Significance
Tumor-associated macrophages are often associated with poor prognosis, but the molecular cues that impart the change in macrophage phenotypes are mostly unclear. Our findings identify c-Jun N-terminal phosphorylation as a key mediator of macrophage education and point to a recruitment of immunosuppressive regulatory T cells as a possible effector protumorigenic mechanism. This cross-talk between adaptive and innate immune responses occurs before overt tumorigenesis, reversing the classic paradigm of initiation and promotion. Our findings raise the possibility that targeting immune checkpoints could be effective for tumor prevention in chronic inflammatory diseases.
Keywords: HCC, c-Jun phosphorylation, M1 macrophages, M2 macrophages, Tregs
Abstract
The inflamed tumor microenvironment plays a critical role in tumorigenesis. However, the mechanisms through which immune cells, particularly macrophages, promote tumorigenesis have only been partially elucidated, and the full scope of signaling pathways supplying macrophages with protumorigenic phenotypes still remain largely unknown. Here we report that germ-line absence of c-Jun N-terminal phosphorylation at serines 63 and 73 impedes inflammation-associated hepatocarcinogenesis, yet deleting c-Jun only in hepatocytes does not inhibit hepatocellular carcinoma (HCC) formation. Moreover, in human HCC-bearing livers, c-Jun phosphorylation is found in inflammatory cells, whereas it is mostly absent from malignant hepatocytes. Interestingly, macrophages in livers of mice with chronic hepatitis gradually switch their phenotype along the course of disease. Macrophage phenotype and density are dictated by c-Jun phosphorylation, in vitro and in vivo. Transition of macrophage phenotype, from antitumorigenic to protumorigenic, occurs before tumorigenesis, resulting in the production of various chemokines, including chemokine (C-C motif) ligand 17 (CCL17) and CCL22. Such signals, emanating from the liver microenvironment, direct the recruitment of regulatory T cells, which are known to facilitate HCC growth. Our findings identify c-Jun phosphorylation as a key mediator of macrophage education and point to the recruitment of immunosuppressive regulatory T cells as a possible protumorigenic mechanism.
The network of interactions among cells that comprise tumors has a major influence on tumor progression. Tumor-associated macrophages (TAMs) are singled out as possibly the most significant ones, other than the malignant cells per se. Importantly, macrophages can assume both antitumorigenic (designated M1 macrophages) and protumorigenic (designated M2) phenotypes, yet the mechanisms that modulate this switch are largely unknown (1, 2). It is generally maintained that this switch is mediated by signals that emanate from the tumor cells, hence the term “tumor-educated macrophages.” Chronic inflammation plays a key pathogenetic role in hepatocellular carcinoma (HCC). However, chronic inflammation is a complex process, which may play both protumorigenic and antitumorigenic roles via the action of many cytokines and various effects of different protumorigenic and antitumorigenic innate and adaptive immune cells (3).
Experimental proof for a protumorigenic role of microenvironment residents was shown for invasive and metastatic tumors. For example, Budhu et al. identified a specific immune signature that drives the metastatic spread of HCC (4), and Qian et al. showed that chemokine (C-C motif) ligand 2 (CCL2) facilitates breast cancer metastasis (5). Furthermore, we and others have shown that inflammatory-cell–induced signaling events in epithelial cells promote carcinogenesis (6, 7). Epithelial–stroma interactions may also be important in the very initial phases of tumor generation (8), and it is possible that immunosurveillance could resist tumorigenesis at these early stages (9). We hypothesized that, in specific pathological states, macrophage education may occur before tumorigenesis, perhaps even as a prerequisite for it. This hypothesis could form the foundation for new preventive treatments aimed at the reeducation of immune cells in individuals susceptible to developing cancer.
Many reports support the existence of immunosurveillance against nonviral cancers (10). A distinction can now be made between protumorigenic inflammation, characterized by tumor infiltration by Th2 cells, Foxp3-positive regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and M2 macrophages, and antitumorigenic inflammation, characterized by infiltration with Th1 cells, CD8+ cytotoxic T lymphocytes, and M1 macrophages (11). Previous studies have identified several factors that are secreted by TAMs and promote tumor progression, including EGF, VEGF, MMP7/9, and CCL18 (12–15), which are thought to affect tumor cell phenotype and tumor vasculature.
Patients with active chronic hepatitis are a unique example of a patient group carrying an extremely high risk for developing HCC, one of the deadliest human tumors (16, 17). Multiple signaling pathways are known to regulate diverse aspects of immune cell phenotype and function; among these pathways, the c-Jun NH2 kinase (JNK)/c-Jun signaling pathway is known to be involved in both inflammatory and cancer processes (18). JNK kinases are responsible for phosphorylating c-Jun at Ser-63 and -73, which are essential for stimulation of c-Jun activity. However, JNK functions in cancer have been difficult to predict because JNK is involved both in cell proliferation and cell survival as well as in cell death (19). The role of JNK kinases in HCC is controversial, with different models showing protumorigenic and antitumorigenic roles for JNK (20). We therefore set out to study the role of c-Jun N-terminal phosphorylation (JNP) in inflammation-associated HCC.
Results
JNP Fosters Inflammation-Associated Hepatocarcinogenesis by Generating a Protumorigenic Microenvironment.
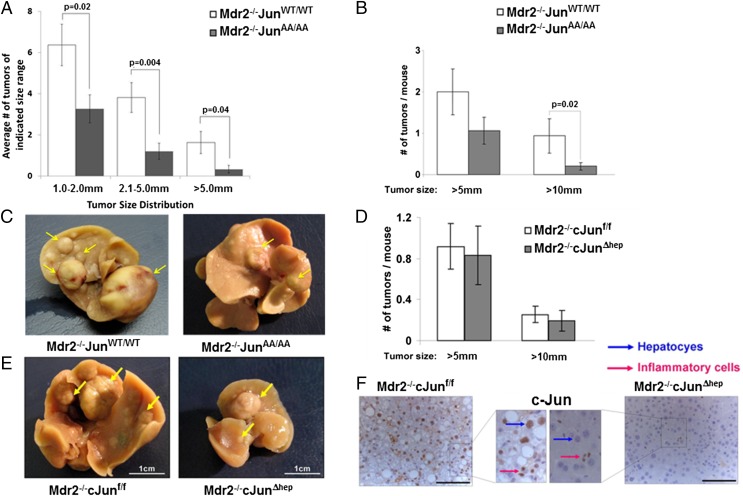
c-Jun is activated by its N-terminal phosphorylation at serines 63 and 73 through JNK proteins. To test the role of JNP in inflammation-induced hepatocarcinogenesis, we crossed Multidrug resistance 2 knockout mice (Mdr2−/−), which spontaneously develop hepatitis followed by HCC and are a mouse model for inflammation-induced HCC (6), with a c-JunAA/AA knock-in mice mutant in which c-Jun cannot be phosphorylated due to replacement of serines 63 and 73 with alanines (21). Inspection of H&E-stained sections and assessment of liver damage markers demonstrated that the resulting Mdr2−/−c-JunAA/AA mice developed chronic hepatitis that was histologically similar to control Mdr2−/−c-JunWT/WT mice (Fig. S1 A and B). Nevertheless, Mdr2−/−c-JunAA/AA mice demonstrated markedly reduced rates of hepatocarcinogenesis: Histological evaluation revealed more than twofold reduction in the number of microscopic tumors (diameter 1–2 mm; P = 0.02), and even more significant reductions for larger tumors [2.1–5 and >5 mm; P = 0.004 and P = 0.04, respectively (Fig. 1A and Fig. S1C)]. Macroscopic inspection confirmed the microscopic analysis (Fig. 1 B and C). These results indicate that JNP facilitates inflammation-induced hepatocarcinogenesis. c-Jun is involved in a wide range of pivotal cellular processes, including cell proliferation and apoptosis; however, we did not find differences in these parameters (Fig. S2).
Fig. 1.
JNP promotes inflammation-dependent hepatocarcinogenesis. (A) Histological quantification of tumor burden in livers from Mdr2−/−c-JunWT/WT and Mdr2−/−c-JunAA/AA mice (n = 15; mean ± SEM). (B) Macroscopic quantification of tumor burden in livers from Mdr2−/−c-JunWT/WT (n = 16) and Mdr2−/−c-JunAA/AA mice (n = 28). Bars show mean ± SEM. (C) Representative whole-liver pictures; arrows indicate tumors. (D) Macroscopic quantification of tumor burden in livers from Mdr2−/−c-Junf/f (n = 11) and Mdr2−/−c-JunΔhep (n = 20) mice (mean ± SEM). (E) Representative whole-liver pictures; arrows indicate tumors and nodules. Note the smaller liver size of Mdr2−/−c-JunΔhep mice. (F) c-Jun immunostains in tumor tissue. Blue arrows, hepatocytes; red arrows, inflammatory cells. (Scale bars: 100 μm.)
In contrast to our findings in Mdr2−/− mice, Eferl et al. have shown that JNP is dispensable for HCC development in mice treated with diethylnitrosamine (DEN), a carcinogen-induced liver cancer model that does not provoke chronic inflammation (22). Noting this different outcome, we hypothesized that in the inflammation-driven model, JNP exerts its protumorigenic effect in inflammatory cells rather than hepatocytes. To this end, we used cross-breeding to introduce floxed c-Jun and Alphafetoprotein–Cre driver (22) into Mdr2−/− mice yielding Mdr2−/−c-JunΔhep mice. Interestingly, Mdr2−/−c-JunΔhep mice display a similar frequency and size distribution of tumors to control Mdr2−/−c-Junf/f mice, which harbor c-Jun in their hepatocytes (Fig. 1 D and E). Because Mdr2−/−c-JunΔhep livers are smaller than those of Mdr2−/−c-Junf/f mice (Fig. 1E), we adjusted the macroscopic measurements of tumors to liver weight as reported for c-JunΔhep mice (22). Immunohistochemical stains for c-Jun confirmed that c-Jun was completely absent in both parenchymal (Fig. S3A) and malignant (Fig. 1F) hepatocytes of Mdr2−/−c-JunΔhep mice, excluding the possibility that Mdr2−/−c-JunΔhep tumors developed due to failure to eliminate c-Jun. However, inflammatory cells in the parenchyma and tumors of Mdr2−/−c-JunΔhep mice showed c-Jun up-regulation (Fig. 1F and Fig. S3A). Together, these findings suggest that in inflammation-induced liver cancer, JNP does not play a role in hepatocytes but, rather, exerts its protumorigenic phenotype via the inflammatory microenvironment.
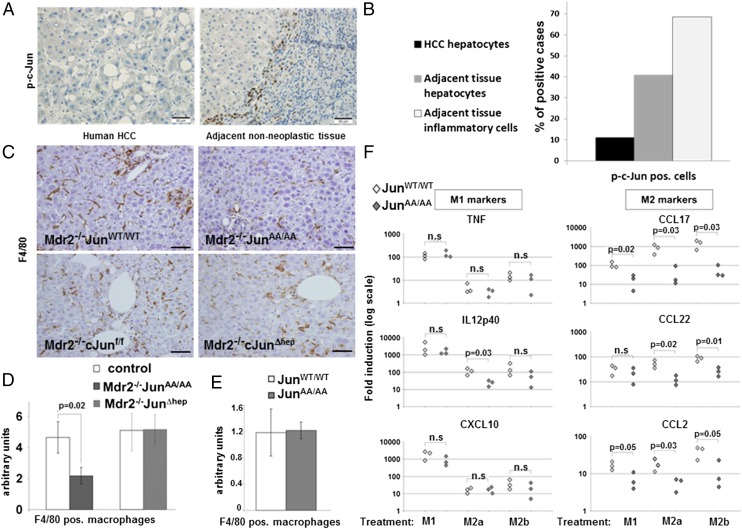
To assess the potential clinical relevance of this finding, we immunostained 27 human HCCs for phosphorylated c-Jun. We detected rare positive staining in malignant hepatocytes of a few HCCs (3 of 27 cases; 11%). However, in adjacent nonneoplastic parenchyma, 40% of the cases demonstrated positive hepatocytes for JNP. Most importantly, in accordance with the mouse data, inflammatory cells positive for JNP were detected in adjacent inflamed liver in most cases (69%), indicating its potential importance in the inflammatory compartment (Fig. 2 A and B).
Fig. 2.
JNP augments macrophage density in livers of Mdr2−/− mice and facilitates in vitro M2 polarization. (A and B) Immunostaining for phosphorylated c-Jun (p-c-Jun) and its quantification in human HCC specimens including surrounding nonneoplastic tissue (n = 27). (C) F4/80 immunostains of liver parenchymas of indicated genotypes. (Scale bars: 125 μm.) (D) Quantification of F4/80 immunostaining (n ≥ 9; mean ± SEM) shown in C. pos, positive. Control indicates either Mdr2−/−c-JunWT/WT or Mdr2−/−c-Junf/f mice, as appropriate. (E) Quantification of F4/80 immunostaining in liver parenchymas of c-JunWT/WT or c-JunAA/AA mice (n = 8; mean ± SEM). (F) Primary peritoneal macrophages (PPMs) from c-JunWT/WT and c-JunAA/AA mice (n = 3) were subjected to either M1- or M2-inducing protocols (M2a and M2b indicate different M2-inducing protocols) or left untreated. Expression of M1 (TNF, IL12p40, and CXCL10) and M2 (CCL17, CCL22, and CCL2) genes is presented as fold increase vs. untreated cells (each data point represents PPMs harvested from a single mouse). Results are representative of three experiments. n.s., not significant.
c-Jun controls hepatocyte proliferation by a p53/p21-dependent mechanism (23). Furthermore, counteracting p53 was found to be the major protumorigenic mechanism of c-Jun in DEN-induced hepatocarcinogenesis (22). In addition, JNK1 expression in hepatocytes was shown to contribute to down-regulation of p21 and up-regulation of c-Myc, as a critical mechanism for DEN-induced HCC (24). Of note, we did not detect differential p53, p21, or c-Myc expression in livers of Mdr2−/− mice with or without hepatocyte c-Jun (Fig. S3 B–D). Collectively, these findings could explain why c-Jun deletion in hepatocytes does not affect hepatocarcinogenesis in Mdr2−/− mice and supports the possibility that, in inflammation-induced HCC, JNP exerts its protumorigenic effect non–cell-autonomously.
c-Jun Phosphorylation Regulates Macrophages Fate.
To analyze which cell types could be responsible for this non–cell-autonomous effect, we first assessed the prevalence of different immune cell types in the livers of the different groups. We revealed a twofold reduction in the number of F4/80-positive macrophages in the parenchyma of 11-mo-old Mdr2−/−c-JunAA/AA mice compared with controls (Fig. 2 C and D; P = 0.02). In contrast, similar macrophage numbers are found in Mdr2−/−c-Junf/f and Mdr2−/−c-JunΔhep mice, in accordance with the lack of difference in tumor burden (Fig. 2 C and D). Comparing resident macrophage (termed Kupffer cells) density in noninflamed livers of c-JunWT/WT and c-JunAA/AA mice, harboring the WT Mdr2 allele, revealed no difference (Fig. 2E and Fig. S4A). Thus, JNP contributes to the accumulation of liver macrophages in chronically inflamed liver, but not in the basal state.
Macrophages can adopt different phenotypes that have opposing effects on tumor growth (2, 25). To study whether JNP affects macrophage phenotype, we subjected primary peritoneal macrophages from c-JunWT/WT and c-JunAA/AA mice to in vitro differentiation protocols (26), inducing either M1 or M2 phenotypes. WT macrophages and JNP-deficient ones did not differ in their ability to up-regulate the expression of the various M1 markers (Fig. 2F). Remarkably, c-JunAA/AA macrophages were markedly impaired in their ability to up-regulate several M2 markers (Table S1) including CCL17, CCL22, and CCL2 (Fig. 2F). These findings reveal that JNP acts in a cell-autonomous manner in macrophages to mediate protumorigenic M2 polarization.
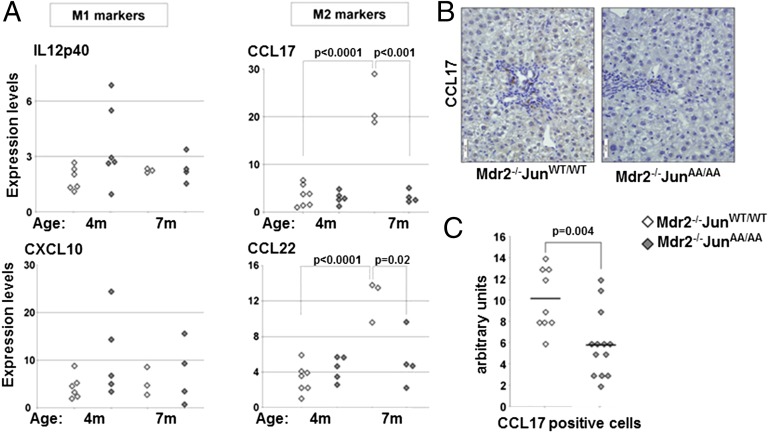
Next, we isolated macrophages from livers of 4- and 7-mo-old Mdr2−/−c-JunAA/AA and control mice. Liver macrophages from both genotypes exhibited comparable expression levels of M1 markers (Fig. 3A). However, whereas Mdr2−/−c-JunWT/WT liver macrophages exhibited an increase of CCL17 and CCL22 expression levels between 4 and 7 mo of age, expression of these chemokines remained low in Mdr2−/−c-JunAA/AA liver macrophages (Fig. 3A). Quantitative PCR (qPCR) analysis of M1 and M2 markers in total liver lysates of 11-mo-old mice showed a similar trend (Fig. S4 B and C). Immunostaining for CCL17 highlighted macrophages that were mostly located near the portal tracts (Fig. 3B). Importantly, the number of macrophages expressing CCL17 was reduced nearly twofold in Mdr2−/−c-JunAA/AA mice (P = 0.004; Fig. 3C). Together, these data show that JNP enhances the expression of M2-related chemokines, without affecting M1 response, in the chronically inflamed liver.
Fig. 3.
JNP bestows a protumorigenic macrophage phenotype in vivo. (A) qPCR analyses of M1 (IL12p40 and CXCL10) and M2 (CCL17 and CCL22) effectors in macrophages isolated from livers of 4- and 7-mo-old Mdr2−/−c-JunWT/WT or Mdr2−/−c-JunAA/AA mice. Each data point represents a single mouse (n ≥ 3). (B) Immunostains for CCL17 in liver parenchymas of 11-mo-old Mdr2−/−c-JunWT/WT and Mdr2−/−c-JunAA/AA mice. (Scale bars: 50 μm.) (C) Quantification of CCL17 immunostains shown in B [Mdr2−/−c-JunWT/WT (n = 9) and Mdr2−/−c-JunAA/AA (n = 13)]. Cross line, geometric mean.
c-Jun Phosphorylation Regulates Recruitment of Treg Cells.
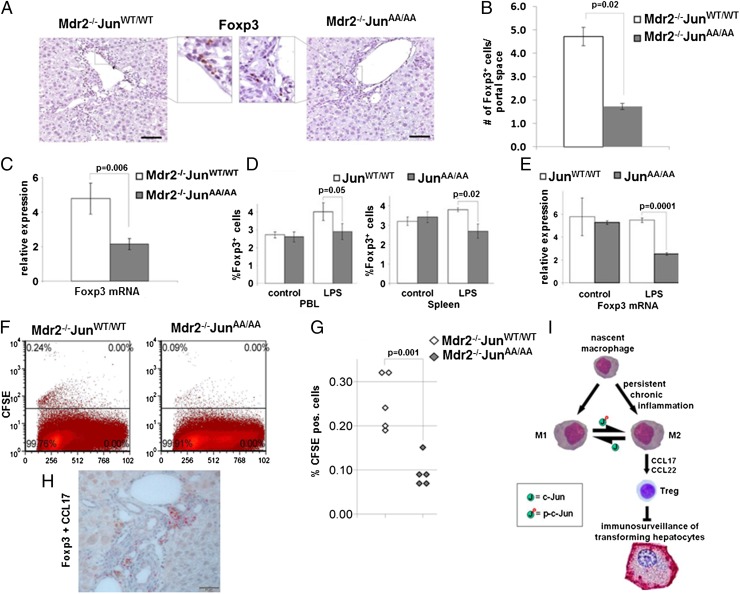
CCL17 and CCL22 chemokines are both chemotactic factors for Treg cells (27). To test whether Mdr2−/−c-JunAA/AA livers harbor fewer Treg cells than controls, we performed immunohistochemical analysis for the Treg marker Foxp3. Quantification using an automated image analysis system revealed a 2.5-fold reduction in the number of Foxp3+ Treg cells in livers of Mdr2−/−c-JunAA/AA mice at 11 mo of age (P = 0.02; Fig. 4 A and B). A similar decrease in total liver Foxp3 mRNA levels was noted (P = 0.006; Fig. 4C). As expected, Mdr2−/−c-JunΔhep mice did not display reduced Treg cell accumulation (Fig. S5), indicating that specific c-Jun deletion in hepatocytes does not affect Treg cell numbers in the liver.
Fig. 4.
JNP facilitates Treg accumulation. (A) Foxp3 immunostains in liver parenchymas of 11-mo-old Mdr2−/−c-JunWT/WT or Mdr2−/−c-JunAA/AA mice. (Scale bars: 100 μm.) Insets show higher magnification of boxed regions. (B) Immunoquantification of Foxp3+ cells, shown in A, by analyzing multiple sections from each sample, representing the entire liver (n = 10, mean ± SEM). (C) qPCR analysis of Foxp3 expression in total liver lysates of 11-mo-old mice (n = 9; mean ± SEM). (D and E) Liposomal LPS-induced chronic inflammation. Results are representative of three experiments. (D) FACS analysis of percentage of CD4+Foxp3+ Treg cells in peripheral blood lymphocytes (PBLs) and spleens (n = 3; mean ± SEM). (E) Foxp3 mRNA levels in splenocytes (n = 3; mean ± SEM). (F and G) Treg cells were isolated from Mdr2−/−c-JunWT/WT mice, labeled with CFSE, and inoculated into 9-mo-old Mdr2−/− mice of the indicated genotype. (F) FACS plots indicating CFSE-positive cells. (G) Percent CFSE-positive cells (n = 5). (H) Coimmunostain for CCL17 (brown) and Foxp3 (red) on liver sections of 11-mo-old Mdr2−/− mice. (Scale bar: 50 μm.) (I) JNP augments tumorigenesis by shaping the inflammatory microenvironment. p-c-Jun, phosphorylated c-Jun.
To study whether JNP is important for Treg cell responses in chronic inflammatory settings in general, we used another model of chronic inflammation characterized by elevated ratios of Treg cells, subjecting mice to liposomal LPS treatment (28). Challenging c-JunWT/WT and c-JunAA/AA mice with liposomal LPS revealed reduced spleen weight in LPS-treated c-JunAA/AA mice (Fig. S6A), without affecting body weight. FACS analysis showed a reduction of Treg cell numbers in the spleens and peripheral blood lymphocytes (PBLs) of c-JunAA/AA mice (Fig. 4D and Fig. S6B). Total Foxp3 mRNA levels in splenocytes corroborated the FACS analysis (Fig. 4E). Thus, in two different chronic inflammation models, Treg accumulation depends on JNP.
It was shown that Foxp3 specifically interacts with the phosphorylated form of c-Jun (29), suggesting that JNP may affect generation of inducible Treg cells. However, we did not reveal differences in the numbers of inducible Treg cells generated from naïve CD4+ CD25− T cells of the two genotypes (Fig. S7). This result led us to test the alternative hypothesis—that reduced production of Treg-recruiting chemokines by macrophages might be the predominant cause for the impaired Treg accumulation in livers of Mdr2−/−c-JunAA/AA mice. To this end, we transferred carboxyfluorescein succinimidyl ester (CFSE)-labeled Treg cells, isolated from spleens of Mdr2−/−c-JunWT/WT mice, to 9-mo-old Mdr2−/−c-JunWT/WT or Mdr2−/−c-JunAA/AA mice. Livers were harvested 48 h after adoptive transfer and dissociated into single cell suspensions, and the percentage of CFSE-labeled cells was analyzed by using FACS. We noted a 2.5-fold reduction in the homing of Treg cells to the livers of Mdr2−/−c-JunAA/AA compared with Mdr2−/−c-JunWT/WT, similar to the magnitude of reduction in Treg cells in these livers (P = 0.001; Fig. 4 F and G). Coimmunostaining for CCL17 and Foxp3 revealed a close spatial relationship between CCL17-expressing macrophages and Treg cells (Fig. 4H and Fig. S8), suggesting that in Mdr2−/− livers Treg cell recruitment is governed by macrophage-derived chemokines.
Discussion
In this work, we used a genetic approach to provide evidence that the JNK/c-Jun signaling pathway is an important molecular mechanism linking chronic inflammation to tumorigenesis by promoting an inflammatory protumorigenic switch. Carcinogen exposure and chronic inflammation are two important underlying conditions for tumor development, the latter accounting for ∼20% of human cancer (30).
The role of the JNK/c-Jun signaling pathway in HCC induced by the carcinogen DEN has been studied by several groups (22, 24, 31, 32). One concept emerging from these studies is that JNK signaling in hepatocytes promotes survival of hepatocytes via abrogating p53-induced apoptosis and up-regulation of c-Myc (22, 24). Interestingly, JNK signaling was shown to play opposing roles in DEN-induced HCC; whereas deletion of JNK1 and JNK2 in both hepatocytes and nonparenchymal cells resulted in reduced tumorigenesis, deletion of these isoforms in hepatocytes alone increased tumorigenesis (20, 31). The JNK1/2 targets that mediate this effect in the nonparenchymal cells were not identified. However, because germ-line c-JunAA mutants do not display reduced hepatocarcinogenesis in the DEN model (22), it is unlikely that c-Jun is the culprit JNK1/2 target in DEN-induced HCC. Indeed, Das et al. implicated the up-regulation of TNF and IL6 by JNK in nonparenchymal cells in facilitating HCC (31).
It is now well appreciated that mouse models of inflammation-induced HCC are significantly different from carcinogen models (20). Tumor-infiltrating inflammatory cells operate in conflicting ways; both tumor-antagonizing and -promoting leukocytes can be found in various proportions in most, if not all, neoplastic lesions (33). Our findings show that JNP shifts the inflammatory microenvironment toward a protumorigenic phenotype; whereas elimination of JNP in all cell types reduced tumor incidence, eliminating c-Jun specifically in hepatocytes had no effect on hepatocarcinogenesis. Pathological examination of human HCC samples revealed JNP in inflammatory cells, whereas malignant hepatocytes were mostly negative, suggesting that also in human HCC JNP may be important in the inflammatory environment. We found that eliminating JNP reduced the number of liver-infiltrating macrophages and changed their phenotype. This finding is most likely a cell-autonomous effect in macrophages, because a similar change was noted in primary peritoneal macrophages. In support of this notion, studies from Glass and coworkers demonstrated that the nuclear receptor corepressor regulates the dynamic range of cultured macrophages’ transcriptional responses to inflammatory signals, in part via modulation of c-Jun activity (34). The nature of the signals that activate JNP in macrophages, to mediate the M1/M2 switch in the chronically inflamed liver, remains to be determined.
The role of immunosurveillance by the adaptive immune system in keeping tumors in check has gained significant support in recent years (10). Treg cells are thought to play a key role in hampering effective antitumor immune responses by inhibiting the activity of cytotoxic T cells (35). The mechanisms that govern Treg cells’ accumulation in tumors include recruitment by chemokines as well as an active conversion of resident T cells into Treg cells (36). Although we could not find evidence that JNP contributes to the conversion of Treg cells in a cell-autonomous way, we found that it plays an important role in the recruitment of Treg cells to the chronically inflamed liver. Furthermore, JNP was necessary for macrophages to assume an M2 phenotype in vitro, including chemokines that are known to be important in Treg recruitment such as CCL17 and CCL22 (Fig. 4I) (27, 35). Together, these results suggest that chronic inflammation switches the macrophage phenotype from antitumorigenic to protumorigenic and that one mechanism affecting protumorigenic activity of M2 macrophages is the up-regulation of Treg-recruiting chemokines, thus hampering tumor immunosurveillance. However, it is possible that other mechanisms could mediate effects of JNP on the premalignant microenvironment.
Dissecting the kinetics of hepatocarcinogenesis and the M1–M2 switch, we revealed that macrophage populations in inflamed livers assumed a protumorigenic phenotype already at 7 mo of age, well before appearance of the earliest tumors. This finding implies that, at least in this model of chronic inflammation-associated HCC, transition to tumor-promoting inflammation precedes the emergence of tumors. Thus, in this case, macrophages are not educated by tumors but, rather, by the chronic inflammatory process that precedes tumorigenesis. Previous studies suggested that immunosurveillance is operative already at the premalignant phases of tumorigenesis (9). If this is indeed the case, any tumor-promoting mechanism that is directed against immunosurveillance is likely to be operative already before tumor initiation, suggesting that immune checkpoint drugs can be effective for tumor prevention.
Methods
Human Liver Tissue.
Human liver biopsy specimens were obtained from resected patients from University Hospital Basel. Biopsy specimens were registered in the University Hospital Basel tissue bank and kept anonymous. The study protocol was approved by the institution ethics committee (no. 310/13) and was in accordance with the Helsinki declaration guidelines. Examination of H&E sections was performed by expert liver pathologists (L.T. and E.P).
Mice.
All animal experiments were performed in accordance with the institutional (Hebrew University of Jerusalem) animal care and use committee (IACUC). All mice were bred (Table S2) and maintained in specific pathogen-free conditions. c-JunAA/AA, c-Junf/f, and c-JunΔhep were back-crossed with Mdr2−/− mice (FVB/N background) for at least seven generations. See SI Methods for more details.
Statistical Analysis.
Results are expressed as means ± SEM. Statistical significance (P < 0.05) was determined by a two-tailed Student t test. Data were processed by using Microsoft Excel.
Supplementary Material
Acknowledgments
We thank Rivka Ben-Sasson, Nina Mayorek, David Knigin, Lipaz Cohen, and Rutie Finkelstein for their assistance. This work was supported by grants from the Israel Science Foundation (ISF) Centers of Excellence (to E.P. and Y.B.-N.); ISF individual research grants (to I.S. and O.P.); European Research Council within the FP-7 Grants 281738 LIVERMICROENV (to E.P.) and 294390 PICHO (to Y.B.-N.); and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. R.E. is supported by the Austrian Science Fund (FWF) DK-plus PhD Program Inflammation and Immunity; FWF Grant P25925-B20; and the Comprehensive Cancer Center Vienna.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1409700111/-/DCSupplemental.
References
- 1.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9(4):259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sica A, Mantovani A. Macrophage plasticity and polarization: In vivo veritas. J Clin Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 4.Budhu A, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10(2):99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Qian BZ, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pikarsky E, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431(7007):461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 7.Greten FR, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118(3):285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Bhowmick NA, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303(5659):848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 9.Finn OJ. Cancer immunology. N Engl J Med. 2008;358(25):2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 10.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 11.Melief CJ, Finn OJ. Cancer immunology. Curr Opin Immunol. 2011;23(2):234–236. doi: 10.1016/j.coi.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103(3):481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dirkx AE, Oude Egbrink MG, Wagstaff J, Griffioen AW. Monocyte/macrophage infiltration in tumors: Modulators of angiogenesis. J Leukoc Biol. 2006;80(6):1183–1196. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- 14.Goswami S, et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65(12):5278–5283. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, et al. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell. 2011;19(4):541–555. doi: 10.1016/j.ccr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 17.Mínguez B, Tovar V, Chiang D, Villanueva A, Llovet JM. Pathogenesis of hepatocellular carcinoma and molecular therapies. Curr Opin Gastroenterol. 2009;25(3):186–194. doi: 10.1097/MOG.0b013e32832962a1. [DOI] [PubMed] [Google Scholar]
- 18.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9(8):537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 19.Tournier C. The 2 Faces of JNK Signaling in Cancer. Genes Cancer. 2013;4(9-10):397–400. doi: 10.1177/1947601913486349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng GS. Conflicting roles of molecules in hepatocarcinogenesis: Paradigm or paradox. Cancer Cell. 2012;21(2):150–154. doi: 10.1016/j.ccr.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Behrens A, Sibilia M, Wagner EF. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet. 1999;21(3):326–329. doi: 10.1038/6854. [DOI] [PubMed] [Google Scholar]
- 22.Eferl R, et al. Liver tumor development. c-Jun antagonizes the proapoptotic activity of p53. Cell. 2003;112(2):181–192. doi: 10.1016/s0092-8674(03)00042-4. [DOI] [PubMed] [Google Scholar]
- 23.Stepniak E, et al. c-Jun/AP-1 controls liver regeneration by repressing p53/p21 and p38 MAPK activity. Genes Dev. 2006;20(16):2306–2314. doi: 10.1101/gad.390506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui L, Zatloukal K, Scheuch H, Stepniak E, Wagner EF. Proliferation of human HCC cells and chemically induced mouse liver cancers requires JNK1-dependent p21 downregulation. J Clin Invest. 2008;118(12):3943–3953. doi: 10.1172/JCI37156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 26.Porta C, et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci USA. 2009;106(35):14978–14983. doi: 10.1073/pnas.0809784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizukami Y, et al. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer. 2008;122(10):2286–2293. doi: 10.1002/ijc.23392. [DOI] [PubMed] [Google Scholar]
- 28.Tarsetti F, et al. Liver carcinogenesis associated with feeding of ethionine in a choline-free diet: Evidence against a role of oval cells in the emergence of hepatocellular carcinoma. Hepatology. 1993;18(3):596–603. [PubMed] [Google Scholar]
- 29.Lee SM, Gao B, Fang D. FoxP3 maintains Treg unresponsiveness by selectively inhibiting the promoter DNA-binding activity of AP-1. Blood. 2008;111(7):3599–3606. doi: 10.1182/blood-2007-09-115014. [DOI] [PubMed] [Google Scholar]
- 30.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das M, Garlick DS, Greiner DL, Davis RJ. The role of JNK in the development of hepatocellular carcinoma. Genes Dev. 2011;25(6):634–645. doi: 10.1101/gad.1989311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakurai T, Maeda S, Chang L, Karin M. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci USA. 2006;103(28):10544–10551. doi: 10.1073/pnas.0603499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Ghisletti S, et al. Cooperative NCoR/SMRT interactions establish a corepressor-based strategy for integration of inflammatory and anti-inflammatory signaling pathways. Genes Dev. 2009;23(6):681–693. doi: 10.1101/gad.1773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127(4):759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 36.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30(5):616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.