Abstract
Consistency in gold chloride staining is essential for anatomical analysis of sensory nerve endings. The gold chloride stain for this purpose has been modified by many investigators, but often yields inconsistent staining, which makes it difficult to differentiate structures and to determine nerve ending distribution in large tissue samples. We introduce additional steps and major changes to the modified Gairns’ protocol. We controlled the temperature and mixing rate during tissue staining to achieve consistent staining and complete solution penetration. We subjected samples to sucrose dehydration to improve cutting efficiency. We then exposed samples to a solution containing lemon juice, formic acid and paraformaldehyde to produce optimal tissue transparency with minimal tissue deformity. We extended the time for gold chloride impregnation 1.5 fold. Gold chloride was reduced in the labrum using 25% formic acid in water for 18 h and in the capsule using 25% formic acid in citrate phosphate buffer for 2 h. Citrate binds gold nanoparticles, which minimizes aggregation in the tissue. We stored samples in fresh ultrapure water at 4° C to slow reduction and to maintain color contrast in the tissue. Tissue samples were embedded in Tissue Tek and sectioned at 80 and 100 μm instead of using glycerin and teasing the tissue apart as in Gairns’ modified gold chloride method. We attached sections directly to gelatin subbed slides after sectioning with a cryostat. The slides then were processed and coverslipped with Permount. Staining consistency was demonstrated throughout the tissue sections and neural structures were clearly identifiable.
Keywords: capsule, fibrous tissue, gold chloride, labrum, nerve endings, shoulder, stain
Gold chloride staining can provide excellent staining of myelinated axons and sensory nerve endings (Boyd 1962, Freud 1884, Gairns 1930, Kahlden 1894, Mann 1929). Although it has been considered a myelin staining method (McNally and Peters 1998), it also stains blood vessels, neurofilaments, elastin and collagen containing structures (Hogervorst and Brand 1998), which often makes it difficult to identify nerve endings specifically. Although the technique has shown promising results, it often has produced inconsistent staining because of inadequate penetration of solutions (Vilensky et al. 2002).
In some studies, Ruffini (type 1 nerve ending) and Pacinian (type 2 nerve ending) corpuscles in different tissues were stained by gold chloride; however, the appearance of these sensory nerve endings varied among reports (Adachi et al. 2002, Bresch and Nuber 1995, Dhillon et al. 2011, Gazza et al. 2006, Georgoulis et al. 2001, Guanche et al. 1999, Hogervorst and Brand 1998, Hasegawa et al. 1999, Katonis et al. 1991, Raunest et al. 1998, Vangsness et al. 1995, Yahia et al. 1988, Zimny et al. 1989). The conflicting identification of Ruffini and Pacinian corpuscles using gold chloride staining is believed to occur because of differences in tissue section thickness (25–100 μm) and infrequent use of serial sections (Hogervorst and Brand 1998).
Several modifications have been made to the gold chloride staining method (Boyd 1962, Silverberg et al. 1989, Zimny et al. 1989). These modifications have improved and standardized some aspects of gold chloride staining, but they have not achieved sufficient dependability to ensure proper identification of sensory nerve endings. We developed major modifications of the Gairns’ gold chloride staining method (Gairns 1930) that produce consistent and reproducible staining throughout a tissue sample and among different samples. In addition, our modifications enabled the identification of type 1 and type 2 nerve endings in fibrous tissue including the cadaveric shoulder capsule and the labrum.
Materials and methods
Reagents
Normal saline was purchased from Baxter Corp. (Deerfield, IL). Sucrose (FW = 342.3), sodium citrate dihydrate (FW = 294.1), 88% formic acid (ACS reagent grade), xylene (histological grade; FW = 106.17), Permount, absolute ethyl alcohol (ACS reagent grade), and 70 and 100% ethyl alcohol (histological grade) were obtained from Fisher Scientific (Pittsburgh, PA). Sakura Tissue Tek O.C.T. compound, sodium phosphate monobasic anhydrous (ACS reagent grade, FW = 137.99) and sodium phosphate dibasic anhydrous (ACS reagent grade, FW = 141.96) were purchased from VWR (Radnor, PA). Aqueous gold chloride (5%) was obtained from Salt Lake Metals (Salt Lake City, UT). Paraformaldehyde (PFA), 1 N sodium hydroxide, porcine skin gelatin (type A), chromium III potassium sulfate dodecahydrate (ACS reagent grade FW = 499.4) and 70% nitric acid (ACS reagent grade, FW = 63.01) were obtained from Sigma (St. Louis, MO). Pure lemon juice purchased from a local store.
Anhydrous sodium phosphate monobasic and dibasic salts were used to prepare 0.2 M phosphate buffer (PB). All sucrose solutions and 4% PFA were prepared in 0.1 M PB diluted from 0.2 M PB. To prepare 4% PFA, sodium hydroxide was added to raise the pH of the PB slowly after powdered PFA was added to allow the PFA to dissolve. A lemon juice solution was prepared using three parts lemon juice, 0.6 parts 4% PFA and 0.4 parts 88% formic acid. Gold chloride (5%) was diluted to 1% using ultrapure water. Alternatively, 1% gold chloride can be purchased from Salt Lake Metals (Salt Lake City, UT). A 0.1 M citrate solution was prepared in ultrapure water. Citrate PB (pH 7) was prepared from 6.5 ml 0.1 M citrate solution and 43.6 ml 0.2 M PB. Absolute ethyl alcohol was added to dry ice to make the freezing bath. In addition, 70 and 100% histological grade ethyl alcohols were used for slide processing. A 2 M nitric acid solution was prepared using 70% nitric acid and water to clean further the pre-cleaned slides. Gelatin chromium solution for subbing gelatin slides contained 5 g gelatin and 0.5 g chromium III potassium sulfate dissolved in ultrapure water.
Materials
Plastic molds and 75 × 50 mm pre-cleaned glass slides were obtained from Fisher Scientific. Gelatin coated 75 x 25 mm slides were obtained from Lab Scientific (Livingston, NJ). Glass jars that hold approximately 50 ml of solution were used.
Tissue preparation
The glenoid capsule is thin, loose fibrous tissue (Hyde 2007, Tovin and Reiss 2007), whereas the labrum is dense cartilaginous fibrous tissue (Hill et al. 2008).
The capsule and labrum specimens harvested from the anteroinferior and posteroinferior regions of shoulder pairs of three male and four female human cadavers. The cadavers ranged in age from 57 to 64 years.
The average size of the tissue specimens of interest was for the capsule 2 × 50 × 50 mm (thickness, width, and length) and for the labrum 9 × 21 × 15 mm (thickness, width, and length). The capsule and labrum are attached in the glenohumeral joint; the labrum serves as an attachment for the capsule. For convenience during staining, the attachment of the capsule and labrum were retained; thus, the maximal tissue length was 65 mm, 50 mm contributed by the capsule and 15 mm by the labrum. The two tissues are not separated until the reduction step of gold chloride staining.
The capsule is 2 mm thick; this thickness is not sufficient to achieve the desired stain color for tissue background and nerve endings. Therefore, additional tissue, primarily, muscle, was allowed to remain attached to the capsule, which increased overall tissue thickness to 5–7 mm. The tissue thickness is important for determining the amount of gold required for the gold impregnation and reduction steps. We tested tissue samples at different thicknesses including 5, 7 and 9 mmand found 7 mm thickness to be optimal.
Immediately after harvest, the specimens were washed twice for 10 min, each time in fresh saline before staining.
Staining
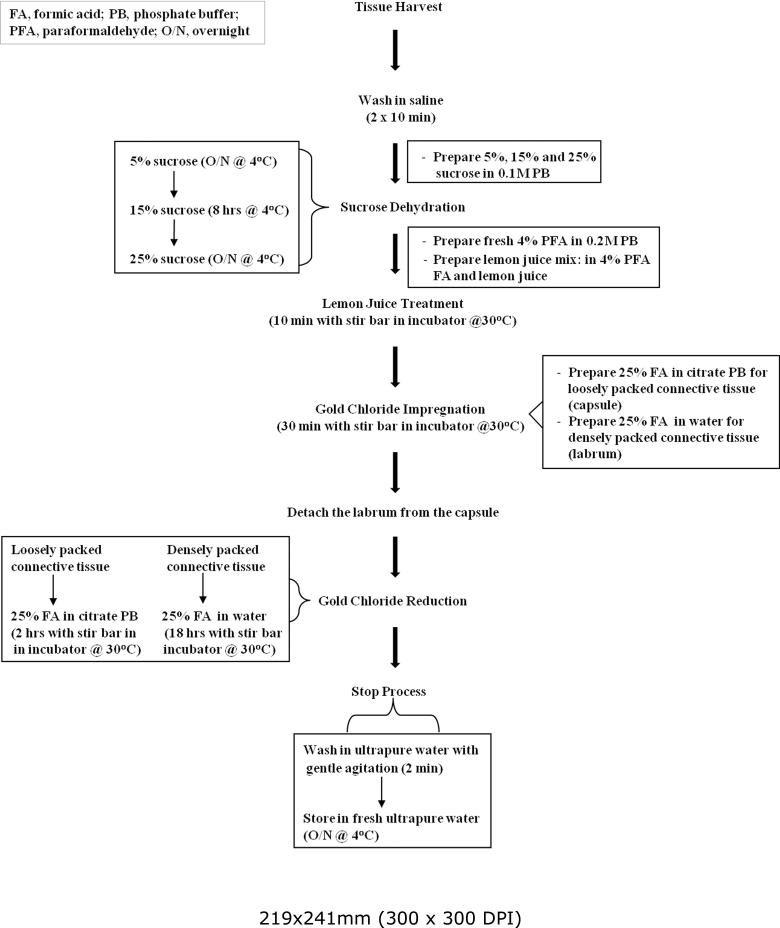
Figure 1 shows the gold chloride staining procedure. Briefly, the tissue was dehydrated with sucrose, treated with lemon juice solution, impregnated with gold chloride and the gold chloride was reduced. For each of these steps, the tissue specimens were completely submerged in 50 ml of the solutions.
Fig. 1.
Flow chart for gold chloride staining procedure.
Unlike other gold chloride protocols, our protocol included a reagent mixing rate of 400-450 rpm using a stir bar and controlled temperature in an incubator. The incubator not only allowed the temperature to be controlled, but also provided a dark environment. Stirring and controlled temperature were constant for each step of the staining process, except during sucrose dehydration. The procedure was carried out at both 20–22° C and 30° C.
Sucrose dehydration
The saline washed specimens were towel blotted and dehydrated in 5, 15 and 25% sucrose at 4° C for a minimum of 8 h at each concentration.
Gairns’ protocol (Gairns 1930) did not include sucrose dehydration. We tested staining with and without sucrose dehydration. Samples subjected to sucrose dehydration either before the lemon juice treatment or immediately after gold chloride reduction.
Tissue transparency
After sucrose dehydration, the capsule and labrum were transferred to 50 ml of the lemon juice solution for 10 min in the dark. Gairns’ protocol required soaking the tissue for 10 min in three parts 100% filtered lemon juice using freshly squeezed lemons and 1 part 88% formic acid in the dark at room temperature (Gairns 1930). Our specimens were tested under five different conditions: 1) three parts lemon juice and one part 88% formic acid (Boyd 1962, Gairns 1930, Silverberg et al. 1989), 2) lemon juice only (Silverberg et al. 1989), 3) three parts lemon juice and one part 4% PFA, 4) 3.6 parts lemon juice and 0.4 parts formic acid, and 5) three parts lemon juice, 0.6 parts 4% PFA and 0.4 parts 88% formic acid. Each lemon juice treatment was tested for 10, 15 and 20 min at both room temperature and at 30° C.
Gold chloride impregnation
The specimens were transferred to 50 ml 1% aqueous gold chloride for 30 min. The 1% aqueous gold chloride was used in the dark according to the original protocol (Gairns 1930). The specimens were not teased as they were in two protocols (Boyd 1962, Gairns 1930). Gold chloride solution can be filtered for use a second time. Both fresh and filtered gold chloride solutions were used. Gold chloride solution was not re-used more than once.
Gold chloride reduction
The capsule and labrum were separated from each other and subjected to two different reduction processes. It is important to use ceramic forceps and blade to separate the labrum from the capsule, because gold reacts with metal. The capsule was immersed in 50 ml 25% formic acid prepared with citrate PB until axons appeared dark purple-black. Our protocol required 1.5 h when using fresh gold chloride and 2 h when filtered gold chloride was used. By contrast to the capsule, reduction of gold chloride in the labrum was performed for 18 h in 25 ml 25% formic acid prepared in ultrapure water.
Reduction of gold was tested under five different conditions including: 1) 25% formic acid in ultrapure water (Gairns 1930), 2) acidified water using acetic acid (pH 3.13) (Silverberg et al. 1989), 3) sodium hydroxide solution (7 drops of sodium hydroxide added to 100 ml water), pH 11.76, 4) 0.1 M citrate PB, then transferred to 25% formic acid in ultrapure water, and 5) 25% formic acid in 0.1 M citrate PB. It is important to note that the conditions for reduction in acidified water or sodium hydroxide solution, by contrast to the other three conditions, did not include controlled temperature and standardized stirring. Reduction was performed for 2, 4, 8, 12, 16, 18 or 24 h.
Stopping the gold chloride reduction
When stained to the desired color, all specimens were washed in ultrapure water and stored in fresh ultrapure water at 4° C overnight or until ready for embedding. In the original protocol of Gairns, tissue was towel blotted, then immersed in glycerin; there was no termination step for gold chloride reduction (Gairns 1930). In our protocol, we slowed gold reduction at 4° C. After washing, samples were transferred to one of the following solutions: 1) 4% PFA for 2 min and 1% sodium thiosulfate for 2 min at room temperature, 2) 4% PFA for 2 min at room temperature, or 3) water at 4° C overnight. Samples that were treated with PFA and/or sodium thiosulfate were transferred to water and stored at 4° C overnight.
Embedding
The labrum and capsule samples were examined after various embedding conditions and in various media including paraffin, gelatin, glycerin gelatin, or Tissue Tek. In earlier protocols, tissue samples were placed in glycerin (Gairns 1930, Boyd 1962). We ultimately chose Tissue Tek for embedding both labrum and capsule samples, although the embedding process differed depending on specimen size. Four freezing techniques were tested for the samples embedded in Tissue Tek. These techniques included using liquid nitrogen, methyl butane with liquid nitrogen, dry ice, or ethanol and dry ice. Ultimately, we selected ethanol and dry ice as the freezing technique for both the labrum and capsule samples.
Labrum samples
Plastic embedding molds were filled carefully with Tissue Tek to avoid introducing air bubbles. The labrums then were towel blotted to remove excess water and immersed in Tissue Tek for at least 30 min. Subsequently, the molds were lowered into an ethanol and dry ice bath to snap-freeze the samples in the Tissue Tek. Care should be taken to prevent the ethanol from contacting the Tissue Tek or sample. Embedment of capsule tissue samples was more complex (see below).
Capsule samples
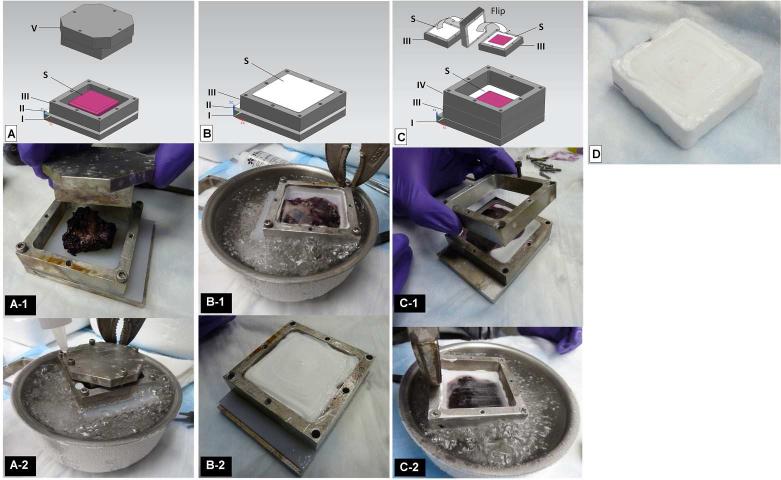
Steps for embedding the capsule tissue was custom designed for and more complex than for the labrum described above (Table 1, Fig. 2). Glass jars were partially filled with Tissue Tek. The capsules were towel blotted and immersed in Tissue Tek for at least 30 min. Because the capsule samples were dome-shaped, they required flattening. To achieve optimal flattening, we removed the specimens from the Tissue Tek and conditioned them by applying downward pressure using an aluminum block. Downward pressure was applied three to five times on the tissue with the surface of interest directed against a strip of polyethylene plastic covered with Kim Wipes. The Kim Wipes removed excess Tissue Tek. If noticeable Tissue Tek remains on the surface of interest after conditioning, a fresh Kim Wipe should be used to remove it. During each repetition of conditioning, the downward pressure was applied to the tissue until the capsule was completely spread, then the pressure was sustained for at least 5 sec. Once conditioning was complete, the samples were ready to be flattened permanently.
Table 1.
Steps for tissue embedding
| Steps | Notes |
|---|---|
| 1. Remove tissue from ultrapure water | |
| 2. Blot tissue using Kim Wipe to remove excess water | |
| 3. Transfer tissue samples to small jars containing Tissue Tek | 30 min at room temperature |
| 4. Start freeze bath using ethanol and dry ice | |
| 5. Condition tissue samples | 3–5 repetitions |
| 6. Refer to the steps in Fig. 3A–D | |
| 7. Wrap frozen block (Fig. 3D) in Parafilm and store @ −20° C until ready for sectioning | For long term storage, samples should be stored at −80° C |
Fig. 2.
Materials and sample processing during embedding. A) Apparatus required for tissue flattening. I, base of aluminum mold; II, polyethylene plastic; III, aluminum mold; V, compression block; S, tissue specimen. A-1) Tissue surface of interest in contact with polyethylene plastic (part II) prior to flattening using the compression block (part V). A-2) Lowering of complete aluminum mold into ethanol and dry ice freeze bath. The white dots represent drops of Tissue Tek that indicate a completely frozen tissue surface. B) Parts required for embedding the exposed half of the tissue specimen. I, base of aluminum mold; II, polyethylene plastic; III, aluminum mold; S, tissue specimen. B-1) Lowering of complete aluminum mold filled with Tissue Tek into ethanol and dry ice bath. I, base of aluminum mold; II, polyethylene plastic; III, aluminum mold; S, tissue specimen. B-2) Mold containing half of specimen embedded and frozen in Tissue Tek. C) Parts S and III are flipped to expose the flattened surface of tissue (part S). I, base of aluminum mold; S and III, frozen specimen in an aluminum mold; IV, additional aluminum mold. C-1) Adding the additional aluminum mold (part IV) after parts S and III have been flipped. C-2) Lowering of complete mold into ethanol and dry ice freeze bath after a layer of Tissue Tek was added over the flattened tissue surface (part S). I, base of aluminum mold; S, frozen specimen; III, aluminum mold; IV, additional aluminum mold (see description in the capsule samples section). D) Frozen tissue block after mold disassembly.
To flatten tissue permanently, we tested different techniques using a custom-made aluminum mold. Various attempts included: 1) pinning the tissue down to partially frozen Tissue Tek, 2) pressing the tissue against the bottom of an aluminum mold using a compression block (part V, Fig. 2A) and 3) using a compression block to press the tissue against polyethylene plastic that was customized to fit the base of the aluminum mold. The best results were obtained by pressing the tissue against polyethylene plastic using a compression block.
The tissue sample was placed in the custom made aluminum mold with the polyethylene plastic at the bottom (Fig. 2A-1) so that the tissue surface of interest was in contact with the plastic. The compression block was tightened against the tissue to produce maximal flatness. The capsule was considered flat when at least ¾ of the length of the screws was screwed into the aluminum mold. The complete aluminum mold including the compression block then was lowered into an ethanol and dry ice freezing bath without allowing ethanol to flow into the mold (Fig. 2A-2).
To determine whether the tissue was completely frozen, we applied two drops of Tissue Tek to the mold. When the Tissue Tek froze, the tissue surface of interest was completely frozen. The compression block then was removed and Tissue Tek was used to fill the area it had occupied, thus embedding the majority of the tissue. The tissue should remain flat against the plastic and should not be disturbed at any point during the remainder of the embedding process. After the mold was filled with Tissue Tek, it was lowered into the ethanol and dry ice freezing bath again (Fig 2B-1). When completely frozen (Fig. 2B-2), the portion of the mold that contained the partially embedded tissue specimen was separated from the base of the mold and flipped to expose the flattened surface of the tissue (Fig. 2C). Another aluminum mold, labeled as additional mold, was stacked on top of the flipped mold (Fig. 2C-1) and both were attached to the base without the plastic. The additional mold was another custom-made aluminum mold similar to the first except it was not designed to accommodate screws with a “round” head, but rather a “countersunk” head. A layer of Tissue Tek was applied to the exposed flattened part of the tissue and the mold was lowered into the ethanol and dry ice freezing bath (Fig. 2C-2) to complete the embedment. The aluminum mold then was disassembled and the block removed (Fig. 2D) and stored at −20° C for the short term or at −80° C for the long term.
Preparation of gelatin subbed slides
Prior to sectioning the embedded samples, 75 × 25 mm gelatin coated and 75 × 50 mm pre-cleaned slides, were subbed with gelatin to improve tissue adherence. Several protocols were combined (Kubke 2011, Larison 2009, NIH) to establish our final protocol for subbing the slides. Slides were placed in glass racks with an open bottom, then placed in glass containers. Pre-cleaned slides were placed in dishwashing solution (approximately 80° F) for 30 min to 1 h with gentle agitation, rinsed under running tap water for 15 min or until all traces of detergent were removed, then rinsed with running distilled water for 5 min. After rinsing, the slides were placed under a fume hood and the glass containers were filled with 2 M nitric acid. Slides remained in the nitric acid solution overnight. The nitric acid then was removed and stored for re-use. Next, the glass racks were rinsed in running tap water for 5–10 min, then rinsed in running distilled water for 5–10 min. The glass containers were covered immediately with glass lids or foil to maintain lint-free slides and transferred to a biological safety cabinet, uncovered,and air dried overnight. Fresh gelatin chromium solution was prepared and slides were dipped in the solution for 1 min, then air dried for 10 min. This coating process was repeated three times. If air bubbles formed, the covered containers with gelatin subbed slides were placed in an incubator at 37° C for 30 min or until no air bubbles remained. Slides then were removed from the glass containers under a biological safety cabinet and air dried overnight. Once dry, the slides were ready for use. The 75 × 25 mm gelatin coated slides were rinsed with running distilled water for 5 min, air dried in the biological safety cabinet. The slides then weredipped in the gelatin chromium solution for 1 min and air dried for 10 min. This coating process was repeated twice.
Sectioning
The procedure for cryosectioning is given in Table 2. The frozen labrum tissue blocks were sectioned at 80 μm in the sagittal plane and capsule samples were sectioned at 100 μm in the contact surface plane. Sectioning of the labrum and capsule samples produced small and large sections, respectively. Therefore, the labrum sections were adhered to 75 × 25 mm gelatin subbed slides directly from the cryostat platform. Capsule sections were positioned, using forceps, on 75 × 50 mm gelatin subbed slides within the cryostat chamber, then the slides were moved outside the chamber so sections could dry on the slides. After sectioning, the slides were air dried at 4° C overnight.
Table 2.
Steps for cryosectioning
| Steps | Notes |
|---|---|
| 1. Set cryostat parameters | Outside temperature = −25° C |
| Chamber temperature = −20° C | |
| Thickness: 80 or 100 μm | |
| 2. Bind specimen to specimen disc in the cryostat chamber using Tissue Tek | Orient tissue as desired |
| 3. Align tissue in cryostat | |
| 4. Section tissue | Labrum samples, 80 μm; Capsule samples, 100 μm |
| 5. Mount on gelatin subbed slides | |
| 6. Air dry overnight at 4° C and 31–33% humidity |
In the original protocol (Gairns 1930), the tissue samples were embedded in glycerin, teased apart and viewed by light microscopy. After embedding, we stored the frozen blocks in the cryostat to permit their acclimation to the cryostat temperature (outside temperature = −25° C, chamber temperature = −20° C). Samples sectioned immediately after freezing were placed in the cryostat for 30 min, whereas samples stored at −80° C prior to freezing were placed in the cryostat for 1 h. Frozen blocks were sectioned at 50, 80, 100, 150 and 200 μm. Samples also were sectioned in the sagittal, coronal, transverse and contact surface planes. The sections were adhered to four types of slides including gold plus (75 × 25 mm), poly-L-lysine (75 × 25 mm), gelatin coated (75 × 25 mm) and gelatin subbed (75 × 25 mm and 75 × 50 mm).
Slide processing
Steps for slide processing are given in Table 3. The day after sectioning, Tissue Tek was completely removed from the capsule sections by immersing them in two ultrapure water baths for at least 30 min (first bath, 15 min; second bath, 15 min) for labrum sections and at least 4 h (first bath, 3 h; second bath, 1 h) for capsule sections based on preliminary tests. The sections then were dehydrated in 70 and 100% ethanol for 1 min (labrum sections) or 2 min (capsule sections) in each solution. Two sequences of alcohol dehydration were tested: 70, 95 and 100% ethanol (Silverberg et al. 1989), and 70 and 100% ethanol; the 70 and 100% dehydration was more effective. The sections then were transferred to two baths of xylene. In the first bath, labrum sections were incubated for 1.5 min and capsulesections were incubated for 3 min. In the second bath, the labrum sections were incubated for 3 min and the capsule sections for 30–45 min. After dehydration, mounting medium was applied. Glycerol, glycerol-gelatin and Permount were tested as mounting media for coverslipping the slides. Permount mounting medium was the medium of choice and was applied to each section (Yahia et al. 1988). After coverslipping, the slides were stored at 4° C until analysis by light microscopy.
Table 3.
Steps for processing slides containing labrum and capsule sections
| Steps | Labrum Sections | Capsule Sections |
|---|---|---|
| 1. Place slides in ultrapure water at room temperature | 30 min–1 h | 4 h minimum, overnight maximum |
| 2. Transfer slides to 70% ethanol | 1 min | 2 min |
| 3. Transfer slides to 100% ethanol | 1 min | 2 min |
| 4. Transfer slides to xylene | 1.5 min | 3 min |
| 5. Transfer slides to fresh xylene | 3 min | 30–45 min |
| 6. Coverslip using Permount mounting medium | ||
| 7. Store slides overnight @ 4° C with humidity of 31–33% | ||
| 8. Observe slides using light microscopy within 1 week of staining |
Results
General procedures
The temperature in the room in which initial trials were performed fluctuated between 18 and 30° C. Temperatures below ambient (< 22° C) prevented complete tissue transparency and limited the reduction of gold chloride to free gold. Despite using a stir bar, fluctuating temperature caused inconsistent staining. Using a stir bar to control the mixing rate in the lemon juice treatment, gold chloride impregnation and reduction steps produced consistent penetration of solution and staining appearance. The lemon juice treatment resulted in inconsistent tissue transparency when a stir bar was not used. Use of a stir bar produced uniform gold chloride impregnation throughout the tissue sample (3 mm depth) as demonstrated by serial sections. Like the lemon juice treatment, complete gold chloride reduction was not consistent in the absence of a controlled mixing rate and temperature. Strict temperature control (27–30° C) and use of a stir bar to control the mixing rate in every step except sucrose dehydration produced uniform penetration of all solutions throughout the tissue samples as well as improved reproducibility.
Sucrose dehydration
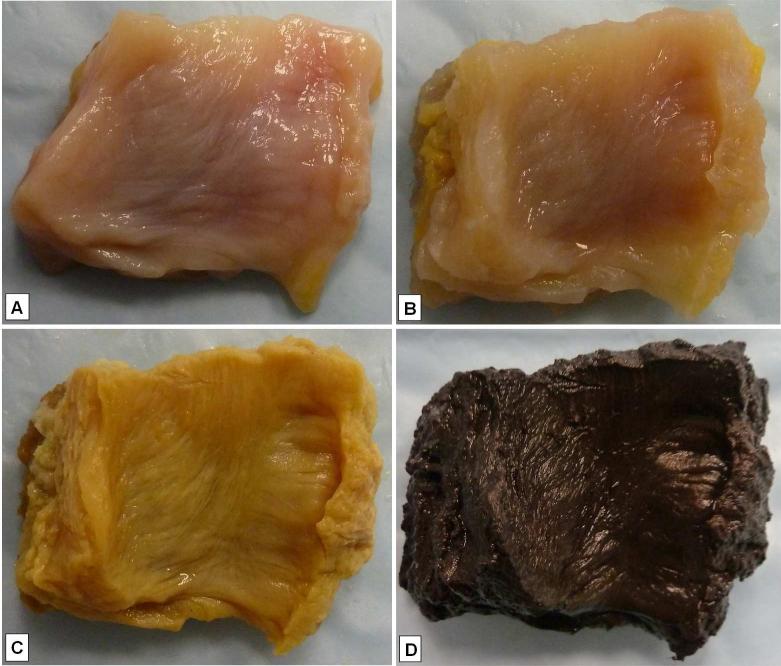
Sucrose dehydration prior to staining enabled smooth cutting of the labrum and capsule without altering the outcome of tissue staining. A specimen obtained after sucrose dehydration is shown in Fig. 3A. The timing of sucrose dehydration was important. The use of sucrose after gold chloride staining produced a pink background and pink myelinated axons. We were unable to differentiate blood vessels and nerve endings; fascicles, however, were identifiable. In the absence of sucrose dehydration, the tissue tore during sectioning.
Fig. 3.
Appearance of the glenoid capsule after each step of the gold chloride staining. A) Harvested tissue after sucrose dehydration. B) Tissue after lemon juice treatment. C) Tissue after gold chloride impregnation. D) Tissue after gold chloride reduction.
Tissue transparency (lemon juice treatment)
Immersing the tissues in pure lemon juice mixed with formic acid as suggested in previous protocols resulted in good transparency, but also extensive tissue deformation. In addition, staining intensity was decreased compared to the use of 100% lemon juice or 100% lemon juice mixed with PFA and formic acid. Lemon juice alone, however, produced adequate tissue transparency. Pure lemon juice combined with 4% PFA and 88% formic acid produced adequate tissue transparency for optimal staining intensity and little or no tissue deformity (Fig. 3B). This treatment yielded the best results after 10 min exposure at 30° C.
Gold chloride impregnation
Our protocol produced a uniform yellow appearance of the tissue (Fig. 3C). The intensity of the yellow color was greater when we used fresh gold chloride compared to gold chloride that had been used earlier and filtered. In the latter case, we compensated for the differences in intensity in the gold chloride reduction step by adjusting the incubation time (see below) to achieve comparable intensity.Tissue samples 5–7 mm thick produced optimal staining results and required 30 min for gold chloride penetration and dispersal throughout the tissue.
Gold chloride reduction
The use of 25% formic acid in ultrapure water as a reducer caused more extensive deformity of the capsule than the labrum. Tissue specimens reduced in acidified water or sodium hydroxide solution suffered little or no tissue deformity, but showed minimal staining intensity. Samples that initially were placed in citrate PB then transferred to 25% formic acid in ultrapure water showed the greatest staining intensity of the entire tissue specimen for all structures (nerve endings, fascicles and blood vessels) compared to using acidified water or sodium hydroxide. The use of 25% formic acid in citrate PB produced optimal staining intensity of the myelinated axons and fascicles, and caused minimal tissue deformity as shown in Fig. 3D. The best staining results were achieved when the labrum was reduced in 25% formic acid in ultrapure water for 18 h and the capsule was reduced in 25% formic acid in citrate PB for 1.5 or 2 h. Capsule specimens exposed to fresh or filtered gold chloride were placed in reducing solution for 1.5 h and 2 h, respectively. Reduction of gold chloride in the capsule using 25% formic acid in citrate PB did not cause extensive tissue deformity, but did cause the tissue to become “dome shaped.”
Stopping the gold chloride reduction
The use of 1% sodium thiosulfate caused a cloudy haze to form around the sensory nerve endings, which made identification of the components of the nerve endings difficult. Using 4% PFA following 1% sodium thiosulfate increased the clarity of the nerve endings. We have not tested the effects of 4% PFA on staining variability, however, and suggest that if 4% PFA is used in this protocol, additional testing should be done. Storing the samples at 4° C dramatically slowed the reduction of gold chloride and was the best way to stop reduction.
Embedding
Paraffin, gelatin, and glycerin-gelatin blocks stiffened, but did not harden, without either formalin or freezing. We were unable to cut sections from the paraffin, gelatin and glycerin-gelatin blocks. Tissue Tek was the best embedding medium for both the capsule and labrum; its use minimized tearing of both tissue types. The aluminum mold we designed for flattening tissue together with the series of steps we developed to embed the capsule, consistently achieved complete or near complete flatness of the surface of interest. The labrum did not require flattening. An ethanol and dry ice bath froze both tissues uniformly without forming ice crystals, unlike using liquid nitrogen, methyl butane with liquid nitrogen, or dry ice alone.
Cryosectioning
Serial sections were collected from 56 capsule and labrum specimens. Infiltration of tissue specimens with Tissue Tek enabled easy sectioning of the tissue with few or no tears or air bubbles. Tissue samples cut at 50 μm did not show the entire structure of sensory nerve endings. Labrum and capsule specimens cut at 80 and 100 μm, respectively, showed both full and partial nerve endings. Sections were cut easily and allowed subsequent slide processing. For the labrum, the structures of interest were more easily identifiable when sectioned at 80 μm than at 100 μm. The 150 μm thick sections cut easily and showed the majority of the structures of interest, but did not permit identification in regions dense with these structures The 200 μm thick sections showed more distinctive structures of sensory nerve endings than 150 μm thick sections, but did not cut easily. Serial sectioning of the specimens allowed us to confirm the identities of most structures.
Slide processing
The original Tissue Tek protocol required the sections to be immersed in water for 10 min, but this time was insufficient for complete removal of Tissue Tek from 80–100 μm thick sections. We compared incubation times in water ranging from 10 min to 5 h and found that 30 min to 1 h for labrum sections and 4 h for capsulesections were optimal.
Labrum sections
Labrum sections detached from gold plus, poly-L-lysine or gelatin-coated slides after 20 min in ultrapure water regardless of surface area. Labrum sections remained attached to the gelatin subbed 75 × 25 mm slides for 30 min to more than 3 h while the Tissue Tek was dissolving in the water. After Tissue Tek removal, minimal curling occurred when the sections were incubated in 70 and 100% ethanol for 1 min and two baths of xylene for 1.5 and 3 min.
Capsule sections
A large amount of residual Tissue Tek remained in the capsule sections after immersion in water for less than 3 h. Excess Tissue Tek on the slide appeared as a white haze across the sections and slide after exposure to ethanol and xylene. The capsule sections did not remain on pre-cleaned slides when immersed in water for 3 h, but they did remain attached to the gelatin subbed slides for more than 3 h. At 3 h, gel-like patches of residual Tissue Tek were observed throughout the capsulesections and the sections appeared distorted under the microscope. A minimum of 4 h was required for complete or near complete removal of Tissue Tek. Two alcohol (70 and 100%) baths instead of three (70, 95 and 100%), after Tissue Tek removal improved the clarity of the sections. To remove the gel-like patches completely, the sections were immersed in a second xylene bath for up to 45 min.
Consistency of tissue staining
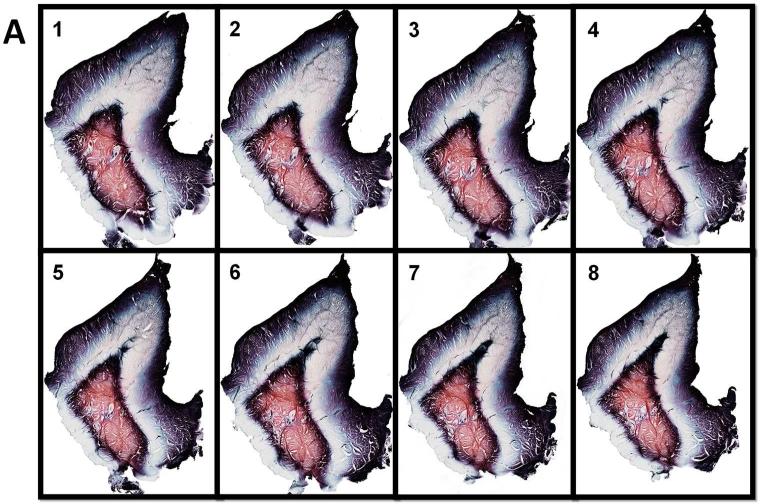
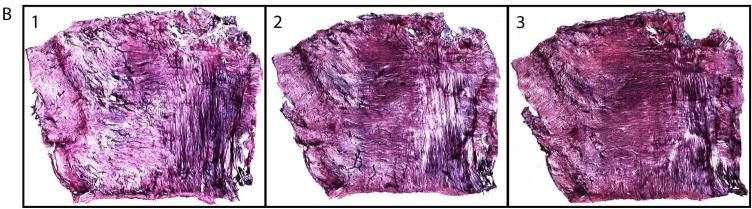
We monitored labrum and capsule staining using light microscopy. Because we sectioned the labrum in a sagittal plane, more sections were collected to sample the entire tissue width (21 mm). For this reason, some specimens yielded up to 263 sections. The sections for the labrum, unlike the capsule, showed patterned tricolor staining that enables one to differentiate the structural zones of the labrum. Moreover, the staining pattern appeared to be the same in all the sections. Figure 4A demonstrates eight serial labrum sections from 263 total sections.
Fig. 4.
Representative serial sections of labrum (A, 10 X) and capsule (B, 4 X) showing consistent gold chloride staining.
The capsule thickness in the contact surface plane was 1.5–2.0 mm; we collected an average of seven 100 μm sections, which corresponded to a tissue depth of 0.7 mm. Once this depth was reached, the color of the stained tissue started to appear different from the previous seven sections in which the staining was less intense and lighter pink. Figure 4B shows consistent staining in three of the seven serial capsule sections.
Appearance of neural structures
Tissue samples that were reduced in 25% formic acid in ultrapure water without using a stir bar showed black myelinated axons, fascicles and blood vessels. The collagenous background appeared transparent. Staining was inconsistent among and throughout the samples. Type 1 nerve endings were easily identifiable. It was difficult, however, to differentiate type 2 nerve endings, fascicles and blood vessels when capsule tissue was reduced in 25% formic acid in ultrapure water. Tissue specimens reduced in acidified water showed results similar to 25% formic acid in ultrapure water, but less staining intensity. Reduction in sodium hydroxide resulted in a light blue collagenous background. The capsules of type 2 sensory nerve endings and blood vessels stained darker blue than collagen, but not as dark as the myelinated axons. The entire structure of type 1 nerve endings stained dark blue and was easily identifiable.
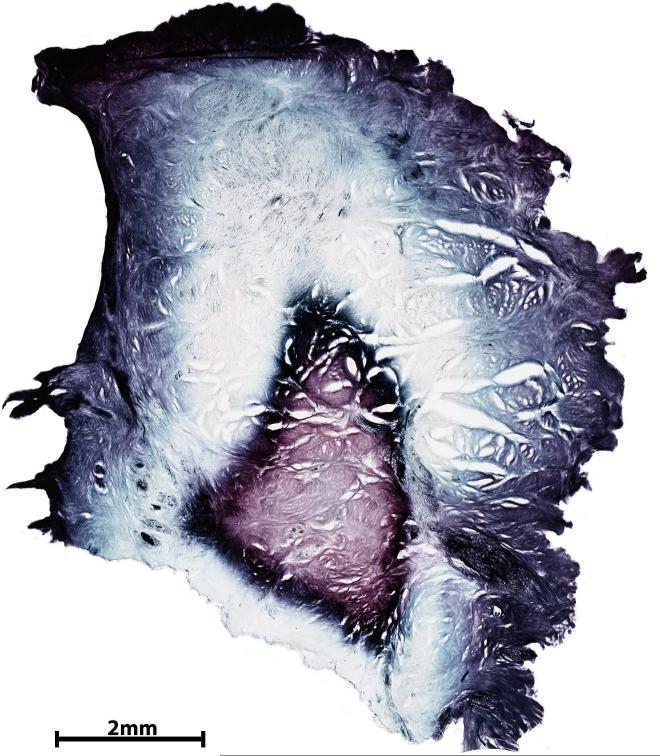
Sections reduced in 25% formic acid in ultrapure water under controlled conditions (temperature and mixing rate) showed a color range within the background of the labrum. Myelinated axons were black and the external capsules of type 2 nerve endings were blue. The background showed three concentric layers. The outermost layer was pink-purple, the second layer was transparent, and the innermost layer was pink (Fig. 5A). When capsule tissue was reduced in 25% formic acid in ultrapure water under controlled conditions, flocculation developed and the desired color contrast between the tissue background and the structures of interest was not achieved by varying exposure time in the reducing solution.
Fig. 5.
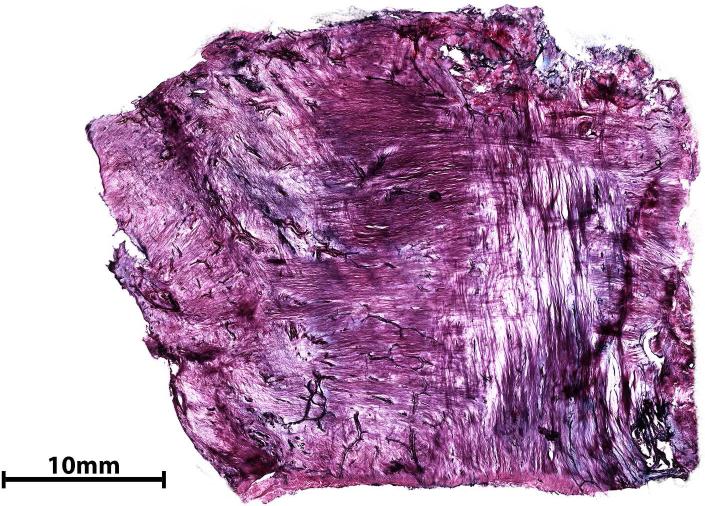
A) Section from labrum tissue reduced in 25% formic acid in ultrapure water showing the transparent to purple range of colors across three concentric layers; neural structures are stained black. B) Section of 100 μm thick glenoid capsule processed using 25% formic acid in citrate phosphate buffer showing pink to purple collagenous background and containing dark purple-black structures identified as type 1 and type 2 nerve endings. A, 10 X; B, 4 X.
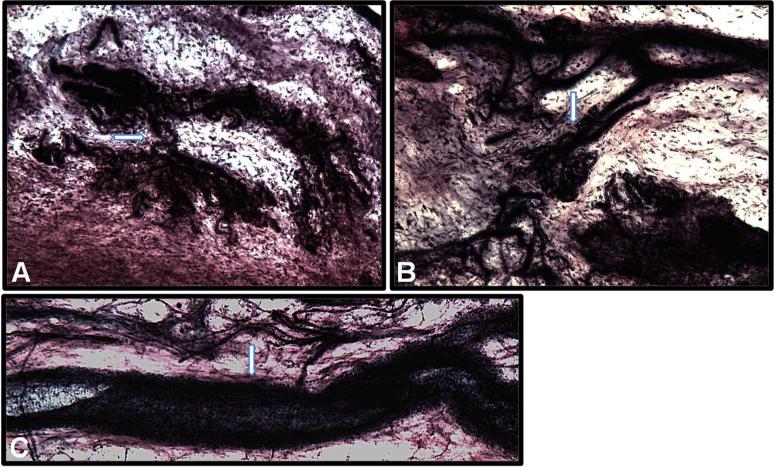
Capsule tissue reduction in 25% formic acid in citrate PB produced optimal color contrast among the collagenous background, type 2 nerve endings, blood vessels and type 1 nerve endings. The use of citrate PB also eliminated flocculation. The collagenous background appeared transparent pink to purple and contained dark purple-black structures (Fig. 5B). The axonal tree-like formation and intertwined cylindrical endings of type 1 sensory nerve endings stained dark purple (Fig. 6A, B). The blood vessels stained darker purple-black with a lumen (Fig. 6C). Myelinated axons stained dark purple. The lamellae of cross sections of the external capsule of type 2 nerve endings showed a light pink concentric pattern and the entire corpuscle had an onion-like appearance (Fig. 6D–F). The lamellae were not defined clearly. Type 2 sensory nerve endings presented with an elongated, conical, pinkish external capsule and a dark purple inner capsule with nuclei in an orbital pattern (Fig. 6D, E, G) when their structure was preserved during sectioning.
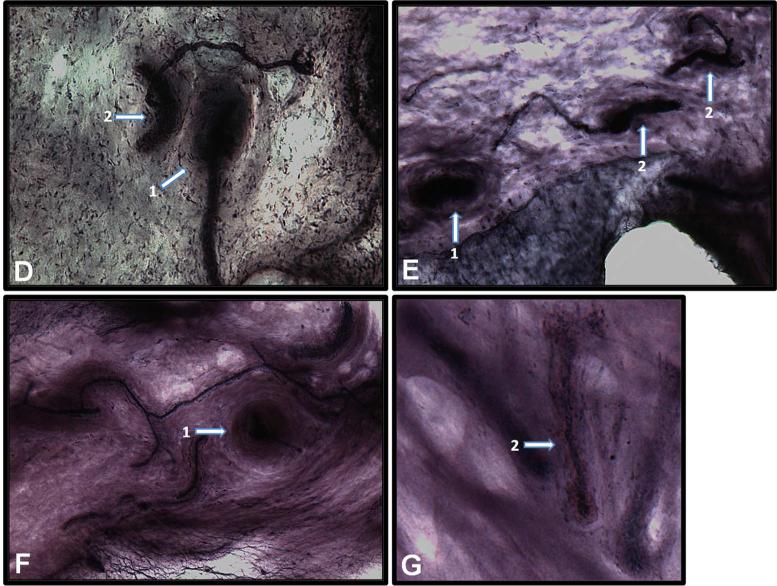
Fig. 6.
Glenoid capsule (white arrows). A) Type 1 sensory nerve ending innervating capsule tissue at the capsule-labrum junction. B) Type 1 sensory nerve ending innervating capsule tissue at the humeral attachment. C) Blood vessel. D– G) Different presentations of type 2 sensory nerve endings innervating capsule tissue including a cross-section of type 2 sensory nerve ending (1) and the entire corpuscle of type 2 nerve ending (2).
Discussion
General observations
Two major difficulties often encountered with earlier staining protocols (Boyd 1962, Gairns 1930, Silverberg et al. 1989, Zimny et al. 1989) include intermittent failure to achieve either tissue transparency or gold reduction, and the absence of uniform staining throughout the depth of the tissue. Temperature and mixing rate of solutions are important for gold nanoparticle synthesis (Beesley 1992). Our results suggest that a temperature controlled environment (27–30° C) and a mixing rate of 400–450 rpm are necessary for consistent staining within the tissue and among samples.
Sucrose dehydration
Because fibrocartilaginous tissues are difficult to section, we added a sucrose dehydration step to our protocol. Specifically, we used sucrose dehydration before the tissue transparency step, because it did not interfere with staining at this stage in our protocol. Sucrose is an excellent cryoprotectant, which prevents formation of ice crystals and preserves optimal morphology (Hayat 2000). We found sucrose dehydration to be especially beneficial when working with the labrum; the capsule was easier to work with and did not require additional treatment.
Tissue transparency
The tissue transparency step is critical for resolving neural structures by light microscopy. Citric acid, a component of lemon juice, is essential for achieving transparency, but 100% lemon juice was more effective than citric acid alone (Silverberg et al. 1989). How transparency is achieved in tissue in unclear, but previous research on the browning of fruits and vegetables offers a clue (Pilizota and Subaric 1998). Tissue pigmentation results from an oxidative reaction. In addition to citric acid, lemon juice contains vitamin C, which is an anti-oxidant that prevents browning of fruits by binding oxygen. Apparently, in this staining process, using lemon juice produced optimal tissue transparency that was caused by citric acid, an acidifier, and vitamin C, an anti-browning reagent. It also is important that in an acidic environment (pH < 3), enzymes responsible for browning are less active (Pilizota and Subaric 1998).
The tissue transparency step in gold chloride staining protocols includes exposure of tissue to both lemon juice and formic acid (Boyd 1962, Gairns 1930), which lowers the pH. When we exposed cadaver tissue to lemon juice only, the tissue color was lightened and under the acidic conditions (pH < 3), the tissue became transparent. The change in tissue pigmentation suggested that lemon juice and formic acid exert similar effects on tissue. We found that lemon juice and formic acid treatment for 10 min at 30° C produces tissue transparency, but should be combined with 4% PFA to minimize tissue deformation.
Gold chloride impregnation
Gold chloride staining depends on deposition of gold chloride, a metallic salt, in the tissue (Mann 1929), a process known as gold impregnation. Boyd noted that the extent of staining depends on the extent of gold impregnation, which is related to the ratio of gold chloride solution to muscle tissue. In our protocol, the extent of gold impregnation depends primarily on the relation of gold to tissue thickness (Boyd 1962) and exposure time. To select the best conditions, we varied the tissue thickness and kept the volume of gold chloride solution constant.
Tissue thickness was critical for staining the capsule. Because the capsule tissue ranged from 1.5–2 mm thick, a layer of fat and muscle was left attached to increase overall tissue thickness, which helped produce optimal staining results. This was true for the reduction step as well. The best results were observed in tissue samples 5–7 mm thick. Boyd (1962) asserted that the duration of tissue exposure to gold chloride plays a negligible role in gold chloride staining. To the contrary, we found that leaving the tissue in gold chloride for 10 min longer than in the original protocol improved its penetration throughout the tissue.
Gold chloride reduction
Gold chloride reduction is the most critical step for structure identification based on color differences among the tissue background, blood vessels, myelinated axons and sensory nerve endings. The type of reduction solution, duration of reduction and temperature together determine the best color contrast among structures. We achieved excellent color contrast when we used 25% formic acid in ultrapure water for 18 h for labrum tissue and 25% formic acid in citrate PB for 2 h for capsule tissue.
The mechanism of gold chloride staining of myelinated axons and sensory nerve endings is not clear. The chemistry of gold in the synthesis of gold nanoparticles and binding of gold to proteins, however, is better understood. Gold clearly plays a role in staining the proteins of myelinated axons and nerve endings. Mann (1929) observed that gold chloride staining was undependable. Freud (1884), in his research on nerve identification using gold chloride staining demonstrated that the intensity of the staining depended on the type of reduction solution used and the duration of exposure. In addition, Freud (1884) found that gold staining depends on the interaction of the metal with an organic compound such as citrate, sucrose, acetic acid, formaldehyde or formic acid. Organic acids, particularly formic acid, develop easily recognized images of the structure(s) of interest (Lenher 1913). This observation supports Gairns’ use of formic acid in his method for gold chloride reduction (Gairns 1930).
When gold chloride is reduced, gold spheres (nuclei) are formed; the process is known as nucleation. As the concentration of gold increases, the atoms cluster to form particles. Flocculation often occurs during gold nanoparticle synthesis. Citrate, commonly used as a reducing agent, stabilizes gold particles by preventing gold nanoparticle aggregation and flocculation (Kumar et al. 2007). Earlier research has suggested that citrate ions have a high affinity for ionic gold. The chelating head group of citric acid interacts with gold ions to form citrate-Au3+ complexes known as citrate-membrane assemblies (Gonzaga et al. 2008). In the presence of sodium citrate and an acid (e.g., tannic acid), the reduction of gold is improved (Beesley 1992). Therefore, the use of formic acid prepared in a citratephosphate buffer not only yields the best staining results, but also minimizes flocculation in capsule tissue.
Stopping the gold chloride reduction reaction
As gold in tissue is reduced over time, its color continues to change until reduction is complete. Depending on the type of reduction solution used, the color varies, so the end colors differ also. We sought to achieve a specific color contrast between the collagen fibers and other structures of the tissue. To do this, it was necessary to stop the reduction process before it was complete. Although storing the samples in ultrapure water at 4° C did not stop the reduction completely, it did slow the process significantly, which allowed us to consistently obtain the desired staining contrast.
Embedding
The capsule, when removed from the reduction solution, shrank unevenly and was deformed into a “dome shape,” which made it difficult to embed. Conditioning, which included three to five repetitions of downward pressure applied to the tissue to achieve tissue flatness, reduced tissue stiffness and ultimately flattened the dome shape; complete or nearly complete tissue flatness (Fig. 3C-1) was achieved in this way. The plastic on the bottom of the aluminum mold prevented extraneous precipitation in the tissue that would occur if the tissue were pressed against metal. Embedding the capsule in a series of steps caused the tissue surface to remain flat during embedment for cryosectioning. In this way, the desired orientation was achieved without distorting shape of the tissue.
Cryosectioning
Two variables that contribute to different results among studies include the thickness of sections and examination of either random or serial sections (Hogervorst and Brand 1998). We selected 80 and 100 μm sections as optimal for labrum and capsule sections, respectively. To confirm the identification of structures, we suggest examining serial sections for anatomical examination.
Slide processing
The time required for complete removal of Tissue Tek from labrum sections (labrum) varied depending on the surface area of the sections and the type of slide used. After Tissue Tek removal, shorter incubation times in ethanol and xylene for labrum sections compared to capsule sections prevented extensive curling of tissue. The extended periods in all solutions for slide processing improved the clarity of the sections and aided the identification of neural structures. The time may vary for different tissue types during each step of slide processing.
Appearance of neural structures
Type 1 sensory nerve endings have a tree-like structure with intertwined cylindrical endings known as capsules (Halata 1977). Type 2 sensory nerve endings include a neurite surrounded by an inner capsule consisting of specialized Schwann cells and squamous cells surrounded by an external collagenous capsule (Bell et al. 1994); however, not all type 2 nerve endings have an external collagenous capsule (Peterson et al. 1972). We identified the substructures of both type 1 and type 2 nerve endings using our staining protocol. We observed that the color contrast between type 1 and type 2 sensory nerve endings, collagenous background and blood vessels varied within the tissues we examined depending on the reducing solution used.
Gold chloride reduction in 25% formic acid in ultrapure water produced excellent results for the labrum, but the staining was inconsistent in the absence of consistent temperature and mixing rate. Controlling these two factors maximized the color range in the tissues and produced the most favorable results.
Under controlled conditions, the labrum exhibited tricolor staining of the three concentric layers consisting of a superficial mesh, dense circumferential braided core and loosely packed peri-core zone (Hill et al. 2008). The background color of the labrum and capsule could be manipulated by changing the amount of time the tissue was exposed to the reducing solutions. Although we could change the background color of the capsule by reducing gold in 25% formic acid in ultrapure water, flocculation still occurred. Therefore, we used citrate PB instead of water. Use of citrate PB both eliminated flocculation and produced the best color contrast between the collagenous background and neural structures.
Gold chloride staining of myelinated axons and nerve endings has been used for many years and has undergone many modifications. Each modification yielded similar results, but none addressed the problem of inconsistent staining. We altered most of the original steps of the Gairns’ protocol (Gairns 1930) to produce more consistent and optimal staining. Our changes included a consistent temperature, mixing rate, sucrose dehydration, altered lemon juice solution, 25% formic acid in citrate PB for reducing gold, the period that the specimens were in solution for each step, and a novel embedding process. Ultimately, our modified protocol enabled us to achieve consistent staining of nerve endings in both the labrum and capsule.
Acknowledgments
We thank Daniel Sisk for assisting with staining specimens, Dr. Takako Chickenji, Mayo Clinic, Rochester, MN, for providing critical knowledge of cryoprotectants for sectioning the labrum, and Damon Mar for building the device for flattening and freezing tissue. This investigation was supported by grants T32HD057850 and HD02528 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Partial support was received from the Marc A. and Elinor J. Asher Orthopedic Research Endowment.
References
- Adachi N, Ochi M, Uchio Y, Iwasa J, Ryoke K, Kuriwaka M. Mechanoreceptors in the anterior cruciate ligament contribute to the joint position sense. Acta Ortho. Scand. 2002;73:330–334. doi: 10.1080/000164702320155356. [DOI] [PubMed] [Google Scholar]
- Beesley JE. Preparation of gold probes. Methods Mol. Biol. 1992;80:163–168. doi: 10.1385/0-89603-204-3:163. [DOI] [PubMed] [Google Scholar]
- Bell JS, Bolanowski S, Holmes M. The structure and function of pacinian corpuscles: a review. Prog. Neurobiol. 1994;42:79–128. doi: 10.1016/0301-0082(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Boyd I. Uniform staining of nerve endings in skeletal muscle with gold chloride. Stain Technol. 1962;37:225–230. doi: 10.3109/10520296209117741. [DOI] [PubMed] [Google Scholar]
- Bresch J, Nuber G. Mechanoreceptors of the middle and inferior glenohumeral ligaments. J. Shoulder Elbow Surg. 1995;1:S63–S64. [Google Scholar]
- Dhillon MS, Bali K, Prabhakar S. Proprioception in anterior cruciate ligament deficient knees and its relevance in anterior cruciate ligament reconstruction. Ind. J. Ortho. 2011;45:294–300. doi: 10.4103/0019-5413.80320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freud S. A new histological method for the study of nerve-tracts in the brain and spinal chord. Brain J. Neurol. 1884;7:86–88. [Google Scholar]
- Gairns F. A modified gold chloride method for the demonstration of nerve-endings. Q. J. Microsc. Sci. 1930;74:151–153. [Google Scholar]
- Gazza F, Botti M, Ragionieri L, Dessole A, Panu R, Palmieri G, Acone F. On the autonomic and sensitive nervous components of digital, metacarpal and metatarsal pads of the coypu (Myocastor Coypus). Ann. Fac. Med. Vet. di Parma. 2006;26:79–90. [Google Scholar]
- Georgoulis AD, Pappa L, Moebius U, Malamou-Mitsi V, Pappa S, Papageorgiou CO, Agnantis NJ, Soucacos PN. The presence of proprioceptive mechanoreceptors in the remnants of the ruptured ACL as a possible source of re-innervation of the ACL autograft. Knee Surg. Sports Traum. Arthrosc. 2001;9:364–368. doi: 10.1007/s001670100240. [DOI] [PubMed] [Google Scholar]
- Gonzaga F, Singh S, Brook MA. Biomimetic synthesis of gold nanocrystals using a reducing amphiphile. Small. 2008;4:1390–1398. doi: 10.1002/smll.200701163. [DOI] [PubMed] [Google Scholar]
- Guanche CA, Noble J, Solomonow M, Wink CS. Periarticular neural elements in the shoulder joint. Orthopedics. 1999;22:615–617. doi: 10.3928/0147-7447-19990601-12. [DOI] [PubMed] [Google Scholar]
- Halata Z. The ultrastructure of sensory nerve endings in the articular capsule of the knee joint of the domestic cat (ruffini corpuscles and pacinian corpuscles). J. Anat. 1977;124:717–729. [PMC free article] [PubMed] [Google Scholar]
- Hasegawa S, Yamamoto H, Morisawa Y, Michinaka Y. A study of mechanoreceptors in fibrocartilage masses in the defect of pars interarticularis. J. Ortho. Sci. 1999;4:413–420. doi: 10.1007/s007760050124. [DOI] [PubMed] [Google Scholar]
- Hayat M, editor. Principles and Techniques of Electron Microscopy: Biological Applications. Cambridge University Press; Cambridge, UK: 2000. Cryoprotectants. pp. 402–404. [Google Scholar]
- Hill A, Hoerning E, Brook K, Smith C, Moss J, Ryder T, Wallace A, Bull A. Collagenous microstructure of the glenoid labrum and biceps anchor. J. Anat. 2008;212:853–862. doi: 10.1111/j.1469-7580.2008.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst T, Brand R. Current concepts review-mechanoreceptors in joint function. J. Bone Joint Surg. 1998;80:1365–1378. doi: 10.2106/00004623-199809000-00018. [DOI] [PubMed] [Google Scholar]
- Kahlden C. The Central Nervous System. In: Fletcher H, editor. Methods of Pathological Histology. MacMillan and Co.; London and New York: 1894. pp. 136–140. [Google Scholar]
- Katonis PG, Assimakopoulos AP, Agapitos MV, Exarchou EI. Mechanoreceptors in the posterior cruciate ligament. Histologic study on cadaver knees. Acta Ortho. Scand. 1991;62:276–278. doi: 10.3109/17453679108993609. [DOI] [PubMed] [Google Scholar]
- Kubke M. Kubke Lab: Protocols/Gelatin Subbing 2011. 2011 Available from http://openwetware.org/wiki/Kubke_Lab:Protocols/Gelatin_Subbing.
- Kumar S, Gandhi K, Kumar R. Modeling of formation of gold nanoparticles by citrate method. Ind. Eng. Chem. Res. 2007;46:3128–3136. [Google Scholar]
- Larison K. Preparing Subbed Slides for Sections. 5th ed. University of Oregon Press; Eugene , OR: 2009. Available from https://wiki.zfin.org/display/prot/Preparing+Subbed+Slides+For+Sections. [Google Scholar]
- Lenher V. Contribution to the chemistry of gold. J. Am. Chem. Soc. 1913;35:546–552. [Google Scholar]
- Mann G. Impregnation Methods. In: Frowde H, editor. Physiological Histology, Methods and Theory. University of Oxford; London and New York: 1929. pp. 261–276. [Google Scholar]
- McNally K, Peters A. A new method for intense staining of myelin. J. Histochem. Cytochem. 1998;46:541–545. doi: 10.1177/002215549804600415. [DOI] [PubMed] [Google Scholar]
- NIH Gelatin Coated Slides. Available from https://science.nichd.nih.gov/confluence/display/mic/Gelatin+Coated+Slides.
- Peterson HA, Winkelmann RK, Coventry MB. Nerve endings in the hip joint of the cat: their morphology, distrubution, and density. J. Bone Joint Surg. Am. 1972;54:333–343. [PubMed] [Google Scholar]
- Pilizota V, Subaric D. Control of enzymatic browning of foods. Food Technol. Biotechnol. 1998;36:219–227. [Google Scholar]
- Raunest J, Sager M, Burgener E. Proprioception of the cruciate ligaments: receptor mapping in an animal model. Arch. Ortho. Trauma Surg. 1998;118:159–163. doi: 10.1007/s004020050338. [DOI] [PubMed] [Google Scholar]
- Silverberg K, Ogilvy C, Borges L. A modified gold chloride technique for optimal impregnation of nerves within corneal whole mounts and dura of the albino rat. J. Histochem. Cytochem. 1989;37:269–271. doi: 10.1177/37.2.2642943. [DOI] [PubMed] [Google Scholar]
- Tovin B, Reiss J. Shoulder. In: Kolt G, Snyder-Mackler L, editors. Physical Therapies in Sport and Exercise. Elsevier's Health Sciences; Philadelphia: 2007. pp. 284–286. [Google Scholar]
- Vangsness C, Ennis M, Taylor J, Atkinson R. Neural anatomy of the glenohumeral ligaments, labrum and subacromial bursa. J. Arthrosc. Relat. Surg. 1995;11:180–184. doi: 10.1016/0749-8063(95)90064-0. [DOI] [PubMed] [Google Scholar]
- Vilensky JA, O'Connor BL, Fortin JD, Merkel GJ, Jimenez AM, Scofield BA, Kleiner JB. Histologic analysis of neural elements in the human sacroiliac joint. Spine. 2002;27:1202–1207. doi: 10.1097/00007632-200206010-00012. [DOI] [PubMed] [Google Scholar]
- Yahia L, Newman N, Rivard C. Neurohistology of lumbar spine ligaments. Acta Ortho. Scand. 1988;59:508–512. doi: 10.3109/17453678809148773. [DOI] [PubMed] [Google Scholar]
- Zimny ML, DePaolo C, Dabezies E. Mechano-receptors in the flexor tendons of the hand. J. Hand Surg. Br. 1989;14:229–231. doi: 10.1016/0266-7681_89_90134-4. [DOI] [PubMed] [Google Scholar]