Significance
How does learning to read affect visual processing? We addressed this issue by scanning adults who could not attend school during childhood and either remained illiterate or acquired partial literacy during adulthood (ex-illiterates). By recording event-related brain responses, we obtained a high-temporal resolution description of how illiterate and literate adults differ in terms of early visual responses. The results show that learning to read dramatically enhances the magnitude, precision, and invariance of early visual coding, within 200 ms of stimulus onset, and also enhances later neural activity. Literacy effects were found not only for the expected category of expertise (letter strings), but also extended to other visual stimuli, confirming the benefits of literacy on early visual processing.
Keywords: reading, brain plasticity, education
Abstract
Learning to read requires the acquisition of an efficient visual procedure for quickly recognizing fine print. Thus, reading practice could induce a perceptual learning effect in early vision. Using functional magnetic resonance imaging (fMRI) in literate and illiterate adults, we previously demonstrated an impact of reading acquisition on both high- and low-level occipitotemporal visual areas, but could not resolve the time course of these effects. To clarify whether literacy affects early vs. late stages of visual processing, we measured event-related potentials to various categories of visual stimuli in healthy adults with variable levels of literacy, including completely illiterate subjects, early-schooled literate subjects, and subjects who learned to read in adulthood (ex-illiterates). The stimuli included written letter strings forming pseudowords, on which literacy is expected to have a major impact, as well as faces, houses, tools, checkerboards, and false fonts. To evaluate the precision with which these stimuli were encoded, we studied repetition effects by presenting the stimuli in pairs composed of repeated, mirrored, or unrelated pictures from the same category. The results indicate that reading ability is correlated with a broad enhancement of early visual processing, including increased repetition suppression, suggesting better exemplar discrimination, and increased mirror discrimination, as early as ∼100–150 ms in the left occipitotemporal region. These effects were found with letter strings and false fonts, but also were partially generalized to other visual categories. Thus, learning to read affects the magnitude, precision, and invariance of early visual processing.
Reading is a cultural activity in which contemporary humans have considerable training. Fluently accessing the sounds and meanings of written words requires very fast and efficient visual recognition of letter strings, at rates exceeding 100 words/min. Neuroimaging studies have begun to show how learning to read modulates the functioning of the visual system, from early retinotopic areas (1, 2) to extrastriate occipital and temporal cortex (1, 3, 4). In particular, a restricted region of the left occipitotemporal cortex, the visual word form area (VWFA), is robustly activated when orthographic stimuli are presented to literate subjects.
This VWFA activation is reproducible across participants and writing systems (5, 6), even when orthographic stimuli are flashed unconsciously (7). Orthographic processing in the VWFA is thought to be very fast, peaking at ∼170–200 ms (8–10), and is colateralized to the dominant hemisphere for language (11, 12). Reading practice enhances activation of the VWFA (1, 13, 14), even in dyslexic children (15). Reading also modulates nonvisual circuits, such as the spoken language network (1, 14, 16, 17).
In addition to these positive effects of learning to read, the theory of neuronal recycling (18) proposes that literacy acquisition also may have a negative “unlearning” effect on the visual system, because it invades cortical territories dedicated to other related functions and shifts their processing mode. In particular, learning to read affects a well-established and advantageous mechanism of the primate visual system for invariant recognition of mirror images (mirror invariance), which allows the prompt recognition of images that are identical up to a left–right inversion (19–21). This mirror invariance mechanism interferes with reading, because a reader needs to distinguish between mirror letters, such as “b” and “d,” to access the correct phonology and semantics of the printed words. Indeed, literacy acquisition is associated with a reduction in mirror invariance (22–24), as well as an enhanced capacity to discriminate mirror images (25).
During literacy acquisition, many children initially find it difficult to discriminate mirror letters, but top-down inputs from phonologic, speech production, and motor areas coding for handwriting gestures may carry discriminative information that ultimately help the visual system to “break its symmetry” (26). Brain imaging and transcranial magnetic stimulation (TMS) studies have shown that in good readers, automatic mirror discrimination is present for letters and words at the VWFA site but is not detected for pictures of objects (27–29), although a small mirror generalization cost can be detected for pictures of faces, houses, or tools using a sensitive same–different behavioral task (23).
The main goal of the present work was to assess the influence of the acquisition of reading ability on the successive stages of visual processing, and to evaluate to what extent early visual processing (<200 ms) is already affected. In our previous work (1), we used functional magnetic resonance imaging (fMRI) to demonstrate the profound influence of reading acquisition on the visual system. By scanning a large group of adult subjects with different literacy levels, we detected a modulation of visual activation as a function of reading ability not only in the VWFA, but also in extrastriate and striate cortex, suggesting an effect on early vision; however, given the low temporal resolution of fMRI, the time course of these effects remained unknown. Literacy acquisition may affect early feedforward processing in the visual cortex, perhaps even including area V1, much like other forms of perceptual learning (30–32); however, it is also possible that the fMRI-detected effects of literacy are related to late top-down interactions with language areas (33).
We determined the timing of literacy effects in literate and illiterate adults by recording event-related potentials (ERPs) from essentially the same sample of participants as in our previous fMRI study and with an identical visual paradigm. To the best of our knowledge, this is the first ERP investigation on the impact of reading on visual system function that includes fully illiterate adults. As in our previous study, we also included “ex-illiterate” subjects, who learned to read in adulthood and achieved variable levels of reading fluency. We obtained valid ERP data from 24 literate, 16 ex-illiterate, and 9 illiterate adult subjects.
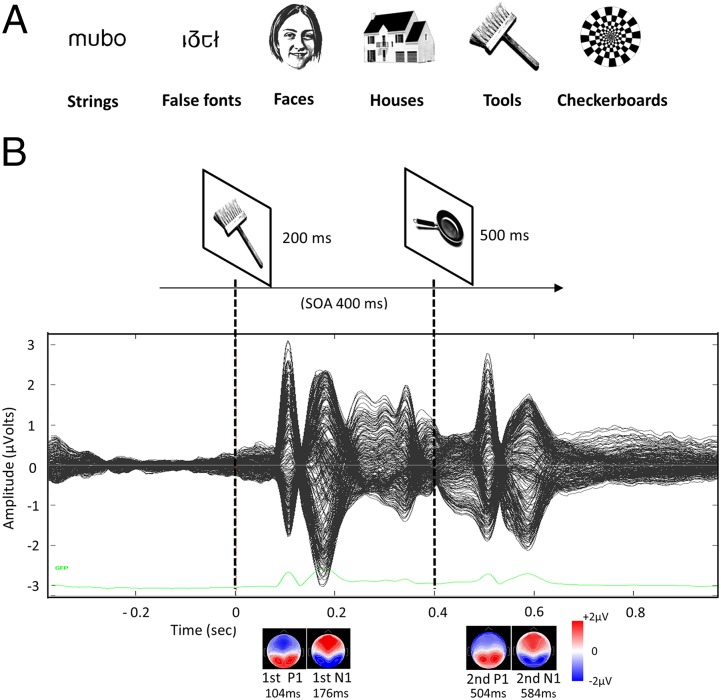
Our visual paradigm consisted of the sequential visual presentation, in separate blocks, of pairs of stimuli from six different categories: strings (pseudowords), false fonts, faces, houses, tools, and checkerboards (Fig. 1 and Methods). The stimuli in each pair were identical, mirror image, or different stimuli from the same category, which allowed us to measure identity and mirror repetition priming. The subjects were simply asked to pay attention and to press a button whenever an odd target picture (a black star) appeared, thereby precluding differences in performance and strategies among subjects of differing literacy levels. Using regression, we evaluated the precise moment at which evoked responses were modulated by reading ability.
Fig. 1.
Stimuli and procedure. (A) Examples of visual categories used in the experiment. (B) Schematic representation of the experimental design. (Top) After a fixation cross, two successive stimuli within the same category were displayed with a 400-ms stimulus-onset asynchrony (SOA). The pairs could be exactly the same, a mirror version of each other or different exemplars (as above). (Middle) ERPs averaged across subjects and conditions. The GFP time course is plotted in green in the lower part of the figure. (Bottom) Scalp maps showing the topographic distribution of P1s and N1s evoked by the first and second stimuli, respectively.
Results
Behavioral performance was highly accurate, with a mean of 96.5% correct detection (95.2% for illiterates, 96.5% for ex-illiterates, and 97.1% for literates) and no significant differences among the groups (F2,43 <1). The less-educated subjects exhibited only slightly slower responses (mean response time: illiterates, 481 ± 34 ms; ex-illiterates, 493 ± 53 ms; literates, 444 ± 58 ms; F2,43= 4.1, P = 0.02), likely owing to lack of familiarity with the time-constrained test situation. Overall, the high accuracy and prompt responses indicate that subjects from all groups were highly attentive during the visual presentation of stimuli; thus, further differences in evoked brain responses are not likely related to differences in attention or task comprehension.
Literacy Enhances Electrophysiological Responses at the Post-P1 Stage of Visual Processing.
We performed a systematic analysis of early ERP components (P1 and N1) in peak and postpeak time windows by averaging electrode voltages from their classical scalp presentations in occipital and occipitotemporal regions, respectively (10 electrodes for each hemisphere and region), over 40-ms intervals. For P1, the window was centered at the P1 peak latency evoked by the first stimulus (S1) in occipital electrodes, which was 104 ms when all subjects and categories were collapsed and averaged together (Fig. 1). Regions of interest (ROIs) are shown in Fig. 2 (occipital) and Fig. 3 (occipitotemporal). To check for possible literacy effects on electrophysiological responses, we regressed the P1 average voltages against the subject’s reading fluency scores (defined as the number of words and pseudowords that could be read in 1 min; see fig. 1 in ref. 1). No significant correlations were found for P1. To confirm the absence of an influence of reading ability on visual responses at the P1 stage, we then performed ANOVA, taking the category of stimuli and hemisphere as within-subject factors and reading ability (estimated through reading fluency scores) as a real-valued between-subject factor, using as the dependent measure the voltage averaged over left and right occipital electrode clusters. We found no main effect of reading ability for the P1 component (F1,47 <1), as well as no significant interaction with category (F5,35 <1) or hemisphere (F1,47 <1).
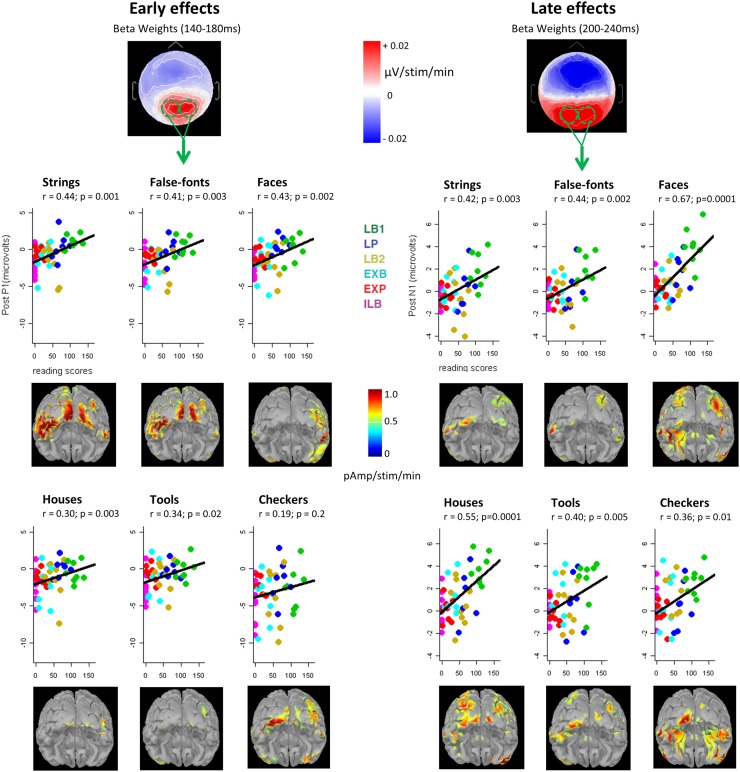
Fig. 2.
Early and late effects of reading ability on electrophysiological responses to visual stimuli. (Left) Early effects of literacy in the post-P1 time window (140–180 ms). The topographic map shows the beta weights of the correlation between scalp voltages and the reading scores of the participants (number of words and pseudowords read per minute), across all categories (in mV/number of additional stimuli read per minute). For each category, voltages from the two occipital clusters collapsed (green lines) are plotted against the subject’s reading scores. (Right) Late effects of literacy in the post-N1 time window (200–240 ms). (Insets) Posterior views of the brain showing the cortical sources of these early and late literacy effects obtained by correlating reading scores with the reconstructed activation at each vertex, at a time point corresponding to the center of the time window (160 and 220 ms, respectively).
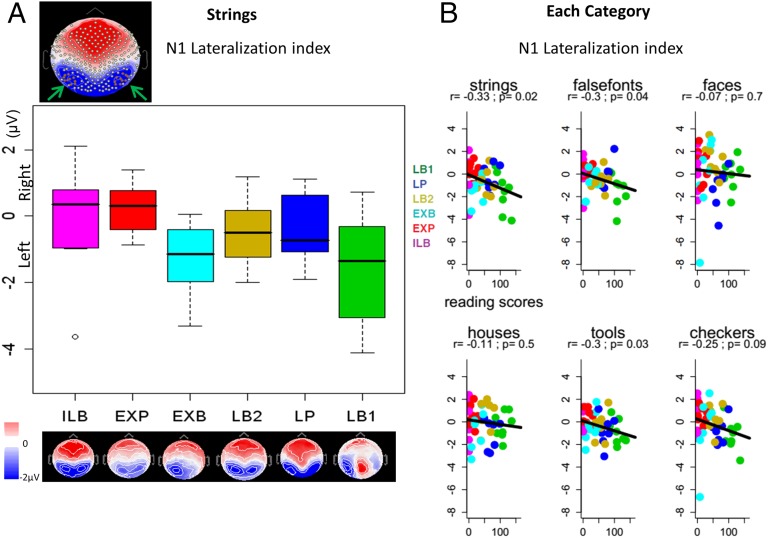
Fig. 3.
Impact of reading ability on the lateralization of N1. (A) Scalp map of the N1 topography at 176 ms after the stimulus in the grand average (all subjects and conditions collapsed). Arrows indicate the symmetric occipitotemporal clusters selected (10 eletrodes for each hemisphere; in red). The boxplots represent the voltages from these two clusters on the left hemisphere minus those from the right hemisphere, calculated for each subject from the activation evoked by letter strings, across a 40-ms window centered on the N1 peak (i.e., left lateralization index of N1). Scalp maps on the N1 peak are plotted for each of the six subgroups of participants with increasing levels of reading ability, from illiterates (ILB) on the left to ex-illiterates (EXP and EXB) in the middle to literates (LB2, LP, and LB1) on the right. (B) Correlation of the N1 lateralization index with the participant’s reading scores for each category.
We then applied the same analysis to a slightly later time window, at the post-P1 stage (140–180 ms). We observed significant positive correlations of voltage amplitudes and reading scores over left and right occipital channels (Fig. 2, Left). Separate regressions for each category confirmed an effect of literacy on posterior visual responses, not only for letter strings as expected, but also for false fonts and all other categories except checkerboards (Fig. 2, Left). ANOVA on these post-P1 voltages revealed a main effect of reading ability (F1,47 = 8.7, P < 0.005), as well as a reading ability × hemisphere × category interaction (F5,235 = 2.7, P < 0.03). Restricting the analysis to each category, we found a main effect of reading ability for all except checkerboards (strings: F1,47 = 11.5, P < 0.002; false fonts: F1,47 = 9.7, P < 0.005; faces: F1,47 = 10.7, P < 0.003; houses: F1,47 = 4.7, P < 0.05; tools F1,47 = 6.0, P < 0.02), confirming a positive and generalized enhancement of early visual responses as a function of reading skill. Moreover, strings was the sole category exhibiting an overall hemisphere effect (F1,47 = 4.1, P < 0.05), with more positive values on the right hemisphere of the scalp. Together with the absence of a significant reading ability × hemisphere interaction for this category, this result suggests that letter strings are processed spontaneously more over the right hemisphere than over the left hemisphere at this early post-P1 stage, as reported previously (7, 34–36), independent of the literacy factor.
To evaluate the impact of early vs. late literacy, we performed ANOVA on post-P1 scalp voltages with a three-level factor of literacy status (i.e., the group to which subjects belonged: literate, ex-illiterate, and illiterate) on the same post-P1 time window, first collapsing all categories together. Literates had higher positive ERPs than illiterates (F1,31 = 7.3, P = 0.01). Ex-illiterates fell in between and did not differ significantly from either literates (F1,38 = 1.8, P = 0.19) or illiterates (F1,23 = 2.6, P = 0.12) . When the analysis was restricted to strings, however, literates exhibited more positive ERPs than illiterates (F1,31 = 6.1, P < 0.02), but not more than ex-illiterates (F1,38 = 1.5, P = 0.24), whereas the difference between ex-illiterates and illiterates barely achieved significance (t1,23 = 1.84; one-tailed P = 0.04). These results suggest that early schooling is not necessary for the early visual effect to emerge, given that it is also seen in unschooled ex-illiterates. A similar difference between ex-illiterates and illiterates was found in the right occipital cortex for all stimuli in our earlier fMRI study (table S2 in ref. 1).
We examined the reproducibility of these results at the time of the second stimuli (S2) on the equivalent post-P1 window (i.e., 140–180 ms post-S2). This complementary analysis confirmed the main effects of reading ability (F1,47 = 4.5, P < 0.04) and of category (F1,47 = 23.3, P < 0.001), but the triple interaction (reading ability × category × hemisphere) became only marginally significant (F1,47 = 2.2, P = 0.056). This reduction in the literacy effect for S2 likely can be explained by the influence of repetition suppression effects (see below).
To confirm the literacy effects on the early ERP components without any predetermined groups of electrodes or temporal windows of interest, we used Fieldtrip software to perform a data-driven permutation-based cluster analysis (SI Methods). This analysis searched for any group of electrodes and consecutive time samples in which a significant correlation between single subject ERPs and reading fluency scores was found. This complementary search fully confirmed our results (Fig. S1); early occipitotemporal effects were found for all categories of stimuli, but these effects never achieved significance before 128 ms after onset.
Although all of the foregoing analyses were performed at the sensor level, we also attempted to estimate the cortical sources of the early-stage effect of literacy by correlating reading scores with the intensity of cortical source activation for each visual category (Methods and Fig. 2). This analysis pointed to classical cortical regions of the ventral occipitotemporal stream of both hemispheres, including bilateral occipital and left ventral occipitotemporal cortex for strings and false fonts, and to a right fusiform region for faces (Fig. 2), compatible with previous fMRI findings (1).
In summary, we found enhanced visual responses in direct proportion to the reading fluency of participants not on the earliest measurable response (P1), but at a slightly later stage of visual processing (140–180 ms). These effects were present not only for the familiar object of visual expertise (i.e., letter strings) or for physically similar stimuli (false fonts), but also for a large set of visual categories.
Literacy Induces Left-Hemispheric Lateralization of the N1.
Previous studies identified the amplitude and left-lateralization of the N1 (∼170 ms) as major correlates of literacy acquisition (13, 37). We analyzed the N1 component of the ERPs by averaging the voltages of occipitotemporal clusters (10 electrodes in each hemisphere; Fig. 3A, Inset) across a 40-ms window centered at the N1 peak evoked by S1 (occurring at 176 ms when all subjects and categories were collapsed), using the same ANOVA procedure as described previously. We found a reading ability × category interaction (F5,235 = 2.6, P = 0.027) driven by checkerboards, the only category for which the N1 exhibited a positive correlation with reading scores (r = 0.28, P = 0.005) when both hemispheres were collapsed. This may be related to the fact that checkerboards exhibited an earlier N1 peak than the other categories (164 ms vs. 172–188 ms); as we report below, other categories showed a strong influence of reading ability on a post-N1 period.
The reading ability × category × hemisphere interaction was not significant (F5,235 <1), but a category × hemisphere interaction was observed (F5,235 = 4.5, P < 0.001), indicating that the lateralization of N1 varied across categories. Significant left-lateralization was found for strings (left hemisphere, −2.09 µV; right hemisphere, −1.49 µV; P = 0.003) and false fonts (left hemisphere, −2.34 µV; right hemisphere, −1.98 µV; P = 0.03), and approached significance for tools (left hemisphere, −1.61 µV; right hemisphere, −1.30 µV; P = 0.051), but not for faces (left hemisphere, −4.31 µV; right hemisphere, −4.53 µV; P = 0.45), houses (left hemisphere, −2.27 µV; right hemisphere, −2.26 µV; P = 0.98), or checkerboards (left hemisphere, −2.71 µV; right hemisphere, −2.50 µV; P = 0.34). Restricting the analysis to each category, we observed a reading ability × hemisphere interaction for letter strings (F1,47 = 5.9, P < 0.02), indicating that reading increases the left-lateralization of electrophysiological responses for this category. The same effect was found for false fonts (reading ability × hemisphere interaction, F1,47 = 4.6, P = 0.04) and tools (F1,47 = 4.7, P = 0.03), but not for faces, houses, or checkerboards.
To confirm these effects, we calculated for each subject a left lateralization index (LLI) by subtracting the averaged voltage value of the occipitotemporal cluster in the right hemisphere from that in the left hemisphere, and regressing it against the subject’s reading scores (Fig. 3). A negative correlation with LLI (r = −0.33, P = 0.02) confirmed that reading fluency is correlated with a left-lateralization of the N1 evoked by letter strings. For literates, higher negatives amplitudes of N1 in response to strings were found in the left hemisphere (LLI, −0.49 µV; P = 0.006). Ex-illiterates showed a marginally significant left-lateralization (LLI, −0.04 µV; P = 0.11), whereas illiterates exhibited a nonsignificant right-lateralization of N1 for strings (LLI, 0.22 µV; P > 0.9). The between-group differences did not reach statistical significance, however, owing to high individual variability within literacy groups. Only the regression approach across 49 subjects, capturing intragroup differences in reading ability, was sufficiently sensitive to reveal hemispheric lateralization effects (Fig. 3B).
We applied the same procedure for N1 scalp voltages evoked by the S2 stimulus. We again found a reading ability × hemisphere interaction (F1,47 = 9.8, P < 0.005), as well as the category × hemisphere interaction (F5,235 = 3.1, P < 0.01; strings: left hemisphere, −1.30 µV, right hemisphere, −1.03 µV, P = 0.10; false fonts: left hemisphere, −1.39 µV, right hemisphere, −1.29 µV, P = 0.61; faces: left hemisphere, −2.45 µV, right hemisphere, −2.80 µV, P = 0.09; houses: left hemisphere, −1.15 µV, right hemisphere, −1.12 µV, P = 0.88 ; tools: left hemisphere, −0.99 µV, right hemisphere, −0.88 µV, P = 0.47; checkerboards: left hemisphere, −2.38 µV, right hemisphere, −2.43 µV, P = 0.81). Significant effects of reading ability on the LLI were found for strings (P = 0.002), false fonts (P = 0.002), and houses (P = 0.036), but not for faces (P = 0.056), checkerboards (P = 0.067), or tools (P = 0.27).
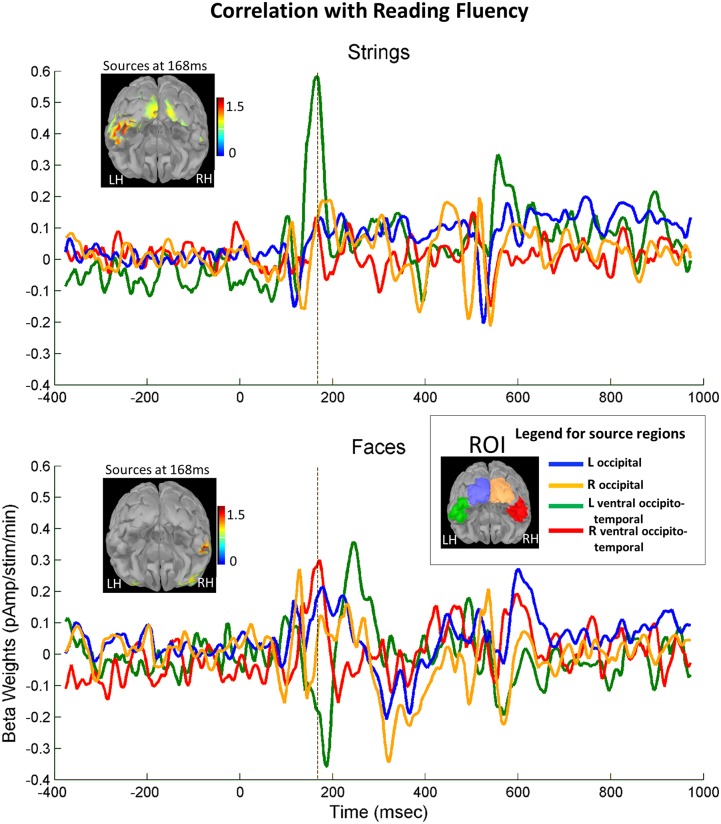
Source reconstruction analysis suggested that reading ability modulates cortical activation to letter strings at the N1 stage, primarily in the left occipitotemporal cortex, at or near the VWFA site, whereas for faces, sources correlated with the literacy score were located in the right occipitotemporal cortex, overlapping the classical fusiform face area (FFA) site (Fig. 4). We plotted the time course of activation and its correlation with literacy in four a priori ROIs covering low-level (occipital) and high-level (occipitotemporal) ventral visual areas in each hemisphere. Activation peaked in the occipitotemporal cortex at ∼170 ms for both strings and faces. Furthermore, for letter strings, source amplitudes at t = 168 ms were positively modulated by reading score in the left occipitotemporal cortex (r = 0.56, P < 0.0001), but not in its right counterpart (r = 0.10, P > 0.4), resulting in a growing leftward asymmetry of responses to strings as a function of literacy (left minus right: r = 0.38, P = 0.009). In contrast, for faces, right occipitotemporal responses were only marginally amplified by literacy (r = 0.23, one-tailed P = 0.059), whereas left occipitotemporal responses exhibited reduced activation, especially just after the peak, that is, 188 ms (r = −0.25, one-tailed P = 0.045). This led to a trend toward a growing right lateralization of face responses with reading score (left minus right calculated on the peak, i.e., 168 ms: r = −0.28, one-tailed P = 0.028). Thus, these results suggest that literacy induces a spatial and temporal focalization of face responses to an increasingly narrow and right-lateralized fusiform peak at 170 ms. They agree with previous fMRI findings of an affect of literacy on the left lateralization of word responses and the right-lateralization of face responses in adults and children (1, 38).
Fig. 4.
Effect of reading ability on the lateralization of occipitotemporal responses at the N1 stage (source analysis). Time course of reconstructed source activity associated with literacy (beta weights of the correlation between source activity and reading scores) for four ROIs of equivalent size (230 vertices) based on individual source reconstruction: (1) left occipital (blue), (2) right occipital (orange), (3) left ventral occipitotemporal (green), and (4) right ventral occipitotemporal (red), separately for strings and faces. (Insets) Estimated cortical activity at 168 ms. For letter strings, a strong peak of literacy-related activity is found at 168ms (N1 stage) in the left ventral occipitotemporal cortex (in green). For faces, a smaller peak of activity occurs in the right occipitotemporal cortex (in red), in parallel with a reduction of activity on the left side, followed by a later enhancement (∼250 ms).
Literacy Enhances Late-Stage Visual Processing.
Does learning to read also influence the propagation of brain responses to later stages of visual processing? To answer this question, we analyzed voltages on a later time window, just after the N1 (200–240 ms after the onset of S1, herein termed post-N1). When performing a regression of ERPs against literacy scores for each electrode, we observed a major enhancement in amplitudes as a function of reading skill on posterior regions (Fig. 2, Right). ANOVA confirmed that occipital voltages were strongly influenced by the literacy factor (F1,47 = 19.1, P < 0.0001). Moreover, a reading ability × category interaction (F5,235 = 4.5, P < 0.001) was also seen for the post-N1 stage.
Restricting the analysis to each category, we found a significant effect of reading ability for each (strings: F1,47 = 10.0, P < 0.005; false fonts: F1,47 = 11.1, P < 0.005; faces: F1,47 = 38.1, P < 0.001; houses: F1,47 = 21.0, P < 0.001; tools: F1,47 = 8.7, P < 0.005; checkerboards: F1,47 = 7.0, P = 0.01). In addition, regression analysis confirmed the highly positive correlations between ERP voltages and reading scores for each visual category (P < 0.01 for all; Fig. 2, Right). The reading ability × category interaction arose in part because faces, which typically evoke higher visual responses than other categories, exhibited greater enhancements than those found for strings, for instance (P < 0.001).
Searching for group differences, we found a main literacy group effect (F2,46 = 3.3, P < 0.05), but nonsignificant differences between literates (mean, 0.43 ± 2.21 µV) and illiterates (mean, −0.49 ± 1.07 µV; P = 0.22) or between literates and ex-illiterates (mean, −0.18 ± 1.2 µV; P = 0.46), owing to high variability within each group. Indeed, restricting the analysis to the literate group still demonstrated a significant effect of reading scores (P = 0.007), suggesting that the actual proficiency level strongly influences late brain responses, over and above any group effect.Cluster analysis confirmed the substantial enhancement of posterior visual responses in relation to subjects’ reading level, especially in this later time window (Fig. S1).
The analysis for S2 in the same window confirmed a main effect of reading ability (F1,47 = 8.9, P < 0.005), but the interaction with category was no longer significant (F5,235 = 1.9, P = 0.09). Regression analysis for S2 showed that reading ability affected the responses to letter strings (r = 0.35, P = 0.01), false fonts (r = 0.30, P = 0.04), faces (r = 0.57, P < 0.0001), and tools (r = 0.30, P = 0.04) and approached significance for checkerboards (r = 0.23, P = 0.1) and houses (r = 0.21, P = 0.1).
In conclusion, our data indicate that reading practice considerably increases electrophysiological responses at a later stage of visual processing (post-N1, 200–240 ms) in a generalized manner for all categories of visual stimuli.
Literacy Improves Exemplar Discrimination over the Left Occipitotemporal Region.
Our experiment was designed to examine repetition suppression and test whether reading expertise enhances the discriminability of visual objects. Repetition suppression was evaluated by subtracting evoked responses to pairs of “different” minus “identical” trials; note that S1 and S2 always belonged to the same category. We found that when averaging across all subjects and conditions, this difference affected the P1 and post-P1 ERPs evoked by S2 (Fig. S2). Furthermore, by regressing these differential voltages for each electrode against the subjects’ reading scores, we identified positive correlations for the left occipitotemporal region in this early time window, that is, 100–148 ms after onset of S2 (Fig. 5A). ANOVA on these differential voltages from left and right occipitotemporal clusters showed a main effect of reading ability (F1,47 = 6.17, P < 0.02) and a highly significant interaction with hemisphere (F1,47 = 17.0, P < 0.0005) in this early window. In fact, only the left hemisphere (F1,47 = 17.8, P < 0.0002), and not the right hemisphere (F1,47 <1), exhibited an increased repetition suppression effect as a function of reading score. Note that repetition suppression itself was present in both the left hemisphere and the right hemisphere (P < 0.0001 for each); however, this effect was modulated by reading ability only in the left hemisphere.
Fig. 5.
Effects of repetition priming as a function of reading ability. For each subject and category, repetition suppression (i.e., different pairs minus identical pairs trials) (A) and mirror repetition priming (i.e., mirror pairs minus identical pairs trials) (B) effects were calculated on the average voltages of a left occipitotemporal cluster of 10 electrodes (green dashed line) in the 100- to 148-ms interval after the second stimuli. Then a regression against reading scores was performed for each category. In each case, the scalp map shows the beta weights of the correlation between repetition suppression and reading scores for all categories collapsed. Below each category, source reconstructions show the correlation with reading score calculated for each vertex at the peak of this interval (i.e., 124 ms). r, Pearson’s correlation coefficient. A positive correlation indicates that as reading fluency increases, the capacity to discriminate between two unrelated items improves (A), as does the capacity to discriminate an item from its mirror image (B). Improvements are seen for letter strings, as well as for faces and, to a lesser degree, false fonts and houses.
Regression analysis confirmed that the left occipitotemporal repetition suppression effect was positively modulated by reading ability for all categories except tools (Fig. 5). No such effect was found in the right hemisphere for any category (P > 0.5 for all). Source reconstruction of this effect pointed to activity in different posterior cortical sectors as a function of category (Fig. 5A).
Analysis based on literacy groups instead of reading scores revealed an interaction of group with hemisphere for the repetition suppression effect (F2,46 = 13.0, P < 0.0001). We reconfirmed that the literacy status effect (group) was present only on the left side of the scalp (left: F2,46 = 7.0, P < 0.005; right: F2,46 = 2.1, P = 0.13). When the analysis was restricted to the left hemisphere, literates exhibited a higher level of repetition suppression than illiterates (mean, 1.30 µV vs. 0.09 µV; F1,31 = 10.0, P < 0.005) or ex-illiterates (mean, 1.30 µV vs. 0.65 µV; F1,38 = 5.2, P = 0.03). In a comparison of ex-illiterates and illiterates, the difference barely reached statistical significance (t1,23 = 1.92; one-tailed P = 0.034), again suggesting that even unschooled adults may enjoy some of the benefits of literacy.
In conclusion, our data indicate that reading acquisition leads to an enhancement of repetition suppression, indicating improved visual discrimination of exemplars within the same category. This effect occurred over the left occipitotemporal region at an early stage of visual processing (100–150 ms).
Literacy Affects Mirror Invariance in the Left Occipitotemporal Region.
To test the influence of literacy on mirror invariance, we subtracted the evoked potential values for mirror minus identical pair trials, thus yielding an index of mirror discrimination. If the illiterate visual system treated mirror images as identical (23, 25), then this contrast should be null in illiterates and should increase with literacy.
Again, for the same early time window as before (100–148 ms post-S2), we found an influence of reading level on the ERP responses to mirror minus identical pairs over the left occipitotemporal region (Fig. 5, Bottom). ANOVA of occipitotemporal clusters showed a main effect of reading ability (F1,47 = 11.2, P < 0.002) and an interaction with hemisphere (F1,47 = 4.3, P < 0.005). Again, only the left hemisphere was influenced by reading ability (left hemisphere: F1,47 = 13.8, P < 0.001; right hemisphere: F1,47 <1). Positive correlations indicated that reading acquisition improved mirror discrimination. Correlations were significant for strings, false fonts, faces, and houses, but not for tools (Fig. 5). Later time windows (centered at 200, 300, 400, and 500 ms), in which other researchers have observed mirror priming in literate adults (39), exhibited no literacy effects on mirror discrimination in our data.
Comparing the literacy groups, we again noted an interaction of literacy group and hemisphere (F2,46 = 5.5, P = 0.007). Only the left hemisphere responses were modulated by literacy group (left hemisphere: F2,46 = 7.3, P = 0.002; right hemisphere: F2,46 = 1.8, P = 0.17). In the left occipitotemporal region, literates exhibited higher mirror discrimination than illiterates (mean, 1.1 µV vs. −0.02 µV; F1,31 = 8.6, P = 0.007) and ex-illiterates (mean, 1.1 µV vs. 0.3 µV; F1,38 = 7.9, P = 0.008). Ex-illiterates did not differ significantly from illiterates (F1,23 = 1.8, P = 0.20).
Finally, we analyzed the electrophysiological responses to the subtraction of different minus mirror trials. This contrast could be provide information on mirror discrimination, considering that the illiterate brain might be expected to treat the mirror stimuli as more similar than the genuinely different stimuli, and this difference would diminish with literacy. We found no correlation with reading ability, however, even when we restricted our analysis to separate hemispheres or categories. The previous analyses suggest that this effect was not significant because reading acquisition jointly improved the capacity to discriminate both mirror and different stimuli.
In summary, our data indicate that reading enhances mirror discrimination (mirror-identical trials) over the left occipitotemporal region at quite an early stage (∼100–150 ms post-S2), not only for strings, but also for almost all of the visual categories.
Discussion
In the present work, we provide evidence that learning to read has a substantial impact on several stages of visual processing. Our study reveals that the ability to read correlates with (i) enhanced early visual responses in the post-P1 time window (∼140–180 ms); (ii) left lateralization of visual processing at the N1 stage over the occipitotemporal region for letter strings and false fonts; (iii) increased activation in the ventral occipitotemporal cortex, left-lateralized for strings and a trend toward right lateralization for faces; (iv) greater repetition suppression (suggesting better exemplar discrimination) in the left occipitotemporal region (∼100–150 ms); (v) increased mirror discrimination at the same time and location; and (vi) enhanced visual responses to all stimuli in a later phase (∼200–240 ms). These literacy effects were found for letter strings and frequently extended to stimuli outside the reading domain (except for the N1 left lateralization for faces).
Correspondence Between fMRI and ERP Findings.
Our present results confirm and extend the findings of our previous fMRI study (1) in which essentially the same subjects were examined with an identical visual paradigm. fMRI revealed that literacy increased the VWFA response to letter strings. Similarly, in the present study, three literacy effects were found in the same left occipitotemporal region: (i) enhanced responses to letter strings (at ∼140–180 ms); (ii) higher levels of repetition suppression, suggesting improved exemplar discrimination; and (iii) enhanced mirror discrimination, at an early stage of visual processing (at ∼100–150 ms). All three effects concur with the findings of several previous neuroimaging studies. The N150 ERP component improved after dyslexics practiced with grapheme–phoneme correspondence (15). Enhanced fMRI activity in the left ventral occipitotemporal cortex was observed after short-term training with a new orthographic system in normal adult readers (14). An efficient capacity for word discrimination was also noted in adult readers at the same site, using both subliminal fMRI repetition priming (7, 40) and multivariate decoding (41). Finally, reduced mirror invariance, specific to the left occipitotemporal cortex, was found with both letters (27) and words (28).
Relative to these earlier findings, the present ERP study provides two important advances: (i) the direct demonstration, through comparison with a rare group of completely illiterate adults, that these effects do indeed arise through the acquisition of literacy, and (ii) an accurate timing of their influence on brain activity, suggesting that they all occur shortly after stimulus onset (∼100–180 ms). In other words, increases in the magnitude, precision, and mirror discrimination of the left occipitotemporal activity all occur at early stages of the visual response, during which automatic processes predominate.
Lateralization of N1.
The impact of literacy on the left hemispheric asymmetry for visual processing of letter strings, previously detected on fMRI as a strong left-lateralized response of the VWFA in the vast majority of right-handed adult readers (12) that increases with literacy (1), was replicated in the present study with ERP measurements. Here this lateralization effect was found at the N1 stage (∼170 ms); whereas illiterates exhibited a tendency to process strings in the right hemisphere, ex-illiterates were slightly more prone to process them in the left hemisphere, and literates exhibited a clear left lateralization.
Left lateralization at the N1 stage was previously reported for familiar orthographic stimuli, and also was found to increase with reading acquisition (13, 37). Nonetheless, although previous findings suggested that this N1 stage effect might not generalize to an unfamiliar and visually distinct script, such as Japanese (37), we observed a partial generalization to other categories, such as false fonts (but not faces).
Competition Between Words and Faces.
We had previously observed that literacy increases the VWFA activation to letter strings and words, but also slightly reduces the fMRI-detected responses to faces in the left hemisphere and strongly increases these responses in the right hemisphere (1). Here we observed again a left lateralization for pseudowords and a nonsignificant trend to right lateralization for faces in proportion to the subjects’ reading ability (Figs. 3 and 4). Several previous studies also reported a left lateralization of visual responses to words and a right hemispheric shift of responses to faces subsequent to reading acquisition (1, 38, 42–45). This pattern of results is suggestive of a competition between words and faces for cortical territory in high-level visual areas. It is compatible with the neuronal recycling hypothesis (18), according to which cultural acquisitions, such as reading, invade cortical territories previously dedicated to other functions, thereby leading to displaced cortical specialization.
Learning to read may force the visual system to dedicate a specific cortical territory to letter recognition, at a specific site (the VWFA) defined in part by its connectivity to language areas (11, 12, 46). Thus, greater anatomic proximity to language areas in the left hemisphere may “attract” reading-related visual responses to the left ventral occipitotemporal cortex and, as a consequence, “push” face processing toward the right hemisphere. This effect may be compounded by additional intrinsic hemispheric properties, such as a right hemispheric bias for low spatial frequencies (47).
Processing Stages Affected by Literacy Acquisition.
Our earlier observation that literacy affects fMRI activation in visual areas (1) remains open to two distinct interpretations. Bottom-up theories suggest that the extensive perceptual training provided by reading leads to a partial or total shift of neuronal tuning curves at several levels of the visual pathway, possibly including area V1 (31, 32). Top-down theories propose that there is in fact no selective tuning of early visual occipitotemporal regions to letters and written words, and that the fMRI responses to written stimuli in the VWFA stem from top-down feedback from language-related cortices owing to an implicit naming, which is absent for other visual stimuli (33).
By revealing an impact of literacy on visual activity as early as 140–180 ms after S1 and on visual repetition effects as early as 100–150 ms after S2, our present results suggest that literacy acquisition has a strong influence on early visual processing. Timing information by itself is insufficient for determining whether this effect arises in a feedforward manner, but some information about the processing stages affected by literacy can be gained by relating our findings to previous observations on the temporal organization of reading-related processes. The vast majority of studies on reading (48–58), including ERP studies of masked priming (53–56), support a classical feedforward model in which information is passed on from visual areas to language areas in a series of stages. Low-level orthographic processing peaks at posterior sites at ∼150 ms post-target onset (35, 36, 53, 59). This processing stage is already letter size-invariant (36), but still case-sensitive (59), font-sensitive (36), and position-sensitive (35). Just after 150 ms, a shift occurs in the orthographic code from position-specific to position-invariant (8, 35), suggesting a transition from low-level visual feature processing to more abstract orthographic processing. In ERPs, lexical effects do not arise until 220 ms (51) or 250 ms (52, 53, 55, 56). A recent study using both magneto-encephalography (MEG) and intracranial signals showed that letter processing (identified by contrasting consonant strings vs. false fonts) occurs at ∼160 ms after stimulus onset, whereas word processing (identified by contrasting real words versus consonant strings) occurs at ∼225 ms (48). This time course fits with our finding of distinct early- and late-stage modulations of visual processing by literacy (Fig. 2). It may be tentatively proposed that the early-stage effects found here correspond to the impact of learning to efficiently process letters and other high-resolution visual stimuli (60, 61), whereas the later-stage effects correspond to the identification of whole words and the interaction of this process with higher cortical language areas (48, 49).
Interpreted in the light of those earlier results, our findings seem most compatible with the hypothesis that literacy refines the early feedforward wave of visual processing, as do other forms of perceptual learning (30, 31, 62). This hypothesis is in accordance with recent behavioral findings of position sensitivity for strings of alphabetic and nonalphabetic symbols in literate subjects, but not in illiterate subjects (63). Additional arguments arise from the fact that an influence of reading acquisition was found on the early visual responses to nonalphabetic visual stimuli, such as faces and houses. Moreover, our task did not require explicit reading or naming, but only a search for an odd target (a black star) among the stimuli presented. During this task, there is no detectable fMRI activation in language areas beyond the VWFA (1). Thus, it seems unlikely that the literacy effects observed in early time windows arise from online top-down effects from language areas.
In general, our present ERP results nicely parallel our previous fMRI findings indicating that literacy influences the activation of bilateral occipital and left occipitotemporal areas (1). One interesting exception is that whereas fMRI revealed an effect of literacy in area V1 (1), in ERPs we found that the P1 window itself (∼100 ms), which reflects the activation of striate and extrastriate retinotopic areas, was unaffected by literacy. This finding could be related to poor sensitivity of our ERP measurements in this time period, or to a genuine absence of a literacy effect. In the latter case, it could indicate that the V1 effects of literacy previously observed on fMRI may be related, at least in part, to top-down influences (64, 65). Accordingly, with ERPs, we also found later literacy-induced enhancement of electrophysiological responses in occipital regions at ∼220 ms, which could indicate either a local reverberation of stimulus-induced activity within the visual system or, by this time, a top-down reverberating circuitry involving even more distant attentional and linguistic areas.
The interpretation of our results as reflecting bottom-up vs. top-down processing must remain tentative, for several reasons. First, the time course of reading remains a matter of debate. Some previous studies, relying on MEG-based source reconstruction techniques, have reported that the first 140–200 ms are sufficient for visual information to contact phonological and lexical codes in the precentral cortex and inferior frontal gyrus (10, 66, 67) and, in turn, send feedback signals to ventral occipitotemporal regions (67). In this case, even early occipital responses at ∼150 ms could be influenced by top-down signals. Second, our present ERP findings were obtained in a block design in which each stimulus category was repeated for seven trials in a row, a design adopted to parallel our previous fMRI study (1). As a result, after the first stimulus, subjects could adopt a distinct top-down attention and task set, possibly amplifying and routing letter strings to left hemisphere circuits in a literacy-dependent manner. Although how this interpretation would explain the effects of literacy on nonreading stimuli, such as faces or houses, is unclear, in the future it will be important to replicate the present results in a randomized study design rather than a block design.
Impact of Literacy on Mirror Invariance.
Does literacy acquisition solely enhance visual processing, or does it also interfere with some processes? As noted in the introductory section, mirror invariance is present in the visual system of infants and nonhuman primates (19–22), and may have to be unlearned to enable the fluent reader to automatically distinguish between, for instance, “b” and “d” (23, 27, 28). Using a behavioral same–different test in a subsample of the same illiterate and literate subjects, we recently demonstrated that literacy reduces mirror invariance for letter strings, and that this effect also generalizes to false fonts and even slightly to images of faces, houses, and tools (23). As their literacy increased, subjects became slightly worse at identifying that mirror images represented the same object, relative to making the same judgment on identical images. This finding suggests that mirror discrimination for letter strings partially generalizes to other visual categories as well. A possible reason for this, supported by both empirical evidence (1) and theoretical models (32), is that letter strings and other visual stimuli, such as faces, are processed in partially overlapping regions of the ventral pathway, and thus are jointly affected by reading acquisition.
Our present findings using mirror repetition priming confirm that literacy enhances mirror discrimination not only for letter strings, but also for other visual categories, particularly faces (Fig. 5). They also clarify the timing of this literacy-induced change in mirror invariance, showing that it occurs at a very early stage of visual processing (100-150 ms after S2). It is plausible that literacy acquisition influences mirror invariance at an early stage, before the peak of N1 responses, when the extraction of abstract letter identities is thought to be completed, typically at ∼170 ms (8, 48). A previous ERP study (39) reported a later effect of mirror priming in literate adults (at ∼400 ms), but we did not observe such an effect in the present study. Differences in paradigms and tasks (the previous study used masked priming and a semantic categorization task) could possibly explain the discrepant results.
Early vs. Late Acquisition of Literacy.
In our previous fMRI study, we performed additional analyses with more restricted group comparisons with the aim of finely separating different factors, such as the impact of early schooling vs. late literacy acquisition (see the supplementary material in ref. 1). In the present study, however, the limited number of subjects with valid ERP data reduced the power for small group comparisons. Thus, we limited our group analysis to the systematic comparison of the three literacy groups (i.e., literates, illiterates, and ex-illiterates). This analysis concurred with our previous fMRI (1) and diffusion tensor imaging (DTI) findings (68) in suggesting that virtually all effects of literacy, early or late, may be obtained at an adult age in unschooled individuals. In all of our measures, the ex-illiterates fell in between the pure illiterates and the early-schooled literates. The difference between ex-illiterates and illiterates, indicating that a specific reading-related improvement that could not be imputed to early schooling achieved significance for the enhanced post-P1 activation to strings (140–180 ms after S1) and the enhanced repetition suppression (100–150 ms after S2). Thus, even early visual events may be influenced by adult literacy training. For other comparisons, we observed trends in the appropriate direction, whose nonsignificance may be related simply to high variability and small group sizes.
Future studies should evaluate the robustness of our findings. Given that the present ex-illiterate sample comprised only individuals with modest reading skills, it would be especially important to investigate the impact of more extensive adult training on plasticity at the earliest stages of vision. Meanwhile, our present data concur with previous studies (1, 31, 60, 69) in demonstrating that, even when acquired in adulthood, literacy can have a deep impact on early visual processing, radically improving the precision with which we perceive and categorize visual inputs.
Methods
Participants.
Among the 63 subjects from Portugal and Brazil included in our fMRI study (1), 18 did not participate in ERP data collection or were excluded at the preprocessing stage because of excessive noise. Four new ex-illiterates from Brazil were included, for a total of 49 subjects (20 males), presenting increasing levels of reading ability (see fig. 1 in ref. 1): illiterates from Brazil (ILB), n = 9; ex-illiterates from Portugal (EXP), n = 8; ex-illiterates from Brazil (EXB), n = 8; literates from Brazil, with socioeconomic status matched to illiterates (LB2), n = 9; literates from Portugal (LP), n = 7; and literates from Brazil, with a medium to high socioeconomic status (LB1), n = 8. The subjects had a mean age of 50.7 ± 8.3 y (range, 32–68 y). There was no difference in mean age among the literacy groups (illiterates, 52.6 y; ex-illiterates, 51.8 y; literates, 49.3 y; F < 1, P = 0.55). Additional details about the subjects have been provided previously (1). Four subjects were excluded from behavioral analysis because button responses were not recorded correctly. All subjects had normal or corrected normal vision. All provided informed consent after careful explanations were provided. For illiterates, the consent form was read aloud.
Design and Procedure.
The present work used a similar paradigm as that in our previous fMRI study (for a full description, see supplementary materials in ref. 1), but with six runs instead of three. In brief, six categories of images—letter strings (pseudowords), false fonts, faces, houses, tools, and checkerboards—were presented to the subjects (Fig. 1A). Pronounceable pseudowords were used instead of real words because the stimuli needed to be equally readable in normal and mirror form, and there were virtually no real words fulfilling this constraint (e.g., “obli/ildo”); all stimuli were written exclusively with the lowercase letter set “bdmnpqiou.”
The subjects were asked to attentively observe each stimulus and to press the button whenever a rare target image (a black star) appeared. The images were displayed in short blocks (10.5 s), each comprising 12 stimuli of the same category. In each trial, a pair of images was presented for a total duration of 1.5 s: 200 ms for the first image (S1), followed by a 200-ms fixation point, followed by the second image (S2) for 500 ms, and finally a fixation point for 600 ms (Fig. 1B). One-third of the trials comprised identical pairs, another one-third comprised different pairs within the same category, and the remaining one-third comprised mirror-inverted versions of the same image.
ERP Methods.
ERPs were recorded at a sampling rate of 250 Hz using an Geodesic NA300 system (EGI) at both the NeuroSpin and Brasilia sites and a 257-electrode geodesic sensor net referenced to the vertex. We corrected for projector and amplifier delays using a photocell and verified that ERP component latencies were as expected in all groups (∼100 ms for P1; 170 ms for N1). We then applied a bandpass filter (0.5–30 Hz), segmented 1,400-ms-long epochs (from 400 ms before to 1,000 ms after S1 onset), and corrected for baseline in the 400-ms interval before S1 onset. We automatically rejected voltages exceeding ±100 µV and electro-oculogram activity exceeding ±70 µV, and complemented artifact rejection with a manual inspection of individual data. Trials with more than 20% bad channels were rejected. Voltage values in the remaining bad channels were replaced by the interpolation of surrounding electrodes values using a spherical splines method. An average reference transform was then applied. All preprocessing was performed using Netstation software (EGI). Data were then exported to Brainstorm (70) (http://neuroimage.usc.edu/brainstorm) for analysis, including source reconstruction. Statistical analyses were performed in R (http://www.R-project.org).
Source Modeling.
Cortical current density mapping was obtained using a distributed model with 15,028 current dipoles, with locations and orientations constrained to the cortical mantle of the Colin27 template brain model from the Montreal Neurological Institute. EEG forward modeling was computed with an extension to EEG using a three-layer symmetric boundary element method surface model from OpenMEEG software (71) (http://www-sop.inria.fr/athena/software/OpenMEEG/), implemented as a Brainstorm plug-in. Individual noise covariance matrices were calculated for each subject across all conditions. Cortical current maps were computed from the EEG time series using a linear inverse estimator (weighted minimum-norm current estimate), and then the resulting absolute values of currents, indexing cortical activation, were correlated with reading scores.
Supplementary Material
Acknowledgments
We thank Alvaro Luiz Portugal Figueiredo for help with data acquisition and François Tadel for help with the Brainstorm software. This work was supported by the Agence Nationale pour la Recherche (Grant CORELEX), the Bettencourt Foundation, the Belgian Fonds de la Recherche Scientifique (Grant FRFC 2.4515.12), and the Belgian Science Policy Office (Interuniversity Attraction Poles Grant 7/33). F.P. was supported by the Fondation pour la Recherche Médicale and Agence Nationale pour la Recherche. R.K. is Research Director of the Fonds de la Recherche Scientifique, Belgium.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1417347111/-/DCSupplemental.
References
- 1.Dehaene S, et al. How learning to read changes the cortical networks for vision and language. Science. 2010;330(6009):1359–1364. doi: 10.1126/science.1194140. [DOI] [PubMed] [Google Scholar]
- 2.Szwed M, et al. Specialization for written words over objects in the visual cortex. Neuroimage. 2011;56(1):330–344. doi: 10.1016/j.neuroimage.2011.01.073. [DOI] [PubMed] [Google Scholar]
- 3.Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat Neurosci. 2003;6(7):767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- 4.Paulesu E, et al. A cultural effect on brain function. Nat Neurosci. 2000;3(1):91–96. doi: 10.1038/71163. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura K, Dehaene S, Jobert A, Le Bihan D, Kouider S. Subliminal convergence of Kanji and Kana words: Further evidence for functional parcellation of the posterior temporal cortex in visual word perception. J Cogn Neurosci. 2005;17(6):954–968. doi: 10.1162/0898929054021166. [DOI] [PubMed] [Google Scholar]
- 6.Baker CI, et al. Visual word processing and experiential origins of functional selectivity in human extrastriate cortex. Proc Natl Acad Sci USA. 2007;104(21):9087–9092. doi: 10.1073/pnas.0703300104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dehaene S, et al. Cerebral mechanisms of word masking and unconscious repetition priming. Nat Neurosci. 2001;4(7):752–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- 8.Cohen L, et al. The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123(Pt 2):291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- 9.Allison T, Puce A, Spencer DD, McCarthy G. Electrophysiological studies of human face perception, I: Potentials generated in occipitotemporal cortex by face and non-face stimuli. Cereb Cortex. 1999;9(5):415–430. doi: 10.1093/cercor/9.5.415. [DOI] [PubMed] [Google Scholar]
- 10.Pammer K, et al. Visual word recognition: The first half second. Neuroimage. 2004;22(4):1819–1825. doi: 10.1016/j.neuroimage.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Cai Q, Paulignan Y, Brysbaert M, Ibarrola D, Nazir TA. The left ventral occipito-temporal response to words depends on language lateralization but not on visual familiarity. Cereb Cortex. 2010;20(5):1153–1163. doi: 10.1093/cercor/bhp175. [DOI] [PubMed] [Google Scholar]
- 12.Pinel P, Dehaene S. Beyond hemispheric dominance: Brain regions underlying the joint lateralization of language and arithmetic to the left hemisphere. J Cogn Neurosci. 2010;22(1):48–66. doi: 10.1162/jocn.2009.21184. [DOI] [PubMed] [Google Scholar]
- 13.Brem S, et al. Brain sensitivity to print emerges when children learn letter-speech sound correspondences. Proc Natl Acad Sci USA. 2010;107(17):7939–7944. doi: 10.1073/pnas.0904402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto R, Sakai KL. Learning letters in adulthood: Direct visualization of cortical plasticity for forming a new link between orthography and phonology. Neuron. 2004;42(2):311–322. doi: 10.1016/s0896-6273(04)00196-5. [DOI] [PubMed] [Google Scholar]
- 15.Spironelli C, Penolazzi B, Vio C, Angrilli A. Cortical reorganization in dyslexic children after phonological training: Evidence from early evoked potentials. Brain. 2010;133(11):3385–3395. doi: 10.1093/brain/awq199. [DOI] [PubMed] [Google Scholar]
- 16.Castro-Caldas A, Petersson KM, Reis A, Stone-Elander S, Ingvar M. The illiterate brain: Learning to read and write during childhood influences the functional organization of the adult brain. Brain. 1998;121(Pt 6):1053–1063. doi: 10.1093/brain/121.6.1053. [DOI] [PubMed] [Google Scholar]
- 17.Petersson KM, Silva C, Castro-Caldas A, Ingvar M, Reis A. Literacy: A cultural influence on functional left-right differences in the inferior parietal cortex. Eur J Neurosci. 2007;26(3):791–799. doi: 10.1111/j.1460-9568.2007.05701.x. [DOI] [PubMed] [Google Scholar]
- 18.Dehaene S, Cohen L. Cultural recycling of cortical maps. Neuron. 2007;56(2):384–398. doi: 10.1016/j.neuron.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Freiwald WA, Tsao DY. Functional compartmentalization and viewpoint generalization within the macaque face-processing system. Science. 2010;330(6005):845–851. doi: 10.1126/science.1194908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rollenhagen JE, Olson CR. Mirror-image confusion in single neurons of the macaque inferotemporal cortex. Science. 2000;287(5457):1506–1508. doi: 10.1126/science.287.5457.1506. [DOI] [PubMed] [Google Scholar]
- 21.Logothetis NK, Pauls J, Poggio T. Shape representation in the inferior temporal cortex of monkeys. Curr Biol. 1995;5(5):552–563. doi: 10.1016/s0960-9822(95)00108-4. [DOI] [PubMed] [Google Scholar]
- 22.Lachmann T, van Leeuwen C. Paradoxical enhancement of letter recognition in developmental dyslexia. Dev Neuropsychol. 2007;31(1):61–77. doi: 10.1207/s15326942dn3101_4. [DOI] [PubMed] [Google Scholar]
- 23.Pegado F, et al. Literacy breaks mirror invariance for visual stimuli: A behavioral study with adult illiterates. J Exp Psychol Gen. 2014;143(2):887–894. doi: 10.1037/a0033198. [DOI] [PubMed] [Google Scholar]
- 24.Duñabeitia JA, Dimitropoulou M, Estévez A, Carreiras M. The influence of reading expertise in mirror-letter perception: Evidence from beginning and expert readers. Mind Brain Educ. 2013;7(2):124–135. doi: 10.1111/mbe.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolinsky R, et al. Enantiomorphy through the looking glass: Literacy effects on mirror-image discrimination. J Exp Psychol Gen. 2011;140(2):210–238. doi: 10.1037/a0022168. [DOI] [PubMed] [Google Scholar]
- 26.Pegado F, Nakamura K, Hannagan T. How does literacy break mirror invariance in the visual system? Front Psychol. 2014;5:703. doi: 10.3389/fpsyg.2014.00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pegado F, Nakamura K, Cohen L, Dehaene S. Breaking the symmetry: Mirror discrimination for single letters but not for pictures in the Visual Word Form Area. Neuroimage. 2011;55(2):742–749. doi: 10.1016/j.neuroimage.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 28.Dehaene S, et al. Why do children make mirror errors in reading? Neural correlates of mirror invariance in the visual word form area. Neuroimage. 2010;49(2):1837–1848. doi: 10.1016/j.neuroimage.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura K, Makuuchi M, Nakajima Y. Mirror-image discrimination in the literate brain: A causal role for the left occpitotemporal cortex. Front Psychol. 2014;5:478. doi: 10.3389/fpsyg.2014.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sigman M, Gilbert CD. Learning to find a shape. Nat Neurosci. 2000;3(3):264–269. doi: 10.1038/72979. [DOI] [PubMed] [Google Scholar]
- 31.Sigman M, et al. Top-down reorganization of activity in the visual pathway after learning a shape identification task. Neuron. 2005;46(5):823–835. doi: 10.1016/j.neuron.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words: A proposal. Trends Cogn Sci. 2005;9(7):335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Price CJ, Devlin JT. The interactive account of ventral occipitotemporal contributions to reading. Trends Cogn Sci. 2011;15(6):246–253. doi: 10.1016/j.tics.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Compton PE, Grossenbacher P, Posner MI, Tucker DM. A cognitive-anatomical approach to attention in lexical access. J Cogn Neurosci. 1991;3(4):304–312. doi: 10.1162/jocn.1991.3.4.304. [DOI] [PubMed] [Google Scholar]
- 35.Dufau S, Grainger J, Holcomb PJ. An ERP investigation of location invariance in masked repetition priming. Cogn Affect Behav Neurosci. 2008;8(2):222–228. doi: 10.3758/cabn.8.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chauncey K, Holcomb PJ, Grainger J. Effects of stimulus font and size on masked repetition priming: An event-related potentials (ERP) investigation. Lang Cogn Process. 2008;23(1):183–200. doi: 10.1080/01690960701579839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maurer U, Zevin JD, McCandliss BD. Left-lateralized N170 effects of visual expertise in reading: Evidence from Japanese syllabic and logographic scripts. J Cogn Neurosci. 2008;20(10):1878–1891. doi: 10.1162/jocn.2008.20125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monzalvo K, Fluss J, Billard C, Dehaene S, Dehaene-Lambertz G. Cortical networks for vision and language in dyslexic and normal children of variable socio-economic status. Neuroimage. 2012;61(1):258–274. doi: 10.1016/j.neuroimage.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 39.Duñabeitia JA, Molinaro N, Carreiras M. Through the looking-glass: Mirror reading. Neuroimage. 2011;54(4):3004–3009. doi: 10.1016/j.neuroimage.2010.10.079. [DOI] [PubMed] [Google Scholar]
- 40.Dehaene S, et al. Letter binding and invariant recognition of masked words: Behavioral and neuroimaging evidence. Psychol Sci. 2004;15(5):307–313. doi: 10.1111/j.0956-7976.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- 41.Nestor A, Behrmann M, Plaut DC. The neural basis of visual word form processing: A multivariate investigation. Cereb Cortex. 2013;23(7):1673–1684. doi: 10.1093/cercor/bhs158. [DOI] [PubMed] [Google Scholar]
- 42.Li S, et al. Neural competition as a developmental process: Early hemispheric specialization for word processing delays specialization for face processing. Neuropsychologia. 2013;51(5):950–959. doi: 10.1016/j.neuropsychologia.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dundas EM, Plaut DC, Behrmann M. The joint development of hemispheric lateralization for words and faces. J Exp Psychol Gen. 2013;142(2):348–358. doi: 10.1037/a0029503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cantlon JF, Pinel P, Dehaene S, Pelphrey KA. Cortical representations of symbols, objects, and faces are pruned back during early childhood. Cereb Cortex. 2011;21(1):191–199. doi: 10.1093/cercor/bhq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossion B, Joyce CA, Cottrell GW, Tarr MJ. Early lateralization and orientation tuning for face, word, and object processing in the visual cortex. Neuroimage. 2003;20(3):1609–1624. doi: 10.1016/j.neuroimage.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 46.Striem-Amit E, Cohen L, Dehaene S, Amedi A. Reading with sounds: Sensory substitution selectively activates the visual word form area in the blind. Neuron. 2012;76(3):640–652. doi: 10.1016/j.neuron.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 47.Woodhead ZVJ, Wise RJS, Sereno M, Leech R. Dissociation of sensitivity to spatial frequency in word and face preferential areas of the fusiform gyrus. Cereb Cortex. 2011;21(10):2307–2312. doi: 10.1093/cercor/bhr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thesen T, et al. Sequential then interactive processing of letters and words in the left fusiform gyrus. Nat Commun. 2012;3:1284. doi: 10.1038/ncomms2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marinkovic K, et al. Spatiotemporal dynamics of modality-specific and supramodal word processing. Neuron. 2003;38(3):487–497. doi: 10.1016/s0896-6273(03)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simos PG, et al. Brain mechanisms for reading words and pseudowords: An integrated approach. Cereb Cortex. 2002;12(3):297–305. doi: 10.1093/cercor/12.3.297. [DOI] [PubMed] [Google Scholar]
- 51.Travis KE, et al. Independence of early speech processing from word meaning. Cereb Cortex. 2013;23(10):2370–2379. doi: 10.1093/cercor/bhs228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dehaene S. Electrophysiological evidence for category-specific word processing in the normal human brain. Neuroreport. 1995;6(16):2153–2157. doi: 10.1097/00001756-199511000-00014. [DOI] [PubMed] [Google Scholar]
- 53.Grainger J, Holcomb PJ. Watching the word go by: On the time-course of component processes in visual word recognition. Lang Linguist Compass. 2009;3(1):128–156. doi: 10.1111/j.1749-818X.2008.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grainger J, Kiyonaga K, Holcomb PJ. The time course of orthographic and phonological code activation. Psychol Sci. 2006;17(12):1021–1026. doi: 10.1111/j.1467-9280.2006.01821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holcomb PJ, Grainger J. On the time course of visual word recognition: An event-related potential investigation using masked repetition priming. J Cogn Neurosci. 2006;18(10):1631–1643. doi: 10.1162/jocn.2006.18.10.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kiyonaga K, Grainger J, Midgley K, Holcomb PJ. Masked cross-modal repetition priming: An event-related potential investigation. Lang Cogn Process. 2007;22(3):337–376. doi: 10.1080/01690960600652471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yum YN, Holcomb PJ, Grainger J. Words and pictures: An electrophysiological investigation of domain specific processing in native Chinese and English speakers. Neuropsychologia. 2011;49(7):1910–1922. doi: 10.1016/j.neuropsychologia.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laszlo S, Federmeier KD. Never seem to find the time: Evaluating the physiological time course of visual word recognition with regression analysis of single item ERPs. Lang Cogn Process. 2014;29(5):642–661. doi: 10.1080/01690965.2013.866259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petit J-P, Midgley KJ, Holcomb PJ, Grainger J. On the time course of letter perception: A masked priming ERP investigation. Psychon Bull Rev. 2006;13(4):674–681. doi: 10.3758/bf03193980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernandes T, Vale AP, Martins B, Morais J, Kolinsky R. The deficit of letter processing in developmental dyslexia: Combining evidence from dyslexics, typical readers and illiterate adults. Dev Sci. 2014;17(1):125–141. doi: 10.1111/desc.12102. [DOI] [PubMed] [Google Scholar]
- 61.Grainger J, Tydgat I, Isselé J. Crowding affects letters and symbols differently. J Exp Psychol Hum Percept Perform. 2010;36(3):673–688. doi: 10.1037/a0016888. [DOI] [PubMed] [Google Scholar]
- 62.Li W, Piëch V, Gilbert CD. Perceptual learning and top-down influences in primary visual cortex. Nat Neurosci. 2004;7(6):651–657. doi: 10.1038/nn1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duñabeitia JA, Orihuela K, Carreiras M. Orthographic coding in illiterates and literates. Psychol Sci. 2014;25(6):1275–1280. doi: 10.1177/0956797614531026. [DOI] [PubMed] [Google Scholar]
- 64.Gilbert CD, Sigman M. Brain states: Top-down influences in sensory processing. Neuron. 2007;54(5):677–696. doi: 10.1016/j.neuron.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 65.Gilbert CD, Li W. Top-down influences on visual processing. Nat Rev Neurosci. 2013;14(5):350–363. doi: 10.1038/nrn3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wheat KL, Cornelissen PL, Frost SJ, Hansen PC. During visual word recognition, phonology is accessed within 100 ms and may be mediated by a speech production code: Evidence from magnetoencephalography. J Neurosci. 2010;30(15):5229–5233. doi: 10.1523/JNEUROSCI.4448-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woodhead ZVJ, et al. Reading front to back: MEG evidence for early feedback effects during word recognition. Cereb Cortex. 2014;24(3):817–825. doi: 10.1093/cercor/bhs365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thiebaut de Schotten M, Cohen L, Amemiya E, Braga LW, Dehaene S. Learning to read improves the structure of the arcuate fasciculus. Cereb Cortex. 2014;24(4):989–995. doi: 10.1093/cercor/bhs383. [DOI] [PubMed] [Google Scholar]
- 69.Szwed M, Ventura P, Querido L, Cohen L, Dehaene S. Reading acquisition enhances an early visual process of contour integration. Dev Sci. 2012;15(1):139–149. doi: 10.1111/j.1467-7687.2011.01102.x. [DOI] [PubMed] [Google Scholar]
- 70.Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM. Brainstorm: A user-friendly application for MEG/EEG analysis. Comput Intell Neurosci. 2011;2011:879716. doi: 10.1155/2011/879716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gramfort A, Papadopoulo T, Olivi E, Clerc M. OpenMEEG: Open source software for quasistatic bioelectromagnetics. Biomed Eng Online. 2010;9:45. doi: 10.1186/1475-925X-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.