2011;65:959–968. [Google Scholar]
- Lapolla JS, Brady SG, Shattuck SO. Phylogeny and taxonomy of the Prenolepis genus-group of ants (Hymenoptera: Formicidae) Systematic Entomology. 2010;35:118–131. [Google Scholar]
- Levings SC, Traniello JFA. Territoriality, nest dispersion, and community structure in ants. Psyche. 1981;88:265–320. [Google Scholar]
- Lewis OT, Martin M, Czaczkes TJ. Effects of trail gradient on leaf tissue transport and load size selection in leaf-cutter ants. Behavioral Ecology. 2008;19:805–809. [Google Scholar]
- Liefke C, Hölldobler B, Maschwitz U. Recruitment behavior in the ant genus Polyrhachis (Hymenoptera: Formicidae) Journal of Insect Behavior. 2001;14:637–657. [Google Scholar]
- Lighton JRB, Bartholomew GA, Feener DH. Energetics of locomotion and load carriage and a model of the energy-cost of foraging in the leaf-cutting ant Atta colombica Guer. Physiological Zoology. 1987;60:524–537. [Google Scholar]
- Lima CA, Della Lucia TMC, Ribeiro MMR, Vianabailez AMM. The role of seasonality on load transport and polymorphism in the grass-cutting ant Atta bisphaerica (Hymenoptera: Formicidae) Sociobiology. 2006;48:175–181. [Google Scholar]
- Lizon A, l’Allemand S, Witte V. A sophisticated, modular communication contributes to ecological dominance in the invasive ant Anoplolepis gracilipes. Biological Invasions. 2010;103:1775–1783. [Google Scholar]
- Lohr B. The pugnacious ant, Anoplolepis custodiens (Hymenoptera: Formicidae), and its beneficial effect on coconut production in Tanzania. Bulletin of Entomological Research. 1992;82:213–218. [Google Scholar]
- Mabelis AA. Distribution of red wood ants (Formica polyctena Först.) over the foraging area of their nest, and the influence of a conspecific neighboring population. Netherlands Journal of Zoology. 1979;29:221–232. [Google Scholar]
- Mailleux AC, Buffin A, Detrain C, Deneubourg JL. Recruitment in starved nests: the role of direct and indirect interactions between scouts and nestmates in the ant Lasius niger. Insectes Sociaux. 2011;58:559–567. [Google Scholar]
- Mailleux AC, Deneubourg JL, Detrain C. How do ants assess food volume? Animal Behaviour. 2000;59:1061–1069. doi: 10.1006/anbe.2000.1396. [DOI] [PubMed] [Google Scholar]
- Mailleux AC, Detrain C, Deneubourg JL. Triggering and persistence of trail-laying in foragers of the ant Lasius niger. Journal of Insect Physiology. 2005;51:297–304. doi: 10.1016/j.jinsphys.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Mailleux AC, Detrain C, Deneubourg JL. Starvation drives a threshold triggering communication. Journal of Experimental Biology. 2006;209:4224–4229. doi: 10.1242/jeb.02461. [DOI] [PubMed] [Google Scholar]
- Mailleux AC, Devigne C, Deneubourg JL, Detrain C. Impact of starvation on Lasius niger’s exploration. Ethology. 2010;116:248–256. [Google Scholar]
- Malsch AKF, Kaufmann E, Heckroth HP, Williams DJ, Maryati M, Maschwitz U. Continuous transfer of subterranean mealybugs (Hemiptera: Pseudococcidae) by Pseudolasius spp. (Hymenoptera: Formicidae) during colony fission? Insectes Sociaux. 2001;48:333–341. [Google Scholar]
- Marsh AC. Behavior of the bearded ant, Ocymyrmex barbiger, in relation to the thermal environment of the Namib desert. South African Journal of Science. 1984;80:192. [Google Scholar]
- McIver JD. Dispersed central place foraging in Australian meat ants. Insectes Sociaux. 1991;38:129–137. [Google Scholar]
- McIver JD, Steen T. Use of a secondary nest in greatbasin desert thatch ants (Formica obscuripes Forel) The Great Basin Naturalist. 1994;54:359–365. [Google Scholar]
- McIver JD, Torgersen TR, Cimon NJ. A supercolony of the thatch ant Formica obscuripes FOREL (Hymenoptera: Formicidae) from the Blue Mountains of Oregon. Northwest Science. 1997;71:18–29. [Google Scholar]
- Mehdiabadi NJ, Schultz TR. Natural history and phylogeny of the fungus-farming ants (Hymenoptera: Formicidae: Myrmicinae: Attini) Myrmecological News. 2010;13:37–55. [Google Scholar]
- Meyer ST, Roces F, Wirth R. Selecting the drought stressed: effects of plant stress on intraspecific and within-plant herbivory patterns of the leaf-cutting ant Atta colombica. Functional Ecology. 2006;20:973–981. [Google Scholar]
- Meyers JM. PhD thesis. Texas A&M University; TX: 2008. Identification, distribution, and control of an invasive pest ant, Paratrechina sp. (Hymenoptera: Formicidae), in Texas; p. 163. [Google Scholar]
- Mill AE. Predation by the ponerine ant Pachycondyla commutata on termites of the genus Syntermes in Amazonian rain forest. Journal of Natural History. 1984;18:405–410. [Google Scholar]
- Moffett MW. Foraging behavior in the Malayan swarmraiding ant Pheidologeton silenus (Hymenoptera: Formicidae: Myrmicinae) Annals of the Entomological Society of America. 1988a;81:356–361. [Google Scholar]
- Moffett MW. Foraging dynamics in the group-hunting myrmicine ant, Pheidologeton diversus. Journal of Insect Behavior. 1988b;1:309–331. [Google Scholar]
- Möglich M, Hölldobler B. Social carrying behavior and division of labor during nest moving in ants. Psyche. 1974;81:219–236. [Google Scholar]
- Moll K, Federle W, Roces F. The energetics of running stability: costs of transport in grass-cutting ants depend on fragment shape. Journal of Experimental Biology. 2012;215:161–168. doi: 10.1242/jeb.063594. [DOI] [PubMed] [Google Scholar]
- Moll K, Roces F, Federle W. Foraging grass-cutting ants (Atta vollenweideri) maintain stability by balancing their loads with controlled head movements. Journal of Comparative Physiology A-Neuroethology Sensory Neural and Behavioral Physiology. 2010;196:471–480. doi: 10.1007/s00359-010-0535-3. [DOI] [PubMed] [Google Scholar]
- Moll K, Roces F, Federle W. How load-carrying ants avoid falling over: mechanical stability during foraging in Atta vollenweideri grass-cutting ants. Public Library of Science One. 2013;8:e52816. doi: 10.1371/journal.pone.0052816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau CS. Inferring ant evolution in the age of molecular data (Hymenoptera: Formicidae) Myrmecological News. 2009;12:201–210. [Google Scholar]
- Moreau CS, Bell CD, Vila R, Archibald SB, Pierce NE. Phylogeny of the ants: diversification in the age of angiosperms. Science. 2006;312:101–104. doi: 10.1126/science.1124891. [DOI] [PubMed] [Google Scholar]
- Mori A, Grasso DA, Visicchio R, Le Moli F. Comparison of reproductive strategies and raiding behaviour in facultative and obligatory slave-making ants: the case of Formica sanguinea and Polyergus rufescens. Insectes Sociaux. 2001;48:302–314. [Google Scholar]
- Morris WF, Wilson WG, Bronstein JL, Ness JH. Environmental forcing and the competitive dynamics of a guild of cactus-tending ant mutualists. Ecology. 2005;86:3190–3199. [Google Scholar]
- Mundim FM, Costa AN, Vasconcelos HL. Leaf nutrient content and host plant selection by leaf-cutter ants, Atta laevigata, in a Neotropical savanna. Entomologia Experimentalis et Applicata. 2009;130:47–54. [Google Scholar]
- Muser B, Sommer S, Wolf H, Wehner R. Foraging ecology of the thermophilic Australian desert ant, Melophorus bagoti. Australian Journal of Zoology. 2005;53:301–311. [Google Scholar]
- Ness JH, Mooney K, Lach L. Ants as mutualists. In: Lach L, Parr CL, Abbott KL, editors. Ant ecology. Oxford University Press; Oxford, UK: 2009 [2010]. pp. 97–114. [Google Scholar]
- Neto JDR, Pinho BX, Meyer ST, Wirth R, Leal IR. Drought stress drives intraspecific choice of food plants by Atta leaf-cutting ants. Entomologia Experimentalis et Applicata. 2012;144:209–215. [Google Scholar]
- Nicholsorians C. The acceptability of young and mature leaves to leaf-cutter ants varies with light environment. Biotropica. 1992;24:211–214. [Google Scholar]
- Nicholsorians CM, Schultz JC. Leaf toughness affects leaf harvesting by the leaf cutter ant, Atta cephalotes (L.) (Hymenoptera: Formicidae) Biotropica. 1989;21:80–83. [Google Scholar]
- Niu YF, Feng YL, Xie JL, Luo FC. Noxious invasive Eupatorium adenophorum may be a moving target: Implications of the finding of a native natural enemy, Dorylus orientalis. Chinese Science Bulletin. 2010;55:3743–3745. [Google Scholar]
- Oliver TH, Leather SR, Cook JM. Macroevolutionary patterns in the origin of mutualisms involving ants. Journal of Evolutionary Biology. 2008;21:1597–1608. doi: 10.1111/j.1420-9101.2008.01600.x. [DOI] [PubMed] [Google Scholar]
- Oster GF, Wilson EO. Caste and ecology in the social insects. Princeton University Press; Princeton, NJ: 1978. p. 352. [PubMed] [Google Scholar]
- Pfeiffer M, Linsenmair KE. Polydomy and the organization of foraging in a colony of the Malaysian giant ant Camponotus gigas (Hymenoptera: Formicidae) Oecologia. 1998;117:579–590. doi: 10.1007/s004420050695. [DOI] [PubMed] [Google Scholar]
- Plowes NJR, Johnson RA, Hölldobler B. Foraging behavior in the ant genus Messor (Hymenoptera: Formicidae: Myrmicinae) Myrmecological News. 2013;18:33–49. [Google Scholar]
- Portha S, Deneubourg JL, Detrain C. Self-organized asymmetries in ant foraging: a functional response to food type and colony needs. Behavioral Ecology. 2002;13:776–781. [Google Scholar]
- Portha S, Deneubourg JL, Detrain C. How food type and brood influence foraging decisions of Lasius niger scouts. Animal Behaviour. 2004;68:115–122. [Google Scholar]
- Quinet Y, Debiseau JC, Pasteels JM. Food recruitment as a component of the trunk-trail foraging behaviour of Lasius fuliginosus (Hymenoptera: Formicidae) Behavioural Processes. 1997;40:75–83. doi: 10.1016/s0376-6357(97)00773-0. [DOI] [PubMed] [Google Scholar]
- Raimundo RLG, Freitas AVL, Oliveira AT. Seasonal patterns in activity rhythm and foraging ecology in the neotropical forest-dwelling ant, Odontomachus chelifer (Formicidae: Ponerinae) Annals of the Entomological Society of America. 2009;102:1151–1157. [Google Scholar]
- Ravary F, Jahyny B, Jaisson P. Brood stimulation controls the phasic reproductive cycle of the parthenogenetic ant Cerapachys biroi. Insectes Sociaux. 2006;53:20–26. [Google Scholar]
- Ribeiro SP, Espírito Santo NB, Delabie JHC, Majer JD. Competition, resources and the ant (Hymenoptera: Formicidae) mosaic: a comparison of upper and lower canopy. Myrmecological News. 2013;18:113–120. [Google Scholar]
- Robinson EJH, Green KE, Jenner EA, Holcombe M, Ratnieks FLW. Decay rates of attractive and repellent pheromones in an ant foraging trail network. Insectes Sociaux. 2008;55:246–251. [Google Scholar]
- Rohe W, Maschwitz U. Carton nest building and trophobiont manipulation in the south-east Asian ant Dolichoderus sulcaticeps (Mayr, 1870) (Hymenoptera: Formicidae) Journal of Natural History. 2003;37:2835–2848. [Google Scholar]
- Roschard J, Roces F. The effect of load length, width and mass on transport rate in the grass-cutting ant Atta vollenweideri. Oecologia. 2002;131:319–324. doi: 10.1007/s00442-002-0882-z. [DOI] [PubMed] [Google Scholar]
- Rudolph SG, Loudon C. Load size selection by foraging leaf-cutter ants (Atta cephalotes) Ecological Entomology. 1986;11:401–410. [Google Scholar]
- Saverschek N, Herz H, Wagner M, Roces F. Avoiding plants unsuitable for the symbiotic fungus: learning and long-term memory in leaf-cutting ants. Animal Behaviour. 2010;79:689–698. [Google Scholar]
- Savolainen R. Colony success of the submissive ant Formica fusca within territories of the dominant Formica polyctena. Ecological Entomology. 1990;15:79–85. [Google Scholar]
- Schafer RJ, Holmes S, Gordon DM. Forager activation and food availability in harvester ants. Animal Behaviour. 2006;71:815–822. doi: 10.1016/j.anbehav.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C. Molecular phylogenetics of ponerine ants (Hymenoptera: Formicidae: Ponerinae) Zootaxa. 2013;3647:201–250. doi: 10.11646/zootaxa.3647.2.1. [DOI] [PubMed] [Google Scholar]
- Schneirla TC. Raiding and other outstanding phenomena in the behavior of army ants. Proceedings of the National Academy of Sciences of the United States of America. 1934;20:316–321. doi: 10.1073/pnas.20.5.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöning C, Csuzdi C, Kinuthia W, Ogutu JO. Influence of driver ant swarm raids on earthworm prey densities in the Mount Kenya forest: implications for prey population dynamics and colony migrations. Insectes Sociaux. 2010;57:73–82. [Google Scholar]
- Seidl T, Wehner R. Visual and tactile learning of ground structures in desert ants. Journal of Experimental Biology. 2006;209:3336–3344. doi: 10.1242/jeb.02364. [DOI] [PubMed] [Google Scholar]
- Shattuck SO, McMillan P. Revision of the species of the Iridomyrmex conifer group (Hymenoptera: Formicidae), with notes on their biology. Australian Journal of Zoology. 1998;46:301–315. [Google Scholar]
- Shepherd JD. Trunk trails and the searching strategy of a leaf-cutter ant, Atta colombica. Behavioral Ecology and Sociobiology. 1982;11:77–84. [Google Scholar]
- Shepherd JD. Adjusting foraging effort to resources in adjacent colonies of the leaf-cutter ant, Atta colombica. Biotropica. 1985;17:245–252. [Google Scholar]
- Shutler D, Mullie A. Size-related foraging behavior of the leaf-cutting ant Atta colombica. Canadian Journal of Zoology-Revue Canadienne de Zoologie. 1991;69:1530–1533. [Google Scholar]
- Silva LVB, Vasconcelos HL. Plant palatability to leaf-cutter ants (Atta laevigata) and litter decomposability in a Neotropical woodland savanna. Austral Ecology. 2011;36:504–510. [Google Scholar]
- Silva PSD, Bieber AGD, Knoch TA, Tabarelli M, Leal IR, Wirth R. Foraging in highly dynamic environments: leaf-cutting ants adjust foraging trail networks to pioneer plant availability. Entomologia Experimentalis et Applicata. 2013;147:110–119. [Google Scholar]
- Sorvari J, Hakkarainen H. Habitat-related aggressive behaviour between neighboring colonies of the polydomous wood ant Formica aquilonia. Animal Behaviour. 2004;67:151–153. [Google Scholar]
- Sousa-Souto L, Schoereder JH, Lima ER. Why do leaf-cutting ants (Hymenoptera: Formicidae) change their foraging pattern? Sociobiology. 2008;52:645–654. [Google Scholar]
- Stradling DJ. Influence of size on foraging in ant, Atta cephalotes, and effect of some plant defence mechanisms. Journal of Animal Ecology. 1978;47:173–188. [Google Scholar]
- Sundstrom L. Foraging responses of Formica truncorum (Hymenoptera: Formicidae) – exploiting stable vs spatially and temporally variable resources. Insectes Sociaux. 1993;40:147–161. [Google Scholar]
- Swartz MB. Predation on an Atta cephalotes colony by an army ant, Nomamyrmex esenbeckii. Biotropica. 1998;30:682–684. [Google Scholar]
- Tanaka HO, Yamane S, Itioka T. Effects of a ferndwelling ant species, Crematogaster difformis, on the ant assemblages of emergent trees in a Bornean tropical rainforest. Annals of the Entomological Society of America. 2012;105:592–598. [Google Scholar]
- Tanner CJ, Adler FR. To fight or not to fight: contextdependent interspecific aggression in competing ants. Animal Behaviour. 2009;77:297–305. [Google Scholar]
- Tilles DA, Wood DL. Foraging behavior of the carpenter ant, Camponotus modoc (Hymenoptera: Formicidae), in a giant sequoia forest. Canadian Entomologist. 1986;118:861–867. [Google Scholar]
- Topoff H, Mirenda J. Army ants do not eat and run – influence of food supply on emigration behavior in Neivamyrmex nigrescens. Animal Behaviour. 1980;28:1040–1045. [Google Scholar]
- Traniello JFA. Colony specificity in the trail pheromone of an ant. Naturwissenschaften. 1980;67:361–362. [Google Scholar]
- Traniello JFA. Social organization and foraging success in Lasius neoniger (Hymenoptera: Formicidae) – behavioral and ecological aspects of recruitment communication. Oecologia. 1983;59:94–100. doi: 10.1007/BF00388080. [DOI] [PubMed] [Google Scholar]
- Traniello JFA. Foraging strategies of ants. Annual Review of Entomology. 1989;34:191–210. [Google Scholar]
- Traniello JFA, Beshers SN. Polymorphism and size pairing in the harvester ant Pogonomyrmex badius – a test of the ecological release hypothesis. Insectes Sociaux. 1991;38:121–127. [Google Scholar]
- Traniello JFA, Levings SC. Intracolony and intercolony patterns of nest dispersion in the ant Lasius neoniger – correlations with territoriality and foraging ecology. Oecologia. 1986;69:413–419. doi: 10.1007/BF00377064. [DOI] [PubMed] [Google Scholar]
- Vanbreda JM, Stradling DJ. Mechanisms affecting load size determination in Atta cephalotes L. (Hymenoptera: Formicidae) Insectes Sociaux. 1994;41:423–434. [Google Scholar]
- Van Wilgenburg E, Elgar MA. Colony structure and spatial distribution of food resources in the polydomous meat ant Iridomyrmex purpureus. Insectes Sociaux. 2007;54:5–10. [Google Scholar]
- Vasconcelos HL. Foraging activity of an Amazonian leaf-cutting ant: responses to changes in the availability of woody plants and to previous plant damage. Oecologia. 1997;112:370–378. doi: 10.1007/s004420050322. [DOI] [PubMed] [Google Scholar]
- Vasconcelos HL, Cherrett JM. The effect of wilting on the selection of leaves by the leaf-cutting ant Atta laevigata. Entomologia Experimentalis et Applicata. 1996;78:215–220. [Google Scholar]
- Vitorio AC, Forti LC, Rodella RA, Costa C, Caldato N, Fujihara RT, Silva MS. Preference of Atta capiguara (Hymenoptera: Formicidae) for different grasses. Sociobiology. 2011;58:341–352. [Google Scholar]
- Ward PS. Taxonomy, phylogenetics, and evolution. In: Lach L, Parr CL, Abbott K, editors. Ant ecology. Oxford University Press; Oxford, UK: 2009 [2010]. pp. 3–17. [Google Scholar]
- Ward PS, Brady SG, Fisher BL, Schultz TR. Phylogeny and biogeography of dolichoderine ants: effects of data partitioning and relict taxa on historical inference. Systematic Biology. 2010;59:342–362. doi: 10.1093/sysbio/syq012. [DOI] [PubMed] [Google Scholar]
- Way MJ. The relationship between certain ant species with particular reference to biological control of the coreid, Theraptus sp. Bulletin of Entomological Research. 1953;44:669–691. [Google Scholar]
- Way MJ, Khoo KC. Colony dispersion and nesting habits of the ants, Dolichoderus thoracicus and Oecophylla smaragdina (Hymenoptera: Formicidae), in relation to their success as biological control agents on cocoa. Bulletin of Entomological Research. 1991;81:341–350. [Google Scholar]
- Wehner R. The architecture of the desert ant’s navigational toolkit (Hymenoptera: Formicidae) Myrmecological News. 2009;12:85–96. [Google Scholar]
- Wetterer JK. Diel changes in forager size, activity, and load selectivity in a tropical leaf-cutting ant, Atta cephalotes. Ecological Entomology. 1990a;15:97–104. [Google Scholar]
- Wetterer JK. Load-size determination in the leaf-cutting ant, Atta cephalotes. Behavioral Ecology. 1990b;1:95–101. [Google Scholar]
- Wetterer JK. Allometry and the geometry of leaf-cutting in Atta cephalotes. Behavioral Ecology and Sociobiology. 1991;29:347–351. [Google Scholar]
- Wetterer JK. Forager polymorphism, size-matching, and load delivery in the leaf-cutting ant, Atta cephalotes. Ecological Entomology. 1994;19:57–64. [Google Scholar]
- Wetterer JK. The ecology and evolution of worker sizedistribution in leaf-cutting ants (Hymenoptera: Formicidae) Sociobiology. 1999;34:119–144. [Google Scholar]
- Wilby A, Shachak M. Harvester ant response to spatial and temporal heterogeneity in seed availability: pattern in the process of granivory. Oecologia. 2000;125:495–503. doi: 10.1007/s004420000478. [DOI] [PubMed] [Google Scholar]
- Wilson EO. Communication by tandem running in the ant genus Cardiocondyla. Psyche. 1959;66:29–34. [Google Scholar]
- Wilson EO. Chemical communication among workers of the fire ant Solenopsis saevissima (F. SMITH) 1. The organization of mass-foraging. Animal Behaviour. 1962;10:134–138. [Google Scholar]
- Wilson EO, Hölldobler B. The rise of the ants: a phylogenetic and ecological explanation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7411–7414. doi: 10.1073/pnas.0502264102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte V, Maschwitz U. Mushroom harvesting ants in the tropical rain forest. Naturwissenschaften. 2008;95:1049–1054. doi: 10.1007/s00114-008-0421-9. [DOI] [PubMed] [Google Scholar]
- Zakharov AA. Observer ants: storers of foraging area information in Formica rufa L (Formicidae: Hymenoptera) Insectes Sociaux. 1980;27:203–211. [Google Scholar]
- Zakharov AA, Tompson LC. Tunnels and territorial structure in polygyne fire ants Solenopsis wagneri (Hymenoptera: Formicidae) Zoologichesky Zhurnal. 1998;77:911–922. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


