Significance
Early postnatal development of the cerebellum involves a number of events that require signaling via the neurotransmitter GABA, which acts on specific receptors anchored in the plasma membrane. GABAergic transmission regulates the proliferation and migration of neuronal precursors of astrocytic lineage. Glial cells are known to express GABA-A receptors that include GABAρ subunits, but their expression pattern, functional properties, and trafficking dynamics remain unknown. This study found that a large number of glial cells express GABAρ in the cerebellum. Functional properties and intracellular trafficking of GABA-A receptors in glial cells grown in vitro suggest a different mechanism of GABAergic control of extrasynaptic transmission.
Keywords: astrocytes, cerebellum, GABA-A receptor, GABAρ receptor, protein trafficking
Abstract
GABA-A receptors mediating synaptic or extrasynaptic transmission are molecularly and functionally distinct, and glial cells are known to express a plethora of GABA-A subunits. Here we demonstrate that GFAP+ cells of the granular layer of cerebellum express GABAρ subunits during early postnatal development, thereby conferring peculiar pharmacologic characteristics to GABA responses. Electron microscopy revealed the presence of GABAρ in the plasma membrane of GFAP+ cells. In contrast, expression in the adult was restricted to Purkinje neurons and a subset of ependymal cells. Electrophysiological studies in vitro revealed that astrocytes express functional receptors with an EC50 of 52.2 ± 11.8 μM for GABA. The evoked currents were inhibited by bicuculline (100 μM) and TPMPA (IC50, 5.9 ± 0.6 μM), indicating the presence of a GABAρ component. Coimmunoprecipitation demonstrated protein–protein interactions between GABAρ1 and GABAα1, and double immunofluorescence showed that these subunits colocalize in the plasma membrane. Three populations of GABA-A receptors in astrocytes were identified: classic GABA-A, bicuculline-insensitive GABAρ, and GABA-A–GABAρ hybrids. Clusters of GABA-A receptors were distributed in the perinuclear space and along the processes of GFAP+ cells. Time-lapse microscopy showed GABAρ2-GFP accumulation in clusters located in the soma and along the processes. The clusters were relatively immobile, with mean displacement of 9.4 ± 0.9 μm and a net distance traveled of 1–2 μm, owing mainly to directional movement or simple diffusion. Modulation of GABAρ dynamics may be a novel mechanism of extrasynaptic transmission regulating GABAergic control of GFAP+ cells during early postnatal development.
The role of GABAergic signaling is fundamental in the cerebellum, not only for influencing cell differentiation and neurotransmitter specification during early postnatal development, but also for controlling precise movements in the adult life (1–3). The expression of ionotropic GABA-A receptors with high affinity for the neurotransmitter is now well recognized, although the source of GABA involved in this process is controversial (4–6).
GABA-A receptors mediating synaptic (phasic) or extrasynaptic (tonic) transmission are molecularly and functionally distinct. In contrast to neurons, the depolarizing effect of astrocytic GABA-A receptors persists through postnatal development, although the response may attenuate with age (7). In cerebellar astrocytes, the array of GABA-A subunits is heterogeneous, and modulation by benzodiazepines is different from that by neurons (8). Indeed, GABA responses of Bergmann cells and ependymal glial cells (EGCs) are insensitive to benzodiazepines or pentobarbital, owing to the assembly of receptors that include GABAδ or GABAρ subunits (9, 10).
GABAρ subunits are part of the ionotropic GABA-A receptor family, which includes 19 identified genes that code for the same number of known proteins: α1–α6, β1–β3, γ1–γ3, δ, ε, Θ, π, and ρ1–ρ3 (11). GABA-A receptors are pentameric heterocomplexes composed of a combination of subunits, most commonly the α1β2γ2 combination, that gate a Cl− channel on activation (11, 12). Other arrays may include δ, ρ, or ε subunits, which confer distinctive functional and pharmacologic properties. GABAρ subunits can combine in homopentameric arrangements that form receptors with high affinity for the neurotransmitter (GABA EC50, 1–5 μM) and a low rate of desensitization, making them suitable for tonic transmission (11, 13, 14). GABAρ subunits are known to be expressed in the retina, where their presence in bipolar neurons controls the glutamatergic output (15, 16); they are present in other areas of the central nervous system as well, including striatum, hippocampus, and cerebellum, but their function there is not fully understood (17–19).
The role of GABAρ in neuronal tonic (extrasynaptic) and phasic (synaptic) transmission has been demonstrated in the Purkinje neurons of the cerebellum (20); however, GABAρ subunits are also expressed in a large fraction of EGCs, specialized, ciliated GFAP+ cells that permit the flow of cerebrospinal fluid circulating in the fourth ventricle (10, 21). GABA-ionic currents in these cells are insensitive to pentobarbital and partially blocked by the GABA-A antagonist bicucculline as well as by (1,2,5,6-Tetrahydropyridin-4-yl)methylphosphinic acid (TPMPA), the first synthesized GABAρ antagonist (22). GABA-A receptors that include GABAρ subunits are also expressed in approximately 30% of the GFAP+ cells present in the striatum (19). Thus, it seems that cells of glial origin, such as EGCs and astrocytes, present a diverse array of ionotropic GABA receptors that include GABAρ subunits and whose functional role remains unidentified.
In the course of recent work on assessing the presence of GABAρ subunits during early postnatal development of cerebellar EGCs, we corroborated their presence in this area but, unexpectedly, found that they are also widely distributed in a large proportion of GFAP+ cells of the granular layer (GL) that appear to be astrocytes. In this paper we report in detail the expression pattern of GABAρ subunits in GFAP+ cells of the cerebellum, the functional characterization of GABA responses of cerebellar astrocytes grown in vitro, and the participation of a GABAρ component in these responses. In addition, we provide evidence of the intracellular trafficking of GABAρ in astrocytes grown in vitro, and speculate about the possible synthesis of proteins in the processes of these cells.
Results
GABA-A Receptors in Cerebellar GFAP+ Cells.
Previous findings indicated that GABAρ subunits are expressed in EGCs of the cerebellum, in the zone facing the fourth ventricle (10) (Fig. S1A), which encouraged us to analyze the expression of these subunits during earlier stages of development in more depth. RT-PCR revealed GABAρ1, GABAρ2, and GABA-α1 expression at postnatal day (P) 5, P10, and P30 (Fig. S1B). Unexpectedly, immunofluorescence revealed the presence of GABAρ1 and GABAρ2 in most, but not all, GFAP+ cells of the subventricular zone (Fig. 1A, rows three and four, arrowheads) and GL (Fig. 1A, rows three and five, arrows). The proportion of GFAP+ cells expressing GABAρ1 was larger at P5 than at P10, in contrast to the expression of GABAρ2, which was more abundant at P10 (Fig. S2 A and B, columns one and two). Nevertheless, expression of GABAρ subunits decreased during the development in GFAP+ cells, such that in the adult it was limited to a few end feet of Bergmann glial cells and EGCs (Fig. S2 A and B, column three).
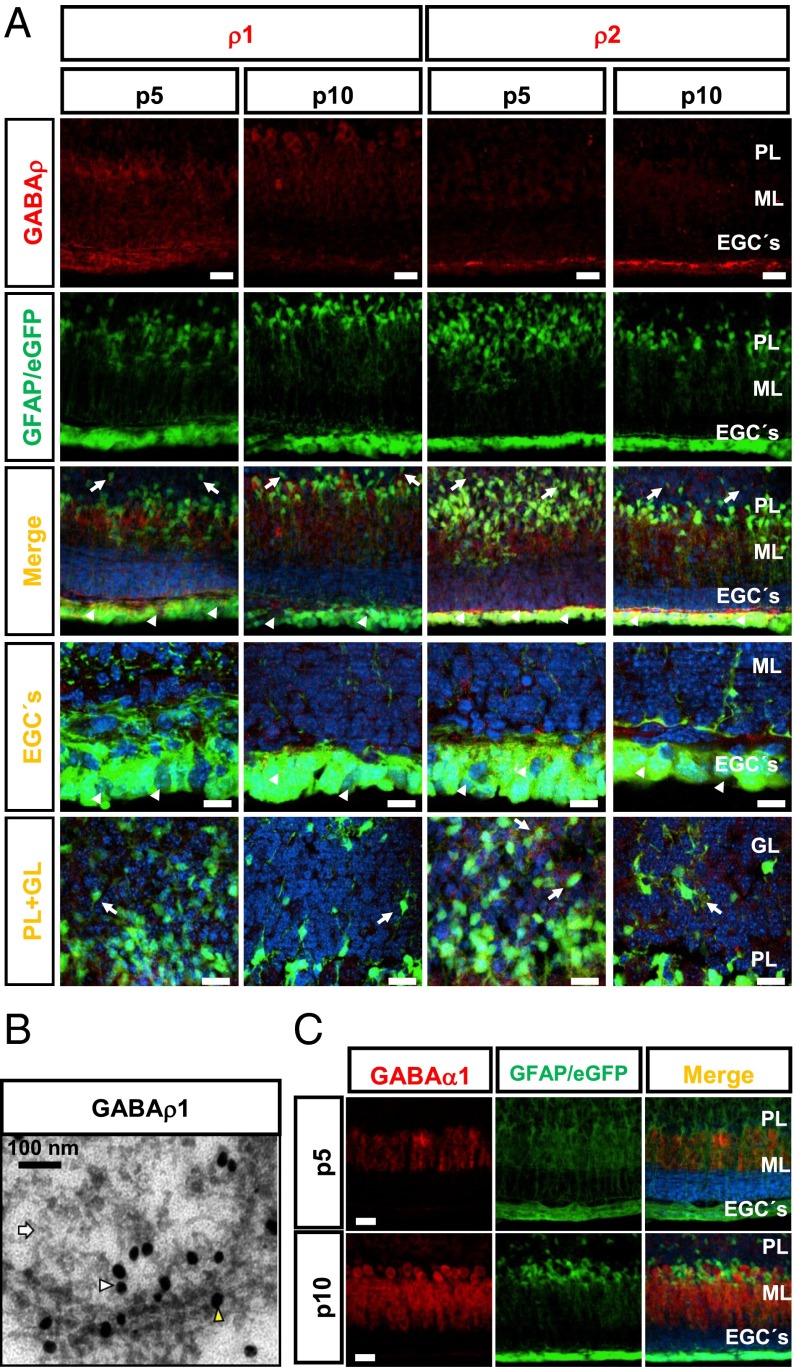
Fig. 1.
Expression of GABA-A receptor subunits in cerebellar GFAP+ cells. (A) GABAρ1 or GABAρ2 (first row) at P5 and P10 in GFAP-GFP transgenic mice (second row). The merged image shows that GFAP+ cells (green) of the subventricular zone (arrowheads) and GL (arrows) express GABAρ1 and GABAρ2 (red). The last two columns show maximizations of EGCs and GFAP+ cells of the GL expressing GABAρ. (Scale bars: 50 μm.) (B) Ultrastructural location of GABAρ1 in glial cells of the GL of cerebellum. The yellow arrowhead points to a gold particle labeling GABAρ1 in the plasma membrane; the white arrowhead, to a GABAρ1 label in submembranous structures. Astrocytes were identified by the presence of filamentous structures (white arrows). (Scale bar: 100 nm.) (C) Expression of GABAα1 (red) at P5, but not at P10, is located in Bergmann glial processes (green). n = 3. ML, molecular layer; PL, Purkinje layer. (Scale bars: 50 μm; 20 μm for maximizations.)
At P5 and P10, between 20% and 30% of GFAP+ cells of the GL were labeled with selective GABAρ1 and GABAρ2 antibodies (Fig. S3C). At P5, the GABAρ1 and GABAρ2 signals were located in soma and processes of GFAP cells, whereas at P10, the label was redistributed to the periphery of these cells (Fig. S3 A and B). We used an immunogold assay and transmission electron microscopy to gain insight into the location of this receptor. Evaluation of several images each from three independent experiments revealed the presence of GABAρ1 at the plasma membrane of cells distinguished by their internal filamentous structures, criteria that unequivocally distinguish astrocytes under electron microscopy (23). These cells were distributed in the GL, and the label was observed along many processes and was associated with intracellular filaments; an example of these cells is shown in Fig. 1B.
Expression of GABAα1 is crucial for proper GABAergic neurotransmission in the cerebellum (24), and this subunit is known to be present mainly in the adult neurons with scattered expression in GFAP+ cells (10). At P5 and P10, immunolabeling for GABAα1 was present mainly in non-GFAP+ cells, although Bergmann glial processes in the close vicinity of Purkinje cells exhibited limited immunolabeling at P5 (Fig. 1C, upper row), but not at P10 (Fig. 1C, lower row).
Cerebellar Ionotropic GABA Receptors Include a GABAρ Component.
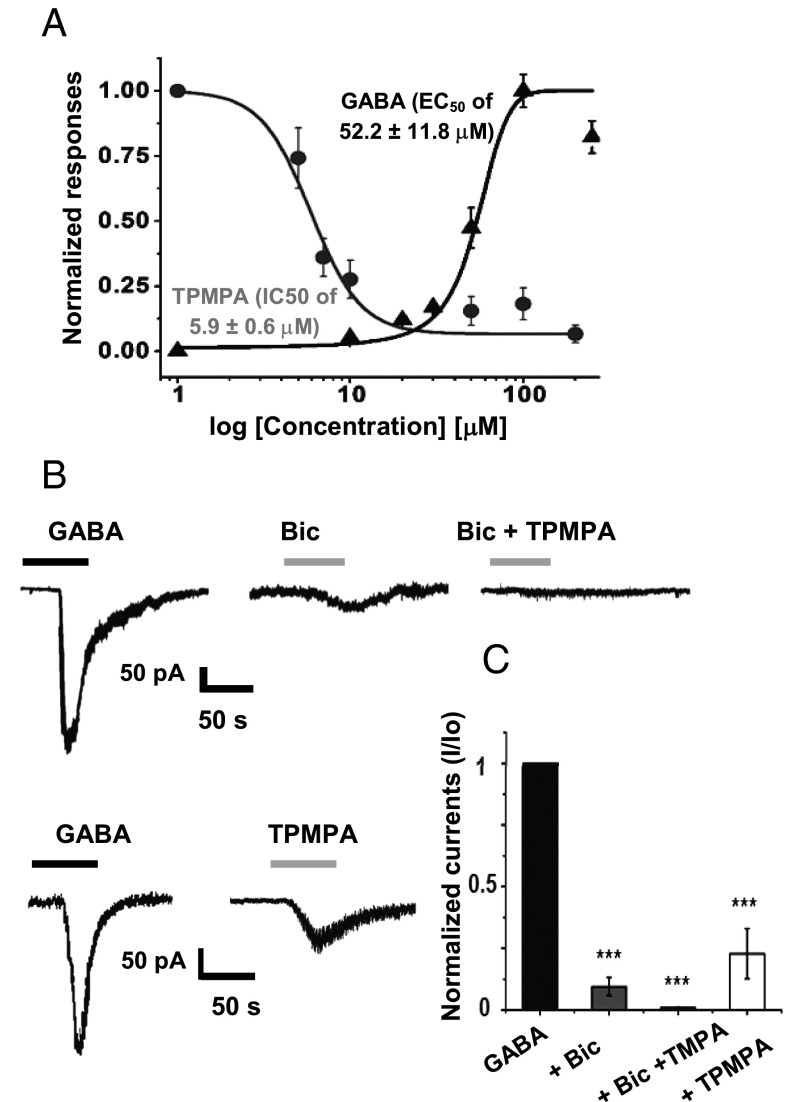
After identifying in situ GABA-A receptors that include a GABAρ component, we cultured astrocytes to study the functional and pharmacologic characteristics of the receptor and to assess under more controlled conditions the intracellular trafficking of the receptor. Cerebellar cells obtained from P5 mice and grown in vitro for 5 d were 91 ± 7.5% GFAP+ (mean ± SE). The cells were divided into three populations according to morphology: flat cells (50 ± 4.9%), flat polygonal cells (45 ± 3.7%), and star-like cells (5 ± 1.4%) (Fig. S4A). GABA currents from these GFAP+ cells exhibited two components, one component that inactivates promptly (τinac = 3.6 ± 0.5 s) and a slowly inactivating component (τinac = 48.6 ± 16.4 s). GABA EC50 was 52.2 ± 11.8 μM (Fig. 2A and Fig. S5A), and the GABA currents were partially blocked by 100 μM bicuculline, which inhibited the GABA responses by 90.5 ± 3.7% (Fig. 2B); these responses were totally abolished by a combination of 100 μM bicuculline and 10 μM TPMPA (Fig. 3B). Consecutive applications of 50 μM GABA consistently reduced the responses; however, a residual nondesensitizing component remained in all of the cells recorded after five applications (Fig. S5B). This component could be attributed to the expression of GABAρ subunits. The desensitizing component reappeared after 28 min of wash perfusion (Fig. S5C). These results indicate that GABA-A receptors from astrocytes are functional and have a GABAρ component.
Fig. 2.
GABA responses of cerebellar astrocytes in culture. (A) GABA and TPMPA dose–response curves (n = 6). (B) GABA responses (from left to right): Bicuculline 100 μM inhibited the GABA response (90.5 ± 3.7%), bicuculline 100 μM plus TPMPA 10 μM blocked 100% of the GABA response (n = 6), and TPMPA 10 μM inhibited the GABA response (81.1 ± 5.9%) (n = 6). (C) Dose–response relationship for GABA and TPMPA blockage. Quantitation of inhibition of GABA responses by bicuculline and GABA was significant. ***P < 0.001, one-way ANOVA.
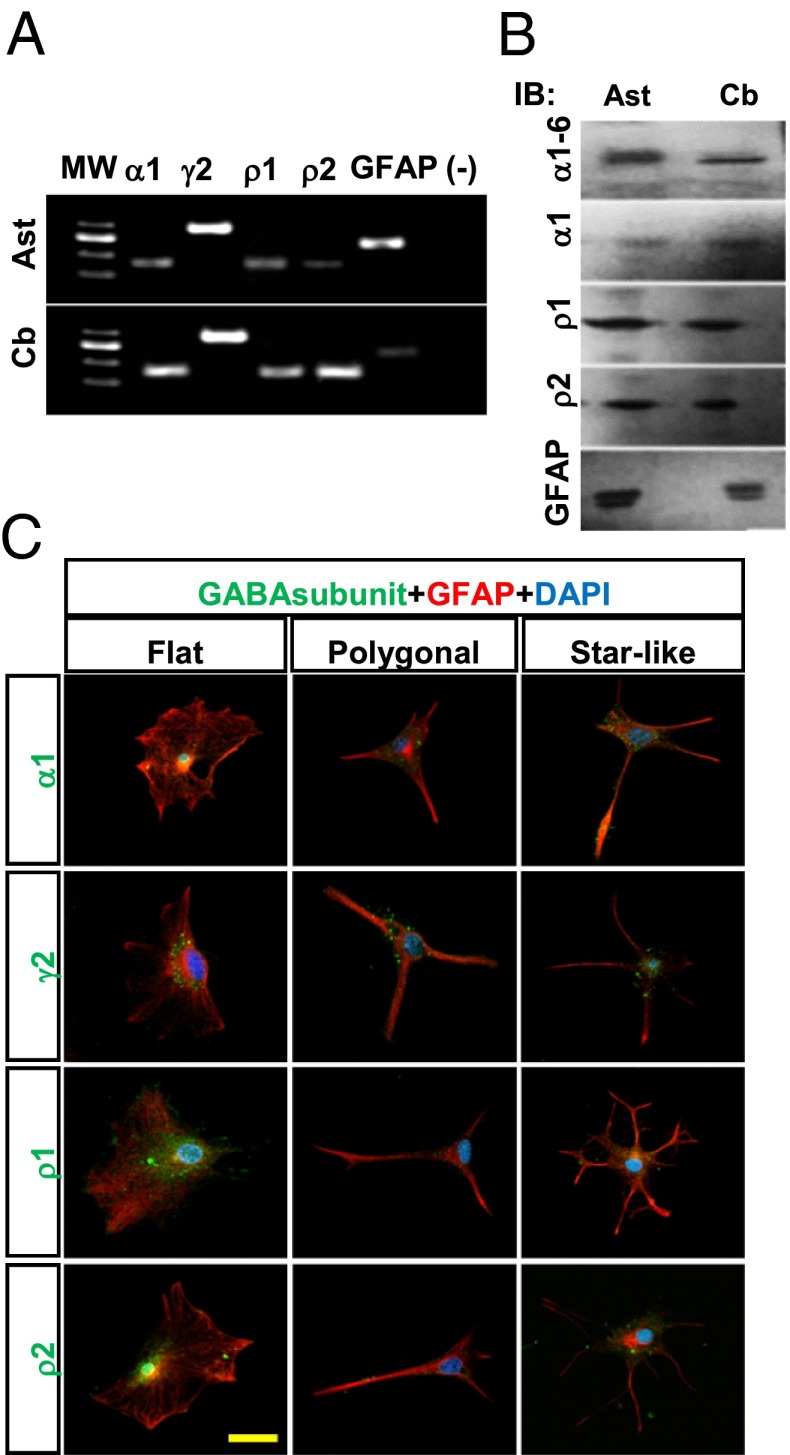
Fig. 3.
GABA-A receptors in cerebellar astrocytes in culture and in cerebellum. (A) Expression of mRNA for GABAα1, GABAγ2, GABAρ1, GABAρ2, and GFAP in cerebellar astrocytes in culture (Ast) and in whole cerebellum (Cb). (B) Western blot analysis for GABAα1–6, GABAα1, GABAρ1, GABAρ2, and GFAP in extracted membranes from astrocytes (Ast) and whole cerebellum (Cb). (C) Distribution of GABAα1, GABAγ2, GABAρ1, and GABAρ2 subunits (green) in GFAP+ cells (red) with different morphologies. Nuclei were counterstained with DAPI (blue). n = 3. (Scale bar: 20 μm.)
Application of 10 μM TPMPA inhibited the GABA response by 81.1 ± 5.9% (Fig. 3B), further supporting the presence of heterogenous populations of ionotropic GABA receptors: “classic” GABA-A, a GABAρ component, and heteromeric GABA-A–GABAρ receptors. This hypothesis is partially supported by the unexpected sensitivity of the ionotropic GABA receptors to TPMPA (IC50, 5.9 ± 0.07 μM) (Fig. 2A and Fig. S5C), which is close to that of homomeric GABAρ receptors (1–5 μM) and far lower than that of classic GABA-A receptors (500 μM) (11).
To test whether the peculiar responses to GABA found in astrocytes correspond to the expression of multiple heterogeneous GABA-A subunits, we confirmed the expression of GABA-A (α1 and γ2), GABAρ1, and GABAρ2 by RT-PCR and Western blot analysis (Fig. 3 A and B). In addition, immunolabeling revealed the presence of GABAα1 in 78 ± 2.5% of GFAP+ cells, GABAγ2 in 93 ± 1.2%, GABAρ1 in 60.0 ± 6.8%, and GABAρ2 in 70.0 ± 4.9%. The receptor subunits were seen to accumulate in the soma and to have a punctate distribution along the processes (Fig. 3C). Clustering and distribution of GABAα1, GABAρ1, and GABAρ2 were similar in the three GFAP+ cell morphologies, whereas GABAγ2 was more likely to accumulate in fewer clusters in the plasma membrane around the nucleus (Fig. S4 B and C).
GABAρ1 Associates with GABAα1.
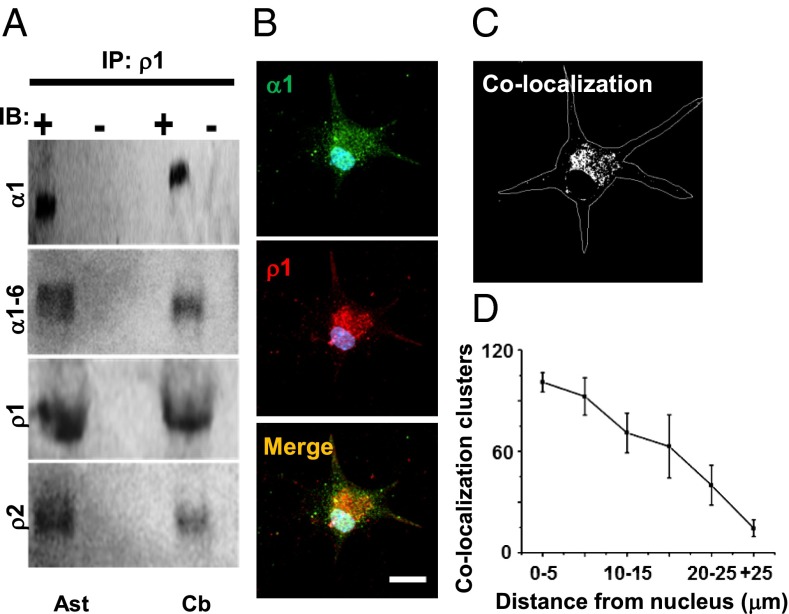
Although the existence of heteromeric GABA-A-GABAρ receptors is indicated by their current kinetics, hybrid pharmacologic properties, and similar distribution in astrocytes in culture, we still needed to demonstrate their physical interaction. For this, we used an antibody selective for GABAρ1 to assess the potential interactions with GABAα and GABAρ1 by coimmunoprecipitation (Fig. 4A). The results indicate that in membranes of GFAP+ cells, an anti-GABAρ1 pulls down at least one GABA-Aα subunit, given that anti-GABAα1–6 and anti-GABAα1 were detected in the immunoprecipitate. In addition, GABAρ2 was recognized by a selective antibody, suggesting that the GABAρ1–GABAρ2 interaction that occurs in the retina (25, 26). These interactions were demonstrated in membranes isolated from cerebellum as well (Fig. 4A).
Fig. 4.
Protein–protein interaction between GABAρ1 and GABAα subunits. (A) Coimmunoprecipitation using antibody against GABAρ1 showing interactions among GABAα1–6-GABAρ1; GABAα1-GABAρ1, and GABAρ1-GABAρ2 subunits in cerebellar astrocytes (Ast) in culture and in adult cerebellum (Cb). Membrane proteins without anti-GABAρ1 were negative. (B) Immunolocalization of GABA-Aα1 (green) and GABAρ1 (red). Merged yellow clusters indicate colocalization of GABAα1 and GABAρ1. (C) Fluorescence from clusters colabeled for GABA-Aα1 and GABAρ1 converged mainly at the plasma membrane around the soma, and to a lesser extent in the distal processes of GFAP+ cells in culture (450 clusters from five cells). (D) Quantification of colabeled clusters.
As shown in Fig. 3, most of the immunolabeling for GABAρ1 and GABA-Aα1 in cultured astrocytes was distributed similarly. To determine the spatial colocalization of these subunits in astrocytes, we used double immunofluorescence analysis of astrocytes in culture. Fig. 4 B and C shows a sample astrocyte in which we used antibodies that recognize the extracellular domain of GABAρ1 or GABA-Aα1. As indicated, these subunits were detected in the area surrounding the soma (101 ± 5.7 clusters; n = 20) and, to a lesser extent, in distal processes (14.5 ± 5 clusters; n = 20) (Fig. 4D).
The foregoing evidence supports the conclusion that cerebellar astrocytes in culture express functional GABA-A receptors, and that some of these receptors are composed of GABAρ subunits that interact with classic GABA-A subunits, such as GABAα1 and GABAρ2.
Distribution and Trafficking of GABAρ2.
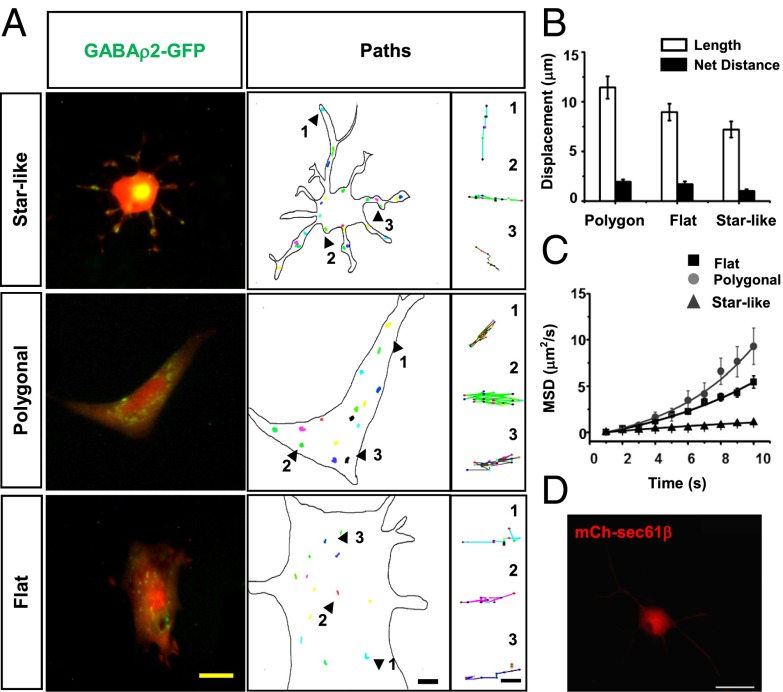
It was previously shown that in astrocytes grown in vitro, GABAρ2 is located in soma and proximal processes (27). In the present study, we reexamined the distribution of this receptor subunit in astrocytes with all three morphologies and corroborated those findings. In 40 cells, the chimeric receptors were located mainly in the soma (42 ± 4.9 clusters) and to some extent in distal processes (8 ± 2 clusters). The previous study also found that GABAρ2 clusters remain stationary for long periods, which does not explain their presence in terminal processes of astrocytes either in culture or in situ. Thus, we examined the trajectories of GABAρ2 tagged with GFP expressed by adenoviral transduction (Fig. S6).
Receptor clusters displayed a mean cumulative displacement of 9.4 ± 0.9 μm over 30 min, but remained practically stationary, with a net distance traveled of only 1.5 ± 0.23 μm (Fig. 5B). No significant differences were observed among cells with different morphologies. A plot of the first 10 points of mean squared displacement (MSD) revealed slow and limited, directed movement by polygonal and flat astrocytes, as illustrated by the exponential fitting of the MSD curves (Fig. 5C). In contrast, MSD curves in star-like astrocytes exhibited a strong linear correlation, suggesting that GABAρ2 is transported by means of free diffusion (Fig. 5C). Thus, it appears that transport of GABAρ2 is not consistent with classic vesicular traffic and may be explained by local synthesis of proteins that gives rise to clustered receptors in the astrocyte processes. Alternatively, the size of the vesicles that transport the receptor might be smaller than the resolution limit of the epifluorescence microscope (∼200 nm in the lateral direction and ∼600 nm in the axial direction) (28).
Fig. 5.
Distribution and trafficking of GABAρ2-GFP. (A) Left to right columns: Fluorescence emitted by GABAρ2–GFP (green) and soluble mCherry (red). GABAρ2-GFP accumulates in clusters located in the soma and along the processes. Arrowheads and numbers indicate samples of isolated tracked paths of GABAρ2-GFP. (Scale bars: 25, 5, and 1 μm, respectively.) (B) Displacement length and net distance traveled of GABAρ2-GFP did not differ significantly among astrocytes with different phenotypes. (C) MSD curves of GABAρ2-GFP fluorescent clusters. Exponential correlation indicates directional movements in soma and processes of flat and polygonal cells, and linear correlation is evidence of simple diffusion in star-like cells (510 clusters from 40 cells). (D) Distribution of mCh-Sec61β in soma and distal processes. (Scale bar: 20 μm.)
To support the first view, we labeled the endoplasmic reticulum (ER) of astrocytes by transiently expressing the protein Sec61β tagged with mCherry. Sec61β is a major component of the translocation apparatus for proteins in the ER (29). Fluorescence was consistently detected around the nucleus of astrocytes in culture, but—important for test of our suggestion—many fluorescent clusters were observed in the processes of astrocytes (Fig. 5D), indicating the presence of ER, the cell compartment containing the ribosomes on which protein synthesis occurs.
Discussion
The periventricular layer of the roof of the fourth ventricle is formed by ependymal cells of glial origin. These cells generate GABA currents on exogenous administration of GABA owing to the presence of an array of GABA-A subunits that include GABAρ. GABA responses are partially blocked by TPMPA or bicuculline and totally abolished by a combination of the two compounds (10). Here we present evidence that during postnatal development of the cerebellum, GABAρ subunits are widely expressed in EGCs and even in GFAP+ cells of the GL and subventricular zone. Expression of GABAρ subunits is down-regulated in young mice, but limited to Purkinje neurons and a small fraction of GFAP+ cells in adults.
The developmental pattern of GABAρ subunit localization partially agrees with earlier reports suggesting expression of GABAρ1 and GABAρ2 at low levels in rat Purkinje and Golgi neurons during early postnatal development (30, 31). Previous studies have unequivocally demonstrated the presence of other GABA-A subunits (αx βx γx) in immature neurons of the cerebellum, and the expression of this receptor is a fundamental element in proper formation of the synaptic circuitry (32–34). Glial cells are also part of the tripartite synapse, and GABA-A subunits have been identified in Bergmann glia (α2 γ1 δ) and EGCs (α1 ρ1) (9, 10, 35), where they might play a key role as extrasynaptic sensors of GABAergic tone, similar to what occurs in astrocyte-like GFAP+ progenitors (36). GABA-A receptors found in cerebellar astrocytes include a bicuculline-resistant nondesesnsitizing component (τ = 48.6 ± 16.4 s), the result of the presence of GABAρ subunits, which are functionally suitable to control tonic transmission.
The detection of GABAρ subunits in glia (Fig. 1 and Figs. S1 and S2) should be attributed to the accurate identification of GFAP+ cells of the transgenic mice used for immunofluorescence (37), a valuable tool that allowed differentiation of the labeled glial cells from other cell linages. Although a role for the GABAρ subunits expressed in GFAP+ cells is not yet evident, these subunits reportedly participate in radial neuronal migration during brain cortex development (38). Immature neurons of the intermediate zone express a combination of classical GABA-A subunits and GABAρ, and it has been inferred that activation of the GABAρ component provides a precise signal for the immature neurons to migrate toward the external zone of the cortex (38). GABAρ may have a similar function in the developing cerebellum, considering that immature GABAergic neurons are known to transiently express GFAP during proliferation, and the possibility exists that the population of cells that we have identified as glia correspond to multipotent cells that will give rise to both interneurons and astrocytes (39).
We also examined the location of GABAρ1 in GFAP+ cells by immunogold assay and transmission electron microscopy, which indicated the presence of the receptors in the plasma membrane of glial cell processes. This position would render those cells with a potent detector for sensing environmental GABA, which is known to modulate important developmental events in the cerebellum and in the nervous system in general (40, 41).
Trafficking of GABA-A receptors in neurons grown in vitro has been widely studied (42, 43). For example, receptor lateral diffusion from perisynaptic loci is known to be relevant for replenishing the receptor pool at inhibitory synapses; this mechanism is subtly dependent on the α subunit that forms the pentameric heterocomplex and seems to be a mechanism of finely controlling GABAergic signaling (44, 45). In contrast, the role and trafficking of ionotropic GABA receptors in glia are not clearly understood. It is evident that hybrid GABA-A–GABAρ complexes are present in vitro and in vivo, and that the positions of the subunits partially converge in the soma of GFAP+ cells in culture. Their dynamic insertion into the plasma membrane was not determined in the present study, but antibodies that detect an extracellular domain of the receptor reveal the presence of GABAρ2 in the plasma membrane of soma and processes. In contrast, our analyses of the intracellular trajectories of GABAρ2 tagged with GFP suggest that the mobility of this subunit is limited, and that differences exist among astrocyte phenotypes. In flat and polygonal cells the receptor movements are slow and directed, whereas in star-shaped cells, the receptors move by simple diffusion, similar to the trafficking of the cannabinoid receptor 1 in flat astrocytes grown in vitro (46).
The foregoing results suggest that temporal regulation of the expression of GABA-A subunits could contribute to the precise regulation of glial development in the cerebellum. The presence of GABAρ subunits either independently or in combination with other GABA-A subunits confers even more complexity to GABAergic signaling during early postnatal life. Although GABA-A receptors are known to reside in GFAP+ cell processes, how they are delivered to the plasma membrane remains undetermined. Possibilities include transport of the receptors in vesicles smaller than the resolution limit and local synthesis of the receptors in the distal processes. Localization of ribosomal complexes and RNA in distal processes of astrocytes would support the latter possibility, which also seems likely because ER was detected in these processes in this and previous studies (47, 48).
Methods
Tissue Processing, Immunofluorescence, and Immunogold Assays.
All experiments were conducted in accordance with the guidelines of the National Institutes of Health’s Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the Universidad Nacional Autónoma de México. For the cerebellum slice preparation, male GFAP-eGFP transgenic mice (37) at P5, P10, and P30 were processed as described previously (18). Astrocytes were grown in vitro (49), and protein extracts and membrane preparations were obtained as reported previously (50). Antibodies were used for Western blot analysis and to locate the GABA-A receptors in cerebellum and in cultured cerebellar astrocytes (51). The antibodies used in this study are listed in Table S1.
Double immunofluorescence was performed to determine GABA-A subunit expression in GFAP+ cells. The cells were counterstained with DAPI and then dehydrated and mounted in Vectashield (Vector Laboratories). Images were obtained using a Zeiss LSM510 Meta confocal microscope, with wavelengths of 561 nm for excitation of Alexa Fluor 594, 488 nm for excitation of Alexa Fluor 488 and eGFP, and 750 nm for excitation of DAPI. For image quantitative analysis, the z-stack images were processed in ImageJ analysis software (Colocalization and Analyzed Particles plug-ins).
Immunogold assays were performed as described previously (19) in P10 CD1 male mice. The primary antibody was 1:50 rabbit IgG anti–GABA-Aρ1 (Santa Cruz Biotechnology), and the secondary antibody was 1:80, 25 nm colloidal gold-conjugated, goat anti-rabbit IgG (25116; Electron Microscopy Sciences). The samples were observed with a JEOL JEM-1010 transmission electron microscope at 80 kV.
RT-PCR.
RNA was isolated from P5, P10, and P30 cerebellum and from cerebellar astrocytes in culture (5 d in vitro) using TRIzol reagent (Invitrogen), following the manufacturer’s instructions. Gene-specific primers designed for the GABA subunits GABAα1, GABAγ2, GABAρ1, GABAρ2, and GFAP are listed in Table S2.
Electrophysiological Recordings.
Electrophysiological recordings of astrocytes in culture were described previously (49). Once the glial identity was verified, the membrane potential was held at −60 mV, and GABA was applied for 30 s. Concentration-response and desensitization curves were determined using application intervals of 7 min. Antagonism of responses elicited by 50 μM GABA was studied by preincubating and coapplying the GABA-A receptor antagonist bicuculline (100 μM) or the GABAρ-selective antagonist TPMPA (10 μM). Finally, dose–response curves for TPMPA were constructed to determine the potency of the drug. Stock solutions of GABA, bicuculline (Sigma-Aldrich), and TPMPA (TOCRIS) were prepared according to the manufacturer´s instructions and then diluted in extracellular bath solution.
Coimmunoprecipitation.
Assays were performed on membranes isolated from either P10 cerebella or astrocytes in culture (5 d in vitro) using a goat primary anti-GABAρ1 antibody, following the basic protocol reported earlier (20). G-Sepharose was added to the mix for 4 h at 4 °C. To remove the resin, samples were centrifuged at 9,500 × g for 10 min at 4 °C, the supernatant was removed, and the pellet was washed and centrifuged three times at 9,500 × g for 5 min. Then 15 μL of loading buffer was added, and the samples were denatured by heating for 5 min at 95 °C. Before PAGE, the samples were centrifuged at 9,500 × g for 3 min and electroblotted onto PVDF membranes; proteins were detected as described previously (20).
Expression of Fluorescent Proteins in GFAP+ Cells in Culture and Analyses of Receptor Trajectories.
Trafficking of GABAρ2 was assessed using AdGABAρ2-GFP (27), and cell morphology was highlighted using AdmCherry (6.4 × 106 pfu) (Vector Biolabs). Fluorescent clusters were tracked under an inverted epifluorescence microscope (Olympus CKX41, objective: LCAch 40×/0.55 Php), and images were obtained at 0.017 Hz at room temperature. The distribution and dynamics of fluorescent particles were analyzed with ImageJ (Analyze Particles and MTrackJ plug-ins), and the motility of fluorescent clusters and MSD were determined and plotted in Origin 8 Pro (46).
For localizing ER in astrocytes in culture, the plasmid mChe-Sec61β (49155; Addgene) (52) was transfected using Lipofectamine PLUS reagent (Life Technologies), and the distribution of the fluorescent protein was observed 48–72 h later (53).
Supplementary Material
Acknowledgments
We thank Prof. H. Kettenmann (Max Delbruck Center) for kindly donating the GFAP-eGFP transgenic mouse strain; E. N. Hernández-Ríos, M. L. Palma-Tirado, J. M. García-Servín, and L. Casanova-Rico for providing technical support; A. E. Espino-Saldaña and M. Ramírez-Romero for providing technical assistance; and Dr. D. D. Pless for editing the manuscript. A. Pétriz and M.A. González-González are doctoral students at the Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México, supported by Consejo Nacional de Ciencia y Tecnología (CONACYT) Fellowships 332889 and 339430, respectively. This work was supported by Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica-Dirección General de Asuntos del Personal Académico Grant IN200913 and CONACYT Grant 220224.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1419632111/-/DCSupplemental.
References
- 1.Root CM, Velázquez-Ulloa NA, Monsalve GC, Minakova E, Spitzer NC. Embryonically expressed GABA and glutamate drive electrical activity regulating neurotransmitter specification. J Neurosci. 2008;28(18):4777–4784. doi: 10.1523/JNEUROSCI.4873-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashimoto M, Hibi M. Development and evolution of cerebellar neural circuits. Dev Growth Differ. 2012;54(3):373–389. doi: 10.1111/j.1440-169X.2012.01348.x. [DOI] [PubMed] [Google Scholar]
- 3.Ito M. The Cerebellum: Brain for an Implicit Self. FT Press Science; Upper Saddle River, NJ: 2012. [Google Scholar]
- 4.Ye Z, McGee TP, Houston CM, Brickley SG. The contribution of δ subunit-containing GABAA receptors to phasic and tonic conductance changes in cerebellum, thalamus and neocortex. Front Neural Circuits. 2013;7:203. doi: 10.3389/fncir.2013.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Succol F, Fiumelli H, Benfenati F, Cancedda L, Barberis A. Intracellular chloride concentration influences the GABAA receptor subunit composition. Nat Commun. 2012;3:738. doi: 10.1038/ncomms1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S, et al. Channel-mediated tonic GABA release from glia. Science. 2010;330(6005):790–796. doi: 10.1126/science.1184334. [DOI] [PubMed] [Google Scholar]
- 7.Vélez-Fort M, Audinat E, Angulo MC. Central role of GABA in neuron–glia interactions. Neuroscientist. 2012;18(3):237–250. doi: 10.1177/1073858411403317. [DOI] [PubMed] [Google Scholar]
- 8.Kettenmann H, Backus KH, Schachner M. Aspartate, glutamate and gamma-aminobutyric acid depolarize cultured astrocytes. Neurosci Lett. 1984;52(1-2):25–29. doi: 10.1016/0304-3940(84)90345-8. [DOI] [PubMed] [Google Scholar]
- 9.Müller T, et al. Developmental regulation of voltage-gated K+ channel and GABAA receptor expression in Bergmann glial cells. J Neurosci. 1994;14(5 Pt 1):2503–2514. doi: 10.1523/JNEUROSCI.14-05-02503.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reyes-Haro D, et al. γ-Aminobutyric acid-ρ expression in ependymal glial cells of the mouse cerebellum. J Neurosci Res. 2013;91(4):527–534. doi: 10.1002/jnr.23183. [DOI] [PubMed] [Google Scholar]
- 11.Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: Classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60(3):243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sigel E, Steinmann ME. Structure, function, and modulation of GABA(A) receptors. J Biol Chem. 2012;287(48):40224–40231. doi: 10.1074/jbc.R112.386664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polenzani L, Woodward RM, Miledi R. Expression of mammalian gamma-aminobutyric acid receptors with distinct pharmacology in Xenopus oocytes. Proc Natl Acad Sci USA. 1991;88(10):4318–4322. doi: 10.1073/pnas.88.10.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martínez-Delgado G, Estrada-Mondragón A, Miledi R, Martínez-Torres A. An update on GABAρ receptors. Curr Neuropharmacol. 2010;8(4):422–433. doi: 10.2174/157015910793358141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones SM, Palmer MJ. Activation of the tonic GABAC receptor current in retinal bipolar cell terminals by nonvesicular GABA release. J Neurophysiol. 2009;102(2):691–699. doi: 10.1152/jn.00285.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sagdullaev BT, McCall MA, Lukasiewicz PD. Presynaptic inhibition modulates spillover, creating distinct dynamic response ranges of sensory output. Neuron. 2006;50(6):923–935. doi: 10.1016/j.neuron.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Didelon F, et al. gamma-Aminobutyric acidA rho receptor subunits in the developing rat hippocampus. J Neurosci Res. 2002;67(6):739–744. doi: 10.1002/jnr.10178. [DOI] [PubMed] [Google Scholar]
- 18.Rosas-Arellano A, et al. The GABA(A)ρ receptors in hippocampal spontaneous activity and their distribution in hippocampus, amygdala and visual cortex. Neurosci Lett. 2011;500(1):20–25. doi: 10.1016/j.neulet.2011.05.235. [DOI] [PubMed] [Google Scholar]
- 19.Rosas-Arellano A, Machuca-Parra AI, Reyes-Haro D, Miledi R, Martínez-Torres A. Expression of GABAρ receptors in the neostriatum: Localization in aspiny, medium spiny neurons and GFAP-positive cells. J Neurochem. 2012;122(5):900–910. doi: 10.1111/j.1471-4159.2011.07621.x. [DOI] [PubMed] [Google Scholar]
- 20.Harvey VL, Duguid IC, Krasel C, Stephens GJ. Evidence that GABA rho subunits contribute to functional ionotropic GABA receptors in mouse cerebellar Purkinje cells. J Physiol. 2006;577(Pt 1):127–139. doi: 10.1113/jphysiol.2006.112482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarez-Morujo AJ, et al. The ependymal surface of the fourth ventricle of the rat: A combined scanning and transmission electron microscopic study. Histol Histopathol. 1992;7(2):259–266. [PubMed] [Google Scholar]
- 22.Ragozzino D, et al. Design and in vitro pharmacology of a selective gamma-aminobutyric acidC receptor antagonist. Mol Pharmacol. 1996;50(4):1024–1030. [PubMed] [Google Scholar]
- 23.Leon W. Histology Cell and Tissue Biology. 5th Ed Elsevier Biomedical; New York: 1983. [Google Scholar]
- 24.Vicini S, et al. GABA(A) receptor alpha1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci. 2001;21(9):3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan Y, Ripps H, Qian H. Random assembly of GABA rho1 and rho2 subunits in the formation of heteromeric GABA(C) receptors. Cell Mol Neurobiol. 2006;26(3):289–305. doi: 10.1007/s10571-006-9001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCall MA, Lukasiewicz PD, Gregg RG, Peachey NS. Elimination of the rho1 subunit abolishes GABA(C) receptor expression and alters visual processing in the mouse retina. J Neurosci. 2002;22(10):4163–4174. doi: 10.1523/JNEUROSCI.22-10-04163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martínez-Delgado G, et al. Dynamics of GABAρ2 receptors in retinal bipolar neurons and cerebellar astrocytes. Neuroreport. 2011;22(1):4–9. doi: 10.1097/wnr.0b013e328340d7d6. [DOI] [PubMed] [Google Scholar]
- 28.Gustafsson MG. Extended resolution fluorescence microscopy. Curr Opin Struct Biol. 1999;9(5):627–634. doi: 10.1016/s0959-440x(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 29.Hartmann E, et al. Evolutionary conservation of components of the protein translocation complex. Nature. 1994;367(6464):654–657. doi: 10.1038/367654a0. [DOI] [PubMed] [Google Scholar]
- 30.Mejía C, et al. Expression of GABArho subunits during rat cerebellum development. Neurosci Lett. 2008;432(1):1–6. doi: 10.1016/j.neulet.2007.11.062. [DOI] [PubMed] [Google Scholar]
- 31.Rozzo A, et al. Expression and dendritic mRNA localization of GABAC receptor rho1 and rho2 subunits in developing rat brain and spinal cord. Eur J Neurosci. 2002;15(11):1747–1758. doi: 10.1046/j.1460-9568.2002.02013.x. [DOI] [PubMed] [Google Scholar]
- 32.Meinecke DL, Rakic P. Developmental expression of GABA and subunits of the GABAA receptor complex in an inhibitory synaptic circuit in the rat cerebellum. Brain Res Dev Brain Res. 1990;55(1):73–86. doi: 10.1016/0165-3806(90)90107-a. [DOI] [PubMed] [Google Scholar]
- 33.Zdilar D, Luntz-Leybman V, Frostholm A, Rotter A. Differential expression of GABAA/benzodiazepine receptor beta 1, beta 2, and beta 3 subunit mRNAs in the developing mouse cerebellum. J Comp Neurol. 1992;326(4):580–594. doi: 10.1002/cne.903260407. [DOI] [PubMed] [Google Scholar]
- 34.Nadler LS, Guirguis ER, Siegel RE. GABAA receptor subunit polypeptides increase in parallel but exhibit distinct distributions in the developing rat cerebellum. J Neurobiol. 1994;25(12):1533–1544. doi: 10.1002/neu.480251206. [DOI] [PubMed] [Google Scholar]
- 35.Riquelme R, Miralles CP, De Blas AL. Bergmann glia GABA(A) receptors concentrate on the glial processes that wrap inhibitory synapses. J Neurosci. 2002;22(24):10720–10730. doi: 10.1523/JNEUROSCI.22-24-10720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8(9):1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nolte C, et al. GFAP promoter-controlled EGFP-expressing transgenic mice: A tool to visualize astrocytes and astrogliosis in living brain tissue. Glia. 2001;33(1):72–86. [PubMed] [Google Scholar]
- 38.Denter DG, et al. GABAC receptors are functionally expressed in the intermediate zone and regulate radial migration in the embryonic mouse neocortex. Neuroscience. 2010;167(1):124–134. doi: 10.1016/j.neuroscience.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 39.Silbereis J, Cheng E, Ganat YM, Ment LR, Vaccarino FM. Precursors with glial fibrillary acidic protein promoter activity transiently generate GABA interneurons in the postnatal cerebellum. Stem Cells. 2009;27(5):1152–1163. doi: 10.1002/stem.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dave KA, Bordey A. GABA increases Ca2+ in cerebellar granule cell precursors via depolarization: Implications for proliferation. IUBMB Life. 2009;61(5):496–503. doi: 10.1002/iub.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spitzer NC. Activity-dependent neurotransmitter respecification. Nat Rev Neurosci. 2012;13(2):94–106. doi: 10.1038/nrn3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petrini EM, et al. Synaptic recruitment of gephyrin regulates surface GABAA receptor dynamics for the expression of inhibitory LTP. Nat Commun. 2014;5:3921. doi: 10.1038/ncomms4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vithlani M, Terunuma M, Moss SJ. The dynamic modulation of GABA(A) receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol Rev. 2011;91(3):1009–1022. doi: 10.1152/physrev.00015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas P, Mortensen M, Hosie AM, Smart TG. Dynamic mobility of functional GABAA receptors at inhibitory synapses. Nat Neurosci. 2005;8(7):889–897. doi: 10.1038/nn1483. [DOI] [PubMed] [Google Scholar]
- 45.Muir J, Kittler JT. Plasticity of GABAA receptor diffusion dynamics at the axon initial segment. Front Cell Neurosci. 2014;8:151. doi: 10.3389/fncel.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osborne KD, Lee W, Malarkey EB, Irving AJ, Parpura V. Dynamic imaging of cannabinoid receptor 1 vesicular trafficking in cultured astrocytes. ASN Neuro. 2009;1(5):AN20090040. doi: 10.1042/AN20090040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pivneva T, et al. Store-operated Ca2+ entry in astrocytes: Different spatial arrangement of endoplasmic reticulum explains functional diversity in vitro and in situ. Cell Calcium. 2008;43(6):591–601. doi: 10.1016/j.ceca.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 48.Volterra A, Liaudet N, Savtchouk I. Astrocyte Ca2+ signalling: An unexpected complexity. Nat Rev Neurosci. 2014;15(5):327–335. doi: 10.1038/nrn3725. [DOI] [PubMed] [Google Scholar]
- 49.Reyes-Haro D, Miledi R, García-Colunga J. Potassium currents in primary cultured astrocytes from the rat corpus callosum. J Neurocytol. 2005;34(6):411–420. doi: 10.1007/s11068-006-8727-z. [DOI] [PubMed] [Google Scholar]
- 50.Miledi R, Eusebi F, Martínez-Torres A, Palma E, Trettel F. Expression of functional neurotransmitter receptors in Xenopus oocytes after injection of human brain membranes. Proc Natl Acad Sci USA. 2002;99(20):13238–13242. doi: 10.1073/pnas.192445299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosas-Arellano A, Ochoa-de la Paz LD, Miledi R, Martínez-Torres A. Brain distribution and molecular cloning of the bovine GABA rho1 receptor. Neurosci Res. 2007;57(3):347–353. doi: 10.1016/j.neures.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 52.Zurek N, Sparks L, Voeltz G. Reticulon short hairpin transmembrane domains are used to shape ER tubules. Traffic. 2011;12(1):28–41. doi: 10.1111/j.1600-0854.2010.01134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moreno C, Sampieri A, Vivas O, Peña-Segura C, Vaca L. STIM1 and Orai1 mediate thrombin-induced Ca(2+) influx in rat cortical astrocytes. Cell Calcium. 2012;52(6):457–467. doi: 10.1016/j.ceca.2012.08.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.