Significance
Many evolutionary–developmental models have attempted to relate development and aging, with one popular hypothesis proposing that healthy age-related brain decline mirrors developmental maturation. But this elegant hypothesis has so far lacked clear and direct data to support it. Here, we describe intrinsic, entirely data-driven evidence that healthy brain degeneration and developmental process mirror one another in certain brain regions. Specifically, a data-driven decomposition of structural brain images in 484 healthy participants reveals a network of mainly higher-order regions that develop relatively late during adolescence, demonstrate accelerated degeneration in old age, and show heightened vulnerability to disorders that impact on brain structure during adolescence and aging. These results provide a fundamental link between development, aging, and disease processes in the brain.
Keywords: brain structure, development, aging, schizophrenia, Alzheimer's disease
Abstract
Several theories link processes of development and aging in humans. In neuroscience, one model posits for instance that healthy age-related brain degeneration mirrors development, with the areas of the brain thought to develop later also degenerating earlier. However, intrinsic evidence for such a link between healthy aging and development in brain structure remains elusive. Here, we show that a data-driven analysis of brain structural variation across 484 healthy participants (8–85 y) reveals a largely—but not only—transmodal network whose lifespan pattern of age-related change intrinsically supports this model of mirroring development and aging. We further demonstrate that this network of brain regions, which develops relatively late during adolescence and shows accelerated degeneration in old age compared with the rest of the brain, characterizes areas of heightened vulnerability to unhealthy developmental and aging processes, as exemplified by schizophrenia and Alzheimer’s disease, respectively. Specifically, this network, while derived solely from healthy subjects, spatially recapitulates the pattern of brain abnormalities observed in both schizophrenia and Alzheimer’s disease. This network is further associated in our large-scale healthy population with intellectual ability and episodic memory, whose impairment contributes to key symptoms of schizophrenia and Alzheimer’s disease. Taken together, our results suggest that the common spatial pattern of abnormalities observed in these two disorders, which emerge at opposite ends of the life spectrum, might be influenced by the timing of their separate and distinct pathological processes in disrupting healthy cerebral development and aging, respectively.
Many phylogenetic or ontogenetic models attempt to relate development and aging at genetic, molecular, or cognitive systems levels (1–4). In neuroscience, one of the most popular hypotheses in this respect postulates that the process of healthy age-related brain decline mirrors developmental maturation. This concept was first introduced in 1881 as a “loi de régression” (Ribot’s law) when Théodule Ribot, a French philosopher, observed that the destruction of memories progresses in reverse order to that of their formation: from the unstable to the stable, from the newly formed memories to older “sensory, instinctive” memories (5). More generally, this hypothesis postulates that the sequence of events associated with brain decline should present itself in reverse order to the series of events related to brain development, with brain regions thought to develop relatively late—at both ontogenetic and phylogenetic levels—also degenerating relatively early (2, 6, 7).
One way of tracking this hypothesized mirroring pattern of development and aging in the human brain is to use the information provided at a macroscopic level by structural MRI in large-scale, lifespan human populations. Although structural MRI does not distinguish between the various cellular mechanisms underpinning development and aging processes [e.g., dendritic and synaptic remodeling, neurogenesis and neuronal death, astrogliosis, (de)myelination], this technique is sensitive to detect the overall contribution of these mechanisms to macroscopic age-related changes in brain structure (8–10).
In 2000, Raz (11) presented for the first time MRI data suggesting that the chronological order of completion of intracortical fibers myelination was associated with age-related differences in cortical volume. In particular, Raz (12) later noted that “the pattern of differential brain aging suggests that phylogenetically newer and ontogenetically less precocious brain structures such as association cortices and the neostriatum show increased vulnerability to the effects of aging … follow(ing) the rule of (phylogenetically and ontogenetically) last-in, first-out” (12). Direct and intrinsic evidence for a clear link between brain structural development and aging lending support to this evolutionary–developmental “retrogenesis” model [or the “last-in, first-out” model as Raz (12) and others call it] is needed, however. Most of the structural imaging studies investigating relationships between development and aging have so far led to different, and sometimes contradictory, results (13, 14). One possible explanation for this inconsistency is that these studies have tested only one specific pattern of age-related change and have focused on age subgroups or on predefined regions of the brain.
Here, we took a purely data-driven approach to assess the intersubject brain structure variability among 484 healthy participants covering most of the lifespan (8–85 y). We analyzed the structural brain images of these healthy subjects using a linked independent component analysis (ICA) (SI Materials and Methods) (15). This approach provides an automatic decomposition of the images into spatial components characterizing the intersubject brain structural variability, i.e., each spatial component represents a mode of variation of brain structure across all participants.
Results
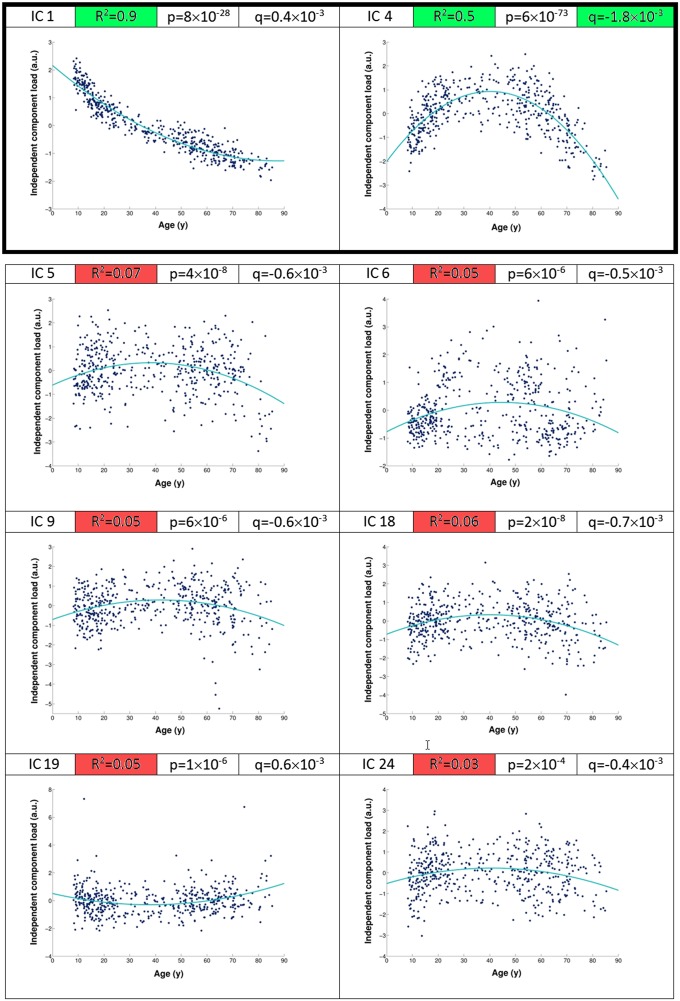
We obtained 70 independent components from this unbiased decomposition that was solely based on the structural information in the gray matter images. As the ICA is, thus, blind to any of the participants’ demographics or cognitive measures, we identified post hoc two components that showed strong statistical, as well as practical, association with age (i.e., significance was measured here using effect magnitude in addition to corrected P values) (Fig. 1). The first independent component (IC1) represented the expected dominant mode of variation showing the monotonic decrease of the whole gray matter with increasing age typically reported in large-scale lifespan studies (16). Spatially, it essentially described the standard deviation across all gray matter images (explaining ∼50% of the structural variance across participants) (SI Materials and Methods), and post hoc analysis revealed that age explained 90% of IC1 variance.
Fig. 1.
Of all eight age-related components, only two achieved clear practical significance. We assessed post hoc the relationship of each of the 70 components with age (using polynomial fit). Of eight statistically significant components (all P < 0.05 corrected for multiple comparisons), only two achieved clear practical significance (IC1 and IC4), as measured by the percentage of age-related variance explained with a quadratic fit (indicated by the R2 values): the “global” dominant mode showing monotonic decrease of the whole gray matter with age (IC1), with 90% of the variance of IC1 across subjects explained by age (R2 = 0.9), and the inverted-U component (IC4), with 50% of IC4 variance explained by age (R2 = 0.5). R2 values for all other components were below 0.1. The inverted-U component IC4 showed a symmetric, strong nonmonotonic relationship with age and presented the strongest quadratic fit as measured by its quadratic coefficient (q = −1.8 × 10−3). a.u., arbitrary unit.
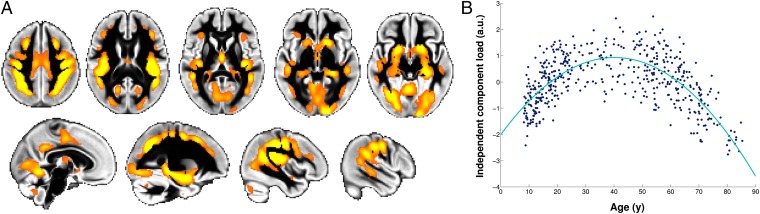
Critically, our data-driven approach made it possible to identify a distinct second age-related component (IC4) accounting for a more subtle part of the structural variance, and showing a nonmonotonic post hoc relationship with age. It defined a spatially specific network of mainly transmodal regions encompassing heteromodal cortex, and limbic and paralimbic regions (17): lateral prefrontal cortex, frontal eye field, intraparietal sulcus, superior temporal sulcus, posterior cingulate cortex, and medial temporal lobe (Fig. 2, Fig. S1, and Table S1). Additional regions included the parietal operculum (especially OP1), crus of the cerebellum, fusiform and lingual gyrus, supplementary motor area (SMA), and a focal, lateral portion of the primary motor cortex (M1). Post hoc analysis revealed that this independent component IC4 had a striking inverted-U relationship with age peaking at 40 y (Figs. 1 and 2 and SI Materials and Methods). While this component accounted for 3% of the structural variance across all 484 participants, it had a strong relationship with age, as age explained 50% of the variance within the IC4 component (Fig. S2).
Fig. 2.
Network of gray matter regions showing the inverted-U relationship with age. (A) Spatial network corresponding to the second age-related independent component IC4 (orange) overlaid on the gray matter average across all 484 healthy participants (thresholded for better visualization at Z > 4). Left is right. (B) Second age-related independent component IC4 load for each of the 484 participants plotted against age (quadratic fit is in turquoise; P = 6 × 10−73) (SI Materials and Methods). a.u., arbitrary unit.
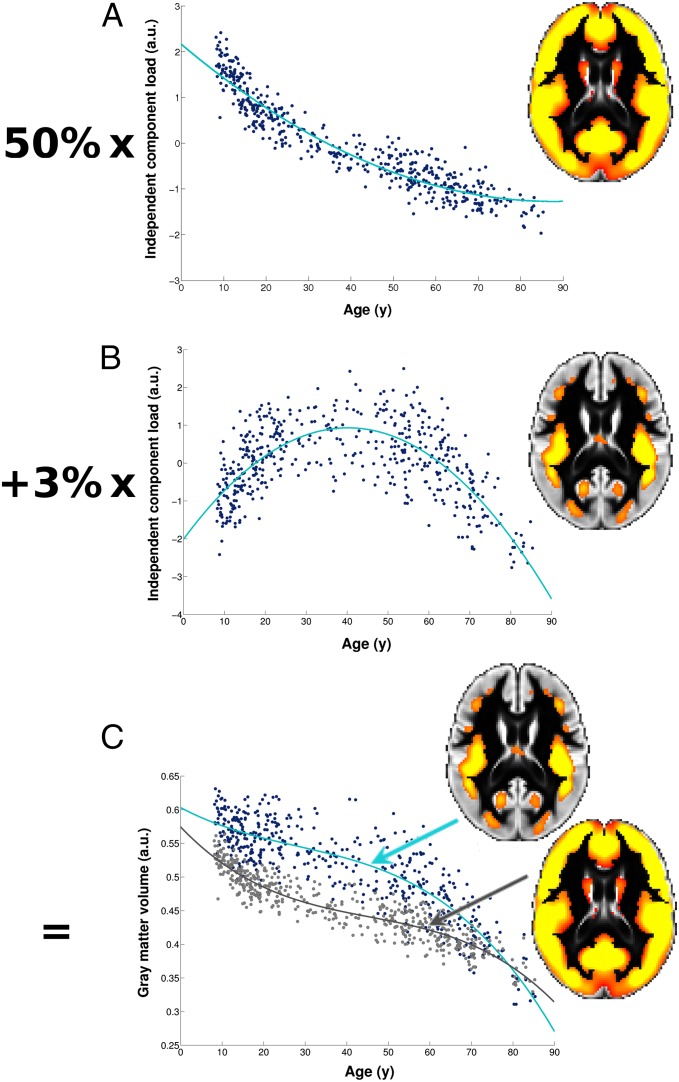
Broadly speaking, each component of an ICA describes a mode of variation—here, specifically, a mode of variation of brain structure—over and above variation associated with all other components. Each voxel of the structural image for every participant can be seen as the sum of each of these components for that same voxel, weighted by the amount of structural variance that they explain. Therefore, in the regions defined by the second age-related component IC4, the variation in brain structure across subjects is explained by the additional effect of this symmetric inverted-U shape on top of the dominant mode of strong monotonic decrease of the whole gray matter with age seen in IC1 (Fig. 3). This inverted-U component IC4 thus describes a network of regions which, compared with the rest of the gray matter, develop relatively late and slowly during adolescence and young adulthood, but show accelerated age-related degeneration in old age (Fig. 3).
Fig. 3.
Regions of the inverted-U network develop relatively slowly during adolescence but present accelerated age-related degeneration at an old age. In the ICA approach, the gray matter volume relationship with age at each voxel is explained by a weighted combination of all ICA components contributing to that voxel. (A) A widespread component including most of the gray matter explains 50% of the structural variation in the images (IC1). (B) The component of interest (IC4) explains 3% of the structural variation across images. (C) The relationship with age in the “core” of the inverted-U network of IC4 (as defined here for visual interpretation by using a threshold of Z > 4; B) is therefore explained by a combination of A and B, meaning that there is an additional effect on top of the dominant pattern of monotonic decrease in whole gray matter volume with increasing age as seen in IC1. As a result, compared with the whole of the gray matter (gray line in C), regions of the inverted-U network of IC4 (turquoise line in C) develop relatively slowly during adolescence and young adulthood (the turquoise line shows a less steep slope than the gray line) but also show accelerated age-related degeneration at old age (the turquoise line shows a steeper slope than the gray line). a.u., arbitrary unit.
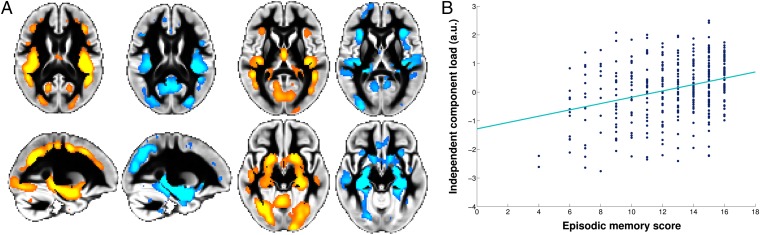
This brain network, characterized at one end of the life spectrum by healthy late development and at the other end by healthy accelerated degeneration, might therefore show particular vulnerability to disorders that impact on brain structure during adolescence (18) and aging (19), regardless of their etiology. To test this hypothesis, we used structural imaging data from a study of Alzheimer’s disease and a study of adolescent-onset schizophrenia, two diseases that serve here as models of unhealthy aging and development, respectively, to compare their spatial pattern of structural vulnerability with the inverted-U spatial network of IC4. It should be noted at this stage that this hypothesis does not suppose that these disorders should have common pathological processes causing brain structural damage. What we are interested in here is whether the timing of such distinct pathologies in disrupting normal brain development and aging, at a time when regions of the inverted-U network are experiencing the greatest change, leads to similar patterns of structural damage in this network. We found that the spatial distribution of this inverted-U component IC4 not only closely matched the gray matter regions which show accelerated atrophy in Alzheimer’s disease (r = 0.55, P < 10−3) (Fig. 4, SI Materials and Methods, and Figs. S1 and S3), but also matched those regions showing an altered developmental trajectory in adolescent-onset schizophrenia (r = 0.48, P < 10−3) (Fig. 5). Direct comparison of the spatial distribution between Alzheimer’s disease and adolescent-onset schizophrenia pattern of macrostructural abnormalities also revealed a good spatial cross-correlation (r = 0.48, P < 10−3) (Fig. S3).
Fig. 4.
The inverted-U component spatially corresponds to the structural pattern of abnormalities in Alzheimer’s disease and correlates with episodic memory in healthy subjects. (A) The spatial network corresponding to the inverted-U component IC4 (orange) closely matches the gray matter found to be atrophic in Alzheimer’s disease compared with healthy elderly (blue; thresholded for better visualization at P < 0.001; n = 120; voxel-by-voxel spatial cross-correlation: r = 0.55; P < 10−3). (B) The inverted-U component load for each of the healthy participants plotted against episodic memory score (CVLT long-delay recall; n = 370; linear fit is in turquoise; r = 0.31; P = 1.2 × 10−9) (SI Materials and Methods). Results presented here have not been age-corrected, as the relationship between episodic memory scores and age was highly nonlinear. In fact, their lifespan trajectory matched that of the inverted-U component (Fig. S6), explaining the linear relationship between the two presented in B. a.u., arbitrary unit.
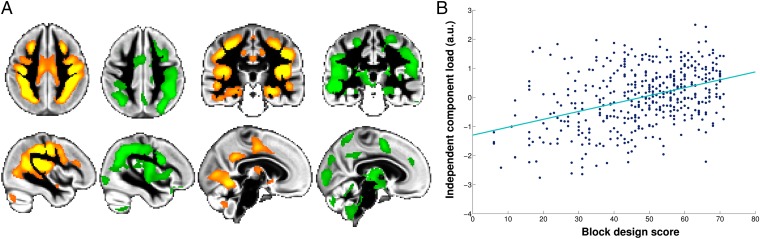
Fig. 5.
The inverted-U component spatially corresponds to the structural pattern of abnormalities in adolescent-onset schizophrenia and correlates with intelligence scale in healthy subjects. (A) The spatial network corresponding to the inverted-U component IC4 (orange) closely matches the gray matter showing altered trajectory in adolescent-onset schizophrenia compared with healthy adolescents (green; thresholded for better visualization at P < 0.05; n = 24; voxel-by-voxel spatial cross-correlation: r = 0.48; P < 10−3). (B) The inverted-U component load for each of the healthy participants plotted against intellectual ability [e.g., block design score (fluid intelligence) from the Wechsler Abbreviated Scale of Intelligence; n = 439; linear fit is in turquoise; r = 0.40; P = 1.8 × 10−18] (SI Materials and Methods and Fig. S5 shows the plot for crystallized intelligence). Results presented here have not been age-corrected, as the relationship between block design scores and age was highly nonlinear. As for episodic memory scores, lifespan trajectory of fluid intelligence matched that of the inverted-U component (Fig. S6), explaining the linear relationship between the two presented in B. a.u., arbitrary unit.
Of all 70 components, this close resemblance to both schizophrenia and Alzheimer’s disease spatial patterns was only specific to the inverted-U mode of variation IC4 and the dominant mode of variation IC1, as the latter simply represents the standard deviation across all subjects (Fig. S2). Although the inverted-U component was purely derived from healthy individuals, discrimination analysis using this spatial network also allowed for good separation of brain scans between patients with either Alzheimer’s disease or schizophrenia and corresponding matched controls (72% and 83% accuracy, respectively) (SI Materials and Methods).
The inverted-U component IC4 also differed between males and females (Fig. S4). Females showed a significantly higher and slightly later peak with age than males (41 versus 39 y, respectively; P = 4.5 × 10−3) (SI Materials and Methods). This few years of difference in lifespan trajectory of a relevant brain structural component could be related to the later age of onset of symptoms observed in females with schizophrenia and Alzheimer’s disease (20, 21).
Finally, additional regression analyses in the large-scale lifespan healthy population showed specific, strong correlations between the strength of the inverted-U component IC4 and episodic memory and intellectual ability, deficits in which are hallmarks of Alzheimer’s disease and schizophrenia, respectively (P << 10−3) (SI Materials and Methods). More specifically, there was a moderate correlation of r = 0.31 between the network strength and long-delay free recall on the California Verbal Learning Test (CVLT), which is known to be the most salient measure of memory deficit in mild cognitive impairment and Alzheimer’s disease (Fig. 4) (22). There was also a good correlation of r = 0.40 between the network strength and fluid intelligence (block design; Wechsler Abbreviated Scale of Intelligence, WASI) (Fig. 5). We found a more modest correlation of r = 0.21 between the network strength and crystallized intelligence (vocabulary; WASI) (Fig. S5). However, when looking at the same relationship only in the healthy participants under 40 y old (age peak of the inverted-U component), we found a very strong correlation of r = 0.52, consistent with the notion that verbal intelligence crystallizes to a plateau in middle age (Figs. S5 and S6).
Discussion
Here, a data-driven analysis of brain structural variation across 484 healthy participants revealed a previously unseen component showing a symmetric inverted-U relationship with age, and spatially characterizing a biologically meaningful network of gray matter regions largely involved in transmodal processing. This network of brain regions not only showed mirroring of healthy developmental and aging processes, but also demonstrated heightened vulnerability to etiologically distinct clinical disorders linked to abnormal adolescent and aging trajectories (schizophrenia and Alzheimer’s disease) and recapitulated the pattern of macrostructural abnormalities seen in both disorders.
Two features of our methodological approach were crucial in revealing this inverted-U component IC4 showing symmetrical developmental and aging processes. First, no constraint—spatial or age-related—was imposed on the data. Second, the method allowed us to detect more subtle modes of variation over and above other global components that dominate the intersubject variability, such as seen in IC1, and that are typically reported in lifespan studies (16). This decomposition approach thus revealed this IC4 component which, while explaining only a modest amount of the structural variance across all 484 healthy subjects (3%), had a strong relationship with age (as age explained 50% of the IC4 variance) and accounted for a substantial part of the spatial variance of Alzheimer’s disease and adolescent-onset schizophrenia patterns of abnormalities (30% and 23%, respectively) (Fig. S2).
These results, intrinsically linking development, aging, and two disorders with very distinct ages of onset of symptoms and neuropathological processes, might seem surprising at first. But they become much more intuitive when taking several considerations into account. First, our network of regions in which development and aging mirror one another includes mainly transmodal regions. The heteromodal cortex (or transmodal cortex when including limbic and paralimbic regions) encompasses the highest synaptic levels of bottom-up processing (17). Because it develops later than the rest of the brain, the transmodal cortex is a strong candidate for showing such retrogenesis or last-in, first-out processes. Second, both Alzheimer’s disease and schizophrenia have been linked, separately in the literature, to a selective damage to the heteromodal cortex. Indeed, neuropathological and neuroimaging findings suggest that primary lesions responsible for the classic clinical feature of schizophrenia occur in the phylogenetically recent heteromodal cortex (23, 24). Separately, it has been suggested that the pattern of vulnerability in Alzheimer’s disease is distributed specifically following “nodes” distributed within the heteromodal cortex (and showing substantial overlap with the default mode network) (25). Interestingly, a retrogenic neuropathological pattern in terms of neuronal cell loss and myelin vulnerability has also been observed in Alzheimer’s disease (7).
Not all regions in the inverted-U spatial network of IC4 were transmodal. However, connectivity of OP1 may predispose it to perform more integrative aspects of somatosensory processing (26), while the crus of the cerebellum is most connected to the heteromodal prefrontal and posterior parietal cortices (27). One region that is known to ontogenetically and phylogenetically develop late, but is absent from our network, is the frontal pole (Brodmann area 10) (28). We did not find any evidence for late maturation in this region whose function is still debated (29, 30), which is in line with findings from the MRI study by Hill et al. (2) elegantly linking high-expanding and slowly maturing cortical regions during evolution and human development. However, the lateral portion of M1, which is not typically thought to develop late or degenerate early, was present in our network, and also within the regions reported in the MRI study by Hill et al. (2). The fact that, using structural MRI, we are probing information at a macroscopic scale, which might encapsulate various cellular mechanisms such as myelination, astrocytosis, or vascularization, might explain these apparent discrepancies. At this resolution, it is not possible to resolve which mechanisms may underlie the macroscopic cortical changes that we observe and peak at 40 y (8). This study also comes with the limitations inherent to large cross-sectional datasets across the lifespan, such as possible cohort effects and selection bias, which might influence the age peak to some degree (31). Various imaging studies support a similar timeline for late development (32–35), however, while recent cellular findings show, for instance, that myelination and remodeling of synaptic spines extend later than previously thought, beyond adolescence and young adulthood (36, 37).
It should be noted that relatively late maturation in the lateral part of the primary motor cortex and small part of lingual gyrus is also seen during healthy adolescence and in adolescent-onset schizophrenia (38, 39). We have previously found that adolescent-onset schizophrenia exhibits an altered maturational pattern compared with matched healthy adolescents (38), with differences in M1, SMA, and lingual gyrus initially present at 16 y, and fading away 2.5 y later. One difference between the inverted-U network of IC4 and the spatial pattern of adolescent-onset schizophrenia was the absence of medial temporal lobe abnormalities in the latter case (Figs. S1 and S3). This apparent discrepancy is likely due to the fact that we observed changes over a relatively short period of time in adolescence (16–18.5 y on average), which did not span to young adulthood when hippocampus volume still changes (40).
Remarkably, the white matter myelination in the frontal lobe, as assessed using diffusion imaging and transverse relaxation rate, reveals a similar inverted-U relationship, peaking at a comparable age (41). It is therefore possible that what we observe here as a subtle effect in the gray matter (on top of the dominating loss of gray matter) relates to the myelination process of intracortical fibers (12). This process might also explain why the spatial distribution of the inverted-U component IC4, and the maps of structural abnormalities in schizophrenia and Alzheimer’s disease, is more prominent in the fundus of the sulci as opposed to gyral crowns. Known histological differences between the two, such as the facts that the cortex in the fundus has a greater cell density and, most relevantly, that fundi have thicker supragranular—phylogenetically newest and latest myelinated—layers (42), might partly explain this topography of the gray matter volume differences. Neuropathological studies also interestingly show that β-amyloid distribution in Alzheimer’s disease and reduced cell density in schizophrenia are both preferentially found in the fundus of sulci (43, 44). We cannot exclude the possibility, however, that the distinct topography of the cortical fundi and subcortical structures, as more “internal” structures in the brain, and their specific histology and fiber orientation interact with the imaging technique, contrast, and limited resolution used here, so that we are more sensitive to capture macroscopic differences—due to the myelination process, for instance—in these specific parts of the cortex. It is therefore possible that the same brain regions of the inverted-U network might exhibit the same age-related changes in the gyri, if higher-resolution and comparative histology were available (45).
Another feature of the inverted-U component IC4 was that its lifespan trajectory matched that of fluid and crystallized intelligence (that is, before it crystallizes) and, to a lesser degree, episodic memory in the 484 healthy subjects (Fig. S6). As a consequence, there was a linear relationship between the inverted-U component load and each of these cognitive measures, very much in line with the above-mentioned quadratic, inverted-U myelination process which followed closely the same age trajectory as a functional performance measure (46). Following these observations, Bartzokis (47) proposed a myelin “development-to-degeneration” model of the human brain, according to which “myelin development, maintenance, and its eventual breakdown are essential to understanding … cognitive and behavioral trajectories through life” and that shed light on Alzheimer’s disease as a developmental disorder “requiring myelination as an essential permissive step” (47). This analogous result reinforces the idea, which cannot be tested with the imaging technique and resolution available for this study, that the effect observed here in the gray matter might be somewhat related to myelination.
Using a data-driven approach, we have therefore been able to characterize a biologically meaningful component intrinsically linking late development, early degeneration, and vulnerability to disease. There is mounting evidence that the pattern of various brain disorders can be explained to some extent by observing the healthy brain. Deviations from normal trajectories of brain maturation have been identified in developmental disorders (38, 48), while some neurodegenerative disorders seem to progress within specific healthy brain networks (23, 49). One recent study has also shown that data-driven decomposition of white matter tractograms in healthy young subjects recapitulates the pattern of abnormalities in dementia (50). Here, we show how the symmetric inverted-U component, while derived without any prior hypothesis from healthy subjects’ brain structure, (i) spatially recapitulates the structural vulnerability of two etiologically distinct disorders emerging at opposite ends of the life spectrum (schizophrenia—aptly named “dementia praecox” until the mid-1950s—and Alzheimer’s disease), (ii) accurately discriminates these two disorders from their matched healthy group, and (iii) is associated, in this large-scale lifespan healthy population, with cognitive functions whose impairment are key symptoms of schizophrenia and Alzheimer’s disease. We thus suggest that the spatial pattern of structural abnormalities common to these disorders might be determined crucially by the timing of the interplay of their specific pathophysiological processes with normal brain development and aging, specifically in regions in which these two processes mirror one another.
Materials and Methods
The study was approved by the Regional Ethical Committee of Southern Norway; 484 right-handed healthy volunteers covering much of the lifespan (age range from 8 to 85 y old; 220 males) underwent the same imaging protocol with structural T1-weighted images performed using a 12-channel head coil on a 1.5 T Siemens Avanto Scanner (Siemens Medical Systems). A linked ICA decomposition into 70 components was run on brain structural information derived from three complementary types of gray matter image processing: gray matter volume obtained from an optimized voxel-based morphometry protocol using FMRIB Sofware Library (FSL-VBM) analysis (51, 52), and vertexwise cortical thickness and surface area measures calculated using FreeSurfer (53). For the purpose of this study, we focused on components showing statistical as well as clear practical significant relationship with age (significance measured using effect magnitude as opposed to P values). We tested for a difference in the peak of the curves between males (n = 224) and females (n = 260) in age and height using bootstrap resampling with replacement. We carried out voxel-by-voxel spatial cross-correlation to quantify the overlap between the spatial map of the age-related independent components and the maps of the structural abnormalities in schizophrenia and Alzheimer’s disease, using the values of all voxels within a brain mask. We assessed the significance of the spatial cross-correlation using a Monte Carlo approach. Linear discriminant analysis and leave-one-out cross-validation were carried out in R. Finally, we correlated the strength of the age-related components with the behavioral measures in MATLAB7.12, correcting for multiple comparisons across all components. More details of the method are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Tom Nichols for his valuable help on statistical questions. We also thank Dr. Peter Keating for helpful comments on this manuscript and further advice on statistical issues. Finally, we thank Prof. André Adoutte for his wonderful and unforgettable lectures in evo-devo. This work was supported by Medical Research Council (MRC) MR/K006673/1 (to G.D.), MRC G0500092 (to A.J.), Research Council of Norway 204966/F20 (to L.T.W.), and Wellcome Trust WT090955AIA (to H.J.-B.). P.M.M. acknowledges research support from the Imperial College Biomedical Research Centre.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.C.P. is a guest editor invited by the Editorial Board.
Data deposition: The Z/t stats 3D maps for all three datasets reported in this paper can be found at www.fmrib.ox.ac.uk/analysis/LIFO+AD+AOS/.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1410378111/-/DCSupplemental.
References
- 1.de Magalhães JP, Church GM. Genomes optimize reproduction: Aging as a consequence of the developmental program. Physiology (Bethesda) 2005;20:252–259. doi: 10.1152/physiol.00010.2005. [DOI] [PubMed] [Google Scholar]
- 2.Hill J, et al. Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci USA. 2010;107(29):13135–13140. doi: 10.1073/pnas.1001229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karama S, et al. Childhood cognitive ability accounts for associations between cognitive ability and brain cortical thickness in old age. Mol Psychiatry. 2014;19(5):555–559. doi: 10.1038/mp.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamnes CK, et al. Alzheimer’s Disease Neuroimaging Initiative Brain development and aging: Overlapping and unique patterns of change. Neuroimage. 2013;68:63–74. doi: 10.1016/j.neuroimage.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribot T. Les Maladies de la Mcamoire. Germer-Baillicre; Paris: 1881. [Google Scholar]
- 6.Raz N, et al. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 7.Reisberg B, et al. Evidence and mechanisms of retrogenesis in Alzheimer’s and other dementias: Management and treatment import. Am J Alzheimers Dis Other Demen. 2002;17(4):202–212. doi: 10.1177/153331750201700411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15(4):528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sagi Y, et al. Learning in the fast lane: New insights into neuroplasticity. Neuron. 2012;73(6):1195–1203. doi: 10.1016/j.neuron.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Sampaio-Baptista C, et al. Motor skill learning induces changes in white matter microstructure and myelination. J Neurosci. 2013;33(50):19499–19503. doi: 10.1523/JNEUROSCI.3048-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raz N. Ageing of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. Handbook of Aging and Cognition – II. Erlbaum; Mahwah, NJ: 2000. pp. 1–90. [Google Scholar]
- 12.Raz N. Ageing and the Brain. 2005 Available at onlinelibrary.wiley.com/doi/10.1038/npg.els.0004063/abstract.
- 13.McGinnis SM, Brickhouse M, Pascual B, Dickerson BC. Age-related changes in the thickness of cortical zones in humans. Brain Topogr. 2011;24(3-4):279–291. doi: 10.1007/s10548-011-0198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30(6):730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groves AR, Beckmann CF, Smith SM, Woolrich MW. Linked independent component analysis for multimodal data fusion. Neuroimage. 2011;54(3):2198–2217. doi: 10.1016/j.neuroimage.2010.09.073. [DOI] [PubMed] [Google Scholar]
- 16.Lemaitre H, et al. Normal age-related brain morphometric changes: Nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiol Aging. 2012;33(3):617–619. doi: 10.1016/j.neurobiolaging.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 18.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fjell AM, et al. Alzheimer Disease Neuroimaging Initiative Accelerating cortical thinning: Unique to dementia or universal in aging? Cereb Cortex. 2014;24(4):919–934. doi: 10.1093/cercor/bhs379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salem JE, Kring AM. The role of gender differences in the reduction of etiologic heterogeneity in schizophrenia. Clin Psychol Rev. 1998;18(7):795–819. doi: 10.1016/s0272-7358(98)00008-7. [DOI] [PubMed] [Google Scholar]
- 21.Bowler JV, Munoz DG, Merskey H, Hachinski V. Factors affecting the age of onset and rate of progression of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1998;65(2):184–190. doi: 10.1136/jnnp.65.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitwell JL, et al. MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology. 2008;70(7):512–520. doi: 10.1212/01.wnl.0000280575.77437.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckner RL, et al. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearlson GD, Petty RG, Ross CA, Tien AY. Schizophrenia: A disease of heteromodal association cortex? Neuropsychopharmacology. 1996;14(1):1–17. doi: 10.1016/S0893-133X(96)80054-6. [DOI] [PubMed] [Google Scholar]
- 25.Buckner RL, et al. Molecular, structural, and functional characterization of Alzheimer's disease: Evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eickhoff SB, et al. Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J Neurosci. 2010;30(18):6409–6421. doi: 10.1523/JNEUROSCI.5664-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 2010;20(4):953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW. Prefrontal cortex in humans and apes: A comparative study of area 10. Am J Phys Anthropol. 2001;114(3):224–241. doi: 10.1002/1096-8644(200103)114:3<224::AID-AJPA1022>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 29.Ramnani N, Owen AM. Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5(3):184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- 30.Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318(5850):594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- 31.Fjell AM, et al. Alzheimer Disease Neuroimaging Initiative Critical ages in the life course of the adult brain: Nonlinear subcortical aging. Neurobiol Aging. 2013;34(10):2239–2247. doi: 10.1016/j.neurobiolaging.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lebel C, et al. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60(1):340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- 33.Westlye LT, et al. Life-span changes of the human brain white matter: Diffusion tensor imaging (DTI) and volumetry. Cereb Cortex. 2010;20(9):2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- 34.Kochunov P, et al. Fractional anisotropy of cerebral white matter and thickness of cortical gray matter across the lifespan. Neuroimage. 2011;58(1):41–49. doi: 10.1016/j.neuroimage.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grydeland H, Walhovd KB, Tamnes CK, Westlye LT, Fjell AM. Intracortical myelin links with performance variability across the human lifespan: Results from T1- and T2-weighted MRI myelin mapping and diffusion tensor imaging. J Neurosci. 2013;33(47):18618–18630. doi: 10.1523/JNEUROSCI.2811-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller DJ, et al. Prolonged myelination in human neocortical evolution. Proc Natl Acad Sci USA. 2012;109(41):16480–16485. doi: 10.1073/pnas.1117943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petanjek Z, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108(32):13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Douaud G, et al. Schizophrenia delays and alters maturation of the brain in adolescence. Brain. 2009;132(Pt 9):2437–2448. doi: 10.1093/brain/awp126. [DOI] [PubMed] [Google Scholar]
- 39.Westlye LT, et al. Differentiating maturational and aging-related changes of the cerebral cortex by use of thickness and signal intensity. Neuroimage. 2010;52(1):172–185. doi: 10.1016/j.neuroimage.2010.03.056. [DOI] [PubMed] [Google Scholar]
- 40.Hedden T, Gabrieli JD. Insights into the ageing mind: A view from cognitive neuroscience. Nat Rev Neurosci. 2004;5(2):87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 41.Bartzokis G, et al. Multimodal magnetic resonance imaging assessment of white matter aging trajectories over the lifespan of healthy individuals. Biol Psychiatry. 2012;72(12):1026–1034. doi: 10.1016/j.biopsych.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Clinton J, Roberts GW, Gentleman SM, Royston MC. Differential pattern of beta-amyloid protein deposition within cortical sulci and gyri in Alzheimer’s disease. Neuropathol Appl Neurobiol. 1993;19(3):277–281. doi: 10.1111/j.1365-2990.1993.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 43.Chance SA, Tzotzoli PM, Vitelli A, Esiri MM, Crow TJ. The cytoarchitecture of sulcal folding in Heschl’s sulcus and the temporal cortex in the normal brain and schizophrenia: Lamina thickness and cell density. Neurosci Lett. 2004;367(3):384–388. doi: 10.1016/j.neulet.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 44.Gentleman SM, et al. Quantitative differences in the deposition of beta A4 protein in the sulci and gyri of frontal and temporal isocortex in Alzheimer’s disease. Neurosci Lett. 1992;136(1):27–30. doi: 10.1016/0304-3940(92)90639-o. [DOI] [PubMed] [Google Scholar]
- 45.Budde MD, Annese J. Quantification of anisotropy and fiber orientation in human brain histological sections. Front Integrative Neurosci. 2013;7(2013):3. doi: 10.3389/fnint.2013.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartzokis G, et al. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging. 2010;31(9):1554–1562. doi: 10.1016/j.neurobiolaging.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bartzokis G. Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiol Aging. 2011;32(8):1341–1371. doi: 10.1016/j.neurobiolaging.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaw P, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA. 2007;104(49):19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raj A, Kuceyeski A, Weiner M. A network diffusion model of disease progression in dementia. Neuron. 2012;73(6):1204–1215. doi: 10.1016/j.neuron.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Douaud G, et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130(Pt 9):2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- 52.Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 53.Fischl B, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.