Abstract
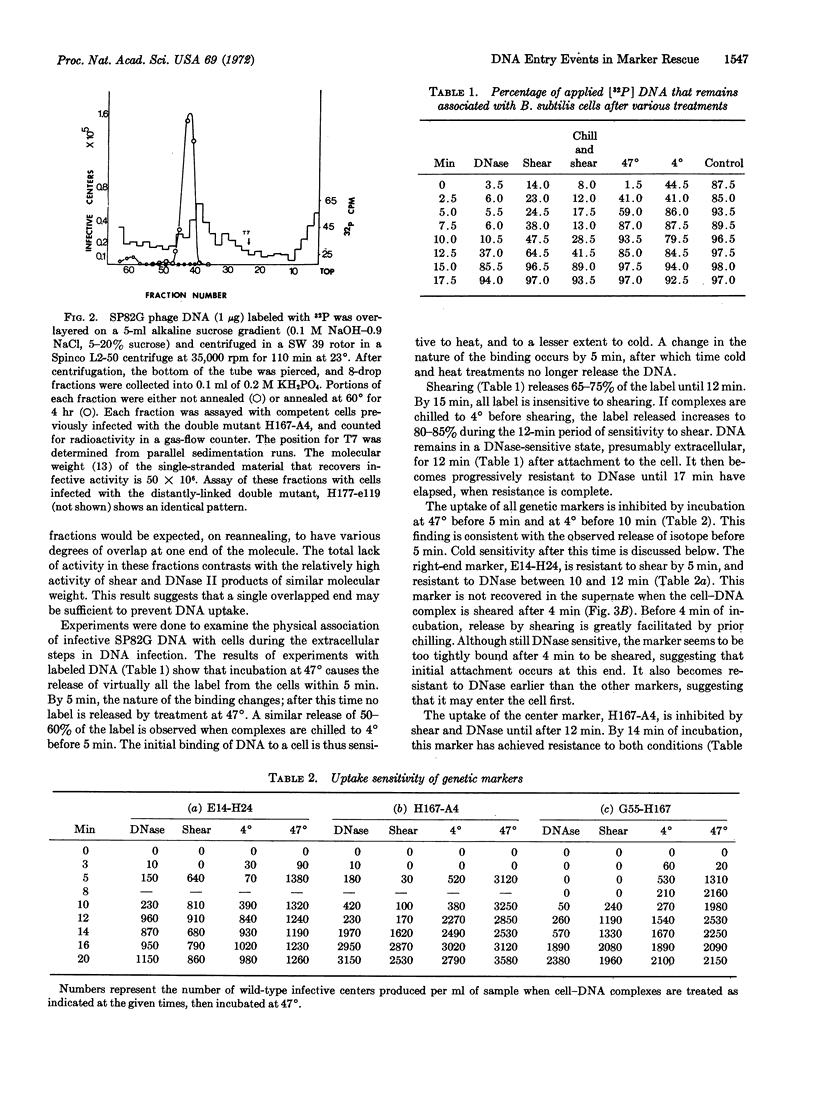
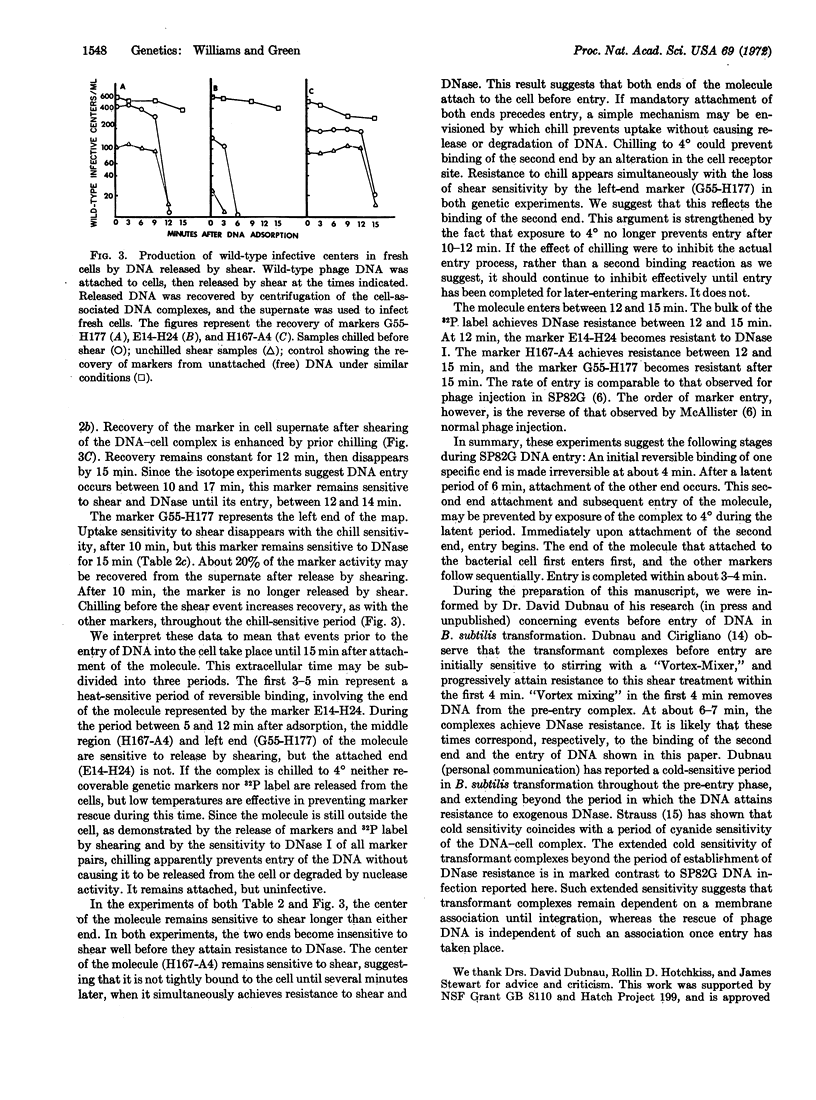
Analysis, by the recovery of specific genetic “markers,” of the effects of DNase I, physical shear, and temperature shock on DNA-cell complexes demonstrates that sequential attachment of both ends of bacteriophage SP82G DNA to Bacillus subtilis precedes entry of the DNA molecule into the cell, and that each attachment is end-and time-specific. The first attachment involves an initial reversible phase, followed by irreversible binding. After a latent period, the second end then attaches to the cell. Entry of the molecule begins immediately after binding of the second end has occurred, and entry is complete within 3 min. The polarity of entry, as judged by attainment of resistance to DNase I, is the reverse of that observed in normal phage injection.
Keywords: “marker rescue”, multi-step binding, polar entry, temperature, shear, DNase sensitivity
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dubnau D., Cirigliano C. Fate of transforming DNA following uptake by competent Bacillus subtilis. IV. The endwise attachment and uptake of transforming DNA. J Mol Biol. 1972 Feb 28;64(1):31–46. doi: 10.1016/0022-2836(72)90319-1. [DOI] [PubMed] [Google Scholar]
- FREIFELDER D. A NOVEL METHOD FOR THE RELEASE OF BACTERIOPHAGE DNA. Biochem Biophys Res Commun. 1965 Jan 4;18:141–144. doi: 10.1016/0006-291x(65)90897-1. [DOI] [PubMed] [Google Scholar]
- GREEN D. M. INFECTIVITY OF DNA ISOLATED FROM BACILLUS SUBTILIS BACTERIOPHAGE, SP82. J Mol Biol. 1964 Dec;10:438–451. doi: 10.1016/s0022-2836(64)80065-6. [DOI] [PubMed] [Google Scholar]
- Gabor M., Hotchkiss R. D. Manifestation of linear organization in molecules of pneumococcal transforming DNA. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1441–1448. doi: 10.1073/pnas.56.5.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan E. A genetic study of temperature-sensitive mutants of the subtilis phage SP82. Virology. 1966 Dec;30(4):650–660. doi: 10.1016/0042-6822(66)90170-x. [DOI] [PubMed] [Google Scholar]
- LEVINE J. S., STRAUSS N. LAG PERIOD CHARACTERIZING THE ENTRY OF TRANSFORMING DEOXYRIBONUCLEIC ACID INTO BACILLUS SUBTILIS. J Bacteriol. 1965 Feb;89:281–287. doi: 10.1128/jb.89.2.281-287.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister W. T. Bacteriophage infection: which end of the SP82G genome goes in first? J Virol. 1970 Feb;5(2):194–198. doi: 10.1128/jvi.5.2.194-198.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NESTER E. W., LEDERBERG J. Linkage of genetic units of Bacillus subtilis in DNA transformation. Proc Natl Acad Sci U S A. 1961 Jan 15;47:52–55. doi: 10.1073/pnas.47.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPIZIZEN J. Genetic activity of deoxyribonucleic acid in the reconstitution of biosynthetic pathways. Fed Proc. 1959 Dec;18:957–965. [PubMed] [Google Scholar]
- STRAUSS N. CONFIGURATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID DURING ENTRY INTO BACILLUS SUBTILIS. J Bacteriol. 1965 Feb;89:288–293. doi: 10.1128/jb.89.2.288-293.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss N. Early energy-dependent step in the entry of transforming deoxyribonucleic acid. J Bacteriol. 1970 Jan;101(1):35–37. doi: 10.1128/jb.101.1.35-37.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss N. Further evidence concerning the configuration of transforming deoxyribonucleic acid during entry into Bacillus subtilis. J Bacteriol. 1966 Feb;91(2):702–708. doi: 10.1128/jb.91.2.702-708.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]



