Abstract
Supramolecular interactions between different hydrogen-bonding guests and poly(2-vinyl pyridine)-block-poly (styrene) can be exploited to prepare remarkably diverse self-assembled nanostructures in dispersion from a single block copolymer (BCP). The characteristics of the BCP can be efficiently controlled by tailoring the properties of a guest which preferentially binds to the P2VP block. For example, the incorporation of a hydrophobic guest creates a hydrophobic BCP complex that forms phase separated nanoparticles upon self-assembly. Conversely, the incorporation of a hydrophilic guest results in an amphiphilic BCP complex that forms spherical micelles in water. The ability to tune the self-assembly behavior and access dramatically different nanostructures from a single BCP substrate demonstrates the exceptional versatility of the self-assembly of BCPs driven by supramolecular interactions. This approach represents a new methodology that will enable the further design of complex, responsive self-assembled nanostructures.
Molecular biology relies on complex and dynamic supramolecular morphology transitions to accomplish complex functions. To perform these tasks, nature is armed with a diverse library of programmed structures derived from phospholipids, polypeptides, and polysaccharides and their supramolecular complexes.[1] Synthetic chemists have long been inspired by biology and many materials have been studied to produce complex assemblies for example; peptide amphiphiles,[2] surfactants,[3] and polymer conjugates,[4–6] amphiphilic polymers,[7] and single polymer chain assemblies.[8–10] We are interested in developing new methods to control macromolecular assembly through supramolecular engineering to synthetically realize some of nature’s complexities in regards to multi-component supramolecular self-assemblies.
Block copolymers (BCPs) have the unique ability to form a rich array of self-assembled nanostructures in the bulk and in solution. In the bulk, complex phase diagrams have been mapped and morphologies including lamellar, cylindrical, and bicontinuous (gyroid) self-assembled structures have been identified.[11–13] Similarly, a variety of structures can be accessed in solution from vesicles to spherical and cylindrical micelles.[14–17] Recently it has been reported that bulk-type morphologies can be accessed in solution processing of BCPs.[18–29] However, despite the potential of supramolecular chemistry to dictate the solution self-assembly of hydrophobic BCPs, systematic design rules to control nanostructures on demand are still limited. Importantly, these responsive assemblies would enable the development of new, complex, and multi-component soft nanostructures.
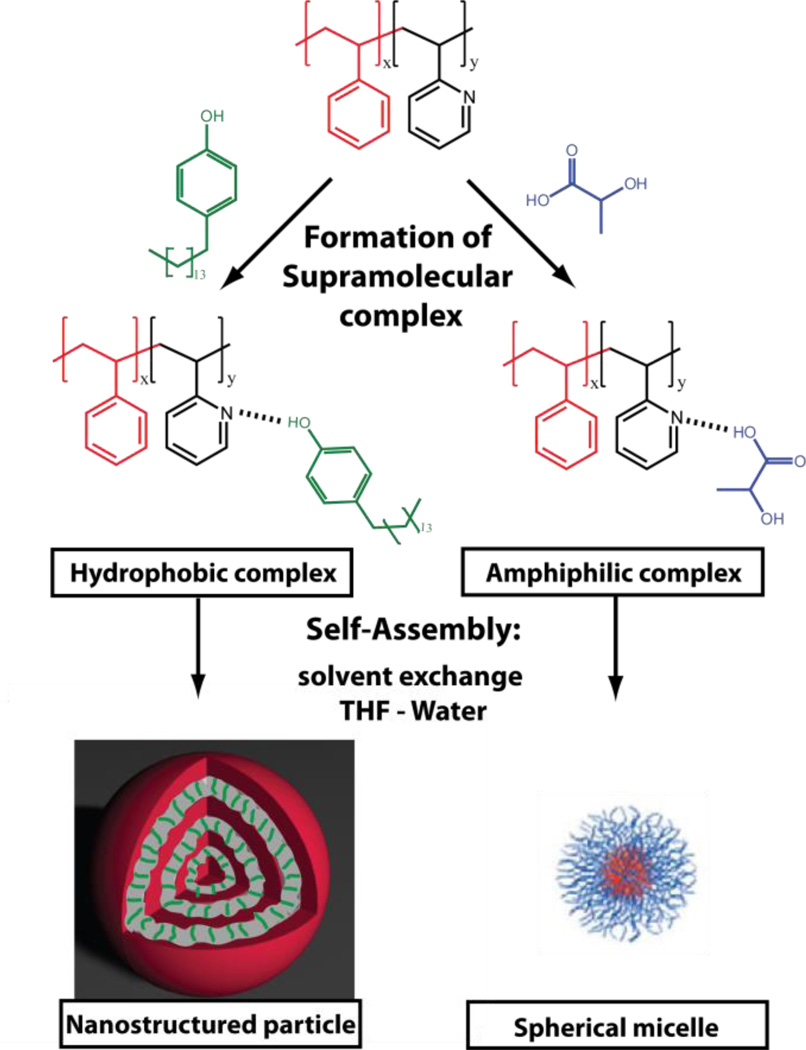
Herein, we demonstrate a versatile strategy to prepare a diverse range of self-assembled nanostructures from the same hydrophobic BCP. Harnessing the power of supramolecular chemistry we demonstrate that nanoparticles with vastly different structures can be prepared. Traditional spherical micelle assemblies can be prepared by utilization of an amphiphilic supramolecular BCP which incorporates a hydrophilic guest. In contrast, nanoparticles with internally phase separated morphologies can be accessed by introducing a hydrophobic guest (Figure 1). Furthermore, we demonstrate that the dynamic supramolecular hydrogen bond can be easily broken to allow the removal of the guest yielding mesostructured porous nanoparticles.
Figure 1.
Schematic representation of the self-assembly of supramolecular block copolymer complexes containing different guests. Diverse nanoparticle structures can be accessed by controlling the properties of the guest and hence the self-assembly properties.
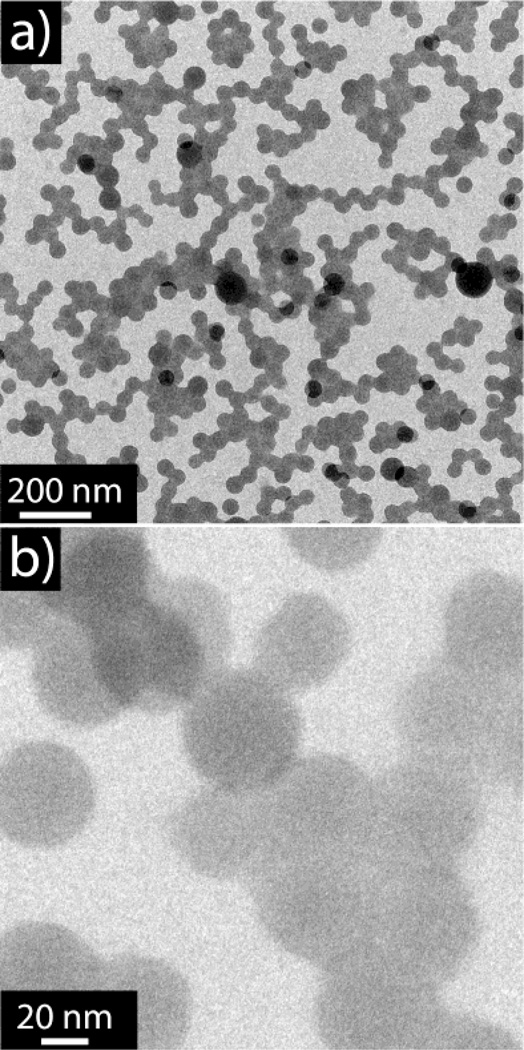
To establish the self-assembly conditions, poly(styrene)-b-poly(2-vinylpyridine) (PS-b-P2VP) phase separated nanoparticles were initially prepared in the absence of a supramolecular guest. The diblock copolymer, prepared by anionic polymerization (Mn = 19 kDa, XP2VP = 25%, PDI = 1.13) was dissolved in 4.5 mL of THF (0.1 mg mL−1) and water (9 mL) was slowly added (1mL min−1) to the solution with stirring. Following complete water addition, THF was evaporated under ambient conditions, resulting in a stable dispersion of nanoparticles with average diameters of 500 nm as measured by DLS (Figure S1). Transmission electron microscopy revealed an ordered morphology of the BCP nanoparticles. The P2VP phase was stained by iodine vapor and appears as the dark regions in the TEM micrographs (Figure 2a and 2b). Interestingly, the same BCP forms ill-defined morphologies in the bulk even after extended annealing,[25] indicating the spherical confinement is important to yield well ordered assemblies. Furthermore this system does not require surfactants to keep the dispersions stable, this simplifies the process and enables facile design of the system compared to traditional emulsion preparations.
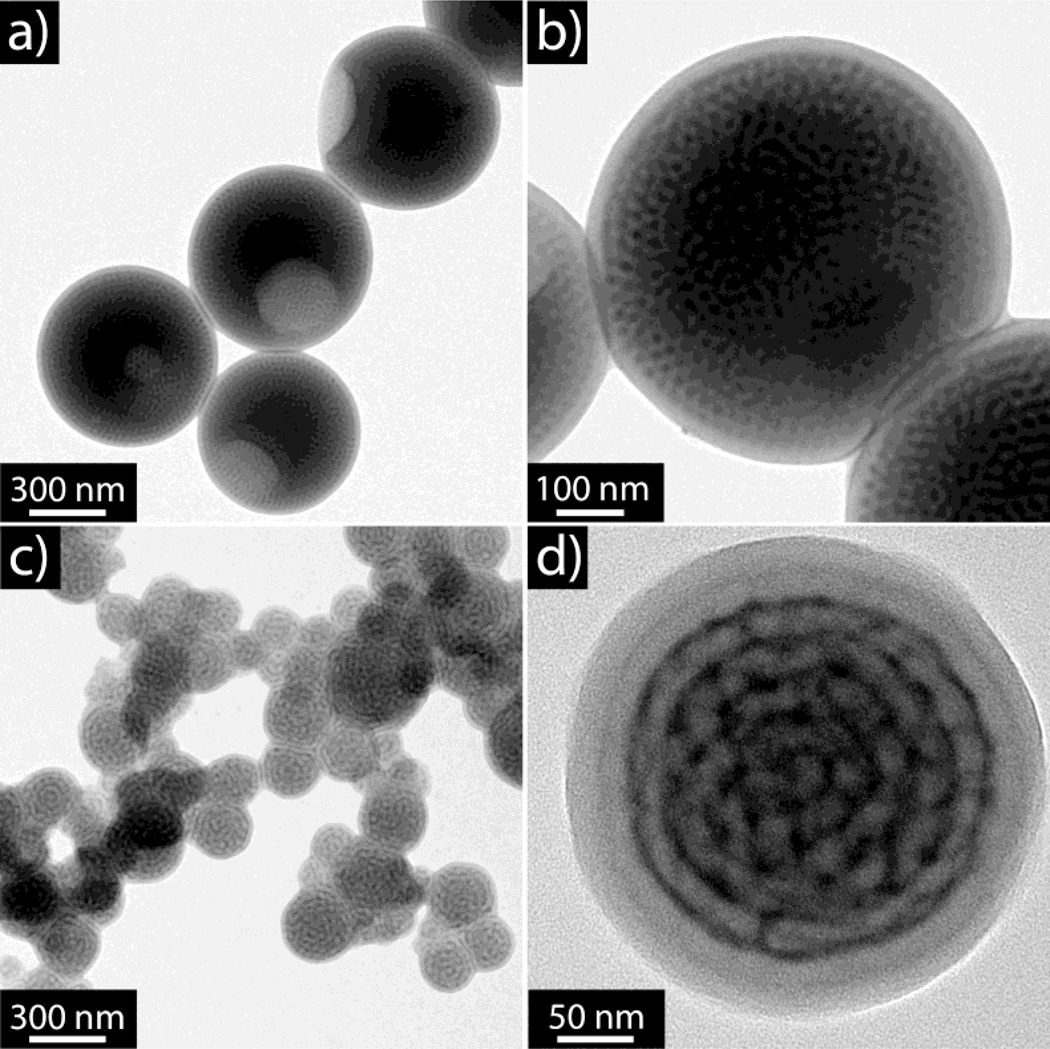
Figure 2.
TEM images of nanoparticles after assembly. a, b) low and high magnification images of poly(styrene)-block-poly(2-vinyl pyridine) (PS-b-P2VP) copolymer in the absence of supramolecular guests; c, d) Low and high magnification images of the hydrophobic supramolecular complex prepared from PS-b-P2VP and an equal-molar incorporation of pentadecyl phenol (PDP). The dark regions correspond to the P2VP phase stained by iodine vapor.
The incorporation of pentadecyl phenol (PDP) into PS-b-P2VP block copolymer assemblies has been well established.[30] Since the seminal work of Ikkala and coworkers [31] there have been numerous reports showing the versatility of this process.[32–34] However, there are many opportunities to incorporate supramolecular guests in dispersed systems. To prepare the complex, equal-molar mixtures of PDP and PS-b-P2VP were dissolved in THF and the complex was characterized by FTIR (see Figure S2 for full spectrum). The solvent exchange procedure was then performed with this complex to drive the self-assembly as described for the pure BCP above. The incorporation of PDP into the particles drastically changed the interior morphology as clearly observed by TEM in Figures 2c and 2d, the new morphology is a radially stacked onion like structure with interconnected layers. Interestingly, the size of the particles was also significantly reduced to around 250 nm, possibly due to the different interfacial energies associated with the supramolecular complex. As a control experiment, pure PDP without any BCP was assembled under identical conditions, resulting in poorly defined precipitates with large polydispersity (Figure S3). This result demonstrates that the BCP is crucial to guide the self-assembly of the supramolecular complex. A range of assemblies were also investigated varying the loading of PDP relative to the 2VP groups, the switch in morphology was triggered after loading more than 30% of the P2VP groups with the PDP supramolecular assembly (Figure S4).
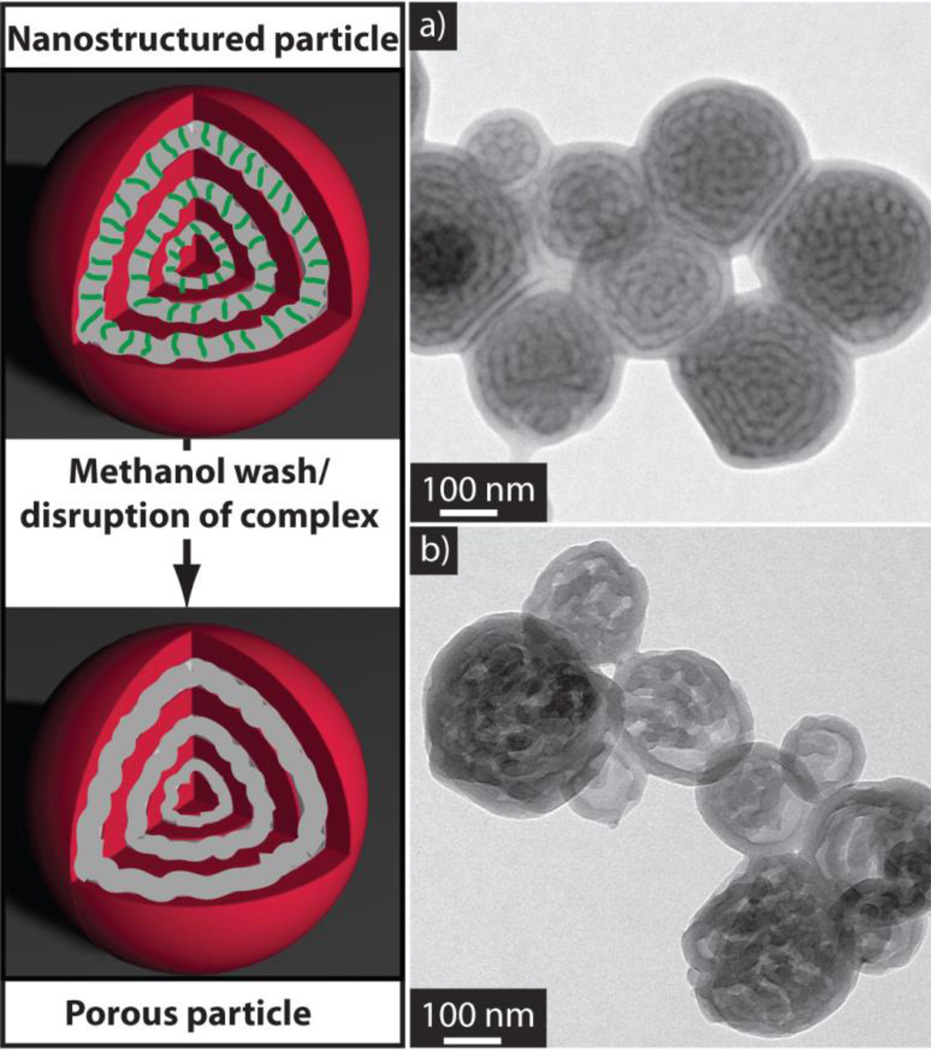
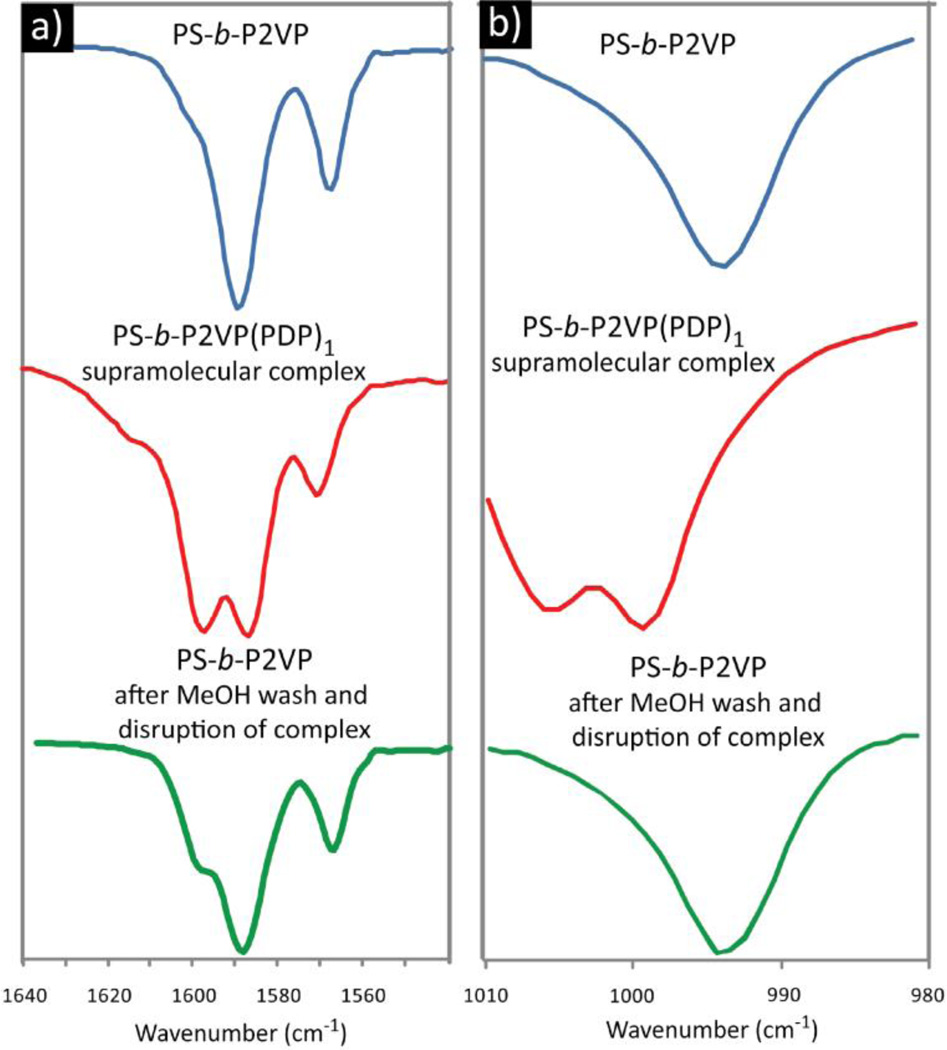
The dynamic nature of the supramolecular interactions creates opportunities to develop responsive materials. In this case, the PDP confined within the BCP nanoparticle can be easily removed by disrupting the supramolecular interaction. To demonstrate the guest release properties of this system, the nanoparticles comprised of the supramolecular complex were dispersed in methanol (illustrated in Figure 3), thereby solubilizing the PDP and releasing it from the particles. The resulting particles are porous in nature suggesting the removal of the hydrophobic guest without disruption to the BCP morphology (Figure 3b). The incorporation and release of the supramolecular guest can also be conveniently monitored by FTIR. The infrared spectra of BCP/PDP mixtures are shown in Figure 4. The original bands at 1590 and 993 cm−1 corresponding to the stretching vibration of the pyridyl group in the non H-bonded state, are observed to shift due to the changed chemical environment upon supramolecular bonding to PDP. In the assembly containing the PDP this change is denoted by the new bands at 1597 and 1006 cm−1 which can be attributed to the stretching vibration of the pyridyl group in the H-bonded state. After the disruption of the supramolecular interactions by washing with methanol thus breaking the hydrogen bonds, the bands shift back to the original state. These results demonstrate the complete release of the PDP guest and the formation of porous nanoparticles, as indicated by the TEM data.
Figure 3.
(left) Schematic representation of the formation of porous particles after disruption of the hydrogen bonds and removal of pentadecyl phenol with methanol. a) TEM image of the hydrophobic supramolecular complex of poly(styrene)-block-poly(2-vinyl pyridine) copolymer with an equal-molar incorporation of pentadecyl phenol. b) TEM image of particles after washing with methanol, demonstrating the removal of pentadecyl phenol and nanoparticles with porous architecture.
Figure 4.
FTIR results demonstrating the interaction of the pyridyl group with pentadecyl phenol. Depicted are the two sections exhibiting the bands corresponding to the 2VP moieties: a) wavenumbers 1640 to 1540 and b) wavenumbers 1010 to 980. The original bands at 1590 and 993 cm−1 corresponding to the non-H-bonded state (blue) are observed to shift to 1597 and 1006 cm−1 respectively, upon supramolecular bonding to PDP (red). The bands are observed to shift back to the original positions after removal of the PDP guest by washing with methanol (green).
The self-assembly of the BCP can be drastically affected by varying the nature of the guest. To demonstrate this, lactic acid was incorporated in a similar manner by mixing equal-molar quantities with respect to the 2-vinyl pyridine composition of the PS-b-P2VP copolymer in THF. The binding of lactic acid to the P2VP block results in an amphiphilic BCP, hence more traditional micelle-like structures are expected from the self-assembly in solution. Indeed, the self-assembly of the amphiphilic complex in water, following an identical procedure as before, results in monodisperse 40 nm spherical micelles (Figure 5a and 5b). This result demonstrates the versatility of this new methodology. Drastically different self-assembled nanostructures can be engineered from the same BCP by simply varying the nature of the supramolecular guest.
Figure 5.
a, b) TEM images at low and high magnification of the amphiphilic supramolecular complex of poly(styrene)-block-poly(2-vinyl pyridine) copolymer with an equal-molar incorporation of lactic acid. Dramatically different structures result from the incorporation of the hydrophilic guest which leads to monodisperse 40 nm micelles.
In summary, we have presented a simple and versatile procedure to access a diverse range of self-assembled nanostructures from a single block-copolymer. Polymer nanoparticles with well-ordered phase separated morphology can be accessed from the solution self-assembly of a hydrophobic PS-b-P2VP BCP. However, the introduction of a hydrophilic guest capable of hydrogen bonding with the pyridine block results in an amphiphilic BCP, thus drastically altering the self-assembly behavior and leading to traditional spherical micelles in water. Furthermore, a hydrophobic guest can be incorporated into the BCP which forms internally nanostructured assemblies in water with the hydrophobic guest entrapped within the nanoparticle. The dynamics of this process were demonstrated by selectively removing this guest resulting in porous BCP particles. The methodologies presented can be used to engineer new systems that incorporate and release guests upon triggered disruption of the supramolecular bonds. Furthermore, the diversity of nanostructures that can be tuned by the incorporation of different guests enables opportunities for outstanding control of the nanoparticle properties.
Supplementary Material
Acknowledgments
Funding from an Australian Research Council International Fellowship (LAC), Australian American Association Sir Keith Murdoch Fellowship (LAC), and the DOE Office of Science Graduate Fellowship Program (MJR) is gratefully acknowledged. This work was also supported by the MRSEC Program of the National Science Foundation under Award DMR-0520415 and DMR-1121053 and in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268201000046C.
References
- 1.Doherty GJ, McMahon HT. Annual Review of Biophysics. 2008;37:65–95. doi: 10.1146/annurev.biophys.37.032807.125912. [DOI] [PubMed] [Google Scholar]
- 2.Cui H, Webber MJ, Stupp SI. Biopolymers. 2010;94:1–18. doi: 10.1002/bip.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Svenson S. Curr. Opin. Colloid Interface Sci. 2004;8:201–212. [Google Scholar]
- 4.Block M, Hecht S. Angew. Chem. Int. Ed. 2005;44:6986. doi: 10.1002/anie.200502436. [DOI] [PubMed] [Google Scholar]
- 5.Chapman R, Jolliffe KA, Perrier S. Polym. Chem. 2011;9:1956–196. [Google Scholar]
- 6.Boyer C, Huang X, Whittaker MR, Bulmus V, Davis TP. Soft Matter. 2011;5:1599–1614. [Google Scholar]
- 7.Tanner P, Baumann P, Enea R, Onaca O, Palivan C, Meier W. Acc. Chem. Res. 2011;44:1039–1049. doi: 10.1021/ar200036k. [DOI] [PubMed] [Google Scholar]
- 8.Hosono N, Gillissen MAJ, Li Y, Sheiko SS, Palmans ARA, Meijer EW. J. Am. Chem. Soc. 2013;135:501–510. doi: 10.1021/ja310422w. [DOI] [PubMed] [Google Scholar]
- 9.Mes T, van der Weegen R, Palmans ARA, Meijer EW. Angew. Chem. Int. Ed. 2011;50:5085–5089. doi: 10.1002/anie.201100104. [DOI] [PubMed] [Google Scholar]
- 10.Terashima T, Mes T, de Greef TFA, Gillissen MAJ, Besenius P, Palmans ARA, Meijer EW. J. Am. Chem. Soc. 2011;133:4742–4745. doi: 10.1021/ja2004494. [DOI] [PubMed] [Google Scholar]
- 11.Bates FS, Fredrickson GH. Annu. Rev Phys. Chem. 1990;41:525–557. doi: 10.1146/annurev.pc.41.100190.002521. [DOI] [PubMed] [Google Scholar]
- 12.Ruzette AV, Leibler L. Nat. Mater. 2005;4:19–31. doi: 10.1038/nmat1295. [DOI] [PubMed] [Google Scholar]
- 13.Bang J, Jeong U, Ryu DY, Russell TP, Hawker CJ. Adv. Mater. 2009;21:4769–4792. doi: 10.1002/adma.200803302. [DOI] [PubMed] [Google Scholar]
- 14.Riess G. Prog. Polym. Sci. 2003;28:1107–1170. [Google Scholar]
- 15.Gohy J-F. Adv. Polym. Sci. 2005;190:65–136. [Google Scholar]
- 16.Hayward RC, Pochan DJ. Macromolecules. 2010;43:3577–3584. [Google Scholar]
- 17.Lin BF, Marullo RS, Robb MJ, Krogstad DV, Antoni P, Hawker CJ, Campos LM, Tirrell MV. Nano Lett. 2011;11:3946–3950. doi: 10.1021/nl202220q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Z, Liu G, Liu F. Macromolecules. 2001;34:8814–8817. [Google Scholar]
- 19.Okubo M, Saito N, Takeoh R, Kobayashi H. Polymer. 2005;46:1151–1156. [Google Scholar]
- 20.Jeon S-J, Yi G-R, Koo CM, Yang S-M. Macromolecules. 2007;40:8430–8439. [Google Scholar]
- 21.Jeon S-J, Yang S-M, Kim BJ, Petrie JD, Jang SG, Kramer EJ, Pine DJ, Yi G-R. Chem. Mater. 2009;21:3739–3741. [Google Scholar]
- 22.Jeon S-J, Yi G-R, Yang S-M. Adv. Mater. 2008;20:4103–4108. [Google Scholar]
- 23.Yabu H, Higuchi T, Ijiro K, Shimomura M. Chaos. 2005;15:047505/1-047505/7. doi: 10.1063/1.2137621. [DOI] [PubMed] [Google Scholar]
- 24.Yabu H, Higuchi T, Shimomura M. Adv. Mater. 2005;17:2062–2065. [Google Scholar]
- 25.Higuchi T, Tajima A, Yabu H, Shimomura M. Soft Matter. 2008;4:1302–1305. doi: 10.1039/b800904j. [DOI] [PubMed] [Google Scholar]
- 26.Higuchi T, Tajima A, Motoyoshi K, Yabu H, Shimomura M. Angew. Chem. Int. Ed. 2008;47:8044–8046. doi: 10.1002/anie.200803003. [DOI] [PubMed] [Google Scholar]
- 27.Higuchi T, Tajima A, Motoyoshi K, Yabu H, Shimomura M. Angew.Chem. Int. Ed. 2009;48:5125. doi: 10.1002/anie.200900002. [DOI] [PubMed] [Google Scholar]
- 28.Robb MJ, Connal LA, Lee BF, Lynd NA, Hawker CJ. Polym. Chem. 2012;3:1618–1628. doi: 10.1039/C2PY20131C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Connal LA, Lynd NA, Robb MJ, Jang SG, Spruell JM, Hawker CJ. Chem. Mater. 2012;24:4036–4042. doi: 10.1021/cm3011524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Zoelen W, Asumaa T, Ruokolainen J, Ikkala O, ten Brinke G. Macromolecules. 2008;41:3199–3208. [Google Scholar]
- 31.Ruokolainen J, ten Brinke G, Ikkala O. Adv. Mater. 1999;11:777–780. [Google Scholar]
- 32.Ikkala O, ten Brinke G. Science. 2002;295:2407–2409. doi: 10.1126/science.1067794. [DOI] [PubMed] [Google Scholar]
- 33.Zhou SQ, Chu B. Adv. Mater. 2000;12:545–556. [Google Scholar]
- 34.Hagaman D, Enright TP, Sidorenko A. Macromolecules. 2012;45(1):275–282. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.