Significance
The use of a liposomal formulation of linolenic acid to kill Helicobacter pylori bacteria in the stomach represents a powerful treatment option for H. pylori infection, as well as its associated gastroduodenal diseases. Because the therapeutic agent is a natural compound found in common vegetable oils, such treatment is expected to be cost-effective compared with the existing antibiotic-based anti-H. pylori therapeutics. More important, the excellent antimicrobial efficacy, marked by significantly reduced in vivo bacterial colonization and inflammation, is achieved through rapid fusion of the nanoformulation with bacterial membrane, a mechanism known to overcome bacterial drug resistance. Similar therapeutic approaches can be developed to treat various types other bacterial infections.
Keywords: nanomedicine, nanotherapeutics, drug delivery, free fatty acid, bacterial infection
Abstract
Helicobacter pylori infection is marked by a vast prevalence and strong association with various gastric diseases, including gastritis, peptic ulcers, and gastric cancer. Because of the rapid emergence of H. pylori strains resistant to existing antibiotics, current treatment regimens show a rapid decline of their eradication rates. Clearly, novel antibacterial strategies against H. pylori are urgently needed. Here, we investigated the in vivo therapeutic potential of liposomal linolenic acid (LipoLLA) for the treatment of H. pylori infection. The LipoLLA formulation with a size of ∼100 nm was prone to fusion with bacterial membrane, thereby directly releasing a high dose of linolenic acids into the bacterial membrane. LipoLLA penetrated the mucus layer of mouse stomach, and a significant portion of the administered LipoLLA was retained in the stomach lining up to 24 h after the oral administration. In vivo tests further confirmed that LipoLLA was able to kill H. pylori and reduce bacterial load in the mouse stomach. LipoLLA treatment was also shown to reduce the levels of proinflammatory cytokines including interleukin 1β, interleukin 6, and tumor necrosis factor alpha, which were otherwise elevated because of the H. pylori infection. Finally, a toxicity test demonstrated excellent biocompatibility of LipoLLA to normal mouse stomach. Collectively, results from this study indicate that LipoLLA is a promising, effective, and safe therapeutic agent for the treatment of H. pylori infection.
As one of the most common bacterial pathogens in the world, Helicobacter pylori infects more than half of the world's population (1, 2). H. pylori infection is responsible for most cases of inflammatory gastritis, peptic ulcer disease, and gastric cancer in the human population (3). Worldwide, the standard treatment of H. pylori infection involves two antibiotics (clarithromycin plus amoxicillin or metronidazole) and a proton pump inhibitor, termed triple therapy, which remains the first line of treatment in the clinic (4). However, H. pylori eradication rates with triple therapy have significantly decreased, varying from 60 to 75%, as a result of an increase in the emergence of H. pylori strains resistant to these antibiotics (5). Specifically, resistance prevalence of H. pylori to metronidazole, which is a key component of the triple-therapy regimen, has increased to ∼40% in developed countries, with an even higher prevalence of ∼90% in developing countries (6). Although a variety of modified antibiotic regimens aimed to overcome drug resistance are under investigation, they have only shown mixed results (7). Furthermore, poor patient compliance, adverse effects, and the high cost associated with multiple antibiotics frequently lead to therapy failure (8). Clearly, new anti-H. pylori treatments with both superior therapeutic efficacy and negligible adverse effects are urgently needed.
Various free fatty acids (FFAs), including lauric acid, myristoleic acid, linoleic acid, and linolenic acid (LLA) with antibacterial activity against a broad range of bacteria, including H. pylori, have recently generated research interest (9, 10). Intriguingly, compared with conventional antibiotics, FFAs induce drug resistance in H. pylori at a much lower frequency (11). In addition, these lipid-like molecules are omnipresent, and as such, they are considered safe. Although promising, inhibition of H. pylori with FFAs continues to be challenging. Specifically, the majority of medium-chain FFAs effective against H. pylori are poorly soluble. Their solubility is further decreased after oral administration because of carboxyl protonation under gastric pH, making these molecules ineffective. In addition, FFAs being subject to oxidation, esterification, and lipid–protein complexation further reduces their bactericidal activity in vivo (9).
Packing FFAs into a nanoparticle formulation has emerged as an attractive and effective approach to overcoming the aforementioned challenges. In particular, the amphiphilic properties of FFAs allow for direct incorporation into the lipid bilayer membrane of phospholipid liposomes with a high loading yield (12). The vast success of liposomes as an approved delivery vehicle bestows excellent opportunities on FFAs for translational testing and clinical use. Among various liposomal FFA formulations, liposomal LLA (LipoLLA) has shown remarkable bactericidal activity against H. pylori (13). When tested with H. pylori Sydney strain 1 (SS1), a laboratory strain of the bacteria, LipoLLA showed an antibacterial efficacy comparable with free LLA predissolved in organic solvent in inhibiting both spiral and coccoid forms of the bacteria. In comparison with amoxicillin, which killed the spiral form but not the coccoid form of the bacteria, LipoLLA was able to kill both forms. In further tests on multiple clinically isolated and metronidazole-resistant strains of H. pylori, LipoLLA eradicated all strains of the bacteria, regardless of their resistance status to metronidazole. More important, the bacteria did not develop drug resistance under the experimental conditions when cultured with LipoLLA at various subbactericidal concentrations, but they acquired resistance to both metronidazole and LLA when cultured with drugs at comparable concentrations. These preliminary in vitro results suggest that LipoLLA holds great potential to become an effective antimicrobial agent to treat H. pylori infection.
To fulfill the therapeutic potential of LipoLLA, we systematically evaluated LipoLLA to treat H. pylori infection in vivo. By using fluorescence-labeled LipoLLA, we examined its distribution in luminal stomach lining and time-dependent retention inside the mouse stomach after oral administration. By using a mouse model of H. pylori infection, we evaluated the therapeutic efficacy of LipoLLA against H. pylori in comparison with the standard triple therapy and LLA. To assess host response to LipoLLA treatment, we also examined the levels of proinflammatory cytokines, including interleukin 1β (IL-1β), IL-6, and tumor necrosis factor alpha (TNFα), during the treatment. In addition, the toxicity profile of LipoLLA in the mouse was evaluated through histological analysis of the gastric tissue and changes in body weight. The findings from this study provide a more clinically related assessment of LipoLLA as an effective and safe anti-H. pylori agent.
Results
LipoLLA Formulation and in Vitro Characterization.
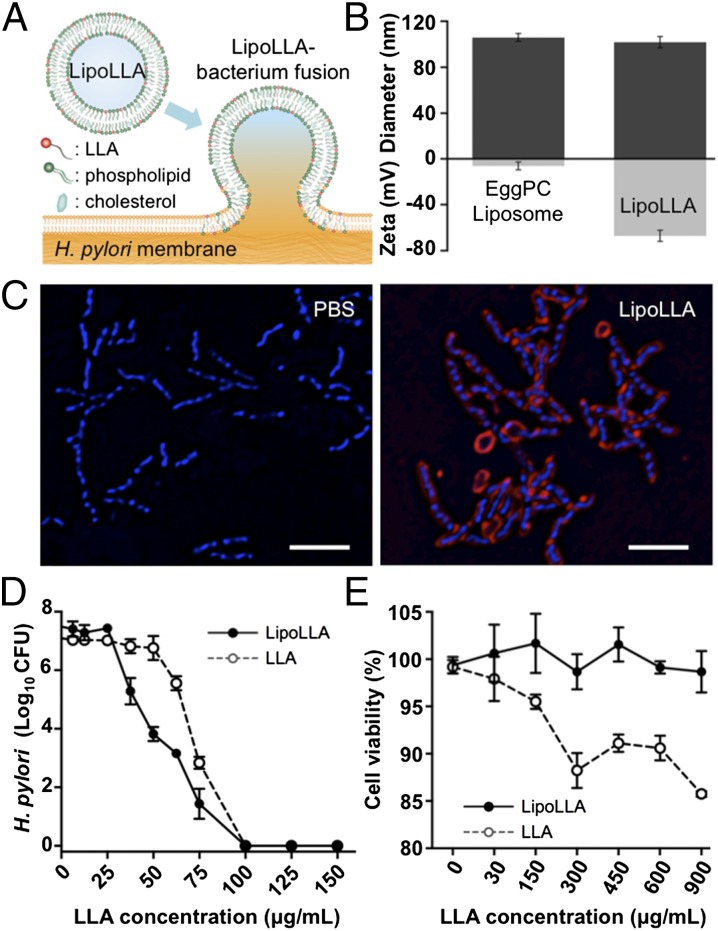
Because of its amphiphilic nature, LLA can be readily loaded into liposomes and can subsequently fuse with bacteria for antibacterial activity (Fig. 1A). In the study, LLA, l-α-phosphatidylcholine (EggPC), and cholesterol were first mixed at a weight ratio of 3:6:1 and then extruded to formulate LipoLLA (14). The resulting LipoLLA had a hydrodynamic diameter of 105.7 ± 0.3 nm, which is comparable to that of the bare liposomes made of EggPC and cholesterol only, at a weight ratio of 9:1 (Fig. 1B). The polydispersity indices of the bare liposomes and LipoLLA were 0.17 ± 0.01 and 0.18 ± 0.01 respectively, indicating the relatively narrow distribution of liposome sizes. Meanwhile, the surface zeta potential of the bare liposomes was −8.7 ± 0.1 mV in deionized water, whereas the zeta potential of LipoLLA was −54.9 ± 1.0 mV. Such a sharp decrease of the surface zeta potential indicates the incorporation of LLA into the lipid bilayers, where the carboxylic acid group is deprotonated to COO− at a near physiologic pH of 7.4.
Fig. 1.
LipoLLA formulation and in vitro characterization. (A) Schematic illustration of LipoLLA fusing with a bacterial membrane for antibacterial activity. (B) Hydrodynamic size (diameter, nm) and surface zeta potential (mV) of EggPC liposome (without LLA) and LipoLLA measured by dynamic light scattering. (C) Fluorescence images confirm the fusion interaction between LipoLLA and H. pylori. LipoLLA was labeled with fluorescent dye RhB (red), and the bacteria were stained with DAPI (blue). Control bacteria were incubated with PBS. (Scale bars, 5 μm.) (D) In vitro bactericidal activity of LipoLLA and LLA at different concentrations against H. pylori. (E) Cellular viability of human gastric carcinoma AGS cells when treated with LipoLLA and LLA at different drug concentrations. In D and E, all concentrations refer to LLA concentration, regardless of the formulations. Error bars represent the SD derived from three independent experiments.
The LipoLLA formulation was further examined for its fusion capability with H. pylori bacteria, a mechanism that could disrupt the integrity of the bacterial membrane for bactericidal activity (13). Here, LipoLLA was labeled with lipophilic 1,2-dimyristoyl-snglycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (ammonium salt) (DMPE-RhB) fluorophore (excitation/emission = 557/571 nm). Untreated bacteria, used as a control, only showed nucleoids stained with DAPI (blue) (Fig. 1C). However, when the bacteria were incubated with LipoLLA containing DMPE-RhB, a strong RhB fluorescence signal surrounding the bacterial nucleoids was observed, suggesting the fusion activity had occurred (Fig. 1C). In addition, the fluorescence signal was exclusively and evenly distributed around the bacterial nucleoids, and the image clearly reflected the characteristic spiral shape of H. pylori bacteria. Therefore, the microscopic observation, consistent with our previous studies (15), confirms the fusion of LipoLLA with H. pylori.
The bactericidal activity of LipoLLA against H. pylori was also evaluated in vitro. Bacteria incubated in broths containing varying concentrations of LipoLLA resulted in a nonlinear correlation between bacterial viability and LipoLLA concentrations, which is consistent with a LipoLLA–H. pylori fusion mechanism (Fig. 1D) (13). For this study, we defined minimal bactericidal concentration as the minimum concentration of the bactericidal agent required to kill 3 logs (99.9%) of the bacteria during a 30-min incubation. Accordingly, the minimal bactericidal concentration values for LipoLLA and LLA were determined to be 65 and 80 μg/mL, respectively. Despite its strong bactericidal activity, LipoLLA showed little toxicity to human gastric carcinoma AGS cells, as exposure to LipoLLA at concentrations between 30 and 900 μg/mL showed negligible lactate dehydrogenase release (Fig. 1E). In contrast, exposure to the free LLA resulted in a concentration-dependent increase of lactate dehydrogenase release. Notably, 5% DMSO is nontoxic to cells; therefore, the observed cytotoxicity is likely a result of the free form of LLA. In fact, free fatty acids including LLA in their free forms have long been known to cause a range of toxic effects (16–18). Compared with free form FFAs, liposomal formulation confines FFA molecules within the lipid bilayers, and thus limits their interference with intracellular activities, thereby lowering their toxic effects.
Retention and Distribution of LipoLLA in Mouse Stomach.
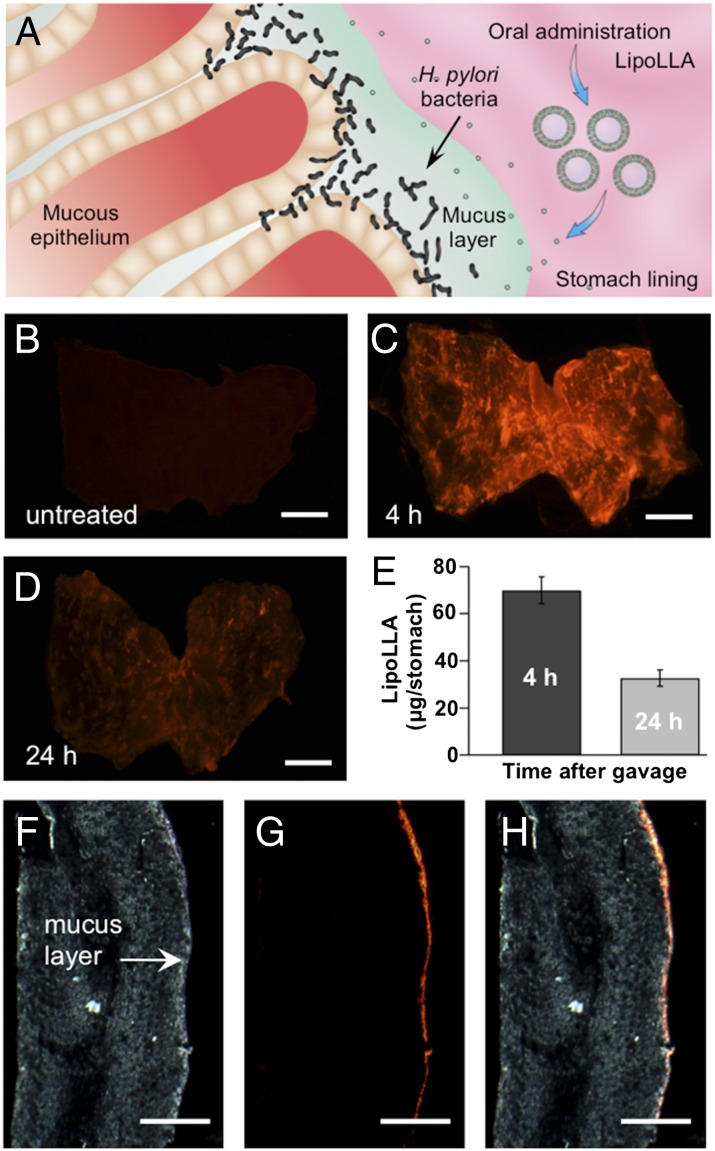
H. pylori mainly resides within the adherent mucus layer close to the epithelial surface (19). Therefore, for effective antibacterial treatment, LipoLLA permeation across the mucus layer and its retention on the stomach wall are critical (Fig. 2A). To study the retention and distribution of LipoLLA in mouse stomach, we administered the mice orally with the fluorescence-labeled LipoLLA. At 4 and 24 h after LipoLLA administration, the whole mouse stomach was excised and opened. Then the luminal lining was rinsed with PBS and flattened for fluorescence imaging. As a control group, gastric tissue obtained from untreated mice showed no detectable fluorescence emission (Fig. 2B). In contrast, strong fluorescence was observed in the gastric tissue collected at 4 h after the oral gavage, which was a longer time compared with the reported gastric emptying times of mice (Fig. 2C) (20). Hence, the apparent presence of the liposomes observed here indicates effective liposome retention in the stomach lining. The image obtained at 24 h after oral gavage also showed evidence of fluorescence signal throughout the entire stomach, even though the fluorescence intensity decreased slightly (Fig. 2D). Further quantification of the gastric retention of LipoLLA revealed that ∼69 μg LipoLLA was retained in the stomach 4 h after treatment; this amount decreased to ∼34 μg at 24 h after treatment (Fig. 2E).
Fig. 2.
Retention and distribution of LipoLLA in mouse stomach. (A) Anatomic illustration of stomach lining infected by H. pylori. (B–D) Fluorescence images of LipoLLA from the luminal lining of freshly excised mouse stomach at 0 (untreated), 4, and 24 h after oral gavage of LipoLLA, respectively. (E) Quantification of LipoLLA retained in the mouse stomach 4 and 24 h after oral gavage. (F–H) Bright-field, fluorescence, and overlay images of a transverse cryosection of a mouse stomach collected 4 h after the oral gavage of LipoLLA. All images are representative of n = 3 mice, and the retention is quantified as the mass of LipoLLA per stomach ± SD (n = 5). (Scale bars, 5 mm in B–D and 1 mm in F–H.)
We further studied LipoLLA tissue distribution by examining transverse cryosections of mouse stomach collected 4 h after the oral gavage. The bright-field image shows the mucus as a thin layer on the luminal side of the stomach (Fig. 2F). The fluorescence image obtained from the same sample shows a continuous thin layer of LipoLLA on the luminal side of the cryosection (Fig. 2G). Overlay of the fluorescence image with the bright-field image reveals a precise colocalization of the two, confirming the diffusion of LipoLLA toward the gastric epithelium and its retention in the mucus layer (Fig. 2H).
Anti-H. pylori Efficacy in Vivo.
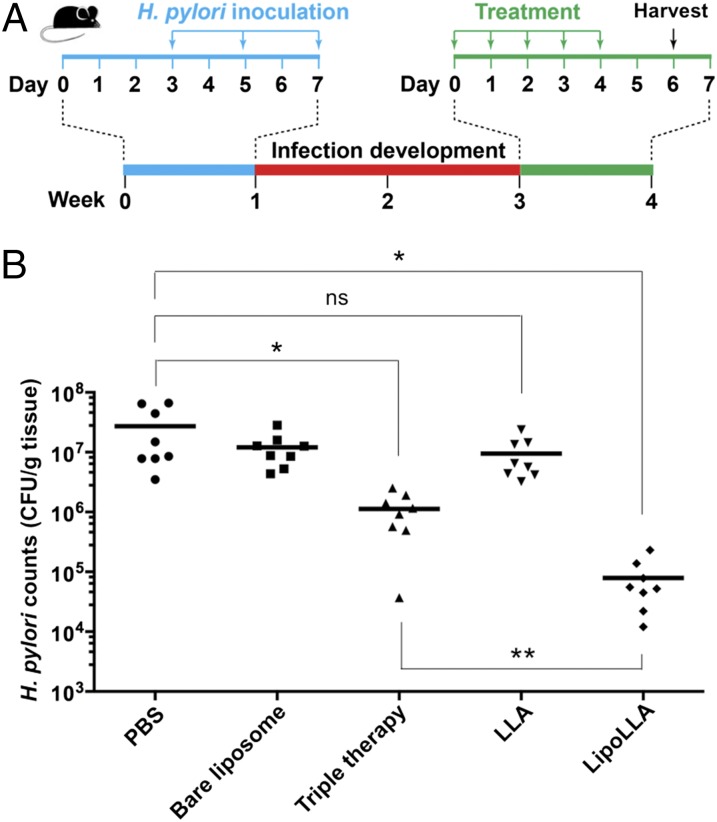
Next, we sought to evaluate the in vivo therapeutic efficacy of LipoLLA against H. pylori. To establish the H. pylori infection model, we infected each C57BL/6 mouse with 3 × 108 CFU H. pylori SS1 in brain–heart infusion (BHI) broth by oral gavage three times at 2-d intervals (Fig. 3A) (21, 22). At 2 wk after inoculation, infected mice were divided into five groups (n = 8) and treated with PBS, bare liposome, triple therapy, LLA, or LipoLLA. Proton pump inhibitor was given to all mice 30 min before the administration of all formulations to neutralize acid in the stomach and prevent potential drug degradation.
Fig. 3.
Anti-H. pylori efficacy in vivo. (A) The study protocol including H. pylori inoculation and infection development in C57BL/6 mice, followed by the treatments. (B) Quantification of bacterial burden in the stomach of H. pylori-infected mice treated with PBS, bare liposome, triple therapy, LLA, and LipoLLA, respectively (n = 8 per group). Bars represent median values. *P < 0.05, **P < 0.001.
In the study, therapeutic efficacy was evaluated by enumerating and comparing H. pylori counts in mouse stomach. After the treatment, quantification of the bacterial burden in the mouse stomach showed 1.6 × 108 and 1.0 × 108 CFU/g of stomach tissue for the two negative control groups treated with PBS and bare liposomes, respectively (Fig. 3B). Mice treated with triple-therapy antibiotics as a positive control showed a bacterial burden of 7.2 × 105 CFU/g, which is a significant reduction compared with negative controls. For the mice treated with LLA predissolved in DMSO, a bacterial burden of 7.5 × 106 CFU/g was quantified. The insignificant decrease of bacterial count in LLA-treated mice compared with the two negative control groups suggests the ineffectiveness of LLA in vivo against H. pylori infection. Such ineffectiveness is likely a result of the poor solubility of LLA and its inability to fuse with bacterial membrane, both of which hinder effective bacterial killing. In contrast, when the mice were treated with LipoLLA, the bacterial burden was assessed to be 5.5 × 104 CFU/g, a significant reduction compared with all other treatment groups. In particular, LipoLLA reduced H. pylori burden in mice compared with in the negative controls by ∼2.5 orders of magnitude, whereas triple-therapy antibiotics only reduced it by ∼1.4 orders of magnitude. The superior efficacy observed with LipoLLA demonstrates its significant potential as an effective anti-H. pylori agent.
Proinflammatory Response to LipoLLA Treatment.
The in vivo acute toxicity of LipoLLA was evaluated by examining changes in the local immune response, using real-time PCR. We focused on expression of cytokines including IL-1β, IL-6, and TNFα, which are known to be up-regulated during infection with H. pylori (23, 24). A section of the gastric tissue obtained from each mouse was used to analyze the effect of LipoLLA on host immune response by real-time PCR. As shown in Fig. 4, H. pylori infection resulted in up-regulation of IL-1β, IL-6, and TNFα, as reported in previous studies (23, 24). However, when infected mice were treated with LipoLLA, mRNA expression of these proinflammatory cytokines was significantly reduced (P < 0.001), indicating that no acute toxicity was found in response to LipoLLA treatment. In fact, these results indicate that LipoLLA had a dampening effect on the assessed proinflammatory cytokines in response to H. pylori infection.
Fig. 4.
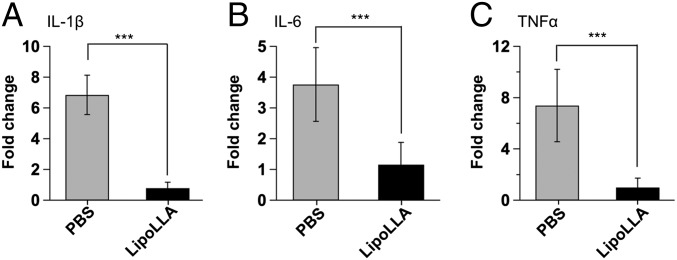
Proinflammatory cytokine production. Expression levels of proinflammatory cytokines, IL-1β, IL-6, and TNFα in H. pylori-infected C57BL/6 mice after treatment with PBS or LipoLLA. Data are expressed as fold change relative to the corresponding proinflammatory cytokine levels in uninfected mice. Error bars represent the SD derived from 8 mice per group. ***P < 0.0001.
LipoLLA Toxicity Evaluation in Vivo.
Last, we evaluated the toxicity of LipoLLA, using uninfected mice. In the study, mice were orally administered PBS buffer or LipoLLA once daily for 5 consecutive days. Mice administered LipoLLA maintained the same body weight compared with mice administered PBS (Fig. 5A). In the 5-d period, all the mice showed no obvious weight change. On day 6, all the mice were killed. The longitudinal sections of gastric tissues obtained from the mice were collected and stained with hematoxylin and eosin. The gastric tissues treated with LipoLLA maintained an undisturbed structure with a clear layer of epithelial cells, which was similar to the gastric samples treated with PBS (Fig. 5 B and C). The LipoLLA toxicity was further evaluated using gastric tissue sections by a terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assay to examine the level of gastric epithelial apoptosis as an indicator of gastric mucosal homeostasis (25). Compared with the PBS control, there was no apparent increase in gastric epithelial apoptosis, as indicated by TUNEL staining, in LipoLLA-treated mice (Fig. 5 D and E). The absence of any detectable gastric histopathologic changes or toxicity within a 5-d treatment suggests orally administered LipoLLA is safe.
Fig. 5.
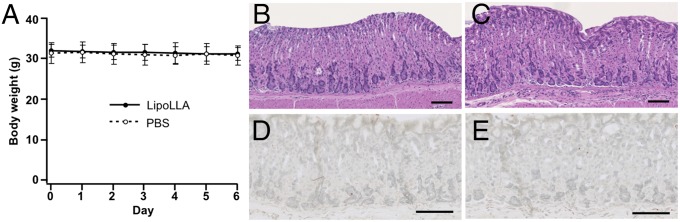
LipoLLA in vivo toxicity. Uninfected mice were orally administered PBS buffer or LipoLLA once daily for 5 consecutive days. (A) Mice administered LipoLLA maintained the same body weight, similar to mice administered PBS. All mice showed no obvious weight changes. (B–E) On day 6, mice were killed and sections of the mouse stomach processed and stained with hematoxylin and eosin (B and C) or TUNEL (D and E). LipoLLA treatment (C and E) showed the same level of safety as PBS (B and D). (Scale bars, 100 µm.)
Discussion
In the present study, we evaluated the anti-H. pylori efficacy of LipoLLA on a mouse model. Our results demonstrated a significantly improved antimicrobial efficacy of LipoLLA in reducing H. pylori bacterial load in mouse stomach compared with other treatment regimens, including triple therapy, the current worldwide standard treatment of H. pylori. Unsaturated fatty acids have been shown to inhibit H. pylori in vitro (11, 26, 27). However, in vivo killing of H. pylori by FFAs has been a challenge until now, as demonstrated by the lack of H. pylori killing when mice were treated with free LLA in the present study.
To address the increasing challenges in treating H. pylori infection, nanotechnology has offered a range of innovative approaches. In particular, a plethora of nanoparticle platforms have been developed that are primarily focused on altering the pharmacokinetics of antibiotics for enhanced potency (28, 29). Some platforms concurrently encapsulate multiple antibiotics (30), some offer mucoadhesion for prolonged drug retention (31), some are conjugated with bacterium-binding ligand for targeted delivery (32), and some respond to the pH gradient between gastric lumen and the adherent mucosal layer for on-site drug delivery (33). Although promising, these strategies all rely on the conventional antibiotic payloads for bioactivity. Inevitably, they also inherit a high susceptibility for drug resistance.
Compared with conventional antibiotics, the superior anti-H. pylori activity conferred by LipoLLA can be explained by the unique capability of LipoLLA to fuse rapidly with bacterial membrane, and subsequently disrupt membrane integrity, a highly destructive mechanism for bacterial killing with low susceptibility for resistance development (13). This mechanism is supported by the sharp decrease in viable bacteria at the minimal bactericidal concentration values and a killing time no longer than 30 min, which is relatively short compared with the 2.5 h needed for H. pylori to complete a replication cycle (9). The fusion mechanism is further supported by the microscopic observation, in which LipoLLA exclusively distributed throughout the bacterial membrane (Fig. 1C). Compared with free LLA, LipoLLA carries LLA within the lipid bilayers. When the liposome is fusing with bacterial membrane, LLA molecules will be directly delivered to the bacterial membrane with limited interference with bacterial intracellular pathways. Such formulation minimizes chemical alterations on biological pathways but promotes physical–structural disruption of cell membrane, a mechanism previously reported to reduce the induction of bacterial drug resistance (13). For in vivo applications, this working mechanism is further ensured by a liposome formulation, in which the lipid bilayers protect LLA molecules from the damaging environment of the stomach and prevent potential oxidation and enzymatic degradation (12, 13).
In this study, LipoLLA also shows promise in targeting the gastric mucosal layer for prolonged retention and effective anti-H. pylori activity. We showed that LipoLLA accumulated within the mucus layer, and a significant portion was retained for up to 24 h. The distinct host environment of H. pylori is marked by limited drug permeation and retention. However, plenty of nanoparticles, both natural and manmade ones, have shown a remarkable capability to transport across the mucus mesh for possible entry to the underlying epithelia and prolonged residence time in the mucus layer (34, 35). A comparison of LipoLLA to various mucus-penetrating nanoparticles suggests that a relatively small size of LipoLLA (∼100 nm in diameter, with a narrow distribution), together with a dense anionic surface charge that minimizes hydrophobic entrapment to mucus, is attributable to the effective LipoLLA retention in mouse stomach (36).
In addition to the better efficacy of LipoLLA compared with the commonly used standard of triple therapy, our data also show that LipoLLA is safe. Treatment with LipoLLA had no effect on mouse body weight, gastric histopathology, or gastric mucosal integrity. Further, treatment of mice with LipoLLA did not elicit the host immune response. Interestingly, mice treated with LipoLLA had significantly reduced H. pylori-induced proinflammatory cytokines compared with H. pylori-infected but untreated mice. Unsaturated fatty acids are reported to play a role in gastric mucosal protection through various pathways, including increased synthesis in prostaglandins (37, 38). The hallmark of H. pylori infection is its persistence through the entire lifetime of the host with the presence of significant inflammatory responses, including phagocyte recruitment. It is also known that the immune response to H. pylori contributes to the disease pathogenesis (39, 40). As such, dampening of the proinflammatory response, as observed here, is expected to reduce the inflammatory reaction responsible for perpetuating tissue injury.
In summary, we evaluated the in vivo therapeutic potential of LipoLLA for the treatment of H. pylori infection. The LipoLLA formulation with a size of ∼100 nm and a surface zeta potential of −54 mV was prone to fusion with bacterial membranes, thereby directly releasing a high dose of LLA into the membranes. LipoLLA penetrated the mucus layer of mouse stomach, and a significant portion was retained in the stomach lining 24 h after the oral administration. In vivo tests confirmed that LipoLLA was able to kill H. pylori and reduce bacterial load in the mouse stomach. The LipoLLA treatment also reduced the levels of proinflammatory cytokines including IL-1β, IL-6, and TNFα, which were otherwise elevated in response to H. pylori infection. Last, a toxicity test demonstrated excellent biocompatibility of LipoLLA to normal mouse stomach. Overall, the results indicate that LipoLLA holds great promise as an effective and safe therapeutic agent for the treatment of H. pylori infection.
Materials and Methods
Preparation and Characterization of LipoLLA.
LipoLLA was prepared by using a standard vesicle extrusion method (14). Briefly, a mixture of EggPC, cholesterol (Avanti Polar Lipids), and LLA (Ultra Scientific) with a weight ratio of 6:1:3 (16 mg total weight) was dissolved in 4 mL chloroform. Then the chloroform was evaporated to form a thin lipid layer, which was rehydrated by adding 2 mL PBS. The mixture was vortexed for 1 min, followed by sonication for 3 min in a bath sonicator (Fisher Scientific FS30D). Then a Branson 450 sonifier with a Ti-probe was used to sonicate the solution at 20 W for 1 min. The resulting lipid vesicles were then extruded through a 100-nm pore-sized polycarbonate membrane 11 times with a miniextruder (Avanti Polar Lipids). Then the suspension was passed through a Sephadex G75 column (Fisher Scientific) to remove the unloaded LLA and was sterilized by filtering through a 0.22-μm filter unit (Millipore). To quantify LLA loading efficiency, LipoLLA or LLA was dried on a rotavapor (Buchi, Model R-124) and then dissolved in methanol and derivatized with phenacylester, following an established protocol (41, 42). The final solutions containing LLA phenacylester derivatives were assayed by reversed-phase high-performance liquid chromatography with a C18 column (Perkin-Elmer). The hydrodynamic size and surface zeta potential of the liposomes were measured by dynamic light scattering, using a Malvern Zetasizer ZS (Malvern Instruments). All characterization measurements were repeated three times at 25 °C.
H. pylori Culture and LipoLLA in Vitro Activity.
H. pylori SS1 was used in this study. The bacteria were maintained on Columbia agar supplemented with 5% (vol/vol) laked horse blood at 37 °C under microaerobic conditions (10% CO2, 85% N2, and 5% O2), as previously described (24). Antimicrobial activity of LipoLLA against H. pylori was performed following a reported protocol (13). Briefly, bacteria were inoculated into BHI broth containing 5% (vol/vol) FBS and cultured overnight. Then the bacteria were harvested by centrifugation at 5,000 × g for 10 min, resuspended in fresh BHI broth, and adjusted to an optical density at 600 nm of 1.0, corresponding to ∼1 × 108 CFU/mL A bacterial suspension containing 1 × 106 CFU bacteria was mixed with LipoLLA at various predetermined concentrations in a 96-well plate and incubated at 37 °C under microaerobic conditions for 30 min, and a series of 10-fold dilutions of the bacterial suspension was inoculated onto Columbia agar plates supplemented with 5% (vol/vol) laked horse blood. The agar plates were then cultured in the incubator for 4 d before enumerating the colonies.
LipoLLA Fusion with H. pylori.
The fusion between LipoLLA and H. pylori was examined with fluorescence microscopy. In the study, DMPE-RhB (0.5 mol%) was mixed with EggPC, LLA, and cholesterol before the preparation of LipoLLA and was subsequently incorporated into the bilayer membrane of LipoLLA (1 mg/mL). Then 1 mL LipoLLA suspension was mixed with 5 × 108 CFU H. pylori. After 10 min incubation, the bacteria were collected by centrifugation at 5,000 × g for 5 min, followed by fixation with 2% (vol/vol) glutaraldehyde in PBS at room temperature for 15 min. The bacteria were then washed and resuspended in 1 mL deionized water. For imaging, 5 μL bacterial suspension was mixed with 5 μL DAPI-containing mounting media and placed on a lysine-coated slide. The sample was then imaged, using a 100× oil immersion objective on an Applied Precision DeltaVision deconvolution scanning fluorescence microscope.
LipoLLA in Vitro Cytotoxicity Study.
The human gastric carcinoma AGS cell line (ATCC CRL 1739) was cultured in RPMI medium 1640 supplemented with 10% (vol/vol) heat-inactivated FBS at 37 °C in a humidified 5% CO2 atmosphere. For the study, 1 × 104 cells per well were grown in 12-well culture plates. After an overnight culture, cells were incubated with LLA or LipoLLA at predetermined concentrations for 24 h. Cell death was assessed by measuring the release of lactate dehydrogenase (Promega) into the culture medium. Untreated cells served as a negative control, and the cells treated with 1% Triton X-100 served as a positive control.
Gastric Retention of LipoLLA.
C57BL/6 male mice at 8 wk of age were randomly assigned to three groups (n = 8) to receive RhB-labeled LipoLLA (4 mg/mL) for 4 or 24 h. One group of mice was left untreated as a control. Each mouse in the other two groups was administered 0.3 mL LipoLLA intragastrically through oral gavage. Mice were killed at the indicated times, and the stomachs were removed from the abdominal cavity. The stomachs were cut open along the greater curvature, the gastric content was removed, and the gastric fluid containing excess liposomes was washed away. Gastric samples of three mice from each group were frozen in optimal cutting temperature compound for confocal imaging. Gastric samples of the remaining five mice from each group were homogenized and the homogenates centrifuged at 5,000 × g for 10 min to remove tissue or cell debris. Supernatants were collected and measured for fluorescence intensity of liposome-bound DMPE-RhB.
Anti-H. pylori Efficacy in Vivo.
Each C57BL/6 male mouse received 0.3 mL of 1 × 109 CFU/mL H. pylori in BHI broth administered intragastrically through oral gavage every 48 h, repeated three times (on days 3, 5 and 7, respectively), and the infection was allowed to develop for 3 wk. The mice were randomly assigned to five treatment groups (n = 8) to receive LipoLLA, LLA, triple therapy, bare liposomes, or PBS. Mice were first administered omeprazole (a proton pump inhibitor) through oral gavage at a dose of 400 μmol/kg, followed by a lag time of 30 min before administration of the assigned treatments. LipoLLA, LLA in 5% (vol/vol) DMSO (both at 24 mg LLA/kg), and triple-therapy formulation (amoxicillin 28.5 mg/kg and clarithromycin 14.3 mg/kg) were administered through oral gavage once daily for a consecutive 5 d. Bare liposomes without LLA and PBS served as two negative control groups. Forty-eight hours after the last administration, mice were killed and the stomach was removed from the abdominal cavity. The stomach was cut along the greater curvature, and the gastric content was removed and rinsed with PBS. The stomachs were cut into three longitudinal sections, and each section was weighed. The sections were used for assessment of bacterial colonization, gene expression, and histology/epithelial apoptosis. For bacterial colonization, a gastric tissue section was suspended in 1 mL PBS and homogenized for H. pylori recovery. The homogenate was serially diluted and spotted onto Columbia agar plate containing Skirrow’s supplement (10 µg/mL vancomycin, 5 µg/mL trimethoprim lactate, 2,500 IU/L polymyxin B; Oxiod). The plates were then incubated at 37 °C under microaerobic conditions for 5 d, and bacterial colonies were enumerated and adjusted for dilutions.
Quantification of Inflammatory Cytokines.
A section of the gastric tissue stored at −80 °C in RNA-stabilizing solution was homogenized in 1 mL TRIzol reagent (Invitrogen), followed by DNase treatment (Ambion) for further purification of RNA samples. RNA concentration was measures using a NanoDrop spectrophotometer (NanoDrop Technologies), and the integrity was checked by electrophoresis on a 1% agarose gel. Two micrograms RNA was reverse-transcribed into cDNA, using the high-capacity cDNA reverse transcription kit (Applied Biosystems). After the transcription, gene expression was determined by real-time PCR, using SYBR Green dye (Eurogentec), as described in our previous studies (43, 44). Expression of IL-1β, IL-6, TNFα, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was performed using the following primers: murine(m) IL-1β-F, 5′-AAAAGCCTCGTGCTGTCGGACC; mIL-1β-R, 5′-TTGAGGCCCAAGGCCACAGGT; mIL-6-F, 5′-AGACAAAGCCAGAGTCCTTCAGAGA; IL-6-R, 5′-GCCACTCCTTCTGTGACTCCAGC; mTNFα-F, 5′-TTCCAGAACTCCAGGCGGTGC; mTNFα-R, 5′-TGAGTGTGAGGGTCTGGGCCAT; GAPDH-F, 5′-TCAACAGCAACTCCCACTCTTCCA; and GAPDH-R, 5′-ACCCTGTTGCTGTAGCCGTATTCA. Real-time PCR conditions consisted of an initial cycle at 95 °C for 5 min, followed by 40 cycles of amplification with denaturation at 95 °C for 15 s, annealing at 60 °C for 20 s, and extension at 72 °C for 40 s. A melting curve was generated for each sample at the end of the reaction to ensure specificity. Gene expression levels were normalized to GAPDH, and the data were analyzed using comparative cycle threshold calculations (∆∆CT; Applied Biosystems). Data were expressed as fold change relative to the corresponding proinflammatory cytokine levels in uninfected mice. Each real-time PCR experiment was run three times.
In Vivo Toxicity Study.
To evaluate LipoLLA toxicity in vivo, C57BL/6 male mice (n = 8) at 6–8 wk of age were orally administered 0.3 mL of 8 mg/mL LipoLLA once daily for 5 consecutive days. Mice administered with PBS were tested in parallel as a negative control. Mouse body weight was monitored during the experiment period by weighing the mice daily. Twenty-four hours after the last oral administration, the mice were killed and the stomachs were removed for histological analysis. The longitudinal sections of gastric tissue were fixed in neutral-buffered 10% (vol/vol) formalin and then embedded in paraffin. The tissue sections were stained with hematoxylin and eosin. Epithelial cell apoptosis was evaluated by TUNEL assay (Boehringer Mannheim). Sections were visualized by Hamamatsu NanoZoomer 2.0HT and the images processed using NDP viewing software.
Acknowledgments
This work is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award R01DK095168.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.De Francesco V, et al. Worldwide H. pylori antibiotic resistance: A systematic review. J Gastrointestin Liver Dis. 2010;19(4):409–414. [PubMed] [Google Scholar]
- 2.McColl KEL. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362(17):1597–1604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 3.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urgesi R, Cianci R, Riccioni ME. Update on triple therapy for eradication of Helicobacter pylori: Current status of the art. Clin Exp Gastroenterol. 2012;5:151–157. doi: 10.2147/CEG.S25416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mégraud F. H pylori antibiotic resistance: Prevalence, importance, and advances in testing. Gut. 2004;53(9):1374–1384. doi: 10.1136/gut.2003.022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaakoush NO, Asencio C, Mégraud F, Mendz GL. A redox basis for metronidazole resistance in Helicobacter pylori. Antimicrob Agents Chemother. 2009;53(5):1884–1891. doi: 10.1128/AAC.01449-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347(15):1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 8.O’Connor A, Molina-Infante J, Gisbert JP, O’Morain C. Treatment of Helicobacter pylori infection 2013. Helicobacter. 2013;18(Suppl 1):58–65. doi: 10.1111/hel.12075. [DOI] [PubMed] [Google Scholar]
- 9.Desbois AP, Smith VJ. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol. 2010;85(6):1629–1642. doi: 10.1007/s00253-009-2355-3. [DOI] [PubMed] [Google Scholar]
- 10.Jarboe LR, Royce LA, Liu P. Understanding biocatalyst inhibition by carboxylic acids. Front Microbiol. 2013;4:Article 272. doi: 10.3389/fmicb.2013.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petschow BW, Batema RP, Ford LL. Susceptibility of Helicobacter pylori to bactericidal properties of medium-chain monoglycerides and free fatty acids. Antimicrob Agents Chemother. 1996;40(2):302–306. doi: 10.1128/aac.40.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prajapati HN, Dalrymple DM, Serajuddin ATM. A comparative evaluation of mono-, di- and triglyceride of medium chain fatty acids by lipid/surfactant/water phase diagram, solubility determination and dispersion testing for application in pharmaceutical dosage form development. Pharm Res. 2012;29(1):285–305. doi: 10.1007/s11095-011-0541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obonyo M, et al. Antibacterial activities of liposomal linolenic acids against antibiotic-resistant Helicobacter pylori. Mol Pharm. 2012;9(9):2677–2685. doi: 10.1021/mp300243w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C-M, et al. Eradication of drug resistant Staphylococcus aureus by liposomal oleic acids. Biomaterials. 2011;32(1):214–221. doi: 10.1016/j.biomaterials.2010.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao W, et al. Hydrogel containing nanoparticle-stabilized liposomes for topical antimicrobial delivery. ACS Nano. 2014;8(3):2900–2907. doi: 10.1021/nn500110a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kharroubi I, et al. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: Role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology. 2004;145(11):5087–5096. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- 17.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281(17):12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 18.Malhi H, Barreyro FJ, Isomoto H, Bronk SF, Gores GJ. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut. 2007;56(8):1124–1131. doi: 10.1136/gut.2006.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schreiber S, et al. The spatial orientation of Helicobacter pylori in the gastric mucus. Proc Natl Acad Sci USA. 2004;101(14):5024–5029. doi: 10.1073/pnas.0308386101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennink RJ, et al. Validation of gastric-emptying scintigraphy of solids and liquids in mice using dedicated animal pinhole scintigraphy. J Nucl Med. 2003;44(7):1099–1104. [PubMed] [Google Scholar]
- 21.Obonyo M, Guiney DG, Harwood J, Fierer J, Cole SP. Role of gamma interferon in Helicobacter pylori induction of inflammatory mediators during murine infection. Infect Immun. 2002;70(6):3295–3299. doi: 10.1128/IAI.70.6.3295-3299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hase K, et al. Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology. 2003;125(6):1613–1625. doi: 10.1053/j.gastro.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 23.Rad R, et al. Toll-like receptor-dependent activation of antigen-presenting cells affects adaptive immunity to Helicobacter pylori. Gastroenterology. 2007;133(1):150–163. doi: 10.1053/j.gastro.2007.04.071. [DOI] [PubMed] [Google Scholar]
- 24.Obonyo M, et al. Deficiencies of myeloid differentiation factor 88, Toll-like receptor 2 (TLR2), or TLR4 produce specific defects in macrophage cytokine secretion induced by Helicobacter pylori. Infect Immun. 2007;75(5):2408–2414. doi: 10.1128/IAI.01794-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Que FG, Gores GJ. Cell death by apoptosis: Basic concepts and disease relevance for the gastroenterologist. Gastroenterology. 1996;110(4):1238–1243. doi: 10.1053/gast.1996.v110.pm8613014. [DOI] [PubMed] [Google Scholar]
- 26.Khulusi S, Ahmed HA, Patel P, Mendall MA, Northfield TC. The effects of unsaturated fatty acids on Helicobacter pylori in vitro. J Med Microbiol. 1995;42(4):276–282. doi: 10.1099/00222615-42-4-276. [DOI] [PubMed] [Google Scholar]
- 27.Gaby AR. Helicobacter pylori eradication: Are there alternatives to antibiotics? Altern Med Rev. 2001;6(4):355–366. [PubMed] [Google Scholar]
- 28.Zhang L, Pornpattananangku D, Hu CMJ, Huang CM. Development of nanoparticles for antimicrobial drug delivery. Curr Med Chem. 2010;17(6):585–594. doi: 10.2174/092986710790416290. [DOI] [PubMed] [Google Scholar]
- 29.Gao W, Hu C-MJ, Fang RH, Zhang L. Liposome-like nanostructures for drug delivery. J Mater Chem B Mater Biol Med. 2013;1(48):6569–6585. doi: 10.1039/C3TB21238F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramteke S, Jain NK. Clarithromycin- and omeprazole-containing gliadin nanoparticles for the treatment of Helicobacter pylori. J Drug Target. 2008;16(1):65–72. doi: 10.1080/10611860701733278. [DOI] [PubMed] [Google Scholar]
- 31.Umamaheshwari RB, Ramteke S, Jain NK. Anti-Helicobacter pylori effect of mucoadhesive nanoparticles bearing amoxicillin in experimental gerbils model. AAPS PharmSciTech. 2004;5(2):e32. doi: 10.1208/pt050232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramteke S, Ganesh N, Bhattacharya S, Jain NK. Triple therapy-based targeted nanoparticles for the treatment of Helicobacter pylori. J Drug Target. 2008;16(9):694–705. doi: 10.1080/10611860802295839. [DOI] [PubMed] [Google Scholar]
- 33.Lin Y-H, et al. Development of pH-responsive chitosan/heparin nanoparticles for stomach-specific anti-Helicobacter pylori therapy. Biomaterials. 2009;30(19):3332–3342. doi: 10.1016/j.biomaterials.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 34.Olmsted SS, et al. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys J. 2001;81(4):1930–1937. doi: 10.1016/S0006-3495(01)75844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nance EA, et al. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci Transl Med. 2012;4:149ra119. doi: 10.1126/scitranslmed.3003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai SK, Wang Y-Y, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61(2):158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das UN, Begin ME, Ells G. Fatty acid changes during the induction of differentiation of human promyelocytic leukemia (HL-60) cells by phorbolmyristate acetate. Prostaglandins Leukot Essent Fatty Acids. 1992;46(3):235–239. doi: 10.1016/0952-3278(92)90077-v. [DOI] [PubMed] [Google Scholar]
- 38.Das UN. Essential fatty acids and their metabolites as modulators of stem cell biology with reference to inflammation, cancer, and metastasis. Cancer Metastasis Rev. 2011;30(3-4):311–324. doi: 10.1007/s10555-011-9316-x. [DOI] [PubMed] [Google Scholar]
- 39.Suarez G, Reyes VE, Beswick EJ. Immune response to H. pylori. World J Gastroenterol. 2006;12(35):5593–5598. doi: 10.3748/wjg.v12.i35.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Algood HMS, Cover TL. Helicobacter pylori persistence: an overview of interactions between H. pylori and host immune defenses. Clin Microbiol Rev. 2006;19(4):597–613. doi: 10.1128/CMR.00006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bodoprost J, Rosemeyer H. Analysis of phenacylester derivatives of fatty acids from human skin surface sebum by reversed-phase hplc: Chromatographic mobility as a function of physico-chemical properties. Int J Mol Sci. 2007;8:1111–1124. [Google Scholar]
- 42.Yang D, et al. The antimicrobial activity of liposomal lauric acids against Propionibacterium acnes. Biomaterials. 2009;30(30):6035–6040. doi: 10.1016/j.biomaterials.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banerjee A, et al. Deficiency of the myeloid differentiation primary response molecule MyD88 leads to an early and rapid development of Helicobacter-induced gastric malignancy. Infect Immun. 2014;82(1):356–363. doi: 10.1128/IAI.01344-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obonyo M, Rickman B, Guiney DG. Effects of myeloid differentiation primary response gene 88 (MyD88) activation on Helicobacter infection in vivo and induction of a Th17 response. Helicobacter. 2011;16(5):398–404. doi: 10.1111/j.1523-5378.2011.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]