Abstract
Background
Platelet concentrates prepared from whole blood in the U.S. are made using the platelet-rich-plasma (PRP) method. The platelet concentrates must be made within 8 hours of blood collection and stored for only 5 days. In Europe and Canada, platelet concentrates are made using the buffy-coat (BC) method from whole blood held overnight at 22°C and storage times may be up to 7 days. Our studies were designed to determine how long BC platelets can be stored in plasma or Plasmalyte while meeting the FDA’s post-storage viability criteria.
Study Design, Materials, And Methods
Normal subjects donated whole blood that was stored at 22°C for 22 ± 2 hours prior to preparation of BC platelets. Platelets were stored for 5 to 8 days in either plasma or Plasmalyte concentrations of 65% or 80%. Radiolabeled autologous stored versus fresh platelet recoveries and survivals were assessed as well as post-storage in vitro assays.
Results
BC platelets stored in either plasma or 65% Plasmalyte met FDA post-storage platelet recovery criteria for 7 days but survivals for only 6 days, while storage in 80% Plasmalyte gave very poor results. Both stored platelet recoveries and survivals correlated with the same donor’s fresh results, but the correlation was much stronger between recoveries than survivals. In vitro measures of extent of shape change, morphology score, and pH best predicted post-storage platelet recoveries, while annexin V binding best predicted platelet survivals.
Conclusion
BC platelets stored in either plasma or 65% Plasmalyte meet FDA’s post-storage viability criteria for 6 days.
Keywords: Buffy-Coat Platelets, Platelet-Rich-Plasma Platelets, Extended Platelet Storage, Plasmalyte Platelet Storage
INTRODUCTION
Platelet concentrates (PC) from whole blood are prepared using two different techniques: either the platelet-rich-plasma (PRP) method; or the buffy-coat (BC) method. The major difference between the two methods is the centrifugation of the whole blood. In the PRP method, a soft centrifugation of the whole blood is performed, the supernatant PRP is transferred to a storage bag, a hard centrifugation of the PRP is done, the majority of the platelet-poor plasma (PPP) is removed, and the PC is re-suspended in 50–60 ml of PPP.(1) In the BC method, the whole blood is hard spun, the supernatant PPP is removed, the BC is transferred to another bag, the BC is soft spun, and the supernatant platelets are removed to a storage bag.(2) In most blood centers, BCs from 4 to 6 whole blood collections are pooled before the soft spin of the BC is performed, and the BCs are stored as pre-storage pools.(3) Most blood centers in Europe prepare PC as BC, and Canada has recently adopted this system. The United States remains the only major country which still uses the PRP method for preparing PC. Because the platelets are never hard spun against the bottom of the bag but only against the red cell layer, it has been assumed that the quality of BC platelets would be better than PRP platelets. However, when the same normal subjects donated whole blood on two separate occasions allowing a direct comparison of 7-day stored PRP or BC platelets from the two donations, there were no differences observed in the radiolabeled autologous platelet recoveries and survivals nor in the in vitro results between the two products.(4)
We have previously reported the in vivo and in vitro results of extended stored PRP concentrates stored in plasma or 80% Plasmalyte.(5) In the current study, we report similar data for extended storage of BC PC stored in plasma or Plasmalyte. Plasmalyte was selected for use in our studies as it was the only physiologic solution that was currently-licensed for intravenous use that had previously been used for platelet storage.(6)
MATERIALS AND METHODS
Study Population
Healthy subjects who met allogeneic blood donor criteria were recruited to participate in these studies, and each signed a study consent. The study protocol and consent forms were approved by the institutional review board of the University of Washington School of Medicine. Between 3 and 12 normal subjects participated in each of the storage studies. Fewer subjects participated in studies in which the initial data suggested the stored platelets would not meet FDA acceptance criteria.
Preparation Of Stored BC Platelets
Each subject donated 500 ml of blood into a blood bag (Teruflex, Terumo Corporation, Tokyo, Japan) with diversion sampling arm (BB* AGQ456A2, Terumo Corporation) using standard whole blood donation procedures. This bag is used routinely at our blood center and is made of polyvinylchloride (PVC) with a citrate plasticizer. A subject participated in each study only once. All stored platelets met the manufacturers’ guidelines for platelet concentration, total platelet count, and storage volume. The blood was held at 22°C for 22 to 24 hours before preparation of the BC PC as this is the routine procedure for preparing BC platelets.(3) The whole blood storage time was considered to be the first day of platelet storage. The whole blood was then sterilely docked (Terumo SCD 312, Terumo Corporation, Somerset, NJ) to a Fresenius top-bottom bag with the anticoagulant removed (Fresenius Compoflex Top/Bottom bag, Fresenius Corporation, Waltham, MA) to allow transfer of the whole blood. As this bag is not licensed in the U.S., the bag was used under an FDA IND #13798. The blood was centrifuged at 3566 xg for 12 minutes and 30 seconds with standard braking (RC3 BP Thermo Scientific, Waltham, MA). A Compomat device was used (Compomat G4, Fresenius Corporation, Waltham, MA) to remove the BC platelets, and 50 mls of plasma was added. The anticoagulant was removed from a cord blood collection bag (Pall Corporation Collection Set 791–88, Pall Corporation, Port Washington, NY), and the cord blood bag was sterile-docked onto the Fresenius system to allow transfer of the BC to the cord blood bag. The BC was allowed to sit undisturbed for 1.5 hours before centrifugation in a special holder to hold the cord blood bag upright, and centrifugation was done at 511 xg for 5 minutes with standard braking to sediment the platelets. A Terumo platelet storage bag was sterile-docked to the cord blood bag, and the supernatant platelets were transferred to the storage bag without disturbing the sedimented RBCs. The procedures used to prepare the buffy coat platelets were determined by preliminary studies to optimize the platelet yield in the buffy coat platelets.
For storage of platelets in Plasmalyte, between 10 and 20 ml of plasma was left with the PC. Eight or 6.5 mls of Plasmalyte were added for every 2 or 3.5 mls of PC to give an expected final concentration of 80% or 65% Plasmalyte with 20% or 35% residual plasma, respectively. Plasmalyte contains 90 mmol/L NaCl, 5 mmol/L KCl, 27 mmol/L Na-acetate, 23 mmol/L Na-gluconate, and 3 mmol/L magnesium chloride.(6) The platelets were allowed to rest undisturbed at room temperature for 1½ hours. After preparation, both the plasma and Plasmalyte platelets were placed in an incubator (Model PF96, Helmer Corporation, Fort Wayne, Indiana) with continuous agitation at 70 cycles/minute during storage at 22 ± 2°C.
For platelets stored in Plasmalyte, albumin concentrations were determined in a sample of the donor’s plasma and in their PC after the addition of Plasmalyte. Based on the albumin dilution, the percentage of Plasmalyte in the PC was calculated.
Preparation Of Fresh Platelets
Fresh platelets were prepared from a 43 ml sample of whole blood drawn from the subject into 9 mls of Acid-Citrate-Dextrose (ACD-A) anticoagulant. The whole blood was transferred into two 50 ml conical screw-top tubes (Becton Dickson, Franklin Lakes, NJ) and rested for 30 minutes. The tubes were centrifuged at 200 xg for 15 minutes to prepare PRP, and the PRP from each tube was transferred to another conical tube. ACD-A equal to 15% of the PRP volume was added, the PRP was centrifuged at 2000 xg for 15 minutes, and the ACD-A added was calculated to give a pH of 6.5 to allow immediate platelet re-suspension.
In Vitro Platelet Assays
Platelet counts were performed on the day following preparation of the platelets and at the end of storage using a Hematology Analyzer (ABX Diagnostics, Irvine, CA). Also, on the last day of storage, several in vitro measurements of platelet quality were determined, that is, glucose concentration, pH at 37°C, extent of shape change (ESC),(7) hypotonic shock response (HSR),(7) annexin V binding, morphology score,(8) and mean platelet volume (MPV). HSR evaluates a platelet’s ability to recover normal volume after exposure to a hypotonic solution of distilled water. ESC measures the ability of platelets to change from discoid to spherical shapes after addition of ADP. Both of these assays use a whole blood aggregometer (Model 500-Ca, Chrono-Log, Havertown, PA) to measure the response photometrically. For the Plasmalyte-stored platelets, residual donor plasma that had been stored at 4°C was warmed to 22°C and added to the platelets to adjust the platelet count to 300 × 109/L before the ESC and HSR measurements were performed.(9) Annexin V binds to phosphatidyl serine, which becomes expressed on the platelet surface with aging. Annexin V is fluorescently labeled to allow its detection using a flow cytometer (FACSCAN, Becton Dickinson, San Jose, CA).
Platelet Radiolabeling
A 43 ml aliquot of the stored PC was radiolabeled with either 111In or 51Cr, and the opposite label was used to radiolabel the donor’s fresh platelets. Radiolabeling was performed by established techniques, and platelet recoveries and survivals were corrected for elution of the label and for residual radioactivity on day 10 post-transfusion that was considered due to contaminating radiolabeled RBCs.(10) The radiolabeled platelet recoveries and survivals were calculated using the multiple hit COST program.(11) Blood samples were drawn from the donor before, at 2 hours post-infusion, and on days 1, 2, 3, 5, 7, and 10 days post-infusion. The radiolabels used for the fresh and stored platelets were rotated with each sequential subject enrolled in a study so that, at the end of each storage experiment, equal numbers of the subjects’ fresh and stored platelets were labeled with each isotope.
Statistical Methods
Summary statistics for in vitro and in vivo measurements of the quality of stored PC were calculated. Observations from individual units have been grouped by duration of storage (days) and the storage solution (plasma or Plasmalyte). Differences between group means and other linear contrasts have been evaluated for significance. Significance levels have been assessed using the t-distribution. Scatter plots are presented to show the degree of association between stored and fresh in vivo platelet recoveries and survivals. Similarly, scatter plots of in vivo versus in vitro measurements are also presented. Backwards stepwise linear regressions of stored platelet recovery and survival measurements on post-storage in vitro measurements have been fit to determine which in vitro measures may predict post-storage platelet viability.
RESULTS
In Vivo Autologous Radiolabeled Platelet Recoveries And Survivals
Platelet Storage In Plasma
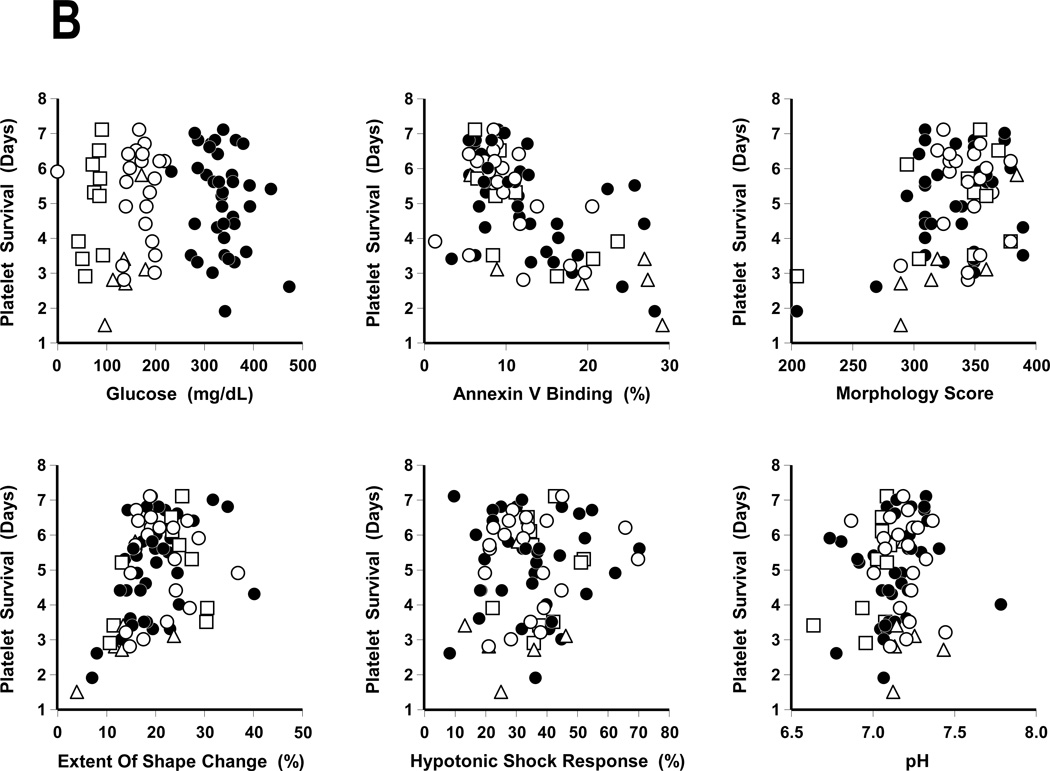
Thirty-eight normal subjects participated in the plasma platelet storage studies. Each subject’s platelets were stored for 5 to 8 days with fresh platelets drawn on the day their stored platelets were transfused. The results are given in Table 1 and Figures 1A and 1B. There were progressive decreases in both platelet recoveries and survivals over storage time.
TABLE 1.
RECOVERIES AND SURVIVALS OF PLATELETS STORED IN PLASMA OR PLASMALYTE
| IN VIVO RADIOLABELED RECOVERIES AND SURVIVALS OF BC PLATELETS STORED IN PLASMA | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Storage Time (Days) |
Observations (#) |
PLATELET RECOVERIES (%) | Stored Recoveries | PLATELET SURVIVALS (Days) | Stored Survivals | ||||
| Fresh | Stored | % of Fresh | 95% LCL | Fresh | Stored | % of Fresh | 95% LCL | ||
| 5 | 10 | 61 ± 13 | 55 ± 16 | 89 ± 10 | 80 | 7.7 ± 1.2 | 5.9 ± 1.1 | 78 ± 16 | 66 |
| 6 | 10 | 67 ± 14 | 54 ± 13 | 80 ± 9 | 74 | 8.1 ± 1.3 | 5.4 ± 1.3 | 67 ± 10 | 60 |
| 7 | 10 | 63 ± 17 | 50 ± 14 | 79 ± 6 | 75 | 8.2 ± 0.8 | 4.8 ± 1.1 | 59 ± 16 | 48 |
| 8 | 8 | 62 ± 12 | 42 ± 14 | 67 ± 13 | 56 | 8.5 ± 0.6 | 3.9 ± 1.4 | 45 ± 15 | 34 |
| IN VIVO RADIOLABELED RECOVERIES AND SURVIVALS OF BC AND PRP PLATELETS STORED IN PLASMALYTE | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Platelets | Plasmalyte Concentration (%) |
Storage Time (Days) |
Observations (#) |
PLATELET RECOVERIES (%) | Stored Recoveries | PLATELET SURVIVALS (Days) | Stored Survivals | ||||
| Fresh | Stored | % of Fresh | 95% LCL | Fresh | Stored | (% of Fresh) | 95% LCL | ||||
| BC | 65 ± 7 | 5 | 6 | 52 ± 8 | 47 ± 10 | 89 ± 11 | 76 | 8.3 ± 0.8 | 5.9 ± 0.7 | 72 ± 13 | 58 |
| BC | 79 ± 4 | 5 | 3 | 56 ± 6 | 46 ± 6 | 83 ± 1 | 76 | 7.1 ± 1.7 | 3.9 ± 1.7 | 53 ± 10 | 30 |
| BC | 67 ± 5 | 6 | 10 | 61 ± 13 | 49 ± 12 | 80 ± 10 | 72 | 8.0 ± 1.5 | 5.5 ± 1.2 | 70 ± 14 | 59 |
| PRP | 65 ± 3 | 6 | 10 | 68 ± 12 | 55 ± 11 | 82 ± 6 | 77 | 8.1 ± 1.4 | 5.0 ± 1.4 | 62 ± 17 | 49 |
| BC | 65 ± 7 | 7 | 5 | 74 ± 3 | 58 ± 3 | 79 ± 5 | 73 | 7.3 ± 1.8 | 4.1 ± 1.6 | 55 ± 10 | 42 |
| BC | 84 ± 3 | 7 | 3 | 59 ± 17 | 29 ± 10 | 49 ± 7 | 28 | 7.9 ± 0.3 | 2.5 ± 1.0 | 32 ± 13 | 1 |
Data reported as average ±1 S.D.
BC – Buffy-coat prepared PC.
PRP – Platelet-rich-plasma prepared PC.
LCL – Lower confidence limit. FDA criteria for 95% LCL for mean stored as a % of fresh - recovery: ≥ 67%; survival: ≥ 58%.
Comparisons of 80% and 65% Plasmalyte stored BC platelets were made and showed recoveries at 5 days of storage were the same, but survivals were significantly less for platelets stored in 80% Plasmalyte (p=0.02). At 7 days of storage, both recoveries and survivals of 80% Plasmalyte stored platelets were significantly less than 65% Plasmalyte stored platelets (both p=0.01).
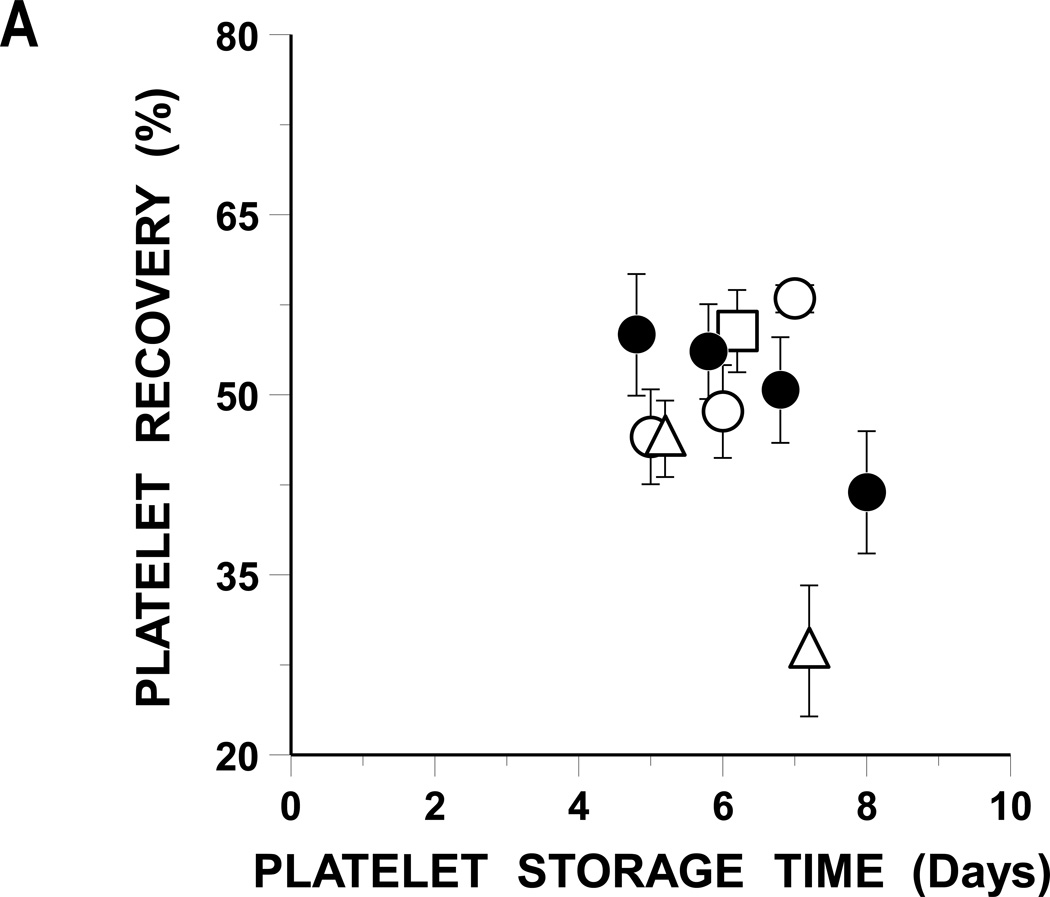
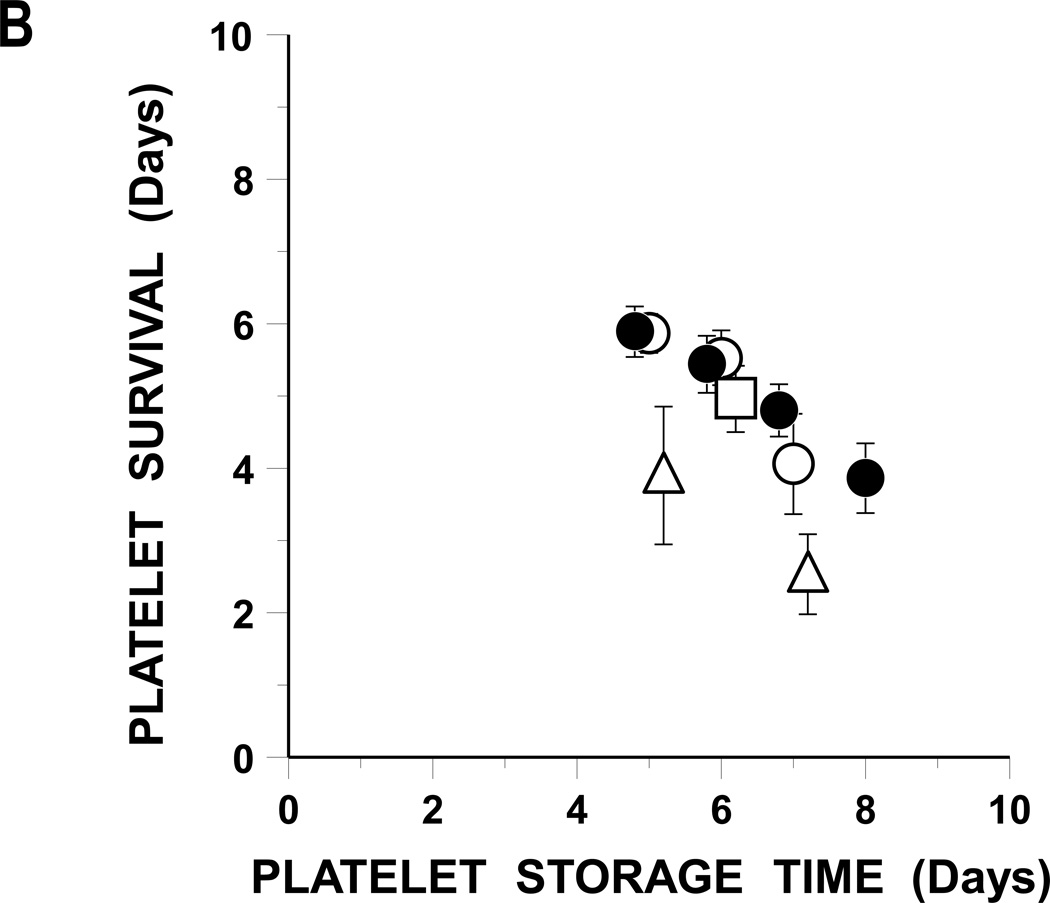
FIGURE 1. Autologous Radiolabeled Platelet Recoveries And Survivals.
A: Mean Platelet Recoveries.
Platelet recoveries (%) are given based on storage time.
B: Mean Platelet Survivals.
Platelet survivals (days) are given based on storage time.
Plasma BC stored platelets (●), BC 65% Plasmalyte (○), BC 80% Plasmalyte (Δ), and PRP 65% Plasmalyte (□). Data are given as the mean ± 1 S.E.
The FDA’s post-storage platelet viability criteria are that the lower 95% confidence limits for mean platelet recoveries as a percentage of the same donor’s fresh recoveries should be ≥67% and for mean survivals should be ≥58% of fresh.(12) Platelet recoveries met FDA post-storage criteria for 7 days, but platelet survivals for only 6 days of storage (Table 1).
Platelet Storage In Plasmalyte
Two different concentrations of Plasmalyte were evaluated; i.e., 80% Plasmalyte with 20% residual plasma or 65% Plasmalyte with 35% residual plasma. Initial studies were done with 80% Plasmalyte, but, because of very poor results, only a few studies were done at this Plasmalyte concentration before switching to a 65% Plasmalyte concentration. In addition, 6-day storage studies were also done with PRP prepared platelets stored in 65% Plasmalyte and 35% residual plasma. The PRP platelets were prepared as previously described except that the whole blood was stored at 22°C for 22 ± 2 hours before preparation of the PC.(5)
Thirty-seven normal subjects participated in the Plasmalyte storage studies, and the results are given in Table 1 and Figures 1 and 2. For these studies, storage times from 5 to 7 days were evaluated. Recoveries and survivals of 65% Plasmalyte-stored platelets were not significantly different from platelets stored in plasma for the same storage times of 5, 6, or 7 days. In addition, 65% Plasmalyte-stored BC platelets met FDA guidelines for 7 days for recoveries and 6 days for survivals, the same as BC plasma-stored platelets. PRP platelets stored in 65% Plasmalyte for 6 days did not differ from BC platelets stored for 6 days in either plasma or Plasmalyte, and these PRP platelets met FDA guidelines for recoveries but not for survivals.
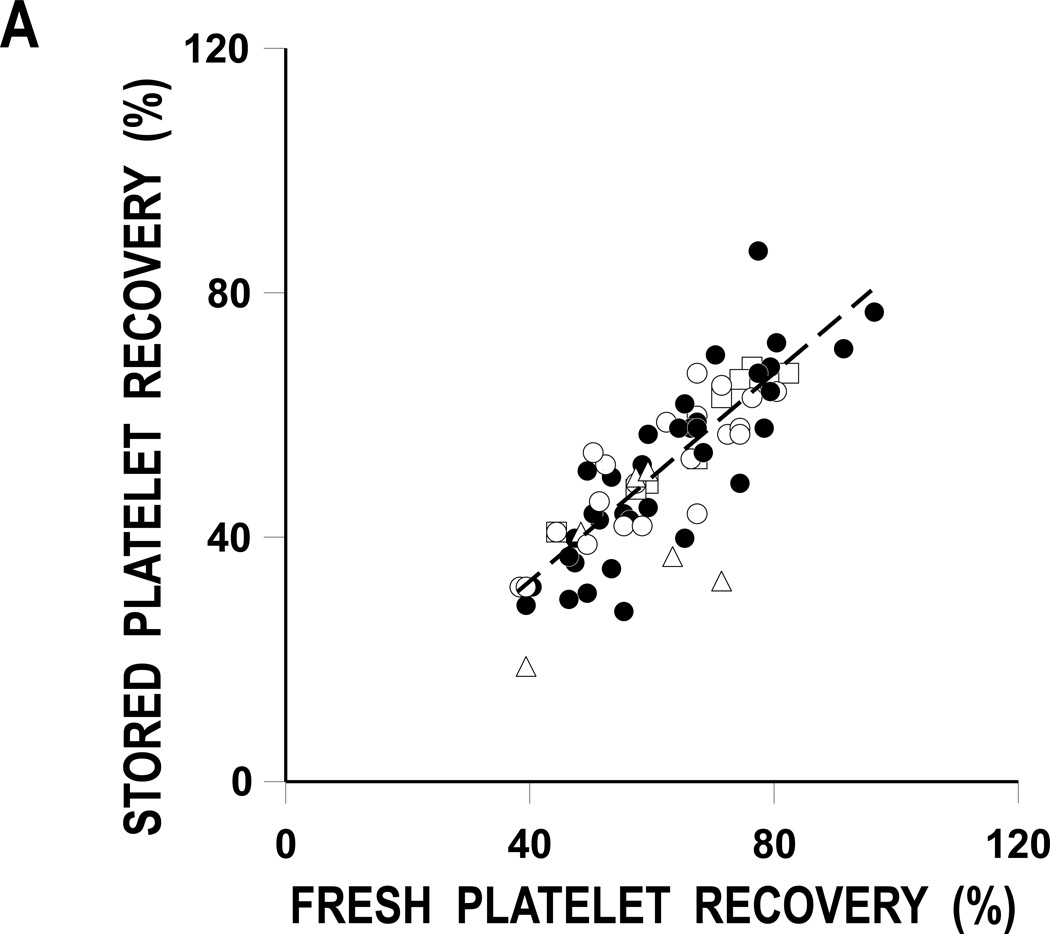
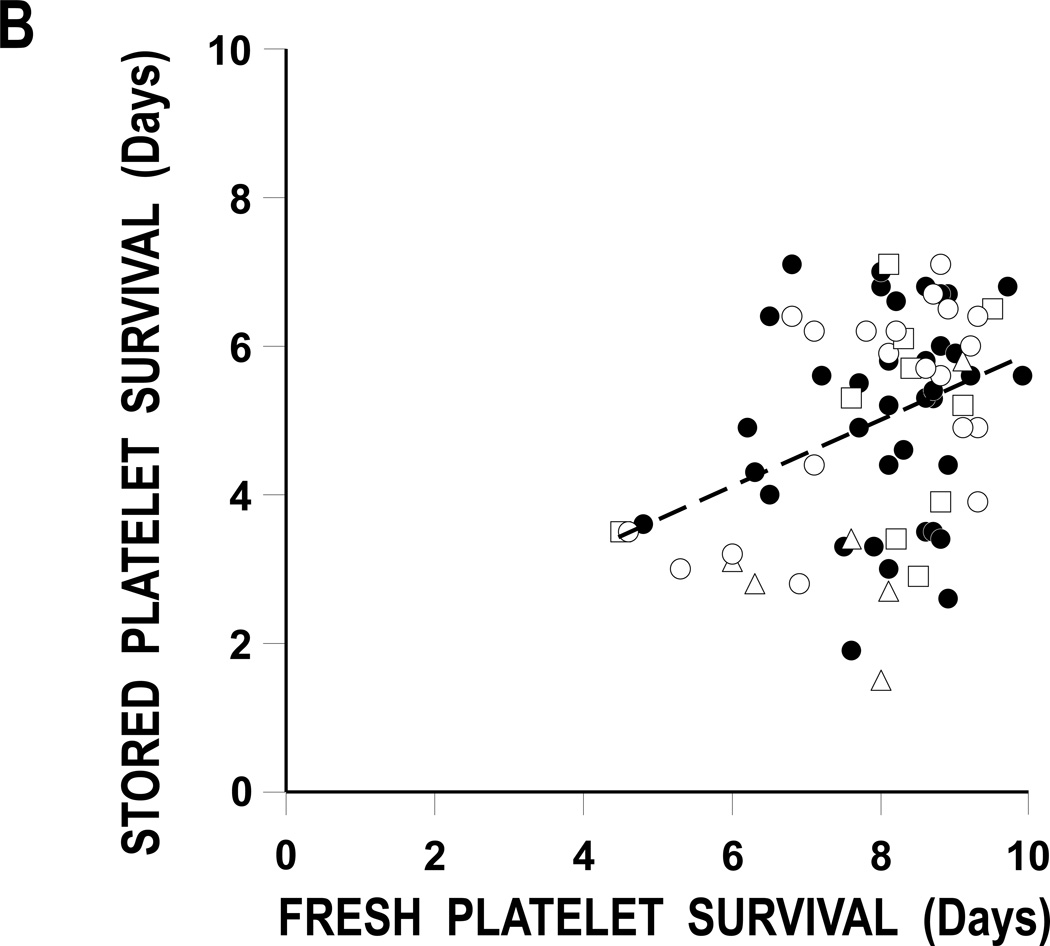
FIGURE 2. Stored Versus Fresh Platelet Recoveries And survivals.
A: Stored Versus Fresh Platelet Recoveries.
B: Stored Versus Fresh Platelet Survivals.
The regression lines for the comparisons of fresh and stored platelet recoveries and survivals are given as the dashed lines. Correlations are 0.83 (p<0.001) and 0.37 (p=0.005) for platelet recoveries and survivals, respectively. Data for BC plasma stored platelets (●), BC 65% Plasmalyte (○), BC 80% Plasmalyte (Δ), and PRP 65% Plasmalyte (□).
BC platelets stored in 80% Plasmalyte for 5 days had recoveries the same as similarly-stored BC platelets in 65% Plasmalyte or in plasma and met FDA guidelines. However, survivals at 5 days of storage were significantly less than both 65% Plasmalyte and plasma-stored PC platelets (p=0.02) and did not meet FDA guidelines. Recoveries and survivals of 80% Plasmalyte-stored BC platelets were markedly less than 65% Plasmalyte or plasma stored platelets after 7 days of storage (both p=0.01) and did not meet FDA storage guidelines.
Correlations Between Fresh And Stored Platelet Recoveries And Survivals
Plots of stored versus fresh platelet recoveries and survivals are given in Figures 2A and B, respectively. The correlations between the recovery and survival variables are 0.83 and 0.37, respectively (p< 0.001 and p=0.005).
In Vitro Platelet Assays
Results of in vitro platelet assays for platelets stored in plasma or Plasmalyte are given in Table 2. Regardless of the method of storage (plasma or Plasmalyte) or the storage time (5 to 8 days), there was no significant loss of platelets during storage. However, the initial platelet counts of the BC PC prepared with 80% Plasmalyte averaged 5.7 × 1010 versus 8.2 × 1010 for 65% Plasmalyte and 9.6 × 1010 for plasma prepared platelets, but these results were not statistically different (p>0.5 and p>0.25 for 80% Plasmalyte platelets versus 65% Plasmalyte and plasma stored platelets, respectively). pHs were all maintained above 6.3 and averaged 7.0 ± 0.1 to 7.3 ± 0.2. MPVs, morphology scores, ESC, and HSR responses were relatively constant over the varying storage times and did not differ between plasma and Plasmalyte-stored platelets for similar storage times. Annexin V binding increased over storage time with no differences between plasma and Plasmalyte stored platelets. As expected, glucose concentrations were significantly less for Plasmalyte- versus plasma-stored platelets at similar storage times (p<0.001). Interestingly, the PRP-platelets stored in 65% Plasmalyte had substantially less residual glucose at the end of 6 days than similarly-stored BC platelets [74 ± 18 mgm/dl versus 174 ± 25 mgm/dl, respectively (p<0.001). However, there were no other in vitro differences between these two products.
TABLE 2.
IN VITRO PLATELET MEASUREMENTS
| STORAGE CONDITIONS | Observations (#) | PLATELET COUNTS | TEST DAY* | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (Days) |
Solution | Plasmalyte Concentration (%) |
Volume of PC (mls) |
Donor (x 108/L) |
Day 1* (x1010) |
Test Day* (x1010) |
pH | MPV | MS | ESC | HSR | Annexin V Binding |
Glucose (mg/dL) |
|
| 5 | Plasma | 86 ± 22 | 10 | 225,000 ± 48,000 | 8.4 ± 2.4 | 8.4 ± 2.7 | 7.3 ± 0.2 | 7.6 ± 0.9 | 343 ± 32 | 23 ± 8 | 36 ± 17 | 11 ± 5 | 340 ± 42 | |
| 5 | Plasmalyte | 67 ± 3 | 75 ± 13 | 7 | 239,000 ± 59,000 | 7.4 ± 2.2 | 7.2 ± 2.3 | 7.2 ± 0.1 | 7.1 ± 0.5 | 352 ± 20 | 21 ± 5 | 45 ± 18 | 9 ± 3 | 157 ± 79 |
| 5 | 79 ± 4 | 66 ± 12 | 3 | 240,000 ± 50,000 | 5.8 ± 1.3 | 5.8 ± 1.1 | 7.2 ± 0.1 | 7.6 ± 1.1 | 353 ± 36 | 17 ± 6 | 33 ± 13 | 14 ± 12 | 155 ± 36 | |
| 6 | Plasma | 80 ± 7 | 10 | 269,000 ± 55,000 | 10.0 ± 2.5 | 9.8 ± 2.4 | 7.1 ± 0.2 | 7.8 ± 0.6 | 344 ± 26 | 20 ± 6 | 32 ± 12 | 9 ± 3 | 332 ± 33 | |
| 6 | Plasmalyte | 67 ± 6 | 99 ± 19 | 10 | 258,000 ± 76,000 | 8.9 ± 2.9 | 8.8 ± 2.7 | 7.2 ± 0.1 | 7.6 ± 0.9 | 345 ± 19 | 21 ± 4 | 33 ± 9 | 8 ± 3 | 174 ± 25 |
| 6 | 65 ± 4 | 93 ± 9 | 10** | 254,000 ± 36,000 | 8.8 ± 1.2 | 9.0 ± 1.2 | 7.0 ± 0.1 | 7.6 ± 1.0 | 332 ± 52 | 22 ± 8 | 39 ± 9 | 12 ± 6 | 74 ± 18 | |
| 7 | Plasma | 96 ± 11 | 10 | 271,000 ± 96,000 | 11.0 ± 3.9 | 10.0 ± 4.3 | 7.1 ± 0.1 | 7.7 ± 0.9 | 338 ± 28 | 20 ± 4 | 42 ± 11 | 14 ± 6 | 331 ± 54 | |
| 7 | Plasmalyte | 65 ± 7 | 83 ± 22 | 5 | 264,000 ± 31,000 | 7.9 ± 1.3 | 7.6 ± 1.1 | 7.2 ± 0.2 | 7.9 ± 0.8 | 332 ± 25 | 20 ± 10 | 27 ± 7 | 16 ± 4 | 156 ± 29 |
| 7 | 84 ± 3 | 62 ± 9 | 3 | 230,000 ± 44,000 | 5.7 ± 1.2 | 5.2 ± 1.3 | 7.2 ± 0.2 | 7.4 ± 0.8 | 300 ± 17 | 10 ± 5 | 25 ± 11 | 25 ± 5 | 124 ± 23 | |
| 8 | Plasma | 99 ± 14 | 8 | 238,000 ± 48,000 | 8.8 ± 2.6 | 8.2 ± 2.5 | 7.1 ± 0.1 | 8.2 ± 0.7 | 311 ± 51 | 14 ± 4 | 29 ± 13 | 17 ± 9 | 349 ± 56 | |
Test day is the last day of storage as given in Column 1.
Data are given as average ±1 S.D.
Data are given for platelet concentrates made on day 1 after whole blood collection.
Platelet-rich-plasma prepared PC. All others are buffy-coat prepared PC.
PC – Platelet Concentrate.
MPV – Mean Platelet Volume.
MS – Morphology Score
ESC – Extent of Shape Change.
HSR – Hypotonic Shock Response.
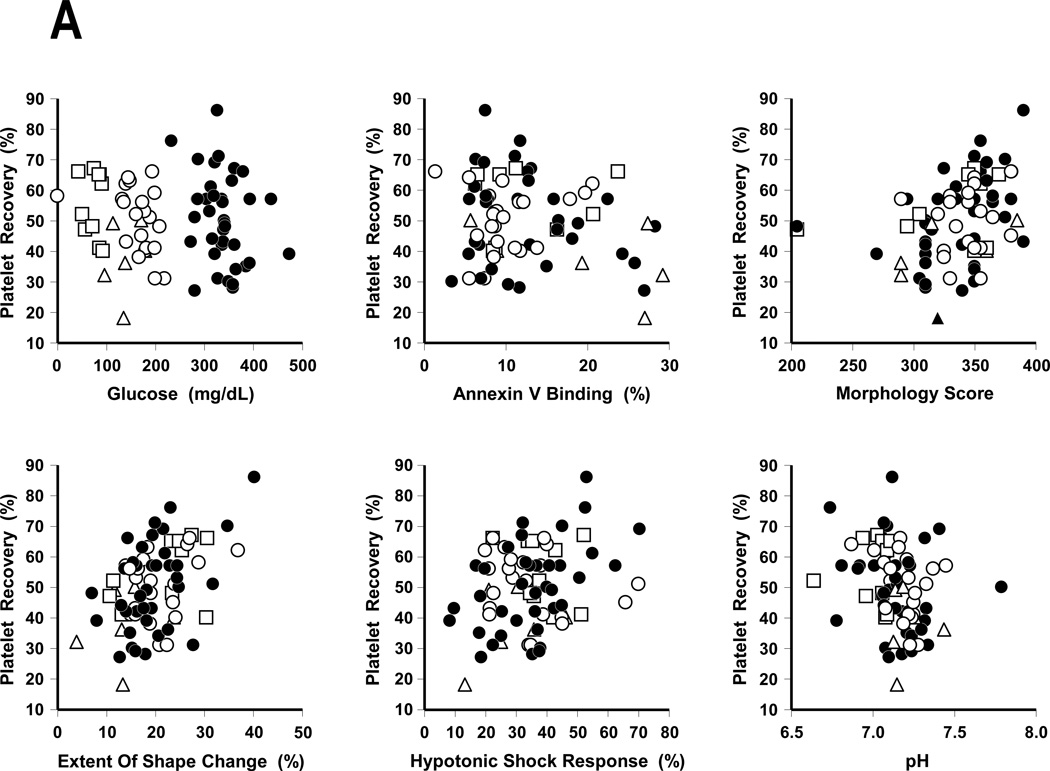
Scatter plots of in vitro variables versus stored platelet recoveries and survivals are given in Figures 3A and B, respectively. Table 3 gives the bivariate correlations between the in vivo variables of platelet recoveries and survivals and the in vitro variables. These values give a numerical summary for the associations seen in Figures 3A and B. The bottom section of the table gives approximate p-values, adjusted for the number of correlations being evaluated.(13) Thus, for example, the estimate of the correlation between morphology score and survival is 0.36, and the approximate p-value for the hypothesis that the correlation between morphology score and survival is 0, is equal to 0.02. In addition to these bivariate models, we fit multivariate regression models to the in vivo variables using backwards, stepwise regression methods to determine which in vitro variables were jointly associated with the in vivo variables. Results for recovery indicate that ESC and pH were jointly associated with it. When three cases with unusual values for in vitro variables (one, pH = 7.79, two with morphology scores = 205) were excluded, the final model also included morphology score. Recovery tends to increase as morphology score and ESC increase, but it tends to decrease as pH increases. When the in vitro variables are considered as a group, these three variables are most strongly associated with platelet recovery. Results from fitting similar models to survival indicate that ESC and annexin V binding are most strongly associated with survival when the in vitro variables are considered as a group. When one case that had a high value of ESC (36.95) and exerted a large influence on the model fit was removed, only annexin V binding was retained, indicating that it is the in vitro variable most strongly associated with survival. As the value of annexin V binding increases, platelet survival tends to decrease.
FIGURE 3. In Vitro Assays Compared to Post-Storage Platelet Recoveries And Survivals.
A: In Vitro Assays Compared To Post-Storage In Vivo Platelet Recoveries.
B: In Vitro Assays Compared To Post-Storage In Vivo Platelet Survivals.
Data for BC plasma stored platelets (●), BC 65% Plasmalyte (○), BC 80% Plasmalyte (Δ), and PRP 65% Plasmalyte (□).
TABLE 3.
RELATIONSHIP BETWEEN IN VITRO AND IN VIVO PLATELET MEASUREMENTS
| CORRELATIONS BETWEEN PLATELET RECOVERY, SURVIVAL, AND IN VITRO MEASURES OF PLATELET QUALITY | |||||||
|---|---|---|---|---|---|---|---|
| Platelet | MPV | Annexin V Binding (%) |
Morphology Score |
ESC (%) | HSR (%) | Glucose (mgm/dl) |
pH |
| Recovery (%) | −0.14 | −0.21 | 0.34 | 0.45 | 0.28 | −0.08 | −0.26 |
| Survival (Days) | −0.39 | −0.59 | 0.36 | 0.39 | 0.10 | 0.05 | 0.10 |
| DUNN-SIDAK PROBABILITIES | |||||||
| Platelet | MPV |
Annexin V Binding (%) |
Morphology Score |
ESC (%) | HSR (%) |
Glucose (mgm/dl) |
pH |
| Recovery (%) | 0.98 | 0.61 | 0.03 | 0.00 | 0.20 | 1.00 | 0.29 |
| Survival (Days) | 0.01 | 0.00 | 0.02 | 0.01 | 1.00 | 1.00 | 1.00 |
MPV = Mean Platelet Volume.
ESC = Extent Of Shape Change.
HSR = Hypotonic Shock Response.
DISCUSSION
These studies have demonstrated that plasma-stored BC platelets meet FDA platelet recovery criteria for 7 days of storage, but platelet survivals for only 6 days. The storage time for all of our studies considered the first day of platelet storage as within the whole blood that was maintained at room temperature for 22–24 hours before platelet concentrate preparation on the day after whole blood collection. These results are the same as we previously reported for PRP plasma-stored PC.(5)
Two different concentrations of Plasmalyte were evaluated in these BC platelet storage studies; i.e., 80% Plasmalyte and 20% residual plasma and 65% Plasmalyte with 35% residual plasma. The use of 80% Plasmalyte was based on experiments using Haemonetics-collected apheresis platelets where concentrations of Plasmalyte between 50% to 82% did not affect either autologous radiolabeled platelet recoveries or survivals for 7-day stored platelets (unreported observations). In our prior studies evaluating PRP PC, the 80% Plasmalyte concentration, which was the only concentration evaluated, resulted in very poor platelet recoveries and survivals for platelets stored for 6 days or longer compared to plasma-stored platelets.(5) For BC platelets, we evaluated both 5 and 7 days of 80% Plasmalyte-stored platelets. The 5-day Plasmalyte-stored platelets had platelet recoveries the same as plasma-stored platelets, but survivals were significantly less (p=0.02). After 7 days of storage, both recoveries and survivals were significantly less than plasma-stored BC platelets (both p=0.01), and neither platelet recoveries nor survivals met FDA post-storage viability criteria.
Because of these very poor results with 80% Plasmalyte stored platelets, we evaluated the 65% platelet additive solution (PAS) concentration with 35% residual plasma that has been used extensively in Europe.(14) Most of the reported studies used a different PAS than Plasmalyte, but these solutions are very similar to Plasmalyte.(14) Using the 65% Plasmalyte concentration, data similar to plasma-stored BC platelets were achieved. Both platelet recoveries and survivals of 65% Plasmalyte stored platelets met FDA criteria for 6 days of storage similar to plasma-stored platelets (Tables 1 and 2 and Figures 1 and 2) Although platelet recoveries met FDA criteria for 7 days of storage, survivals did not.
As the results with 80% Plasmalyte used for storing PRP PC in our prior study were also very poor, we evaluated PRP platelets stored in 65% Plasmalyte for 6 days which was the longest time that 65% Plasmalyte stored BC platelets met FDA criteria. The PRP post-storage platelet recoveries and survivals were the same as BC platelets stored in 65% Plasmalyte or plasma for 6 days. However, these platelets met FDA criteria for recoveries but not survivals.
There is known to be a substantial amount of heterogenicity among donors, even for their fresh, autologous platelet recoveries and survivals.(15) Therefore, Dr. Scott Murphy suggested that, when evaluating stored platelet viability data, each donor should serve as their own control; i.e., each donor’s fresh recoveries and survivals should be compared to their stored.(16) When fresh and stored platelet recoveries and survivals were compared to each other, there was substantial correlations between recoveries (r2 = 0.83, p<0.001) but less so between survivals (r2 = 0.37, p = 0.005) (Figures 3A and 3B, respectively). These correlations between each donor’s fresh and stored data suggest this may be a valid way to determine the quality of stored platelets.
Overall, the results of storing BC platelets in plasma or Plasmalyte were not different than storing PRP platelets for similar times. These data are not different than other studies that compared radiolabeled autologous BC versus PRP platelets in normal volunteers(4,17) and thrombocytopenic patients.(18–20) These data suggest that BC platelets would pass FDA licensing criteria for 6 days of storage and could be used in the U.S. interchangeably with PRP platelet concentrates.
Regarding the in vitro measurements (Table 2), when outliers in the in vitro results were excluded (3 observations for recoveries and 1 for survivals), higher values of ESC and morphology score were associated with improved recoveries, while higher pH values reduced platelet recoveries (Table 3 and Figure 4A). Only annexin V binding was associated with platelet survivals (Table 3 and Figure 4B) with an inverse relationship between these two measurements. We elected not to measure p-selectin levels as a prior study had demonstrated ESC and HSR assays showed better correlation with platelet recoveries and survivals than p-selectin.(21) There were no significant differences between the in vitro measurements of similarly stored plasma and Plasmalyte BC platelets except for lower glucose concentrations for the Plasmalyte stored platelets. Although it has been suggested that enough residual plasma needs to be present to maintain adequate glucose levels, there was no association between glucose levels and either platelet recoveries and survivals; i.e., residual glucose levels did not explain the improved recoveries and survivals of platelets stored in 65% Plasmalyte versus 80% Plasmalyte. Morphology scores, pH, Mean Platelet Volume, ESC, and HSR values were relatively stable over storage times of 5 to 7 days in both plasma and 65% Plasmalyte.
ACKNOWLEDGEMENTS
We gratefully acknowledge the support of Kate Sivertson of Fresenius Kabi who kindly supplied the Fresenius top-bottom bags and Compomat device to allow preparation of the buffy-coat platelets. Dr. Dana Devine of Canadian Blood Services, Vancouver, BC provided information on the centrifugation conditions required to prepare the buffy coat platelets. Also, thanks to Ed Nelson at Pall Corporation who supplied the cord blood bags for centrifuging the buffy coat platelets.
The authors are also grateful to our administrative assistant, Ginny Knight, for her excellent assistance in preparing the manuscript including tables and figures.
This research study was supported by a grant from the National Heart, Lung and Blood Institute, National Institutes of Health (1 P50 HL081015).
Footnotes
Disclaimers: S.J. Slichter: None
D. Bolgiano: None
J. Corson: None
M.J. Jones: None
T. Christoffel: None
S.L. Bailey: None
E. Pellham: None
Conflict of Interest: The authors certify that they have no affiliation with or financial involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in this manuscript.
REFERENCES
- 1.Slichter SJ, Harker LA. Preparation and storage of platelet concentrates. I. Factors influencing the harvest of viable platelets from whole blood. Br J Haematol. 1976;34(3):395–402. doi: 10.1111/j.1365-2141.1976.tb03586.x. [DOI] [PubMed] [Google Scholar]
- 2.Pietersz RN, Reesink HW, Huijgens PC, van Oers MH. Preparation of leucocyte-poor platelet concentrates from buffy coats. Vox Sang. 1988;55:129–132. doi: 10.1111/j.1423-0410.1988.tb05078.x. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Pujol S, Lozano M, Perea D, Mazzra R, Ordinas A, Escolar G. Effect of holding buffy coats 4 or 18 hours before preparing pooled filtered PLT concentrates in plasma. Transfusion. 2004;44:202–209. doi: 10.1111/j.1537-2995.2004.00641.x. [DOI] [PubMed] [Google Scholar]
- 4.Dumont LJ, Dumont DF, Unger ZM, Siegel A, Szczepiorkowski ZM, Corson JS, Jones MK, Christoffel T, Pellham E, Bailey SL, Slichter SJ for the BEST Collaborative. A randomized controlled trial comparing autologous radiolabeled in vivo platelet recoveries and survivals of 7-day stored platelet-rich plasma and buffy coat platelets from the same subjects. Transfusion. 2011;51(6):1241–1248. doi: 10.1111/j.1537-2995.2010.03007.x. [DOI] [PubMed] [Google Scholar]
- 5.Slichter SJ, Bolgiano D, Corson J, Jones MK, Christoffel T. Extended storage of platelet-rich plasma prepared platelet concentrates in plasma or Plasmalyte. Transfusion. 2010;50(10):2199–2209. doi: 10.1111/j.1537-2995.2010.02669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rock G, White J, Labow R. Storage of platelets in balanced salt solutions: A simple platelet storage medium. Transfusion. 1991;31:21–25. doi: 10.1046/j.1537-2995.1991.31191096179.x. [DOI] [PubMed] [Google Scholar]
- 7.Holme S, Moroff G, Murphy S. A multi-laboratory evaluation of in vitro platelet assays: The tests for extent of shape change and response to hypotonic chock. Biomedical Excellence for Safer Transfusion Working Party of the International Society of Blood Transfusion. Transfusion. 1998;38:31–40. doi: 10.1046/j.1537-2995.1998.38198141495.x. [DOI] [PubMed] [Google Scholar]
- 8.Kunicki TJ, Tuccelli M, Becker GA, Aster RH. A study of variables affecting the quality of platelets stored at “room temperature“. Transfusion. 1975;15:414–421. doi: 10.1046/j.1537-2995.1975.15576082215.x. [DOI] [PubMed] [Google Scholar]
- 9.VandenBroeke T, Dumont LJ, Hunter S, Nixon J, Murphy S, Roger J, Herschel L, AuBuchon JP, Gulliksson H, Dengler T, Hornsey V, Prowse C for the Biomedical Excellence for Safer Transfusion Working Party of the International Society of Blood Transfusion. Platelet storage solution effects on the accuracy of laboratory tests for platelet function: a multi-laboratory study. Vox Sang. 2004;86:183–188. doi: 10.1111/j.0042-9007.2004.00412.x. [DOI] [PubMed] [Google Scholar]
- 10.The Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Platelet radiolabeling procedure. Transfusion. 2006;46(Suppl 3):59S–66S. [Google Scholar]
- 11.Lotter MG, Rabe WL, Van Zyl JM, Heyns AD, Wessels P, Kotze HF, Minnaar PC. A computer program in compiled BASIC for the IBM personal computer to calculate the mean platelet survival time with the multiple hit and weighted mean methods. Comput Biol Med. 1988;18:305–315. doi: 10.1016/0010-4825(88)90006-6. [DOI] [PubMed] [Google Scholar]
- 12.Vostal JG. Efficacy evaluation of current and future platelet transfusion products. J Trauma. 2006;60(6 Suppl):S78–S82. doi: 10.1097/01.ta.0000199921.40502.5d. [DOI] [PubMed] [Google Scholar]
- 13.Shaffer JP. Multiple Hypothesis Testing. Ann Rev Psychology. 1995;46:561–584. [Google Scholar]
- 14.Gulliksson H. Additive solutions for the storage of platelets for transfusion. Transfus Med. 2000;10:257–264. doi: 10.1046/j.1365-3148.2000.00262.x. [DOI] [PubMed] [Google Scholar]
- 15.Rock G, Tittley P, Adams GA. Donor variables affecting survival of autologous platelets. Transfusion. 1986;26:30–36. doi: 10.1046/j.1537-2995.1986.26186124027.x. [DOI] [PubMed] [Google Scholar]
- 16.Murphy S. Radiolabeling of PLTs to assess viability: a proposal for a standard. Transfusion. 2004;44(1):131–133. doi: 10.1111/j.0041-1132.2003.00607.x. [DOI] [PubMed] [Google Scholar]
- 17.Cardigan R, Williamson LM. The quality of platelets after storage for 7 days. Transfus Med. 2003;13:173–187. doi: 10.1046/j.1365-3148.2003.00449.x. [DOI] [PubMed] [Google Scholar]
- 18.Keegan T, Heaton A, Holme S, Owens M, Nelson E, Carmen R. Paired comparison of platelet concentrates prepared from platelet-rich plasma and buffy coats using a new technique with 111In and 51Cr. Transfusion. 1992;32:113–120. doi: 10.1046/j.1537-2995.1992.32292180138.x. [DOI] [PubMed] [Google Scholar]
- 19.Shanwell A, Larsson S, Aschan J, Ringdén O. A randomized trial comparing the use of fresh and stored platelets in the treatment of bone marrow transplant recipients. Eur J Haematol. 1992;49:77–81. doi: 10.1111/j.1600-0609.1992.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 20.Bertolini F, Rebulla P, Riccardi D, Cortellaro M, Ranzi ML, Sirchia G. Evaluation of platelet concentrates prepared from buffy coats and stored in a glucose-free crystalloid medium. Transfusion. 1989;29:605–609. doi: 10.1046/j.1537-2995.1989.29789369678.x. [DOI] [PubMed] [Google Scholar]
- 21.Holme S, Sweeney JD, Sawyer S, Elfath MD. The expression of p-selectin during collection, processing, and storage of platelet concentrates: relationship to loss of in vivo viability. Transfusion. 1997;37:12–17. doi: 10.1046/j.1537-2995.1997.37197176945.x. [DOI] [PubMed] [Google Scholar]