Abstract
There is growing evidence that the radiation effects at low doses are not adequately described by a simple linear extrapolation from high doses, due, among others, to bystander effects. Though several studies have been published on this topic, the explanation of the mechanisms describing the bystander effects remains unclear. This study aims at understanding how the bystander signals are or can be propagated in the cell culture, namely if the number of irradiated cells influences the bystander response. An A549 cell line was exposed to several doses of α-particles, being the bystander response quantified in two non-irradiated areas. The radius of irradiated areas differs by a factor of 2, and the non-irradiated areas were optimally designed to have the same number of cells. Our results show evidence for bystander effects occurring in cells far away from the irradiated ones, meaning that bystander signals can easily spread throughout the cell culture. Additionally, our study highlights that the damage caused by radiation on the surrounding of irradiated areas could be different according to the number of irradiated cells, i.e., for the same dose value; the overall cellular damage could be different.
Keywords: Low dose, spatial distribution, bystander effects, A549 cells, alpha-radiation
INTRODUCTION
Exposures to low dose radiation are ubiquitous, arising both from natural radiation, mainly from radon gas exposure, as well as in occupational exposures, especially in medical and industrial applications, and also in patient exposures in the framework of medical imaging procedures using ionizing radiation for diagnostic purposes.
No doubts exist about the biological effects arising from exposure to high doses of ionizing radiation. However, a lack of understanding persists about the biological effects induced from exposures to low dose radiation. At low doses there is concern about the uncertainties on the risk versus dose relationship, which translates in a significant lack of predictive power about the detrimental or protective effects of exposure to low ionizing radiation doses.
The commonly used approach to model these effects is the linear no-threshold (LNT) model. Although the LNT hypothesis has proven to be suitable for regulatory purposes, it is based on epidemiological data, in the range from 0.2 to 2.5 Sv. This dosimetric range is much higher than the worldwide annual exposures to natural radiation sources estimated in the range 1 to 10 mSv/year, with 2.4 mSv being the present estimate of the central dose (UNSCEAR, 2000). Besides the lack of epidemiological studies at low doses, several biological studies show growing evidence for several low-dose effects which compromise a simply extrapolation from higher doses (Tubiana et al., 2006; Brenner and Sachs, 2006). Harbron (2012) suggested that, at present, there is insufficient evidence to allow for a complete rejection of the LNT model, although the concept has serious limitations. Though radiation-induced bystander effects have been well demonstrated using several biological endpoints in human cell lines (Shao et al., 2004; Suzuki et al., 2004; Sawant et al., 2001), the kinetics and mechanisms by which they occur remains unknown, especially the time and spatial effects of how the bystander factors released in the culture medium are propagated. Hu et al. (2006) reported that increased double strand breaks (DSBs) in irradiated and unirradiated bystander areas could be visualized 2 min after radiation and reached its maximum 30 min after radiation. Moreover, they showed that the bystander signal could be transferred to anywhere in the dish and the percentage of DSBs in the bystander cells were not dependent on dose. Belyakov et al. (2001) reported an increase in micronuclei (MN) formation in an unexposed quadrant of the dish, when scored 3 days later, even though only a single cell is targeted. Some other studies show increase of cell killing among unirradiated areas at 3 days post-irradiation with X-rays (Schettino et al., 2003). These studies suggest a time and distance dependence on the bystander response. Hu et al. (2006) studied how early bystander factors could induce a cellular damage and shown also that the percentage of DSBs in non-irradiated bystander cells was not dependent on the dose delivered. However, our previous studies (Belchior et al., 2012) demonstrated that, using medium transfer technique, dose dependence exists, not only for targeted cells abut also for untargeted ones.
The most relevant cellular lesion, to evaluate the radiation effects, is the DNA DSBs (Hoeijmakers 2001). H2AX is a minor histone residing in the chromosomes and is rapidly phosphorylated upon induction of DSBs (Bonner et al., 2008). This phosphorylation is followed by rapid propagation of H2AX phosphorylation in Megabase areas of DNA flanking the DSB. Usage of a fluorescent labeled antibody (FITC-H2AX Ab) allows detection of these fluorescent foci (H2AX foci) with fluorescence microscopy, which are indicators of DSBs. The induction of DSBs could be visualized 1 min after irradiation and reaches its maximum 10–30 min after irradiation, decreasing approximately 30% after that time (Rogakou and Pilch, 1999).
The aim of the present study was to investigate how the bystander signals released after low-doses of α-radiation are influenced by the number of irradiated cells. For this purpose, a human adenocarcinoma lung epithelial cell line (A549) was exposed to α-particles emitted from 210Po, at doses from 5 up to 100 mGy. A549 cells were chosen as the epithelial lining of the airways forms the first line of defense of the organism against toxic agents (Fujii et al., 2001). Some of the deleterious effects induced in these cells include changes in cell morphology (Bayram et al., 1998), release of inflammatory cytokines (Ohtoshi et al., 1998) and alterations in cellular functions (Stringer and Kobzik, 1998). Epithelial cells are of extreme relevance to evaluate the cellular damage and survival induced at low doses, namely due to natural sources exposures, so, in this work, we choose a 210Po α-source for emitting the same energy as radon particles. In order to investigate if bystander effects are affected by the number of irradiated cells, two irradiated areas were used, differing by a factor of 2 in radius and two distinct bystander areas were defined containing the same number of cells in each. The radiation induced extranuclear/extracellular assay, γ-H2AX, allows us to quantify cellular damage, by means of DSBs, in situ and immediately after irradiation. As a consequence, this assay was applied to evaluate the spatial distribution of bystander effects.
Our results show evidence for bystander effects occurring in cells far away from the irradiated ones, meaning that bystander signals can easily spread throughout the cell culture. Additionally, our study highlights that the damage caused by radiation on the surrounding of irradiated areas could be different according with the number of irradiated cells, i.e., for the same dose; the overall cellular damage could be different.
MATERIALS AND METHODS
Cell culture and 210Po irradiation
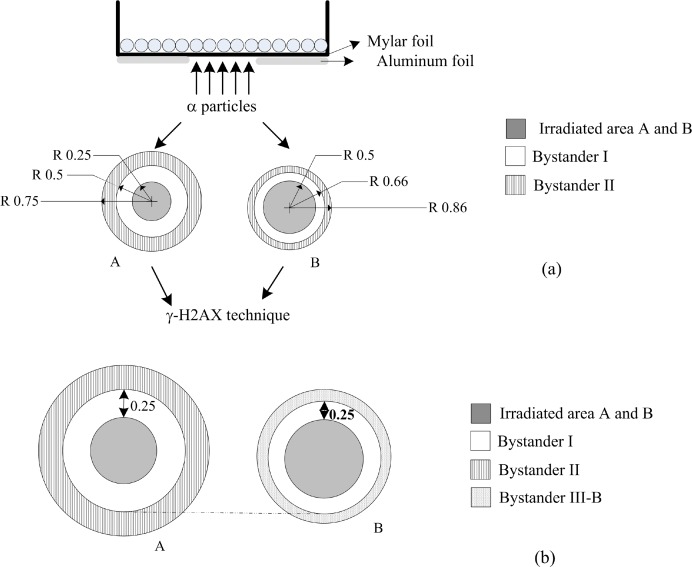
A549 cells were cultured at 37°C with 5% CO2 in Dulbecco’s Modified Eagle’s medium containing 10% fetal bovine serum and 1% penicillin-streptomycin solution (Sigma-Aldrich, St. Louis, USA). Log-phase cells were seeded onto 3.5 cm diameter culture dishes with 6.3 μm of Mylar® foil base, 24 hours before irradiation. Confluent culture cells were exposed to a monoenergetic 210Po source developed by Szabó et al (2002) and characterized by emitting 5.297 MeV α-particles with an average LET of 156 keV/μm (Belchior et al., 2010) and a dose rate of 10 mGy/min. During irradiation, part of the culture dish was shielded using 25 μm of aluminum, and in the unshielded area the cells were irradiated with the average cellular doses of 5, 10, 50 and 100 mGy (Figure 1). Negative control dishes (0 mGy) were exposed to the same conditions of irradiation but 100% shielded. The petri dishes, where the cells were cultured, have 3.5 cm of diameter, and, as mentioned above, some parts of it were covered in order to have distinct unirradiated areas. In both experiments, A and B, the studied areas, irradiated area A, irradiated area B, bystander I and II (referred in Figure 1 as irradiated area A and B, bystander I, II), have an area of 0.19, 0.78, 0.59 and 0.98 cm2, respectively. According to Jiang et al (2010) the A549 cells have a cellular area approximately equal to 700 μm2, which means that an average of 1.3×106 cells is present in the irradiation dish. Assuming this, the number of hits per cell is 16, 32, 160 and 320 for 5, 10, 50 and 100 mGy, respectively.
FIGURE 1.
Schematic view of the culture dish for irradiation. The dimensions are in cm and not at scale. a) The cell dish has 3.5 cm of diameter and depending on the experiment (A) or (B); approximately, 98 or 92% of this area was shielded by aluminum foils, respectively. Unshielded area was exposed to 5, 10, 50 and 100 mGy of α–particles emitted by a 210Po source. b) Schematic view of the bystander III-B area. Immediately after-irradiation the γ-H2AX assay was performed.
Immunofluorescence staining
Immediately after irradiation the cells were washed with phosphate-buffered saline solution (PBS) and fixed with 4% formaldehyde in PBS for 15 minutes. After being washed with PBS, cells were permeabilized with Triton X-100 (0.5%) at room temperature for 5 minutes, washed twice with 1% Bovine Serum Albumin (BSA) (Sigma-Aldrich, St. Louis, USA) and blocked during 1 hour with BSA 4%. Then, cells were incubated with the γ-H2AX primary antibody (mouse anti-γ-H2AX (ser139), Stressgen, bioreagents Corp., Canada) at 2 μg/ml for 2 hours, washed twice with BSA 1%, incubated with FITC-conjugated anti-mouse second antibody (Santa Cruz Biotechnology, USA) at 1mg/ml for 1 hour, washed three times more and incubated with Hoechst (Sigma-Aldrich, St. Louis, USA) at 1μg/ml for 5 minutes and finally mounted with anti-fade (Vectashield mounting medium H-100, Vector Laboratories, Burlingame, Canada). Cells were analyzed at 64x magnification in a fluorescence microscope. Images were randomly obtained in each slide. Image analysis of γ-H2AX foci was performed by the freeware Cellprofiler (Carpenter et al., 2006). At least 100 nuclei were analyzed per experiment per dose.
Treatment with lindane or dimethyl sulfoxide
Prior to irradiation, cells were pretreated for 2h with lindane (Sigma-Aldrich, St. Louis, USA) to inhibit gap junctional intercellular communication (GJIC). Control cells were treated with 0.25% dimethyl sulfoxide (DMSO), an effective scavenger of reactive oxygen species, and 2% PBS as the solvent for lindane. Immediately after irradiation, γ-H2AX assay was performed. The concentration of the two chemicals used has previously been shown to be non-toxic and non-genotoxic to the cells under the condition used in the presented studies (Harada et al., 2009).
Statistical analysis
Data are presented as means ± SEM (standard error of the mean). Significance levels are assessed using the t-student test. Statistical analysis between three independent groups was perfomed by ANOVA.
RESULTS
Induction of DSBs in irradiated cells
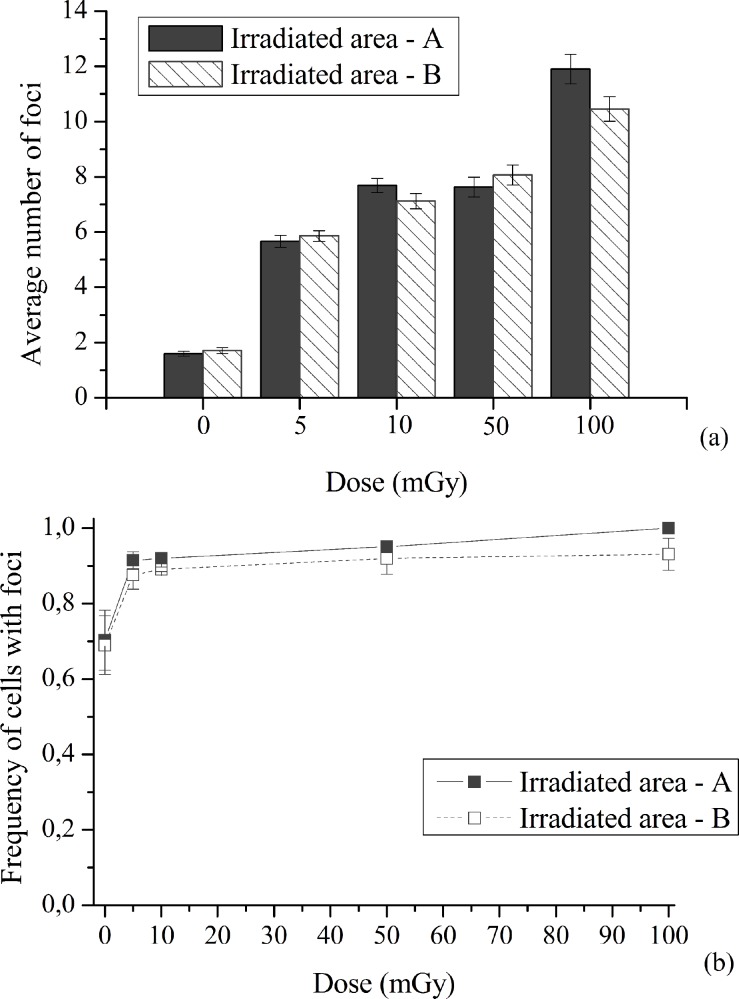
Figure 2 shows; a) the average number of foci per cell in irradiated areas A (lower number of irradiated cells) and B (higher number of irradiated cells), represented in Figure 1 as irradiated areas, for different doses and b) the fraction of cells with foci.
FIGURE 2.
Quantification of nuclear γ-H2AX foci. A549 cells were exposed to 5 to 100 mGy of α-radiation. (a) Induction of DSBs in irradiated cells, by means of foci number, after 5, 10, 50 and 100 mGy of α-radiation. Data were collected from three independent experiments. Error bars represent the SEM. (b) Fraction of A549 cells containing damaged cells, i.e. cells with foci. Data were collected from three independent experiments. Different scales were used to a better visualization.
At both irradiated areas, A and B, the number of foci increased with increasing dose values compared to the matched controls, as shown in Figure 2 (a). When both irradiated areas are compared, the average number of foci per cell is not statistically different. The obtained results seem to indicate that, in the low-dose region, the trend of damage with respect to dose values is not linear, since in the region from 5 up to 50 mGy the induced damage is quite similar (p=0.27). From Figure 2 (b) it is noticeable that A549 cells have a background level of spontaneous damage significantly high, as also observed by Yu et al (2006). The fraction of cells with foci increases with increasing dose values, as a consequence, it can be inferred that a possible situation of overlapped foci, due to a possible clustered DNA damage, has no great implication in our study. As aforementioned, the basal level of foci number per cell is prominent in A549 cells. Even not shown, the main contribution occurs from cells with one focus. As dose increases, it is observable, a higher frequency of cells having more foci per nucleus.
Induction of DSBs in bystander cells
To understand the role of the number of irradiated cells in the damage induced in untargeted cells, the non-irradiated area was divided in two contiguous areas, referred in the sequence as “bystander I” and “bystander II” (see Figure 1). Each bystander areas, I and II, were designed to have the same number of cells.
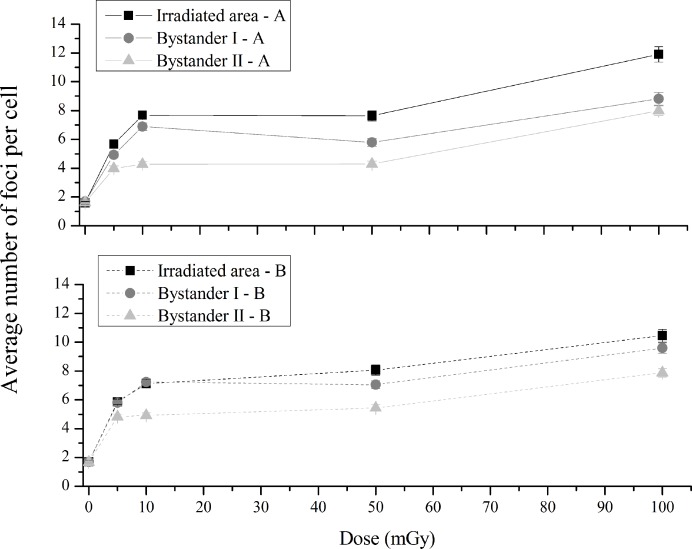
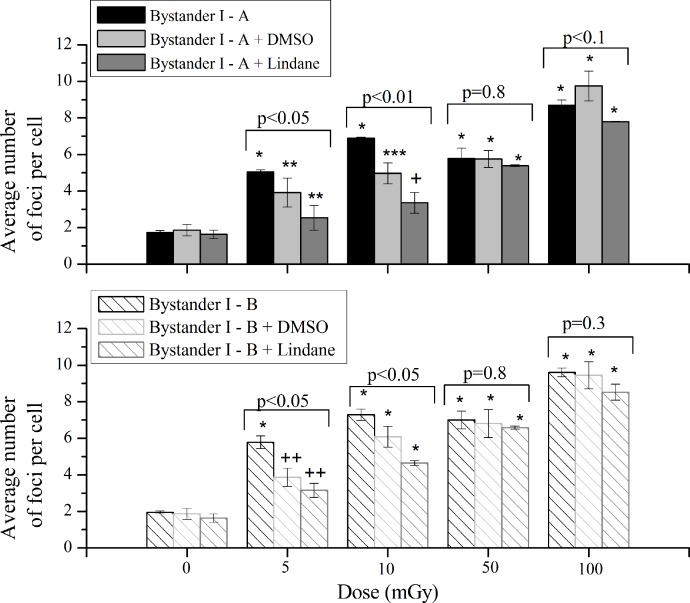
As can be observed in Figure 3 (up), for experiment A, as one moves away from the irradiated area, the induced cellular damage in bystander cells decreases. When irradiated and bystander I areas were compared by dose, the number of foci was significantly lower in bystander I at 5, 10, 50 and 100 mGy (p<0.005, p<0.05, p<0.005 and p<0.05, respectively). Comparing bystander I and II areas, the number of foci decreased in bystander II area at 5 up to 50 mGy (p<0.005, p<0.005 and p<0.1, respectively) and was similar at 100 mGy (p=0.69). Following the same trend as irradiated cells, bystander cells at I - A area also show a radio-hypersensitivity effect. Besides this, the plateau observed, also in bystander areas, between 10 and 50 mGy highlights the non-linear response to radiation.
FIGURE 3.
Average number of foci per cell in the experiment A (up) and B (down) with a lower and a higher number of irradiated cells, respectively. Irradiated – A and B refers to the irradiated area, Bystander I – A and B, the closest to the irradiated cells, refers to the first non-irradiated area and bystander II – A and B to the second non-irradiated area. Data were collected from three independent experiments. Error bars represents the SEM. Note: The lines are purely to guide the eye.
Figure 3 (down) depicts, for experiment-B the average number of foci per cell in the irradiated area, labeled as Irradiated – B, and in the non-irradiated areas, labeled as bystander I - B and II - B, in the case of a higher number of irradiated cells.
When a higher number of cells are irradiated, the trend of the cellular damage, as a dependence of dose, is the same as the one observed for a lower number of irradiated cells. Also, the number of foci decreases with the distance to irradiated area and a hyper-sensitivity is suggested at 10 mGy. When irradiated cells and bystander I – B were compared by dose, the number of foci is similar for all doses (p=0.73 for 5 mGy, p=0.29 for 10 mGy, p=0.36 for 50 mGy and p=0.14 for 100 mGy). By comparing bystander I-B and II-B a statistically significantly decreasing of foci is observed in the II-B area at all dose values (p<0.1, p<0.005, p<0.05 and p<0.05).
Bystander I
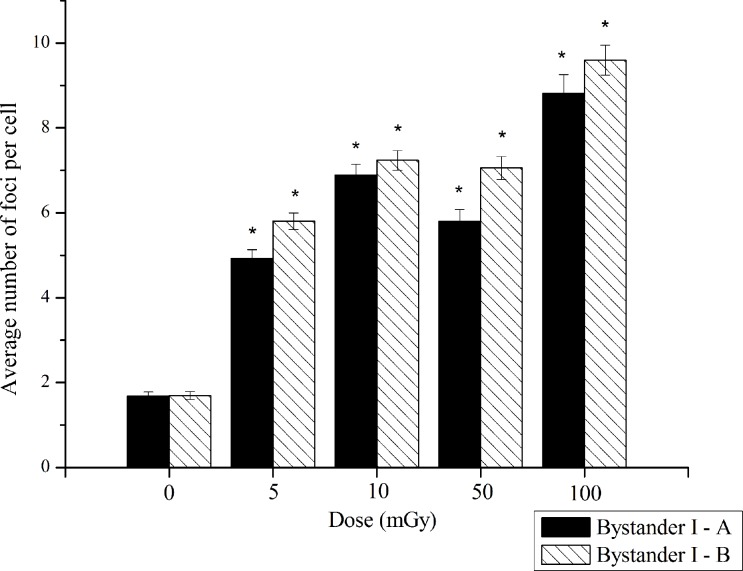
Figure 4 displays the average number of foci per cell in “bystander I-A” and “bystander I-B” areas, i.e. considering a lower and a higher number of irradiated cells, respectively.
FIGURE 4.
Induction of DSBs in the “bystander I-A” and “bystander I-B” areas corresponding to non-irradiated cells in the areas nearest to the irradiated cells, in case of a lower and a higher number of irradiated cells, respectively. Data were collected from three independent experiments. Error bars represents the SEM. *p<0.005 represents the statistically significance between each group and its own controls.
Comparing the number of foci in “bystander I-A” and “bystander I-B” areas (Figure 4), per dose values, it is perceived a tendency for a lower number of DSBs in area I-A, being the statistical differences the following: 5 mGy (p<0.2), 10 mGy (p=0.29), 50 mGy (p<0.2) and 100 mGy (p<0.1).
These results seem to indicate that the number of irradiated cells affects the bystander response near the irradiated area. Due to the largest possibility of cell-to-cell contact at the experiment I-B, as showed in Figure 1, and being the number of DSBs higher at this area, we investigate the role of gap-junctional intercellular communication to further understand the depicted results in Figure 5.
FIGURE 5.
Average number of foci per cell of bystander cells with or without treatment with lindane or DMSO. The data are plotted with the means ± SEM of three independent experiments. *p<0.005, **p<0.02, ***p<0.01, +p<0.1 and ++p<0.05 represents the statistically significance between each group and its own controls The statistical analysis, per dose value, of the data obtained for the three independent treatments was performed by ANOVA analysis.
Role of gap-junctional intercellular communication
Bystander effects are demonstrated in cells that are descendents of irradiated cells either directly or via media transfer or in cells that have communicated with irradiated cells. In this study both situations are allowed, i.e. the bystander signals can be propagated by the medium or the cells can communicate via GJIC. The irradiation of a higher number of cells (experiment B) involves a higher area of cell-to-cell contact between irradiated and non-irradiated cells. The results pinpointed in Figure 5 demonstrated that the induced bystander effects are slightly higher in experiment-B. So, we investigate the role of GJIC in the areas closest to irradiation cells, using Lindane, a GJIC-suppressing agent.
At lower doses (5 and 10 mGy), the number of DSBs was significantly different when cells treated with DMSO or lindane are compared with bystander cells without treatment. At 50 mGy, the number of DSBs was almost the same, in both experiments, with and without treatment. Finally, for 100 mGy the difference between treated and untreated cells is small but in the experiment labeled as “bystander I-B” the same trend of lower doses, i.e., the number of DSBs decrease with DMSO or lindane, with the exception for cells exposed to DMSO is visible. Comparing the number of DSBs, per dose value, obtained after treatment with lindane, an increase is observed at I-B for 10 and 50 mGy (p<0.1 and p<0.005, respectively) and a similarity observed at 5 and 100 mGy (p=0.47 and p=0.18, respectively). With DMSO, the difference between the induced DSBs at each dose value was almost the same at all dose values.
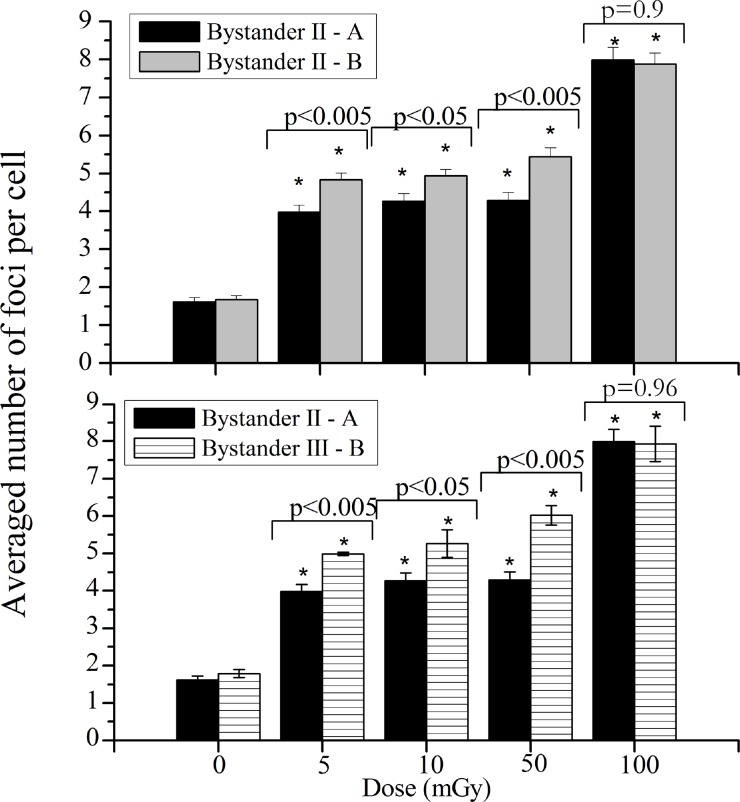
Bystander II
Figure 6 (up) displays the average number of foci per cell in bystander II-A and B areas for the different irradiation dose values.
FIGURE 6.
Induction of DSBs in the “bystander II-A” and “bystander II-B” areas (up) and “bystander II – A” and “bystander III – B” areas (down), corresponding to non-irradiated cells in the areas more distant o the irradiated cells, in case of a lower and a higher number of irradiated cells, respectively. Data were collected from three independent experiments. Error bars represents the SEM and *p<0.005.
Analyzing Figure 6 (up), it can be concluded that the number of irradiated cells influences the bystander response. As can be seen, the cellular damage induced, measured using the foci per cell, in cells located in bystander II-B is higher when compared with bystander II-A, except for 100 mGy.
However, as previously mentioned, in order to maintain the same number of cells in non-irradiated areas, the distance between exposed and bystander cells is different in experiment A and B, i.e., the distance between irradiated areas and bystander II-A (or B) is 0.25 and 0.16 cm, respectively. For this reason, in order to verify that the number of irradiated cells influences the bystander response, a third non-irradiated area, that will be referred to as “bystander III-B” was considered, placed at 0.25 cm from irradiated area.
Figure 6 (down) shows the comparison between non-irradiated cells considering the same distance between irradiated and non-irradiated cells at the position more far away from the exposed area. The comparison of the DSBs induced in non-irradiated areas, II-A and III-B, allows to conclude that the number of irradiated cells influences the bystander effects, since, as obtained in Figure 6 (down), the number of DSBs induced in III-B region is higher when compared with the II-A, except for 100 mGy.
DISCUSSION
One of the main contributions, from natural sources, is related to the exposure to radon and its decay products. After inhalation, the alpha particles from the radon progeny can reach the bronchial epithelial cells. Several studies demonstrated that the spatial distribution of the energy deposited by the alpha particles is non-homogeneous (Balásházy et al., 2002; Balásházy et al., 2009; Farkas and Balásházy, 2008). In our study we used two different irradiation apparatus design in order to investigate whether the number of irradiated cells influences the bystander effect in A549 cells. For this purpose, the A549 cells were exposed to a set of low doses (5, 10, 50 and 100 mGy). A strictly linear relationship between the dose and the DNA damage requires not only that the radiation induced damage in cells follows a mechanism which is independent of DNA damage initiation, but also that every lesion invokes the same trend for malignancy, being independent of cell type or type of radiation. In agreement with Aurengo (2005) who argues that the importance and contribution of each cellular mechanism varies with dose level, our work show that the level of inducible DSBs, in A549 cells, vary with dose and in a non-linear way. As shown in Figure 2, the fraction of A549 cells containing damaged cells rapidly reaches a plateau, after 5 mGy, indicating an altered DNA repair capacity. At 10 mGy, a higher number of DSBs is observed. This effect known as low-dose hypersensitivity effect is described in several human cell lines using different types of radiation (Joiner et al., 1996; Joiner et al., 2001; Marples et al., 2004; Leonard and Lucas, 2009) and is explained mainly by DNA repair capacity. Studies led by Rothkamm and Lobrich (2003) and Grudzenski et al (2010) demonstrate the persistence of DSBs after very low doses of radiation, while lesions induced by higher doses can be rapidly repaired. They concluded that the DSBs repair after very low doses is substantially compromised indicating a total lack of repair below 1-2 mGy and extremely inefficient below 20 mGy. Our results seem to corroborate these studies, showing a similar response between 5 and 50 mGy that can be explained by the aforementioned inefficient repair mechanisms at these doses values. Moreover, in our studies we observed a higher sensitivity to radiation at 10 mGy showing that besides the inefficient repairing capacity of DNA, the cell irradiation itself might not be the unique process to induce cell damage/killing. In cancer cells, as A549 cell line, this higher sensitivity could be, also, explained by an increased oxidative damage as a consequence of mitochondrial oxidative phosphorylation. This will be subject of future work.
Nowadays, the bystander effects play an important role to quantify the low-dose effects and assessment of risk, being extensively reported in the literature. The ever increasing number of studies about these effects shows a lack of knowledge of risk assessment at low-doses, supporting the idea that the models used to estimate the population risk after exposure to low dose radiation must be revised. Following the studies (Nagasawa et al., 2003) suggesting that different metabolic repair systems play an important role in bystander effects, therefore depending on the cell line, we analyzed the behavior of A549 cells in terms of bystander response to α-radiation. The induction of bystander effects require that hit and non-hit cells communicate, which can occur via gap junction intercellular communication (Azzam et al., 1998; Azzam et al., 2001) and/or via the release of various factors into cell culture medium (Iyer et al., 2000; Narayanan et al., 1997). Our results pointed out that the bystander effects at low doses are not negligible when compared to the matched negative controls (p<0.05). Hu et al. (2006) reported that the bystander signal could be transferred to anywhere in the cell culture and the percentage of DSBs in the bystander cells were not dependent on dose. In this work, we also observed that the bystander signals released after cell irradiation can spread easily by the cell culture, and, in addition, influenced by the number of irradiated cells. Moreover, our previous work (Belchior et al., 2013) using a medium transfer technique to quantify the earlier and delayed effects, showed that the cellular damage, in bystander cells, is dependent of dose values and time of incubation after irradiation. As one moves away from the area of irradiation, the bystander effects become less pronounced (Figure 3), but still significantly higher when compared to its own controls. Using a human non-small cell lung cancer cell line, McKenzie (2011) identified the conditions for the 3 types of bystander effects in radiotherapy. Among other results, they found that a higher cell density in the donor flasks (irradiated cells) produces an increased response in the receiver flasks (bystander cells) for the same volume of medium transferred. Using a different technique, medium transfer, to quantify the bystander effects, they found the same results as those obtained for us.
This finding highlights a very important effect revealing, for the same dose value, a different cellular damage in the surrounding of irradiated cells. This may be very important to understand, for instance, the biological effects of radon exposure considering that the deposition of radon progenies’ in lungs is non-homogeneous. Indeed, the dose is calculated taking into account the concentration of radon inhaled during a certain number of hours/days. But, if there are hot-spots, i.e. a higher number of particles deposited in a certain area, resulting from the energy deposition of particles onto bronchial epithelial cells, our results show evidences for a different overall cellular damage.
The results obtained in this work, using an A549 cell line, emphasize that the risks attributable to low dose radiations encompass a complex variable cellular response and cannot simply be extrapolated from higher doses. Besides the evidences for bystander effects occurring in cells far away from the irradiated ones, there are also evidences for low-dose hypersensitivity, supporting the hypothesis that the shape of the curve to estimate the risks attributable to low dose exposure might be non linear.
Acknowledgments
This work was developed in the radiobiology laboratories of the Radiological Protection and Safety Unit (UPSR) at IST/ITN, Instituto Superior Técnico, Universidade Técnica de Lisboa. The work was partially supported by the Portuguese Foundation for Science and Technology (FCT) grant No. SFRH/BD/42172/2007, by IST/ITN and by the IBEB/FCUL project PEst-OE/SAU/UI0645/2011.
REFERENCES
- Aurengo A, Averbeck D, Bonnin A, Le Guen B, Masse R, Monier R, Tubiana M, Valleron AJ. Dose-effect relationships and estimation of the carcinogenic effects of low doses of ionizing radiation. National Academy of Medicine 2005 [Google Scholar]
- Azzam EI, de Toledo SM, Gooding T, Little JB. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluencies of alpha particles. Radiat. Res. 1998;150:497–504. [PubMed] [Google Scholar]
- Azzam EI, Toledo SM, Little JB. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha-particle irradiated to nonirradiated cells. Proc. Natl. Acad. Sci.USA. 2001;98:473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balásházy I, Hofmann W, Farkas A, Szoke I. Modelling carcinogenic effects of low doses of inhaled radon progenies. J. Radiol. Prot. 2002;22:A89–A93. doi: 10.1088/0952-4746/22/3a/316. [DOI] [PubMed] [Google Scholar]
- Balásházy I, Farkas A, Madas B, Hofmann W. Non-linear relationship of cell hit and transformation probabilities in a low dose of inhaled radon progenies. J. Radiol. Prot. 2009;29:147–162. doi: 10.1088/0952-4746/29/2/003. [DOI] [PubMed] [Google Scholar]
- Bayram H, Devalia JL, Sapsford RJ, Ohtoshi T, Miyabara Y, Sagai M, Davies RJ. The effect of diesel exhaust particles on cell function and release of inflammatory madiators from human bronchial epithelial cells in vitro. Am. J. Respir. Cell Mol. Biol. 1998;18:441–448. doi: 10.1165/ajrcmb.18.3.2882. [DOI] [PubMed] [Google Scholar]
- Belchior A, Peralta L, Almeida P, Vaz P. Calibration of an alpha particle irradiator for in vitro cells irradiation. International Journal of Low Radiation. 2010;7(6):500–510. [Google Scholar]
- Belchior A, Monteiro Gil O, Almeida P, Vaz P. Dose and time dependence of targeted and untargeted effects after very low doses of α-particle irradiation of human lung cancer cells. Dose Response. 2013;1(1):431–446. doi: 10.2203/dose-response.12-036.Belchior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyakov OV, Malcolmson AM, Folkard M, Prise KM, Michael BD. Direct evidence for a bystander effect of ionizing radiation in primary human fibroblasts. Br. J. Cancer. 2001;84:674–679. doi: 10.1054/bjoc.2000.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W, Redon C, Dickey J, Nakamura A, Sedelnikova O, Solier S, Pommier Y. γH2AX and cancer. Nat. Rev. Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D, Sachs R. Estimating radiation-induced cancer risks at very low doses: rationale for using a linear no-threshold approach. Radiat. Environ. Biophys. 2006;44:253–256. doi: 10.1007/s00411-006-0029-4. [DOI] [PubMed] [Google Scholar]
- Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, Golland P, Sabatini DM. Cellprofiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas Á, Balásházy I. Quantification of particle deposition in asymmetrical tracheobronchial model geometry. Comput. Biol. Med. 2008;38:508–518. doi: 10.1016/j.compbiomed.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Fujii T, Hayashi S, Hogg JC, Vincent R, Vincent SF. Particulate matter induces cytokine expression in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2001;25:265–271. doi: 10.1165/ajrcmb.25.3.4445. [DOI] [PubMed] [Google Scholar]
- Grudzenski S, Raths A, Conrad S, Rube C, Lobrich M. Inducible response required for repair of low dose radiation damage in human fibroblasts. Cell Biol PNAS. 2010;107:14205–14210. doi: 10.1073/pnas.1002213107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K, Nonaka T, Hamada N, Sakurai H, Hasegawa M, Funayama T, Kakizaki T, Kobayashi Y, Nakano T. Heavy-ion-induced bystander killing of human lung cancer cells: Role of gap junctional intercelular communication. Cancer Sci. 2009;100(4):684–688. doi: 10.1111/j.1349-7006.2009.01093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbron RW. Cancer risks from low dose exposure to ionizing radiation – Is the linear no-threshold model still relevant? Radiography. 2012;18:28–33. [Google Scholar]
- Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- Hu B, Wu L, Han W, Zhang L, Chen S. The time and spatial effects of bystander response in mammalian cells induced by low dose radiation. Carcinogenesis. 2006;27:245–251. doi: 10.1093/carcin/bgi224. [DOI] [PubMed] [Google Scholar]
- Iyer R, Lehnert BE, Svensson R. Factors underlying the cell growth-related bystander responses to alpha particles. Cancer Res. 2000;60:1290–1298. [PubMed] [Google Scholar]
- Jiang RD, Shen H, Paio Y. The morphometrical analysis on the ultrastructure of A549 cells. Romanian Journal of Morphology and Embryology. 2010;51(4):663–667. [PubMed] [Google Scholar]
- Joiner MC, Marples B, Lambin P, Short SC, Turesson I. Hypersensitivity to very-low single radiation doses: its relationship to the adaptive response and induced radioresistance. Mutat. Res. 1996;358:171–183. doi: 10.1016/s0027-5107(96)00118-2. [DOI] [PubMed] [Google Scholar]
- Joiner MC, Marples B, Lambin P, Short SC, Turesson I. Low-dose hypersensitivity: current status and possible mechanisms. Int. J. Radiat. Oncol. Biol. Phys. 2001;49:379–389. doi: 10.1016/s0360-3016(00)01471-1. [DOI] [PubMed] [Google Scholar]
- Leonard BE, Lucas AC. LDR brachytherapy: can low dose rate hypersensitivity from the “inverse” dose rate effect cause excessive cell killing to peripherial connective tissues and organs? Br. J. Radiol. 2009;82:131–139. doi: 10.1259/bjr/66381835. [DOI] [PubMed] [Google Scholar]
- Marples B, Wouters BG, Collis SJ, Chalmers AJ, Joiner MC. Low-dose hyper-radiosensitivity: a consequence of ineffective cell cycle arrest of radiation damaged G2-phase cells. Radiat. Res. 2004;61:247–255. doi: 10.1667/rr3130. [DOI] [PubMed] [Google Scholar]
- McKenzie D. EPSM-ABEC conference. August, Darwin, Northern Territory; Australia: 2011. Bystander effects in radiotherapy of non-small cell lung cancer; pp. 14–18. [Google Scholar]
- Nagasawa H, Huo L, Little JB. Increased bystander mutagenic effect in DNA double-strand break repair-deficient mammalian cells. Int. J. Radiat. Biol. 2003;79:35–41. [PubMed] [Google Scholar]
- Narayanan PK, Goodwin EH, Lehnert BE. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res. 1997;57:3963–3971. [PubMed] [Google Scholar]
- Ohtoshi T, Takizawa H, Okazaki K, Kawasaki S, Takeuchi N, Ohta K, Ito K. Diesel exhaust particles stimulate human airway epithelial cells to produce cytokines relevant to airway inflammation in vitro. J. Allergy Clin. Immunol. 1998;101:778–785. doi: 10.1016/S0091-6749(98)70307-0. [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR. Magabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell. Biol. 1999;146:905–915. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkamm K, Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low X-rays doses. Proc. Natl. Acad. Sci. USA. 2003;100:5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant SG, Randers-Pehrson G, Geard CR, Brenner DJ, Hall EJ. The bystander effect in radiation oncogenesis: I Transformation in C3H 10T1/2 cells in vitro can be initiated in the unirradiated neighbors of irradiated cells. Radiat. Res. 2001;155:397–401. doi: 10.1667/0033-7587(2001)155[0397:tbeiro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Schettino G, Folkard M, Prise KM, Vojnovic B, Held KD, Michael BD. Low-dose studies of bystander cell killing with targeted soft X-rays. Radiat. Res. 2003;160:505–511. doi: 10.1667/rr3060. [DOI] [PubMed] [Google Scholar]
- Shao C, Furusawa Y, Kobayashi Y, Funayam T. Targeted cytoplasmic irradiation induces bystander responses. Proc. Natl. Acad. Sci. USA. 2004;101:13495–13500. doi: 10.1073/pnas.0404930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer B, Kobzik L. Environmental particulate-mediated cytokine production in lung epithelial cells (A549): role of preexisting inflammation and oxidant stress. Journal of Toxicology and Environmental Health, Part A. 1998;55:31–44. doi: 10.1080/009841098158601. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Zhou H, Geard CR, Hei TK. Effect of medium on chromatin damage in bystander mammalian cells. Radiat Res. 2004;162:264–269. doi: 10.1667/rr3226. [DOI] [PubMed] [Google Scholar]
- Szabó J, Fehér J, Pálfalvi J, Balásházy I, Dám AM, Polonyi I, Bogdándi EN. In vitro cell irradiation systems based on 210Po alpha source: construction and characterization. Radiation Measurements. 2002;35:575–578. doi: 10.1016/s1350-4487(02)00089-6. [DOI] [PubMed] [Google Scholar]
- Tubiana M, Aurengo A, Averbeck D, Masse R. The debate on the use of a linear no threshold for assessing the effects of low doses. J. Radiol. Prot. 2006;26:317–324. doi: 10.1088/0952-4746/26/3/N01. [DOI] [PubMed] [Google Scholar]
- UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation) Sources and effects of ionizing radiation Report to the General Assembly with scientific annexes. New York: United Nations; 2000. [Google Scholar]
- Yu T, MacPhail SH, Banáth JP, Klokov D, Olive PL. Endogenous expression of phosphorylated histone H2AX in tumors in relation to DNA double-strand breaks and genomic instability. DNA repair. 2006;5:935–946. doi: 10.1016/j.dnarep.2006.05.040. [DOI] [PubMed] [Google Scholar]