Abstract
Thousands of articles have been published on the topic of ischemic conditioning. Nevertheless, relatively little attention has been given to assessment of conditioning’s dose-response characteristics. Specifically, the consequences of multiple conditioning episodes, what we will term “hyperconditioning”, have seldom been examined. We propose that hyperconditioning warrants investigation because it; (1) may be of clinical importance, (2) could provide insight into conditioning mechanisms, and (3) might result in development of novel models of human disease. The prevalence of angina pectoris and intermittent claudication is sufficiently high and the potential for daily ischemia-reperfusion episodes sufficiently large that hyperconditioning is a clinically relevant phenomenon. In basic science, attenuation of conditioning-mediated infarct size reduction found in some studies after hyperconditioning offers a possible means to facilitate further discernment of cardioprotective signaling pathways. Moreover, hyperconditioning’s impact extends beyond cytoprotection to tissue structural elements. Several studies demonstrate that hyperconditioning produces collagen injury (primarily fiber breakage). Such structural impairment could have adverse clinical consequences; however, in laboratory studies, selective collagen damage could provide the basis for models of cardiac rupture and dilated cardiomyopathy. Accordingly, we propose that hyperconditioning represents the dark, but potentially illuminating, side of ischemic conditioning - a paradigm that merits attention and prospective evaluation.
Keywords: angina pectoris, collagen, hyperconditioning, infarct size, intermittent claudication, ischemic conditioning
INTRODUCTION
Ischemic conditioning is the intriguing phenomenon whereby brief and transient episodes of ischemia that, by themselves, do not cause tissue necrosis, instead render tissues and organs resistant to a sustained ischemic insult. Three conditioning paradigms have been described so far. The conditioning stimulus in these three differs in terms of the timing or site or both; (1) preconditioning, where one or more episodes of brief ischemia-reperfusion are applied in the same tissue before the sustained ischemic episode (Murry et al. 1986), (2) postconditioning, where the protective stimulus is applied in the same tissue immediately after relief of sustained ischemia (Zhao et al. 2003), and (3) remote conditioning, where the conditioning stimulus can be applied in a distant tissue or organ before, during, or immediately after sustained ischemia; remote pre-, per-, and postconditioning respectively (Przyklenk et al. 1993; Whittaker and Przyklenk 1994; Kerendi et al. 2005; Schmidt et al. 2007). The positive effects of conditioning are well-established in the heart, with reduction of myocardial infarct size serving as the acknowledged gold standard. Importantly, however, the relevance of ischemic conditioning extends beyond the myocardium; conditioning-induced protection has been extensively documented in other organs including (but not limited to) kidney, brain, liver, mesentery and skeletal muscle (Przyklenk 2013).
There are myriad reviews of the conditioning phenomenon. Generally, such reviews focus on the many experiments detailing the cellular and molecular mechanisms involved in eliciting protection (Hausenloy and Yellon 2007; Cohen and Downey 2011; Przyklenk 2013) and also the potential for clinical application (Heusch 2013; Ovize et al. 2013). In comparison, the dose-response aspects of conditioning have received less attention. Some studies have sought to determine the minimum “dose” of ischemia-reperfusion episodes required to provoke protection. Others have examined the effect of the duration of the ischemia-reperfusion episodes and also their temporal relationship to the subsequent prolonged ischemic event. In addition, there has been some interest in what happens when a large number of ischemia-reperfusion episodes occur. Nevertheless, the overarching theme in such dose-response assessments appears to be that conditioning is beneficial and that the worst that can happen is that the protection provided wanes as the “dose” increases and may eventually disappear.
Our goal in this review is to assess the effect of many episodes of ischemia-reperfusion; a phenomenon we term ‘hyperconditioning’. We also aim to illustrate that such over-dosing, in the form of a large number of ischemia-reperfusion episodes, can have adverse effects. To achieve these goals, we will consider both the hallmark of conditioning (protection of cardiac muscle cells from ischemia-reperfusion injury and reduction of infarct size), and another crucial component of the cardiovascular system seldom considered in conditioning studies; collagen.
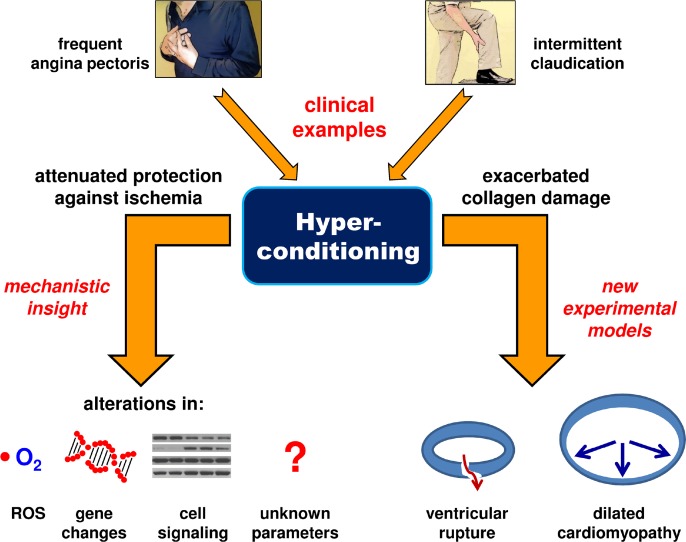
Although few studies have examined hyperconditioning, and some of these did not appreciate the conditioning-related aspects of their results, we propose that now is an opportune time to examine this facet of conditioning. The rationale is two-fold. First, multiple periods of ischemia-reperfusion do indeed occur clinically and perhaps to a greater extent than is often appreciated. Second, investigation of the mechanisms responsible for the conditioning effects, or lack thereof, found after multiple episodes of ischemia-reperfusion could provide new insight and open up novel areas of research in conditioning (Fig. 1).
FIG 1.
Summary of the proposed hyperconditioning paradigm. Clinically, hyperconditioning may occur with angina pectoris and intermittent claudication. Experimentally, hyperconditioning could be used to provide insight into the mechanisms of conditioning and also to provide new models of cardiac disease. [ROS – reactive oxygen species].
In this review, we will use the term conditioning to encompass ischemic preconditioning, perconditioning, postconditioning, and also remote conditioning.
CLINICAL RELEVANCE OF REPEATED EPISODES OF ISCHEMIA-REPERFUSION
Clinical trials of conditioning protocols typically employ no more than a single set of four brief periods of ischemia-reperfusion. No ill-effects have been reported from laboratory studies using such protocols and so it seems equally unlikely that adverse events would occur in clinical application. As far as we are aware, planned multiple episodes of conditioning in humans have only been used in studies designed to assess arterial endothelial function via measurement of flow-mediated dilation (Kimura et al. 2007; Luca et al. 2013; Jones et al. 2014), rather than the gold standard of cardioprotection. These conditioning episodes, administered to the arm by inflation of a standard blood pressure cuff, ranged from three or four daily cycles of five minutes of ischemia-reperfusion applied for seven days to a single, five-minute occlusion, applied six times a day for four weeks. The benefits conferred by conditioning upon endothelial function were maintained or even enhanced after the multiple exposures. However, the generalizability of the results may be limited because all of the studies employed healthy, young (∼20–30 years old), male volunteers.
Nevertheless, there are also circumstances where people are “naturally” exposed to a large number of brief periods of ischemia-reperfusion. In addition, such exposure may occur weekly, daily, or even multiple times per day; for example, in angina pectoris, during transient ischemic attacks (TIA) in the brain, and in intermittent claudication produced by peripheral vascular disease. Although these phenomena are all relatively common, we should first consider the prevalence of high frequency ischemia-reperfusion occurrences in each of these pathologies.
For patients with angina pectoris, frequent episodes are indeed commonplace. For example, the Australian CADENCE (Coronary Artery Disease in General Practice) study, a cross-sectional survey of patients with angina pectoris using a cluster-stratified sampling of 207 general practices throughout the country, found that 29% (95% confidence intervals, 26% to 31%) of 2,031 patients experienced episodes at least once a week (Beltrame et al. 2009). This number included 7% with daily episodes. Daily angina pectoris was also reported to occur in 5% (259) of 5,460 patients four months after hospital admission for acute coronary syndromes in the MERLIN (Metabolic Efficiency with Ranolazine for Less Ischemia in Non-ST-Elevation ACS)-TIMI 36 multinational randomized trial (Arnold et al. 2009).
Transient ischemic attacks (TIA) occur in several hundred thousand people in the United States annually and often presage subsequent stroke (Nguyen-Huynh et al. 2003; Gorelick 2004; Sonni and Thaler 2013). However, a frequently used definition of TIA (“a neurologic deficit lasting less than 24 hours that is attributed to focal cerebral or retinal ischemia” (Johnston 2002)) is not necessarily compatible with the concept that periods of conditioning ischemia should be brief; i.e., a few minutes at most. Moreover, despite positive results from animal experiments and some observational clinical studies, the preconditioning effect of TIA has been questioned (Johnston 2004; Weber et al. 2011). For these reasons, we will not consider TIA further.
Perhaps the largest potential contributor to frequent conditioning episodes comes from limb ischemia caused by peripheral artery disease (PAD). PAD is symptomatically often indicated by the presence of intermittent claudication; leg pain or discomfort induced by walking and relieved by rest. One of the first survey studies to assess PAD in the general population found that the prevalence of intermittent claudication was 4.5% (95% confidence intervals, 3.5% to 5.5%) in 2,720 patients aged 55 to 74 years randomly selected from ten general practices in Edinburgh, Scotland (Fowkes et al. 1991). A multinational Working Group estimated (based upon large population studies) the weighted mean prevalence of intermittent claudication to be 2–3% for the entire population and that it increased to >5% in people older than 65 years (Norgren et al. 2007). However, because PAD is frequently asymptomatic, the total prevalence is likely much higher than indicated by intermittent claudication alone. Because the pain or discomfort associated with intermittent claudication is usually resolved after resting, it is possible to envision that a person could experience multiple episodes of ischemia-reperfusion every day.
Despite the obvious fact that conditioning and intermittent claudication both involve repeated periods of ischemia-reperfusion, there has been little research to connect the two. One recent study documented ‘increased initial claudication distance’ (the walking distance at which patients first experience symptoms) after conditioning ischemia was applied to the arm (Saes et al. 2013). In contrast, this remote conditioning protocol did not increase total walking distance versus an untreated control group of patients also with intermittent claudication (Saes et al. 2013). Of course, it is possible that such studies are confounded by “self-conditioning”; i.e., patients in both groups may have experienced an episode of intermittent claudication before their treadmill test.
Intermittent claudication itself as a trigger of conditioning or remote conditioning has also received little attention. A prospective study of 14 patients with intermittent claudication reported increased (versus baseline) pain-free treadmill walking distance (15% increase; P<0.001) and total walking distance (23% increase; P<0.01) measured after completion of a conditioning protocol comprised of five submaximal treadmill exercises each separated by five minutes of rest (Capecchi et al. 1997). For remote conditioning, the only instance that we are aware of is a letter to the editor (Ozeke et al. 2011). The authors proposed intermittent claudication-mediated conditioning as a potential explanation for why a retrospective observational study (van Straten et al. 2010) of 1,222 patients with PAD found that the disease was not an independent predictor of early mortality (within 30 days) after coronary artery bypass grafting, even though it was a predictor of long-term mortality.
In summary, both angina pectoris and intermittent claudication appear likely to produce multiple conditioning episodes; events that may even occur daily. Moreover, the prevalence of both conditions suggests the potential for a heretofore under-appreciated clinical phenomenon, hyperconditioning, which warrants further investigation.
INFARCT SIZE REDUCTION AFTER HYPERCONDITIONING
There is evidence from laboratory studies to suggest that there may be a limit to the number of conditioning episodes that can be applied before protection is diminished. Some studies, but not all, report that conditioning-mediated protection is reduced or lost after multiple conditioning episodes. For example, in rabbits subjected to 45 minutes of sustained coronary artery occlusion, preconditioning with one or four, five-minute periods of ischemia, each interrupted with ten minutes of reperfusion, had a significant infarct-sparing effect; infarct size expressed as a percentage of the at-risk myocardium averaged ∼20% versus 60% in controls (Iliodromitis et al. 1997). In contrast, infarct size after six and eight cycles of conditioning ischemia averaged 42% and 47% of the area-at-risk, respectively, with the latter result not differing statistically from the control group. The authors also documented that the eight cycle protocol alone did not result in any myocardial necrosis (Iliodromitis et al. 1997).
Cohen and colleagues, also used a rabbit model – in this case, conscious closed-chest rabbits – to examine the effect of multiple conditioning episodes (Cohen et al. 1994). Animals were surgically instrumented with a pneumatic occlusion device encircling a dominant branch of the left coronary artery. After recovery, five-minute periods of coronary artery occlusion were applied every 30 minutes for eight hours per day over three to four days by inflation of the occluder. The total number of conditioning episodes averaged 58 per heart (range 40 to 65). A 30-minute coronary occlusion was then applied ten minutes after the final conditioning episode. Infarct size averaged less than 6% of the area-at-risk in hearts that received a single five-minute episode of preconditioning ischemia, but was ∼27% of the risk region in the multiple exposure group, and ∼38% of the risk region in controls. Finally, in a separate cohort of rabbits, the authors established that the larger infarcts observed in the hyperconditioned group was not due to necrosis caused by the 40–65 episodes of repeated ischemia per se. Thus, in both of the aforementioned studies, the protection achieved by one to four conditioning episodes was markedly attenuated when hearts were exposed to multiple conditioning episodes. It should be noted that neither study examined the mechanism for the apparent attenuation of protection. Tachyphylaxis (i.e., increased tolerance to the effect of an intervention) was suggested as a mechanism; however, the identity of the receptors or effectors that might be involved was neither assessed nor discussed.
In contrast, other studies found preserved protection even after multiple conditioning periods. For example, Li et al. examined the effect of one, six, and twelve conditioning episodes (each of five minutes’ duration followed by ten minutes of reperfusion) in canine hearts prior to a one hour occlusion of the left circumflex artery (Li et al. 1990). Infarct size was similar in all three conditioned groups (3.9%, 0.4%, and 2.9% of the risk region respectively) and was significantly lower than the average of 29.8% found in the control group.
In clinical studies, episodes of angina pectoris have been used as a proxy for conditioning episodes. The issue of multiple conditioning episodes and subsequent loss of protection has been examined in one small prospective clinical study (Papadopoulos et al. 2005). Consecutive patients (n=86) with a first acute myocardial infarction were divided on the basis of whether or not they experienced angina pectoris in the 48 hours before hospital admission. Patients who reported chest pain were further divided into those with one to four episodes versus those with more than four (average 7.3 episodes). Peak plasma creatine kinase-MB concentration (mean ± standard deviation) was used as a measure of injury and was higher in patients who reported no angina pectoris (172±13 IU/L) than in either the 1–4 episode group (75±25 IU/L) or the multi-episode group (65±22 IU/L). Therefore, protection did not appear to be lost when multiple conditioning episodes occurred.
Implications: We recently advocated the merits of examining conditioning “on the edge”; that is, insight may be derived from situations where conditioning is on the border between success and failure (Przyklenk and Whittaker 2013). Hyperconditioning may well provide such an opportunity. For example, if cardioprotection is lost after a certain number of conditioning episodes, what changes occur in terms of inter- and intra-cellular signaling? At the time when the above-mentioned basic science studies were done (1980s and 1990s), the emphasis was on characterization rather than interrogation of molecular mechanisms. Thus, mechanistic studies of hyperconditioning represent an as-yet unexplored opportunity for investigation. One of the few studies to examine potential mechanistic aspects of multiple periods of brief ischemia focused on the quantitative analysis of reactive oxygen species (ROS) (Bolli et al. 1995); although they did so in the context of myocardial stunning rather than conditioning. Canine hearts underwent ten, five-minute episodes of left anterior descending coronary artery occlusion each separated by ten minutes of reperfusion, with ROS production assessed during the first, fifth, and tenth reperfusion interval. ROS generation was greater during the first versus the fifth reperfusion; however, there was no difference between the first and tenth reperfusion periods (samples were only collected at these three times). The mechanisms and implications for the apparent failure of conditioning to prevent ROS formation are unknown.
The effects of repeated ischemia have also been assessed in the conscious pig model; in this case on infarct size and gene regulation (Depre et al. 2010). Three protocols were examined; (1) a single set of two ten-minute coronary artery occlusions separated by ten minutes of reperfusion, (2) repeated sets of occlusions (as described in (1)) applied six times at 12 hour intervals, and (3) 90-minute periods of coronary artery stenosis (flow reduced to 55–65% of baseline) also applied six times at 12 hour intervals. All of these protocols were cardioprotective; infarct size was reduced versus control (no intervention). In contrast, the groups differed with respect to gene response. Specifically, the proportion of up and down-regulated genes shared between the repetitive and single-set occlusion models was lower (43% and 18%) than that shared between the two repetitive models (86% and 72%). Thus, even though six repeated conditioning episodes did not attenuate infarct size reduction, the study illustrated a potentially interesting difference between single and repeated applications of conditioning.
In summary, the effects of hyperconditioning have attracted little attention in terms of mechanistic investigation, especially related to the potential loss of cardioprotection. We propose that this represents a potentially fruitful area of investigation for both basic science and clinical research.
COLLAGEN AND HYPERCONDITIONING
As discussed in the previous section, as many as 60 brief five-minute episodes of ischemia and reperfusion did not result in cardiac muscle necrosis. Nonetheless, muscle is not the only component of the myocardium (and other tissues) that merits consideration as the beneficiary, or victim, of conditioning’s influence. For instance, collagen is an important structural element that has received virtually no attention in conditioning studies. A limited number of studies have examined the potential for conditioning to accelerate or enhance healing and scar formation post-infarction and, as part of this, have examined collagen. For example, remote conditioning failed to enhance healing, as indicated by changes in tissue hydroxyproline content, after bowel anastomosis surgery in rats (Colak et al. 2007; Holzner et al. 2011). Conversely, post-conditioning was reported to exert a favorable effect on repair at one and six weeks after myocardial infarction in rats by reduction of the area occupied by collagen and an attenuation in expression of collagen types I and III (Wang et al. 2013). However, histological assessment of collagen content was performed with trichrome staining which is known to underestimate collagen content (Whittaker et al. 1994).
A remote postconditioning study in rats used daily application of four, five-minute cycles of hind limb ischemia and five minutes of reperfusion, administered repeatedly for 28 days after a 45-minute left coronary artery occlusion (Wei et al. 2011). In addition, the rats received a four cycle remote perconditioning treatment during the coronary occlusion. Daily conditioning attenuated the increase in collagen content found in the region adjacent to the infarct with perconditioning alone; however, tri-chrome staining was again used to quantify collagen content.
It is important to recognize that evaluation at this late period (28 days) after infarction assesses the effects of conditioning on collagen production (or lack thereof) rather than the direct and acute effect on the existing collagen matrix. Indeed, the potential direct effect of conditioning episodes on collagen also merits consideration. Despite its mechanical strength and its frequently perceived inert nature, collagen is damaged during prolonged ischemia (Sato et al. 1983; Takahashi et al. 1990). For example, using electron microscopy, Sato and colleagues examined myocardial collagen at 20, 40, and 120 minutes after coronary artery occlusion in pigs (Sato et al. 1983). Changes were not apparent until 40 minutes post-occlusion. At this time, glycoproteins associated with collagen and elastin fibrils began to be lost. When the period of sustained ischemia was prolonged to 120 minutes, collagen loss increased and there was also disruption of collagen fibrils. At the level of the light microscope, we observed a loss in birefringence of picrosirius red-stained collagen fibers (an optical property indicative of decreased molecular anisotropy) within the infarct at one day after permanent coronary artery occlusion in rat hearts (Whittaker et al. 1991a). This observation is consistent with structural degradation of collagen molecules. Furthermore, several studies have found that loss of so-called ‘collagen struts’ (fibers that provide lateral connections between muscle cells (Robinson et al. 1987)) is associated with dilation of the heart (Weber et al. 1988; Whittaker et al. 1991a; Caulfield et al. 1992) and that pronounced collagen loss was associated with cardiac rupture (Factor et al. 1987).
These observations of collagen damage (and its attendant deleterious consequences) with prolonged ischemia raise the obvious question; can brief periods of ischemia-reperfusion produce similar injury? Two studies, conducted in different laboratories, examined 12, five-minute coronary artery occlusion each separated by ten minutes of reperfusion (Whittaker et al. 1991b; Charney et al. 1992). One measured collagen content using hydroxyproline assay and found regional reductions of ∼12–15% in the midmyocardium and subepicardium versus unexposed controls (Charney et al. 1992). However, there was no reduction when the entire ischemic region was sampled (3.62±0.71 μg/mg dry weight versus 3.96±1.02 μg/mg in control tissue; P = 0.094) despite a measured increase in collagenase. Our group, using histological rather than biochemical assessment to measure collagen content, found no loss in hearts subjected to 12 repeated occlusion-reperfusion episodes (9.5±0.7%) versus either single occlusion-reperfusion (10.5±0.4%) or control tissue (8.5±0.8%) (Whittaker et al. 1991b). Nevertheless, there was some apparent breakage and disruption of collagen fibers and loss of birefringence detected in silver-stained, but not in picrosirius red-stained sections. This latter difference was attributed to loss of proteoglycans associated with the collagen fibers; silver binds to proteoglycans, whereas Sirius red dye molecules are bound by collagen. Some have suggested that proteoglycans play an important role in collagen mechanics (Silver et al. 2003) and, consistent with this premise, we found that disruption of proteoglycan-collagen binding was associated with mitral valve prolapse (Whittaker et al. 1987). However, the importance of proteoglycans in mediating the mechanical properties of collagen has recently been questioned (Fessel and Snedeker 2009). In any case, such changes in the collagen matrix were seen only after multiple brief episodes of ischemia-reperfusion and never after a single episode of ischemia-reperfusion. In summary, although neither study was designed to assess conditioning (and despite the methodological differences in the assessment of collagen), both are consistent with multiple conditioning episodes causing collagen damage.
Implications: The collagen injury caused by numerous brief ischemic episodes is likely to provoke subsequent inflammation and healing. We speculate that the resultant production of additional collagen could adversely affect both systolic and diastolic function and may even hasten the onset of heart failure. However, we are unaware of any epidemiological or clinical studies to support this concept.
Although collagen damage and ensuing remodeling could have negative clinical consequences, there is also potential utility to this type of injury in terms of developing animal models of human pathology. For example, the damage could be exploited to produce models of cardiac rupture, dilated cardiomyopathy, and fibrotic heart failure. Exacerbated collagen injury produced by repeated brief ischemic episodes prior to an appropriately delayed longer occlusion (to prevent preconditioning-induced myocyte protection) or permanent occlusion could be sufficient to provoke ventricular rupture. At present, permanent left coronary artery occlusion in the mouse represents the only animal model of cardiac rupture, which typically occurs 3–5 days later (Gao et al. 2012). However, the majority of human cases of cardiac rupture occur within 48 hours of myocardial infarction and so the etiology likely differs; i.e., the mouse model may require additional collagen injury. Second, in hearts with higher baseline collagen content than the ∼1% found in the mouse (for example, the rat; ∼4%), multiple brief ischemic episodes might be used to promote ventricular dilation through breakage of collagen struts and other fibers. Typically, models of dilated cardiomyopathy rely on either chronic ventricular pacing in large animals or genetic manipulation in mice (Recchia and Lionetti 2007). Third, collagen remodeling and increased collagen content caused by the inflammatory/healing response to repeated ischemia-reperfusion episodes could provide a novel model of fibrosis-mediated heart failure. Increased fibrosis typically occurs in the presence of cardiac hypertrophy and hypertension (Creemers and Pinto 2011) and therefore a model absent of these two potential confounding factors might prove useful, especially in the evaluation of fibrosis reduction strategies. We have only considered effects on myocardial collagen; however, this concept is potentially relevant to other tissues and organs. For example, intermittent claudication may have unfavorable consequences on collagen in skeletal muscle. Finally, there is the possibility of systemic effects mediated via activation of matrix metalloproteinases, which are known to be increased after ischemic episodes (Tziakas et al. 2004; Zayani et al. 2013).
CONCLUSION
The effect of hyperconditioning has received little attention even though it appears to be a potentially frequent clinical occurrence. Data from basic science studies indicate that conditioning ischemia exhibits some characteristics of hormesis; i.e., a biphasic dose-response curve (Calabrese 2013). Specifically, benefits (protection against ischemia) are found at low doses; but, loss of these benefits, and even adverse effects (collagen damage), occur at high doses.
Although the “negative” portion of this type of dose-response curve is usually something to avoid, we are reminded of the words of the 19th Century Scottish author and physician Samuel Smiles; “we learn wisdom from failure much more than from success”. Therefore, in the case of ischemic conditioning, we propose that such high-dose failures could yield considerable wisdom. First, mechanistic insight into conditioning may be derived by comparison of cellular response to successful low-dose treatment versus unsuccessful high-dose treatment. Furthermore, the observed adverse effects of high doses on collagen might be exploited to produce novel animal models of human disease. Therefore, although Smiles went on to write, “…and probably he who never made a mistake never made a discovery”, we will make a small edit and suggest that examination of failure leads to discovery.
REFERENCES
- Arnold SV, Morrow DA, Lei Y, Cohen DJ, Mahoney EM, Braunwald E, Chan PS. Economic impact of angina after an acute coronary syndrome: insights from the MERLIN-TIMI 36 trial. Circ Cardiovasc Qual Outcomes. 2009;2:344–353. doi: 10.1161/CIRCOUTCOMES.108.829523. [DOI] [PubMed] [Google Scholar]
- Beltrame JF, Weekes AJ, Morgan C, Tavella R, Spertus JA. The prevalence of weekly angina among patients with chronic stable angina in primary care practices: The Coronary Artery Disease in General Practice (CADENCE) Study. Arch Intern Med. 2009;169:1491–1499. doi: 10.1001/archinternmed.2009.295. [DOI] [PubMed] [Google Scholar]
- Bolli R, Zughaib M, Li XY, Tang XL, Sun JZ, Triana JF, McCay PB. Recurrent ischemia in the canine heart causes recurrent bursts of free radical production that have a cumulative effect on contractile function. A pathophysiological basis for chronic myocardial “stunning”. J Clin Invest. 1995;96:1066–1084. doi: 10.1172/JCI118093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ. Biphasic dose responses in biology, toxicology and medicine: accounting for their generalizability and quantitative features. Environ Pollut. 2013;182:452–460. doi: 10.1016/j.envpol.2013.07.046. [DOI] [PubMed] [Google Scholar]
- Capecchi PL, Pasini FL, Cati G, Colafati M, Acciavatti A, Ceccatelli L, Petri S, de Lalla A, Di Perri T. Experimental model of short-time exercise-induced preconditioning in POAD patients. Angiology. 1997;48:469–480. doi: 10.1177/000331979704800601. [DOI] [PubMed] [Google Scholar]
- Caulfield JB, Norton P, Weaver RD. Cardiac dilatation associated with collagen alterations. Mol Cell Biochem. 1992;118:171–179. doi: 10.1007/BF00299396. [DOI] [PubMed] [Google Scholar]
- Charney RH, Takahashi S, Zhao M, Sonnenblick EH, Eng C. Collagen loss in the stunned myocardium. Circulation. 1992;85:1483–1490. doi: 10.1161/01.cir.85.4.1483. [DOI] [PubMed] [Google Scholar]
- Cohen MV, Downey JM. Is it time to translate ischemic preconditioning’s mechanism of cardioprotection into clinical practice? J Cardiovasc Pharmacol Ther. 2011;16:273–280. doi: 10.1177/1074248411407071. [DOI] [PubMed] [Google Scholar]
- Cohen MV, Yang XM, Downey JM. Conscious rabbits become tolerant to multiple episodes of ischemic preconditioning. Circ Res. 1994;74:998–1004. doi: 10.1161/01.res.74.5.998. [DOI] [PubMed] [Google Scholar]
- Colak T, Turkmenoglu O, Dag A, Polat A, Comelekoglu U, Bagdatoglu O, Polat G, Kanik A, Akca T, Aydin S. The effect of remote ischemic preconditioning on healing of colonic anastomoses. J Surg Res. 2007;143:200–205. doi: 10.1016/j.jss.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Creemers EE, Pinto YM. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc Res. 2011;89:265–272. doi: 10.1093/cvr/cvq308. [DOI] [PubMed] [Google Scholar]
- Depre C, Park JY, Shen YT, Zhao X, Qiu H, Yan L, Tian B, Vatner SF, Vatner DE. Molecular mechanisms mediating preconditioning following chronic ischemia differ from those in classical second window. Am J Physiol Heart Circ Physiol. 2010;299:H752–762. doi: 10.1152/ajpheart.00147.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor SM, Robinson TF, Dominitz R, Cho SH. Alterations of the myocardial skeletal framework in acute myocardial infarction with and without ventricular rupture. A preliminary report. Am J Cardiovasc Pathol. 1987;1:91–97. [PubMed] [Google Scholar]
- Fessel G, Snedeker JG. Evidence against proteoglycan mediated collagen fibril load transmission and dynamic viscoelasticity in tendon. Matrix Biol. 2009;28:503–510. doi: 10.1016/j.matbio.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1991;20:384–392. doi: 10.1093/ije/20.2.384. [DOI] [PubMed] [Google Scholar]
- Gao XM, White DA, Dart AM, Du XJ. Post-infarct cardiac rupture: recent insights on pathogenesis and therapeutic interventions. Pharmacol Ther. 2012;134:156–179. doi: 10.1016/j.pharmthera.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Gorelick PB. Epidemiology of transient ischemic attack and ischemic stroke in patients with underlying cardiovascular disease. Clin Cardiol. 2004;27:II4–11. doi: 10.1002/clc.4960271403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: united at reperfusion. Pharmacol Ther. 2007;116:173–191. doi: 10.1016/j.pharmthera.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet. 2013;381:166–175. doi: 10.1016/S0140-6736(12)60916-7. [DOI] [PubMed] [Google Scholar]
- Holzner PA, Kulemann B, Kuesters S, Timme S, Hoeppner J, Hopt UT, Marjanovic G. Impact of remote ischemic preconditioning on wound healing in small bowel anastomoses. World J Gastroenterol. 2011;17:1308–1316. doi: 10.3748/wjg.v17.i10.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliodromitis EK, Kremastinos DT, Katritsis DG, Papadopoulos CC, Hearse DJ. Multiple cycles of preconditioning cause loss of protection in open-chest rabbits. J Mol Cell Cardiol. 1997;29:915–920. doi: 10.1006/jmcc.1996.0328. [DOI] [PubMed] [Google Scholar]
- Johnston SC. Clinical practice. Transient ischemic attack. N Engl J Med. 2002;347:1687–1692. doi: 10.1056/NEJMcp020891. [DOI] [PubMed] [Google Scholar]
- Johnston SC. Ischemic preconditioning from transient ischemic attacks? Data from the Northern California TIA Study. Stroke. 2004;35:2680–2682. doi: 10.1161/01.STR.0000143322.20491.0f. [DOI] [PubMed] [Google Scholar]
- Jones H, Hopkins N, Bailey TG, Green DJ, Cable NT, Thijssen DH. Seven-day remote ischemic preconditioning improves local and systemic endothelial function and microcirculation in healthy humans. Am J Hypertens. 2014;27:918–925. doi: 10.1093/ajh/hpu004. [DOI] [PubMed] [Google Scholar]
- Kerendi F, Kin H, Halkos ME, Jiang R, Zatta AJ, Zhao ZQ, Guyton RA, Vinten-Johansen J. Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res Cardiol. 2005;100:404–412. doi: 10.1007/s00395-005-0539-2. [DOI] [PubMed] [Google Scholar]
- Kimura M, Ueda K, Goto C, Jitsuiki D, Nishioka K, Umemura T, Noma K, Yoshizumi M, Chayama K, Higashi Y. Repetition of ischemic preconditioning augments endothelium-dependent vasodilation in humans: role of endothelium-derived nitric oxide and endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2007;27:1403–1410. doi: 10.1161/ATVBAHA.107.143578. [DOI] [PubMed] [Google Scholar]
- Li GC, Vasquez JA, Gallagher KP, Lucchesi BR. Myocardial protection with preconditioning. Circulation. 1990;82:609–619. doi: 10.1161/01.cir.82.2.609. [DOI] [PubMed] [Google Scholar]
- Luca MC, Liuni A, McLaughlin K, Gori T, Parker JD. Daily ischemic preconditioning provides sustained protection from ischemia-reperfusion induced endothelial dysfunction: a human study. J Am Heart Assoc. 2013;2:e000075. doi: 10.1161/JAHA.112.000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Nguyen-Huynh MN, Fayad P, Gorelick PB, Johnston SC. Knowledge and management of transient ischemic attacks among US primary care physicians. Neurology. 2003;61:1455–1456. doi: 10.1212/01.wnl.0000094204.11766.cc. [DOI] [PubMed] [Google Scholar]
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, Group TIW. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- Ovize M, Thibault H, Przyklenk K. Myocardial conditioning: opportunities for clinical translation. Circ Res. 2013;113:439–450. doi: 10.1161/CIRCRESAHA.113.300764. [DOI] [PubMed] [Google Scholar]
- Ozeke O, Gungor M, Ozer C. Is remote ischemic preconditioning triggered by intermittent claudication secondary to peripheral arterial disease responsible for preventing early mortality after coronary artery bypass surgery? Ann Thorac Surg. 2011;91:333–334. doi: 10.1016/j.athoracsur.2010.06.049. [DOI] [PubMed] [Google Scholar]
- Papadopoulos CE, Karvounis HI, Parharidis GE, Louridas GE. Multiple episodes of ischemic preconditioning are not associated with loss of benefit: preliminary clinical experience. Can J Cardiol. 2005;21:1291–1295. [PubMed] [Google Scholar]
- Przyklenk K. Reduction of myocardial infarct size with ischemic “conditioning”: physiologic and technical considerations. Anesth Analg. 2013;117:891–901. doi: 10.1213/ANE.0b013e318294fc63. [DOI] [PubMed] [Google Scholar]
- Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–899. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- Przyklenk K, Whittaker P. Genesis of remote conditioning: action at a distance—’hypotheses non fingo’? J Cardiovasc Med (Hagerstown) 2013;14:180–186. doi: 10.2459/JCM.0b013e328358c8eb. [DOI] [PubMed] [Google Scholar]
- Recchia FA, Lionetti V. Animal models of dilated cardiomyopathy for translational research. Vet Res Commun 31 Suppl. 2007;1:35–41. doi: 10.1007/s11259-007-0005-8. [DOI] [PubMed] [Google Scholar]
- Robinson TF, Factor SM, Capasso JM, Wittenberg BA, Blumenfeld OO, Seifter S. Morphology, composition, and function of struts between cardiac myocytes of rat and hamster. Cell Tissue Res. 1987;249:247–255. doi: 10.1007/BF00215507. [DOI] [PubMed] [Google Scholar]
- Saes GF, Zerati AE, Wolosker N, Ragazzo L, Rosoky RM, Ritti-Dias RM, Cucato GG, Chehuen M, Farah BQ, Puech-Leao P. Remote ischemic preconditioning in patients with intermittent claudication. Clinics (Sao Paulo) 2013;68:495–499. doi: 10.6061/clinics/2013(04)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Ashraf M, Millard RW, Fujiwara H, Schwartz A. Connective tissue changes in early ischemia of porcine myocardium: an ultrastructural study. J Mol Cell Cardiol. 1983;15:261–275. doi: 10.1016/0022-2828(83)90281-x. [DOI] [PubMed] [Google Scholar]
- Schmidt MR, Smerup M, Konstantinov IE, Shimizu M, Li J, Cheung M, White PA, Kristiansen SB, Sorensen K, Dzavik V, Redington AN, Kharbanda RK. Intermittent peripheral tissue ischemia during coronary ischemia reduces myocardial infarction through a KATP-dependent mechanism: first demonstration of remote ischemic perconditioning. Am J Physiol Heart Circ Physiol. 2007;292:H1883–1890. doi: 10.1152/ajpheart.00617.2006. [DOI] [PubMed] [Google Scholar]
- Silver FH, Freeman JW, Seehra GP. Collagen self-assembly and the development of tendon mechanical properties. J Biomech. 2003;36:1529–1553. doi: 10.1016/s0021-9290(03)00135-0. [DOI] [PubMed] [Google Scholar]
- Sonni S, Thaler DE. Transient ischemic attack: omen and opportunity. Cleve Clin J Med. 2013;80:566–576. doi: 10.3949/ccjm.80a.12141. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Barry AC, Factor SM. Collagen degradation in ischaemic rat hearts. Biochem J. 1990;265:233–241. doi: 10.1042/bj2650233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziakas DN, Chalikias GK, Parissis JT, Hatzinikolaou EI, Papadopoulos ED, Tripsiannis GA, Papadopoulou EG, Tentes IK, Karas SM, Chatseras DI. Serum profiles of matrix metalloproteinases and their tissue inhibitor in patients with acute coronary syndromes. The effects of short-term atorvastatin administration. Int J Cardiol. 2004;94:269–277. doi: 10.1016/j.ijcard.2003.05.013. [DOI] [PubMed] [Google Scholar]
- van Straten AH, Firanescu C, Soliman Hamad MA, Tan ME, ter Woorst JF, Martens EJ, van Zundert AA. Peripheral vascular disease as a predictor of survival after coronary artery bypass grafting: comparison with a matched general population. Ann Thorac Surg. 2010;89:414–420. doi: 10.1016/j.athoracsur.2009.11.036. [DOI] [PubMed] [Google Scholar]
- Wang ZF, Wang NP, Harmouche S, Philip T, Pang XF, Bai F, Zhao ZQ. Postconditioning promotes the cardiac repair through balancing collagen degradation and synthesis after myocardial infarction in rats. Basic Res Cardiol. 2013;108:318. doi: 10.1007/s00395-012-0318-9. [DOI] [PubMed] [Google Scholar]
- Weber KT, Pick R, Janicki JS, Gadodia G, Lakier JB. Inadequate collagen tethers in dilated cardiopathy. Am Heart J. 1988;116:1641–1646. doi: 10.1016/0002-8703(88)90763-6. [DOI] [PubMed] [Google Scholar]
- Weber R, Diener HC, Weimar C, German Stroke Study C. Why do acute ischemic stroke patients with a preceding transient ischemic attack present with less severe strokes? Insights from the German Stroke Study. Eur Neurol. 2011;66:265–270. doi: 10.1159/000331593. [DOI] [PubMed] [Google Scholar]
- Wei M, Xin P, Li S, Tao J, Li Y, Li J, Liu M, Li J, Zhu W, Redington AN. Repeated remote ischemic postconditioning protects against adverse left ventricular remodeling and improves survival in a rat model of myocardial infarction. Circ Res. 2011;108:1220–1225. doi: 10.1161/CIRCRESAHA.110.236190. [DOI] [PubMed] [Google Scholar]
- Whittaker P, Boughner DR, Kloner RA. Role of collagen in acute myocardial infarct expansion. Circulation. 1991a;84:2123–2134. doi: 10.1161/01.cir.84.5.2123. [DOI] [PubMed] [Google Scholar]
- Whittaker P, Boughner DR, Kloner RA, Przyklenk K. Stunned myocardium and myocardial collagen damage: differential effects of single and repeated occlusions. Am Heart J. 1991b;121:434–441. doi: 10.1016/0002-8703(91)90709-q. [DOI] [PubMed] [Google Scholar]
- Whittaker P, Boughner DR, Perkins DG, Canham PB. Quantitative structural analysis of collagen in chordae tendineae and its relation to floppy mitral valves and proteoglycan infiltration. Br Heart J. 1987;57:264–269. doi: 10.1136/hrt.57.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker P, Kloner RA, Boughner DR, Pickering JG. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol. 1994;89:397–410. doi: 10.1007/BF00788278. [DOI] [PubMed] [Google Scholar]
- Whittaker P, Przyklenk K. Reduction of infarct size in vivo with ischemic preconditioning: mathematical evidence for protection via non-ischemic tissue. Basic Res Cardiol. 1994;89:6–15. doi: 10.1007/BF00788673. [DOI] [PubMed] [Google Scholar]
- Zayani Y, Allal-Elasmi M, Jacob MP, Zidi W, Zaroui A, Feki M, Mourali S, Mechmech R, Kaabachi N. Peripheral blood levels of matrix and inflammatory mediators are elevated in Tunisian patients with acute coronary syndromes. Clin Lab. 2013;59:169–175. doi: 10.7754/clin.lab.2012.120223. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]