Abstract
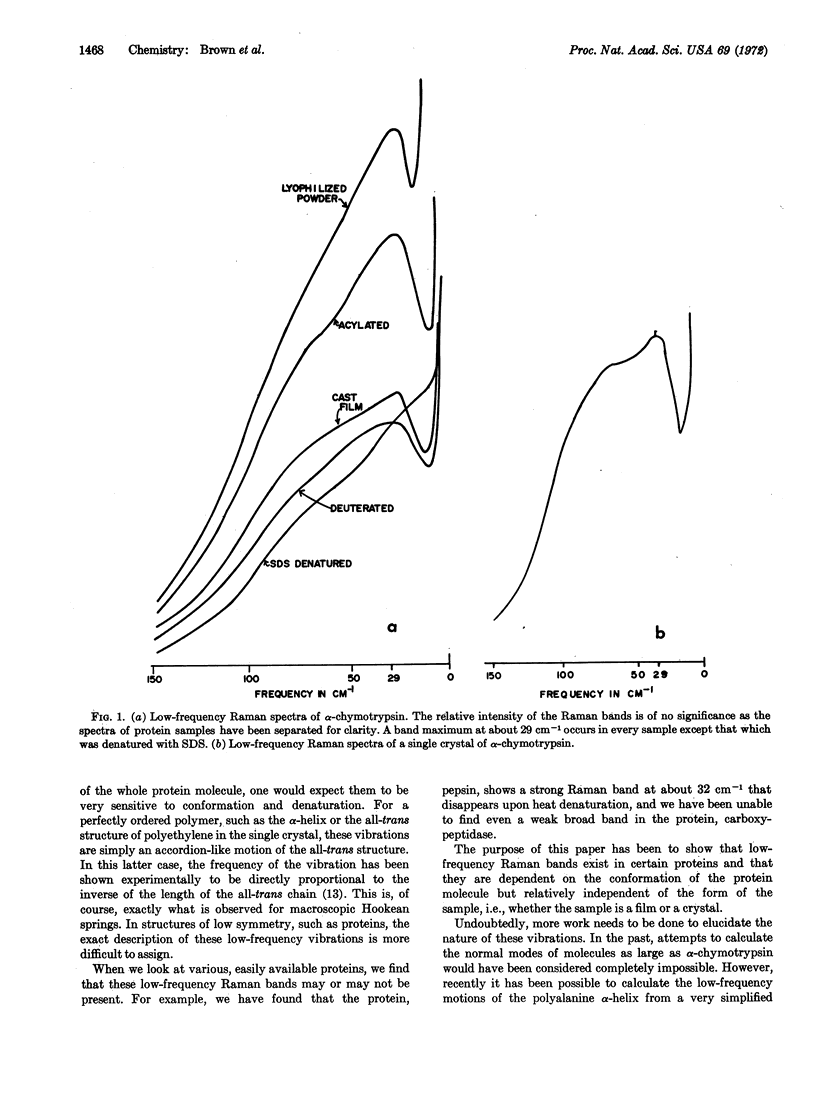
Low-frequency Raman bands (lower than 50 cm-1) exist in certain proteins. They are dependent upon the conformation of the protein molecule, but are relatively independent of the form of the sample, i.e., whether it is a film or a crystal.
Low-frequency Raman spectra were obtained from samples of α-chymotrypsin that had been prepared in several ways. A peak at about 29 cm-1 was found for all samples except the one that had been denatured with sodium dodecyl sulfate. Such low frequency motions must arise from vibrations that involve all, or very large portions, of the protein molecule.
Keywords: α-chymotrypsin, protein conformation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fanconi B., Peticolas W. L. Simplified force field calculations of the low-frequency motions of the alpha-helix. Biopolymers. 1971 Nov;10(11):2223–2229. doi: 10.1002/bip.360101115. [DOI] [PubMed] [Google Scholar]
- Fanconi B., Small E. W., Peticolas W. L. Phonon dispersion curves and normal coordinate analysis of -poly-L-alanine. Biopolymers. 1971;10(8):1277–1298. doi: 10.1002/bip.360100804. [DOI] [PubMed] [Google Scholar]
- Ito K., Shimanouchi T. Vibrational frequencies and modes of alpha-helix. Biopolymers. 1970;9(4):383–399. doi: 10.1002/bip.1970.360090402. [DOI] [PubMed] [Google Scholar]
- Lord R. C., Yu N. T. Laser-excited Raman spectroscopy of biomolecules. I. Native lysozyme and its constituent amino acids. J Mol Biol. 1970 Jun 14;50(2):509–524. doi: 10.1016/0022-2836(70)90208-1. [DOI] [PubMed] [Google Scholar]
- Lord R. C., Yu N. T. Laser-excited Raman spectroscopy of biomolecules. II. Native ribonuclease and alpha-chymotrypsin. J Mol Biol. 1970 Jul 28;51(2):203–213. doi: 10.1016/0022-2836(70)90137-3. [DOI] [PubMed] [Google Scholar]
- Rossi G. L., Bernhard S. A. On the relationship between the conformation and the catalyzed reactivity of acyl-chymotrypsin. J Mol Biol. 1971 Jan 28;55(2):215–230. doi: 10.1016/0022-2836(71)90193-8. [DOI] [PubMed] [Google Scholar]
- Sigler P. B., Jeffery B. A., Matthews B. W., Blow D. M. An x-ray diffraction study of inhibited derivatives of alpha-chymotrypsin. J Mol Biol. 1966 Jan;15(1):175–192. doi: 10.1016/s0022-2836(66)80219-x. [DOI] [PubMed] [Google Scholar]
- Small E. W., Fanconi B., Peticolas W. L. Raman spectra and the phonon dispersion of polyglycine. J Chem Phys. 1970 May 1;52(9):4369–4379. doi: 10.1063/1.1673659. [DOI] [PubMed] [Google Scholar]
- Tobin M. C. Raman spectra of crystalline lysozyme, pepsin, and alpha chymotrypsin. Science. 1968 Jul 5;161(3836):68–69. doi: 10.1126/science.161.3836.68. [DOI] [PubMed] [Google Scholar]
- Walton A. G., Deveney M. J., Koenig J. L. Raman spectroscopy of calcified tissue. Calcif Tissue Res. 1970;6(2):162–167. doi: 10.1007/BF02196195. [DOI] [PubMed] [Google Scholar]



