The innate immune response is initiated upon recognition of pathogen-associated molecular patterns (PAMPs) by germ-line encoded pattern-recognition receptors (PRRs). PRRs including transmembrane receptors, typified by the Toll-like receptors (TLR) and cytoplasmic receptors, including the RIG-I–like receptors (RLRs), trigger signal transduction cascades culminating in the activation of transcription factors that regulate innate antimicrobial immune responses and induction of adaptive immunity. Members of the NF-κB and IFN regulatory factor (IRF) families of transcription factors are the key targets of PRR ligation and together regulate transcription of a broad array of antimicrobial effectors, including defensins, chemokines, and cytokines that orchestrate the immune response to pathogen invasion. Two articles in PNAS (1, 2) identify a new mechanism of cross-talk between the NF-κB and IRF immune signaling pathways and position the kinase IKKβ as a master regulator of innate immunity.
The mammalian NF-κB family consists of five Rel proteins: p65 (RelA), c-Rel, p52, p50, and RelB (3). In the steady state, NF-κB homo- or heterodimers are sequestered in the cytoplasm through binding to inhibitor of κB (IκB) proteins. When a PRR is engaged by a cognate PAMP, a signaling cascade results in activation of the IκB kinase (IKK) complex (Fig. 1). The IKK complex consists of two kinase subunits, IKKα and IKKβ, and a regulatory subunit, NF-κB essential modulator (NEMO). The dominant pathway triggered by PRR activation is the canonical NF-κB pathway, where IKKβ-dependent phosphorylation of IκBα or IκBβ leads to their ubiquitination and degradation by the proteasome, resulting in nuclear translocation of the bound p65-, c-Rel–, and p50-containing heterodimers. The nuclear NF-κB dimers bind to κB DNA sites and activate a transcriptional program that includes numerous effectors of the innate immune response.
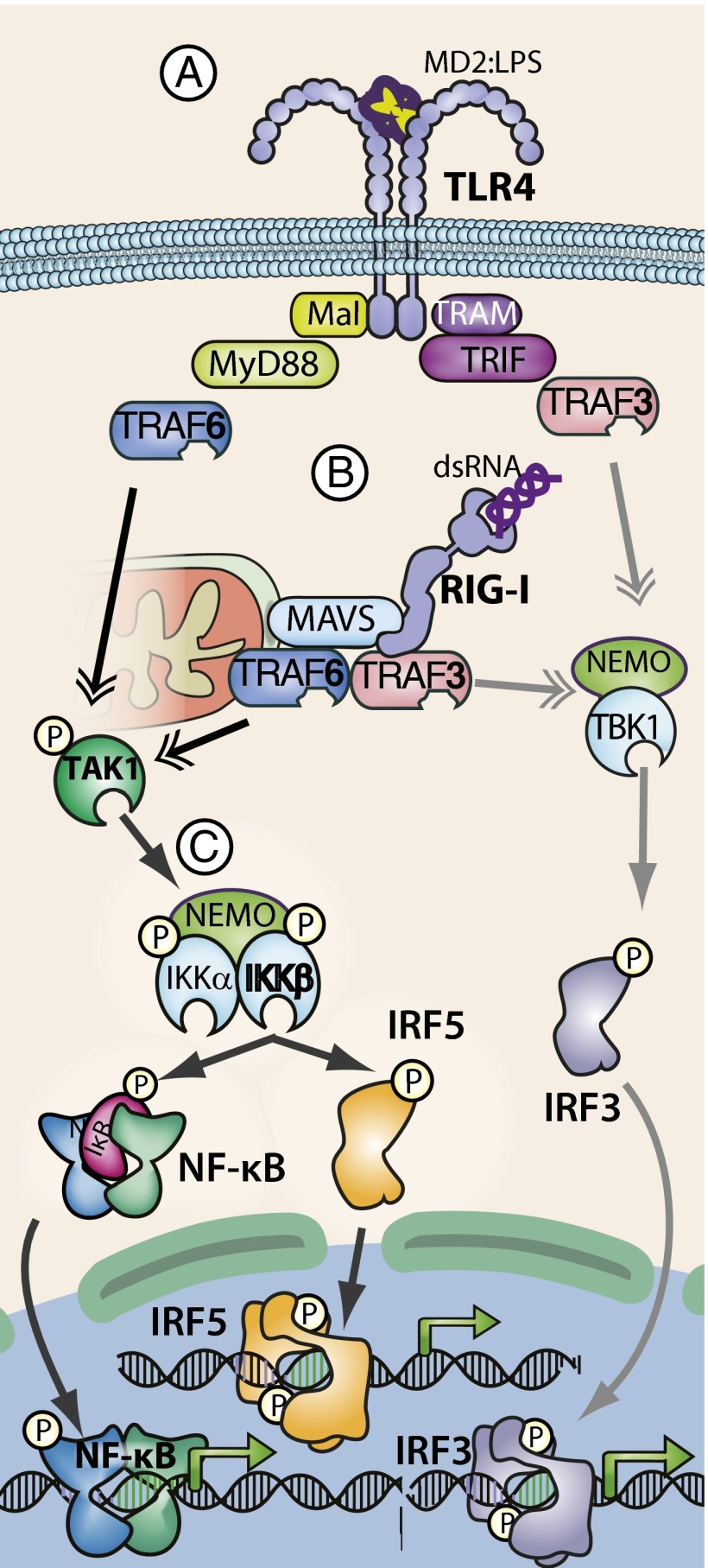
Fig. 1.
TLRs and RLRs activate IRF5 and NF-κB through IKKβ. (A) Upon binding to LPS, TLR4 undergoes dimerization and conformational changes, resulting in the recruitment of intracellular adaptor molecules. Mal/MyD88 (myeloid differentiation primary response gene 88) recruitment leads to TAK1 and IKK complex activation through a TRAF6-dependent mechanism. TRAM (TRIF-related adaptor molecule)/TRIF (TIR domain-containing adapter-inducing IFNβ) adaptor binding to MyD88 leads to TRAF3 and NEMO-dependent activation of TBK1/IKKε, which phosphorylates IRF3, leading to dimerization and IRF3-dependent gene expression. (B) Upon binding to dsRNA, RIG-I binds the mitochondria-associated adaptor protein MAVS. MAVS-dependent recruitment of TRAF6 ultimately results in activation of the TAK1 and IKK complexes. MAVS also induces TRAF3 and NEMO-dependent TBK1/IKKε activation leading the IRF3 activation. (C) TRAF6/TAK1-mediated IKK complex activation downstream of either RLR or TLR signaling results in IKKβ-dependent phosphorylation of IκBα, leading to IκBα ubiquitination and degradation and activation of NF-κB and IRF5, leading to IRF5 dimerization and nuclear localization. Activated IRF3, IRF5, and NF-κB act independently and cooperatively to regulate expression of proinflammatory cytokines, type I IFNs, and antimicrobial effectors.
The mammalian IRF family of transcription factors consists of nine members (IRF1–9) that regulate the expression of a variety of genes, including genes encoding type I IFN (4). IRF3 and IRF7 have been the most widely studied and their mechanisms of activation downstream of PRR engagement and target genes are well characterized. Upon ligation of a TLR or RLR, signaling leads to activation of the IKK-family kinases TANK binding kinase 1 (TBK1) or IKKε (5). TBK1 is the dominant regulator of IRF3 and IRF7 activation in vivo, although there is partial redundancy with IKKε (6–9). TBK1/IKKε phosphorylate IRF3 or IRF7, resulting in a conformational change that promotes homodimerization, nuclear translocation, and expression of target genes.
The work of Ren et al. and Lopez Pelaez et al. implicate IKKβ as a master regulator of innate immunity with a direct role in the activation of IRF5.
IRF5 is also an important regulator of gene expression downstream of PRR signaling, although the contribution of IRF5 to type I IFN expression is unclear. IRF5 is important for TLR-induced expression of proinflammatory cytokines, including TNF, IL-12, and IL-6 (10). However, the kinase responsible for IRF5 phosphorylation and activation had not been identified.
Identification of IKKβ as the IRF5 Kinase
Two independent studies in PNAS (1, 2) identify IKKβ as the IRF5 serine 446 [murine S445, referred to as S462 by Lopez-Pelaez et al. (2)] kinase and a key regulator of IRF5 activity. Using siRNA and pharmacological inhibitors, the Cohen group had previously identified a surprising role for IKKβ in the TLR7-induced production of IFN-β in the human plasmacytoid dendritic cell line, Gen2.2 (11). To understand how IFN-β production was regulated in Gen2.2 cells, Lopez-Pelaez et al. (2) knocked down IRF3, -5, and -7 and found that IRF5 was selectively required for IFN-β, but not IFN-α1, induction. Mass spectrometric analysis showed that IRF5 was phosphorylated upon TLR7 ligation in Gen2.2 cells. Using specific inhibitors of IKKβ and in vitro kinase assays, Lopez-Pelaez et al. found IKKβ directly phosphorylated IRF5 in vitro and was required for IRF5 phosphorylation upon TLR7 ligation (2).
Ren et al. examined the role of IRF5 in innate immune signaling in the human monocytic cell line THP1 (1). Using shRNA, they found IRF5 was required for full induction of TNF, IL-12, and IFN-β by a variety of TLR agonists. Furthermore, overexpression of IRF5 in HEK293 cells promoted TNF and IFN-β production in response to viral infection or viral PAMPs. Because IKKβ overexpression promoted IRF5-enhanced TNF production in this system, Ren et al. directly tested the role of IKKβ in IRF5 activation. IKKβ phosphorylated IRF5 in vitro specifically on serine 445 of murine IRF5, and IKKβ overexpression induced—and IKKβ inhibition prevented—TLR-induced IRF5 dimerization (1). Both groups established the importance of S446 using serine to alanine mutants. S446A IRF5 was defective in nuclear localization upon TLR stimulation (2), failed to undergo IKKβ-induced dimerization, and did not promote expression of IFN-β or IL-12 like its wild-type counterpart (1). Taken together, these data provide convincing evidence that IRF5 is phosphorylated at a single site by IKKβ, an event that is required for dimerization, nuclear localization, and IRF-5–mediated IFN-β and proinflammatory cytokine expression upon TLR or RLR signaling.
PRR Signaling to IRF5
The pathways leading to IKKβ in the context of NF-κB activation downstream of TLR and RLR signaling have been intensely studied (3), and it appears that activation of IRF5 occurs via these established pathways (Fig. 1). TAK1, which is required for IKKβ-dependent NF-κB activation, was also required for IRF5 S446 phosphorylation (2). TRAF6 (TNF receptor-associated factor 6), which acts upstream of TAK1 and the IKK complex, was required for MAVS (mitochondrial antiviral signaling)-induced IRF5 dimerization, as was the IKK complex regulatory subunit NEMO (1). Thus, it appears that the canonical IKK complex is responsible for IRF5 phosphorylation and that upstream components previously shown to be required for IKK complex and canonical NF-κB activation are also likely to be required for activation of IRF5 (Fig. 1).
Implications of IKKβ-Mediated Regulation of IRF5
The work of Ren et al. (1) and Lopez-Pelaez et al. (2) implicate IKKβ as a master regulator of innate immunity with a direct role in the activation of IRF5. Loss of IKKβ has usually been considered synonymous with loss of canonical NF-κB activation. The identification of IRF5 as a bona fide IKKβ target should prompt reinterpretation of some of these studies. This is particularly true given that IRF5 and NF-κB can coordinately regulate many proinflammatory genes (12). It is generally thought that the more severe phenotype exhibited by IKKβ-deficient cells, in comparison with cells lacking canonical NF-κB, is because of partial redundancy between NF-κB subunits. Although it is likely that NF-κB subunits can exhibit some functional redundancy, it is important to consider whether lack of IRF5 activation may also contribute to some of the discrepancies between IKKβ and NF-κB knockouts.
Several questions regarding IRF5 regulation by IKKβ remain to be addressed. For example, although the data presented by Ren et al. (1) and Lopez-Pelaez et al. (2) clearly demonstrate phosphorylation of IRF5 by IKKβ, the activation of IRF5 in IKKβ knockout cells has not yet been examined. Given that IKKα can phosphorylate IRF5 in vitro (2), it will be crucial to examine IRF5 phosphorylation in IKKβ knockout cells. The requirement for NEMO should, likewise, be interrogated in knockout cells. The current studies have also examined a limited number of cell types and stimuli and it will, therefore, be important to determine whether IKKβ is required for IRF5 activation under all circumstances. Future efforts should also examine whether IRF5 is activated by other non-PRR stimuli or in malignancies or other disease states in which IKKβ is constitutively active.
Footnotes
References
- 1.Ren J, Chen X, Chen ZJ. IKKβ is an IRF5 kinase that instigates inflammation. Proc Natl Acad Sci USA. 2014;111:17438–17443. doi: 10.1073/pnas.1418516111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez-Pelaez M, et al. Protein kinase IKKβ-catalyzed phosphorylation of IRF5 at Ser462 induces its dimerization and nuclear translocation in myeloid cells. Proc Natl Acad Sci USA. 2014;111:17432–17437. doi: 10.1073/pnas.1418399111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayden MS, Ghosh S. NF-κB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012;26(3):203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol. 2008;26:535–584. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- 5.Sharma S, et al. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300(5622):1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 6.Perry AK, Chow EK, Goodnough JB, Yeh WC, Cheng G. Differential requirement for TANK-binding kinase-1 in type I interferon responses to Toll-like receptor activation and viral infection. J Exp Med. 2004;199(12):1651–1658. doi: 10.1084/jem.20040528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McWhirter SM, et al. IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc Natl Acad Sci USA. 2004;101(1):233–238. doi: 10.1073/pnas.2237236100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemmi H, et al. The roles of two IkappaB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J Exp Med. 2004;199(12):1641–1650. doi: 10.1084/jem.20040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzgerald KA, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4(5):491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 10.Takaoka A, et al. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434(7030):243–249. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- 11.Pauls E, et al. Essential role for IKKβ in production of type 1 interferons by plasmacytoid dendritic cells. J Biol Chem. 2012;287(23):19216–19228. doi: 10.1074/jbc.M112.345405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saliba DG, et al. IRF5:RelA interaction targets inflammatory genes in macrophages. Cell Reports. 2014;8(5):1308–1317. doi: 10.1016/j.celrep.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]