Membrane-associated guanylate kinases (MAGUKs) constitute a family of scaffold molecules involved in diverse cellular processes, such as cell–cell communication, cell polarity, and signal transduction (1). MAGUKs are multidomain proteins with a core structure consisting of PSD-95/DLG/ZO-1 (PDZ), Src homolgy 3 (SH3), and enzymatically inactive guanylate kinase (GK) domains. Although full-length MAGUKs generally display a high degree of binding specificity, binding studies with isolated PDZ domains often show the ability to interact with numerous partners (2). In PNAS, Li et al. (3) provide a conclusive structural answer to this discrepancy. The authors have solved the crystal structure of a PALS1/Crb complex that demonstrates why the PDZ–SH3–GK supramodule of PALS1, but not the isolated PDZ domain, binds with an extraordinarily high affinity to the C terminus of Crb (3). Domains in the supramodule are arranged in a way that a peptide comprising the last 17 residues of Crb binds simultaneously to the PDZ, the SH3 and, most surprisingly, to the GK domain using two distinct sites. The C-terminal fixation in the PDZ binding groove follows an expected main chain connectivity of PDZ/peptide complexes. Interestingly, residues of the SH3 domain collaborate in peptide binding by building a kind of a clasp. Using site-directed mutagenesis, Li et al. (3) prove that the clasp accounts for the high affinity and specificity of Crb binding to PALS1. This discovery has immediate consequences for other MAGUKs, for example at synapses in the brain, because the authors propose that sequence variations within the clasp-loop are responsible for defining their specificity. As proof-of-principle, Li et al. show that the C terminus of neurexin does not bind to the isolated PDZ but to the PDZ–SH3–GK supramodule of calcium/calmodulin-dependent serine protein kinase (CASK).
Unexpectedly, the PALS1/Crb structure shows an additional participation of the GK domain in Crb binding (3). Crb binds in a manner comparable to the complex of a phosphorylated peptide p-LGN with the GK domain of DLG1/SAP97 (4). In SAP97/p-LGN, a phosphate group at Ser401 binds to conserved residues that coordinate guanosine monophosphate (GMP) in SAP95 (5). Mutations of Ser to Asp or to Glu are widely used as a tool to generate constitutively active kinases (6) because the carboxyl group of the acidic residues mimics a phosphate ion by negative charge and connectivity. This process is exactly what appears to happen naturally in the PALS1/Crb complex, in which Crb binds with Glu, instead of a phosphate, to the GMP binding pocket. As for Li et al.’s (3) example of synaptic MAGUKs, it is yet undetermined if binding of neurexin to CASK also involves the GK domain in addition to PDZ–SH3, but it has been shown that CASK phosphorylates the C terminus of neurexin (7). This finding implies that CASK may not only bind to the C terminus of neurexin via the PDZ–SH3 complex, but might strengthen its interaction by phosphorylation of a serine within the last 17 residues. The case of neurexin/CASK demonstrates that the structural data by Li et al. (3) not only provide insight into PALS1 interactions but have far-reaching implications on research into MAGUKs: additional clues for binding specificity can now be expected from the clasp-loop of particular SH3 domains and from presence of phosphorylatable Ser and Thr, or “constitutively active” Asp or Glu residues, upstream of the PDZ binding motif in interacting peptides. This result allows a refined assessment of potential PDZ binding proteins by their binding motifs (8), as well as a new classification of MAGUKs based on variations in SH3 clasp sequences.
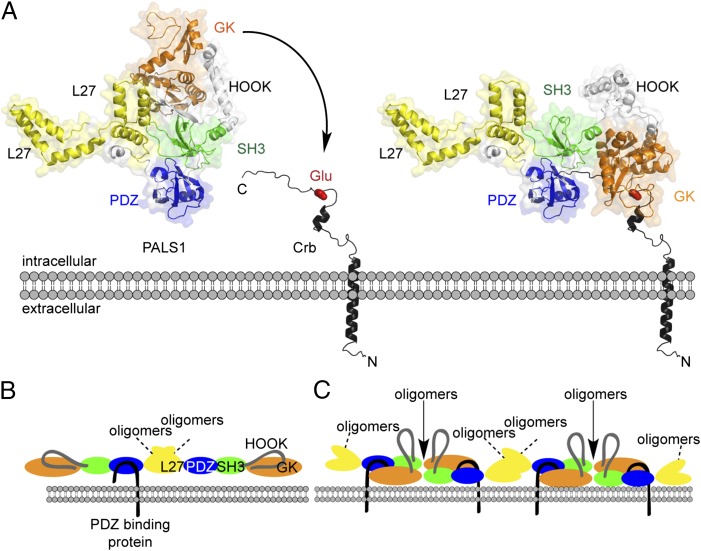
A particular highlight of the PALS1/Crb structure is the opening of the cis-binding of SH3 to GK to allow an interaction in trans-configuration. It has long been suggested that MAGUKs undergo conformational changes (9–14), including the opening of the cis-interacting SH3–GK complex (11, 13). In the current structure, the trans-interaction seems to be induced by Crb, which cross-links the PDZ to the GK domain of the same PALS1 monomer, and thereby exposes the contact epitopes of SH3 and GK to other MAGUKs. In their crystal structure, PALS1/Crb forms a compact symmetric dimer (3). This does not exclude, however, the formation of open dimers, already suggested for MAGUKs (13). In an open dimer, the SH3 of one monomer could interact with an open SH3–GK tandem of another monomer, but would leave the remaining domains free to build even longer line-ups of MAGUK proteins (Fig. 1). Previously, binding of calmodulin to the HOOK region has been suggested to induce a change of the HOOK region from a loop (5) to an extended helical conformation and to lift off the GK domain (13). The idea is supported by the observation that the contact area of SH3 to the GK domain is minimal and opening of the SH3–GK is possible by ligands binding to the HOOK (5, 11, 15), thereby enabling oligomerization of MAGUKs (13). However, these models have not been validated by structural analysis thus far. The structure of PALS1/Crb now identifies peptide binding to PDZ and GK as an unanticipated trigger to open the SH3–GK module. This finding also suggests a different scenario for calmodulin binding to the HOOK than described previously (13). It is more likely that opening of the cis-SH3–GK module by cross-linking of PDZ and GK is required before the binding of calmodulin or other ligands to the HOOK. Because cross-linking of PDZ and GK only works with phosphorylated Ser/Thr or with a constitutively active acidic residue, established MAGUK-based interactions should be revisited for the possibility of oligomerization.
Fig. 1.
Crb-triggered oligomerization of PALS1. (A) Structural model of full-length PALS1 with the PDZ–SH3–GK supramodul in extended conformation (Left). On appearance of Crb C-terminal tail (black) with Glu at position −14 (red), PDZ and SH3 domains bind the tail with high affinity, and GK lifts off the SH3 domain to interact with Glu (arrow), accompanied by reorientation of the HOOK region (Right). (B) MAGUKs such as PALS1 can form oligomers, for example by using N-terminal L27 domains independent of a peptide binding to PDZ. (C) Cross-linking of PDZ and GK as shown by Li et al. (3) results in higher-order complexes because of free SH3 and GK contact sites (arrow) for oligomerization. The PALS1/Crb has been modeled using the data from Li et al. (3) and published structures (PDB ID codes 3UIT, 3UAT, and 1JXM).
As for Li et al.’s (3) example of a synaptic interaction of a MAGUK, we have earlier shown that intracellular trafficking of neurexin is critically dependent on their canonical PDZ binding motif -YYV (16). Although it is still undetermined if CASK is the PDZ protein binding neurexins during trafficking
The structural data by Li et al. not only provide insight into PALS1 interactions but have far-reaching implications on research into MAGUKs.
through the secretory pathway, MAGUKs themselves may require helper proteins to reach the synapse like GKAP (11). The study by Li et al. (3) implies, however, that CASK may integrate neurexin into the active zone by oligomerization. In such a scenario, phosphorylation of neurexins (7) might induce oligomerization and set free SH3, PDZ, and GK domains of CASK for interaction with additional partners. Similar events may be relevant for postsynaptic MAGUKs. Members of the PSD95 protein family are likely to bind initially to NMDA or AMPA receptors with their PDZ1–PDZ2 tandem, which is flexibly linked to the PDZ3–SH3–GK supramodule (12). It is possible that association of cytosolic PDZ binding proteins, like CRIPT, triggers oligomerization of PSD95 by dissociating PDZ3 from SH3-GK (14). Based on the current study, the alternative way of cross-linking PDZ3 and GK of PSD95 is worth considering: for example, postsynaptic neuroligin1, which forms a transsynaptic complex with neurexin and is the best ligand for the PDZ3 (17), is phosphorylated upstream of the PDZ binding motif (18) and is possibly capable of inducing the cross-linking. Through subsequent oligomerization, a single PSD95 could simultaneously cluster neurotransmitter receptors and neuroligin1, while allowing the association of additional cytosolic PDZ binding proteins.
Although the latter aspects need further experimentation, they emphasize the remarkable properties of MAGUKs. The study by Li et al. (3) tells us that MAGUKs are not simply promiscuous scaffolding proteins but are able to selectively participate in signal transduction pathways based on an intricate conformational code built into their structure.
Footnotes
The authors declare no conflict of interest.
See companion article on page 17444.
References
- 1.Funke L, Dakoji S, Bredt DS. Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu Rev Biochem. 2005;74:219–245. doi: 10.1146/annurev.biochem.74.082803.133339. [DOI] [PubMed] [Google Scholar]
- 2.Lee HJ, Zheng JJ. PDZ domains and their binding partners: Structure, specificity, and modification. Cell Commun Signal. 2010;8:8. doi: 10.1186/1478-811X-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, et al. Structure of Crumbs tail in complex with the PALS1 PDZ–SH3–GK tandem reveals a highly specific assembly mechanism for the apical Crumbs complex. Proc Natl Acad Sci USA. 2014;111:17444–17449. doi: 10.1073/pnas.1416515111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu J, et al. Guanylate kinase domains of the MAGUK family scaffold proteins as specific phospho-protein-binding modules. EMBO J. 2011;30(24):4986–4997. doi: 10.1038/emboj.2011.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tavares GA, Panepucci EH, Brunger AT. Structural characterization of the intramolecular interaction between the SH3 and guanylate kinase domains of PSD-95. Mol Cell. 2001;8(6):1313–1325. doi: 10.1016/s1097-2765(01)00416-6. [DOI] [PubMed] [Google Scholar]
- 6.Mansour SJ, et al. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265(5174):966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 7.Mukherjee K, et al. CASK Functions as a Mg2+-independent neurexin kinase. Cell. 2008;133(2):328–339. doi: 10.1016/j.cell.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tonikian R, et al. A specificity map for the PDZ domain family. PLoS Biol. 2008;6(9):e239. doi: 10.1371/journal.pbio.0060239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fomina S, et al. Self-directed assembly and clustering of the cytoplasmic domains of inwardly rectifying Kir2.1 potassium channels on association with PSD-95. Biochim Biophys Acta. 2011;1808(10):2374–2389. doi: 10.1016/j.bbamem.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Reese ML, Dakoji S, Bredt DS, Dötsch V. The guanylate kinase domain of the MAGUK PSD-95 binds dynamically to a conserved motif in MAP1a. Nat Struct Mol Biol. 2007;14(2):155–163. doi: 10.1038/nsmb1195. [DOI] [PubMed] [Google Scholar]
- 11.Wu H, et al. Intramolecular interactions regulate SAP97 binding to GKAP. EMBO J. 2000;19(21):5740–5751. doi: 10.1093/emboj/19.21.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCann JJ, et al. Supertertiary structure of the synaptic MAGuK scaffold proteins is conserved. Proc Natl Acad Sci USA. 2012;109(39):15775–15780. doi: 10.1073/pnas.1200254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGee AW, et al. Structure of the SH3-guanylate kinase module from PSD-95 suggests a mechanism for regulated assembly of MAGUK scaffolding proteins. Mol Cell. 2001;8(6):1291–1301. doi: 10.1016/s1097-2765(01)00411-7. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Lewis SM, Kuhlman B, Lee AL. Supertertiary structure of the MAGUK core from PSD-95. Structure. 2013;21(3):402–413. doi: 10.1016/j.str.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Paarmann I, Lye MF, Lavie A, Konrad M. Structural requirements for calmodulin binding to membrane-associated guanylate kinase homologs. Protein Sci. 2008;17(11):1946–1954. doi: 10.1110/ps.035550.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fairless R, et al. Polarized targeting of neurexins to synapses is regulated by their C-terminal sequences. J Neurosci. 2008;28(48):12969–12981. doi: 10.1523/JNEUROSCI.5294-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irie M, et al. Binding of neuroligins to PSD-95. Science. 1997;277(5331):1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- 18.Bemben MA, et al. CaMKII phosphorylation of neuroligin-1 regulates excitatory synapses. Nat Neurosci. 2014;17(1):56–64. doi: 10.1038/nn.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]