Abstract
Objectives
Chelating iron may be a promising new therapy to eliminate Pseudomonas aeruginosa biofilms in the lungs of cystic fibrosis (CF) patients. Here, we investigate whether ALX-109 [a defined combination of an investigational drug containing lactoferrin (an iron-binding glycoprotein) and hypothiocyanite (a bactericidal agent)], alone and in combination with tobramycin or aztreonam, reduces P. aeruginosa biofilms grown on human CF airway epithelial cells.
Methods
P. aeruginosa (PAO1 and six clinical isolates of Pseudomonas) biofilms grown at the apical surface of confluent monolayers of CF airway epithelial cells were treated with ALX-109, either alone or in combination with tobramycin or aztreonam. Bacterial cfu remaining after treatment were determined by plate counting.
Results
ALX-109 alone reduced PAO1 biofilm formation, but had no effect on established biofilms. ALX-109 enhanced the ability of tobramycin and aztreonam to inhibit PAO1 biofilm formation and to reduce established PAO1 biofilms. ALX-109 and tobramycin were additive in disrupting established biofilms formed by six clinical isolates of P. aeruginosa obtained from the sputum of CF patients. Mucoid P. aeruginosa isolates were most susceptible to the combination of ALX-109 and tobramycin. In addition, ALX-109 also enhanced the ability of aztreonam to reduce established PAO1 biofilms.
Conclusions
Inhalation therapy combining hypothiocyanite and lactoferrin with TOBI® (tobramycin) or Cayston® (aztreonam) may be beneficial to CF patients by decreasing the airway bacterial burden of P. aeruginosa.
Keywords: OSCN−, P. aeruginosa, mucoviscidosis
Introduction
Cystic fibrosis (CF), a disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene,1,2 afflicts ∼70 000 individuals worldwide and is still, to this day, irremediable. Mutations in the CFTR gene result in pancreatic and digestive dysfunction as well as chronic respiratory infections and excessive inflammation in the lungs.3 Despite improvement in therapies and a considerable increase in longevity over the past decades,4 chronic airway infections in CF patients, dominated by Pseudomonas aeruginosa for ∼80% of the adult CF population,5,6 still represent a therapeutic challenge. Clinical data suggest that P. aeruginosa forms antibiotic-resistant biofilms in the CF lung,7,8 which hinder the efficacy of currently available antibiotics and preclude the eradication of P. aeruginosa. Previously, we demonstrated that P. aeruginosa biofilms grown on CF airway epithelial cells develop a dramatically heightened level of resistance to antibiotics,9,10 and that iron released from CF airway epithelial cells is a contributing factor to the development of those biofilms.9 Remarkably, for reasons that are largely unknown, the iron concentration in the airway surface liquid (ASL) of CF patients is 400-fold higher than that in the ASL of non-CF patients,11–13 and reducing iron concentrations in the CF lung has emerged as a potential therapeutic target to eliminate CF airway infections. We also reported previously that the combined use of FDA-approved iron chelators with tobramycin, one of the most frequently used inhaled antibiotics in the CF clinic, dramatically reduced P. aeruginosa biofilm formation on CF airway epithelial cells.14 Importantly, we reported that iron chelators are most effective, in combination with tobramycin, in reducing P. aeruginosa infections when added to the ASL overlying airway epithelia cells.14 Unfortunately, the existing FDA iron chelators have only been approved for systemic, not inhalational, use in patients and cannot be co-administered with inhaled antibiotics.
Because FDA-approved antibiotics do not eliminate bacterial airway infections in CF patients, there is a continuing need to develop new therapies. ALX-009 is an investigational drug developed by Alaxia (Lyon, France) that is composed of lactoferrin (an iron-binding glycoprotein) and hypothiocyanite (OSCN−; a bactericidal agent), and has been designed as an orphan drug by both the FDA and the EMA. The data described in this article were generated with ALX-109, one of the numerous combinations of OSCN− and lactoferrin currently in development.
Both lactoferrin and OSCN− are natural components of the innate immune response in the airways, and both lactoferrin and OSCN− concentrations are reduced in the CF airways.15,16 Lactoferrin, one of the most abundant antimicrobial proteins in normal airway secretions,17 is an iron-binding glycoprotein that restricts iron availability to bacteria, and thereby limits biofilm formation.18 Lactoferrin also increases the membrane permeability of Gram-negative bacteria, resulting in bacterial death.19,20 In the CF lung, and even more so for P. aeruginosa-infected CF patients, increased activity of cathepsins results in the proteolytic cleavage of lactoferrin, efficiently reducing its anti-biofilm properties.15 Additionally, lactoferrin concentrations in CF airways are further reduced by the proteolytic action of bacterial elastase secreted by P. aeruginosa biofilms.21
OSCN−, an antimicrobial compound, is also reduced in CF airways.16 In the airway lumen, lactoperoxidase catalyses the formation of OSCN− from thiocyanate (SCN−) and H2O2.22,23 The antimicrobial action of OSCN− is linked to the ability of HOSCN, the protonated form of OSCN−, to diffuse through microbial membranes and reduce bacterial metabolism by enhancing the oxidation of sulphydryl groups of essential enzymes.24–26 SCN−, a pseudohalide acquired principally from dietary sources and the OSCN− precursor, enters airway epithelial cells through the Na+–I− symporter on the basolateral side of airway cells and is released into the ASL through the CFTR.27 The concentration of SCN− in the ASL is 460 μM, a ∼3- to ∼50-fold higher concentration than in serum.28,29 In CF, the lack of CFTR-mediated SCN− secretion negatively impacts the lactoperoxidase innate immune system. CF airway epithelial cells are unable to secrete SCN−, thereby preventing the production of OSCN− and resulting in the accumulation of harmful H2O2 in the airways.16,30 Recently, OSCN− and OSCN− derivatives used in combination with gentamycin or vancomycin were shown to selectively inhibit bacterial growth in vitro.31
Here, we investigated the efficacy of ALX-109, alone and in combination with tobramycin or aztreonam, antibiotics currently used to treat lung infections in CF patients, to disrupt and to eliminate P. aeruginosa biofilms grown on human CF airway epithelial cells. Inhaled tobramycin (TOBI®, Novartis) and inhaled aztreonam (Cayston®, Gilead), the only two inhaled antibiotics approved to treat P. aeruginosa infections in CF patients, are rapidly eliminated from the CF lung. The concentration of both inhaled antibiotics quickly decreases in sputum, and 2 h post-inhalation their concentration in sputum is <10% of the inhaled dose.32,33 We report that ALX-109 alone reduced PAO1 biofilm formation, but had no effect on established biofilms. Importantly, ALX-109 enhanced the ability of tobramycin and aztreonam to inhibit PAO1 biofilm formation and to reduce established PAO1 biofilms. ALX-109 and tobramycin were also additive in disrupting established biofilms formed by six clinical isolates of P. aeruginosa obtained from the sputum of CF patients. Mucoid P. aeruginosa isolates were the most susceptible to the combination of ALX-109 and tobramycin. In addition, ALX-109 also enhanced the ability of aztreonam to reduce established PAO1 biofilms.
Materials and methods
Materials
Tobramycin (Sigma, St Louis, MO, USA) and aztreonam (MP Biomedical, Solon, OH, USA) were dissolved in sterile water immediately before use. Tobramycin and aztreonam were used at 5–500 and 700 mg/L, respectively. The MICs of aztreonam and tobramycin were previously determined for each of the bacterial strains used in this study.10 ALX-109 was prepared according to Alaxia's recommendations (Lyon, France) and was composed of 8 g/L apo-bovine lactoferrin and 100 μM OSCN− prepared with proprietary Alaxia (Lyon, France) technology.
Bacterial strains
P. aeruginosa strain PAO1 and P. aeruginosa clinical strains (SMC1585, SMC1587, SMC1595, SMC1596, SMC5450 and SMC5451) isolated from the sputum of six independent CF patients at the Dartmouth–Hitchcock Medical Center (Hanover, NH, USA) were grown in rich medium (Lysogeny Broth, LB) at 37°C.10 Isolates SMC1585, SMC5450 and SMC5451 are mucoid, as determined previously on sheep blood agar, MacConkey agar and Mueller–Hinton agar.10 For co-culture biofilm assays, P. aeruginosa overnight cultures in LB were washed twice in cell-growth medium and suspended in cell-growth medium without antibiotics or phenol red (see below).
Cell line and cell culture
Human bronchial epithelial cells (CFBE41o-), hereafter called CFBE cells, were originally isolated from a CF patient homozygous for the ΔF508-CFTR mutation and further transduced to stably express ΔF508-CFTR.34,35 Cells were a generous gift from Dr J. P. Clancy at the University of Alabama. Cells were maintained in MEM supplemented with 10% FBS, 2 mM l-glutamine, 50 U/mL penicillin, 50 g/L streptomycin, 5 g/L plasmocin and 2 g/L puromycin in a 5% CO2–95% air incubator at 37°C. For co-culture biofilm studies, cells were seeded at 0.2 × 106 cells/well in 12-well plates (Corning Incorporated, Corning, NY, USA) and grown for 7–9 days at 37°C to establish confluent monolayers and tight junctions.34,35 Penicillin, streptomycin, plasmocin and puromycin were removed immediately before experiments.
ALX-109 cytotoxicity
ALX-109 was applied to the apical side of CFBE cells for 1, 2, 3, 4, 6 or 16 h. In addition, the ability of ALX-109 to reduce the cytotoxic effects of PAO1 on CFBE cells was also examined. At the end of treatment, the medium was isolated for measurements of lactate dehydrogenase (LDH) and CFBE cells were disrupted with multiple freeze–thaw cycles to measure the total amount of LDH in cells. LDH concentrations were measured using the Cyto Tox96® non-radioactive cytotoxicity assay (Promega, Madison, WI, USA) according to the manufacturer's instructions. Cytotoxicity was expressed as the LDH released into the medium divided by the amount of LDH in cell lysates × 100, and expressed as the percentage LDH released by CFBE cells. Assays were performed in triplicate and experiments were performed three times with independent preparations of ALX-109.
Static co-culture biofilm assays
The efficacy of treatments at either preventing biofilm formation (prevention assay) or disrupting established biofilms (disruption assay) was assessed using a well-described co-culture biofilm model developed by our laboratory.36,37 Briefly, P. aeruginosa was applied at the apical surface of confluent monolayers of CFBE cells previously washed with MEM to eliminate antibiotics and allowed to attach to the cells for 1 h. CFBE monolayers were consequently washed to remove any unattached bacteria. For biofilm prevention assays, ALX-109 alone (8 g/L apo-bovine lactoferrin and 100 μM OSCN−) or in combination with tobramycin (5 mg/L, a concentration determined by us to modestly reduce P. aeruginosa biofilms, see Figure 2) or aztreonam (700 mg/L, the concentration achieved in the CF lung)38 was applied immediately after washing and maintained for 5 h in the presence of 0.4% arginine as described previously.36,37 At the end of treatment, CFBE monolayers were visually inspected using phase-contrast microscopy to assess whether their integrity had been compromised as described previously.37 Triton X-100, 0.1%, was then added for 15 min to lyse the airway cells and isolate bacteria. The lysate was then vortexed for 3 min and cfu were determined by serial dilution and spot titre onto LB plates. The detection limit of the assay was ∼200 cfu/well. Alternatively, for biofilm disruption assays, P. aeruginosa was applied at the apical surface of confluent monolayers of CFBE cells and allowed to attach to the cells for 1 h, whereupon unattached bacteria were removed by changing the cell culture medium.36,37 Thereafter, biofilms were allowed to fully develop on the airway cells for 5 h before being exposed to ALX-109 (8 g/L apo-bovine lactoferrin and 100 μM OSCN−) alone or in combination with tobramycin or aztreonam. At 16 h after the addition of drugs, CFBE monolayers were visually inspected and cfu were determined by spot titre as described above.
Figure 2.
Prevention of P. aeruginosa biofilm formation by ALX-109 alone or in combination with tobramycin or aztreonam. ALX-109 (8 g/L apo-bovine lactoferrin and 100 μM OSCN−), tobramycin (5 mg/L) and aztreonam (700 mg/L) were used alone or in combination (ALX-109 and tobramycin or ALX-109 and aztreonam) to prevent the formation of P. aeruginosa (PAO1) biofilms on the apical surface of human airway epithelial cells in a co-culture assay. The cfu remaining at the end of the 5 h drug treatment were counted by serial dilution and plating. CFBE monolayers were visually inspected by phase-contrast microscopy and remained intact with or without ALX-109 or antibiotic treatment in the biofilm prevention assay. Experiments were performed in triplicate and results are presented as mean ± SEM. Data were log-transformed and analysed by one-way ANOVA and Bonferroni's multiple comparison test. *P < 0.05 versus the untreated control. **P < 0.05 versus the indicated comparison. CTRL, control; ALX, ALX-109; TOB, tobramycin; ATM, aztreonam.
Statistical analysis
Data were analysed with GraphPad Prism 4.0 for Macintosh (GraphPad Software Inc.; San Diego, CA, USA). Data were log-transformed and analysed by one-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparison test. Data are expressed as mean ± SEM. A P value <0.05 was considered significant. Statistical significance is presented in the figures.
Results and discussion
ALX-109 reduces PAO1-induced cytotoxicity
ALX-109 is a defined combination of an investigational drug composed of lactoferrin and OSCN−, both part of the normal innate immune defence of the lungs in response to bacterial infection. Purified lactoferrin is well tolerated by mammalian cells and is undergoing clinical trials in patients with sepsis.39 Here, we investigated whether ALX-109 was cytotoxic to the human airway cells used in our studies. ALX-109 alone had no effect on LDH release by CFBE cells after 1, 2, 3, 4 or 6 h. However, after 16 h ALX-109 significantly increased LDH release by CFBE cells (Figure 1). As reported previously, PAO1 significantly increased LDH release by CFBE cells; however, ALX-109 significantly reduced the PAO1-stimulated LDH release. Thus, ALX-109 reduced the cytotoxic effects of PAO1 on CFBE cells.
Figure 1.
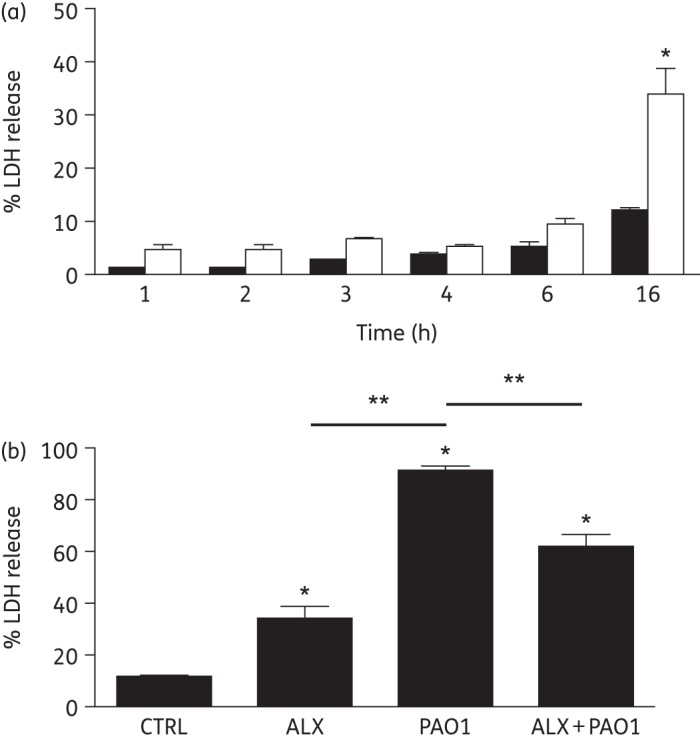
ALX-109 reduces PAO1-induced cytotoxicity. (a) Airway cell growth medium alone (black bars) or medium supplemented with ALX-109 (white bars) was applied to the apical side of human airway epithelial cells. ALX-109 had no effect on LDH release after 1, 2, 3, 4 or 6 h. However, after 16 h ALX-109 significantly increased LDH release by CFBE cells. (b) Mature PAO1 biofilms grown on CF airway epithelial cells for 6 h were treated with medium (PAO1) or ALX-109 (ALX+PAO1) for an additional 16 h, in a biofilm disruption assay. PAO1 significantly increased LDH release, and ALX-109 reduced PAO1-induced LDH release. Experiments were performed in triplicate and results are presented as mean ± SEM. *P < 0.05 versus the untreated control at the same timepoint. **P < 0.05 versus the indicated comparison. CTRL, control; ALX, ALX-109.
ALX-109 prevents P. aeruginosa biofilm formation on human airway epithelial cells
The anti-biofilm properties of ALX-109, alone or in combination with tobramycin or aztreonam, were tested using a well-characterized static co-culture model developed by our laboratory.36,37 In this model, confluent, polarized monolayers of CFBE cells are inoculated with P. aeruginosa applied to the apical side of the airway cells. Bacteria are given 1 h to attach to the cells, after which time unattached bacteria are removed and the co-culture system is treated with ALX-109 alone (8 g/L apo-bovine lactoferrin and 100 μM OSCN−) or in combination with tobramycin (5 mg/L) or aztreonam (700 mg/L) for 5 h, as described in the Materials and methods section. The efficacy of treatment in preventing the development of P. aeruginosa biofilms was quantified by cfu counting. ALX-109 alone reduced PAO1 biofilm formation by 0.7 log units, corresponding to a killing of ∼60% of the bacteria compared with the vehicle-treated control (Figure 2). Tobramycin alone reduced PAO1 cfu by 3.9 log units, while, in combination with ALX-109, it reduced PAO1 cfu by 4.4 log units compared with the untreated control (Figure 2). Thus, the combination of ALX-109 and tobramycin reduced cfu by 0.5 log units compared with tobramycin alone.
Aztreonam, a recently approved antibiotic for CF patients, alone reduced PAO1 cfu by 4 log units, and the combination of ALX-109 and aztreonam reduced PAO1 cfu by an additional 1 log unit (Figure 2). These data demonstrate that the combination of ALX-109 with either tobramycin or aztreonam has an additive effect on preventing PAO1 biofilm formation on human airway epithelial cells.
ALX-109 potentiates the efficacy of tobramycin and aztreonam at disrupting mature PAO1 biofilms
When PAO1 biofilms are grown on human airway cells, they rapidly become highly resistant to tobramycin and several other antibiotics.9,10,36 To test the efficacy of ALX-109 to enhance the ability of tobramycin or aztreonam to disrupt established biofilms, PAO1 biofilms were grown on the apical side of CFBE cells for 6 h, after which time ALX-109 (8 g/L apo-bovine lactoferrin and 100 μM OSCN−) was applied alone or in combination with tobramycin (5 mg/L) or aztreonam (700 mg/L) for another 16 h. Subsequently, bacterial cfu were determined as described above. ALX-109 alone had no effect on established biofilms (Figure 3). Tobramycin alone reduced the cfu of established biofilms by 3 log units. Despite the lack of effect of ALX-109 alone on established biofilms, the combination of ALX-109 and tobramycin decreased established biofilms by 7 log units, compared with untreated controls, an effect significantly larger than that observed with tobramycin alone (i.e. 3 log units, Figure 3).
Figure 3.
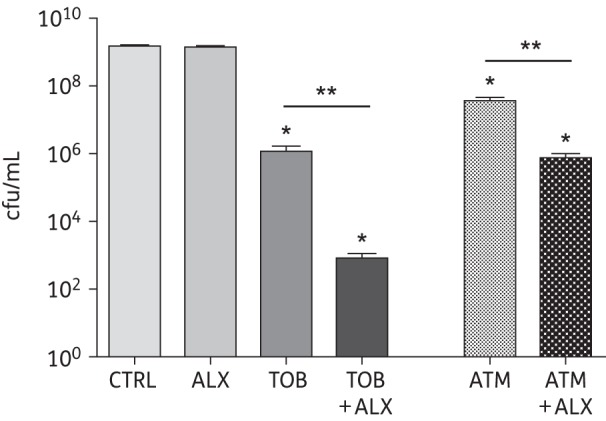
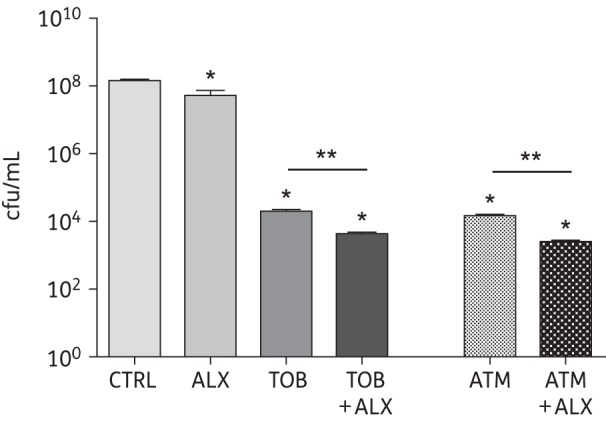
Combined treatment with ALX-109 and tobramycin or aztreonam reduces mature P. aeruginosa biofilms. Mature PAO1 biofilms grown on CF airway epithelial cells for 6 h were treated with ALX-109 (8 g/L apo-bovine lactoferrin and 100 μM OSCN−), tobramycin (5 mg/L) or aztreonam (700 mg/L) alone or in combination (ALX-109 and tobramycin or ALX-109 and aztreonam), for an additional 16 h, in a co-culture disruption assay. In the absence of antibiotics and even in the presence of ALX-109, PAO1 developed biofilms that destroyed the integrity of CFBE monolayers overnight as assessed by visual inspection. ALX-109 alone was unable to disrupt established biofilms, whereas ALX-109 and tobramycin together maintained the integrity of CFBE monolayers and decreased established PAO1 biofilms, an effect significantly larger than that of tobramycin alone. Similarly, an additive effect was observed between ALX-109 and aztreonam, although not as strong as the one observed with ALX-109 and tobramycin. ALX-109 and aztreonam also maintained the integrity of CFBE monolayers. Experiments were performed in triplicate and results are presented as mean ± SEM. Data were log-transformed and analysed by one-way ANOVA and Bonferroni's multiple comparison test. *P < 0.05 versus the untreated control. **P < 0.05 versus the indicated comparison.
Aztreonam alone reduced established biofilms by 1.5 log units, and the combination of aztreonam and ALX-109 reduced established biofilms by 3 log units (Figure 3). Taken together, the data presented in Figure 3 demonstrate that ALX-109 enhances the ability of tobramycin and aztreonam to disrupt established PAO1 biofilms growing on human CF airway cells. The additive effect observed between ALX-109 and tobramycin was more robust than the one observed between ALX-109 and aztreonam.
ALX-109 combined with tobramycin or aztreonam is efficacious against biofilms of clinical isolates of P. aeruginosa
The efficacy of ALX-109, alone and in combination with tobramycin or aztreonam, was also examined on established biofilms formed by six clinical P. aeruginosa strains isolated from the sputum of CF patients. These strains form highly antibiotic-resistant biofilms on CFBE cells.10 Strains SMC1585, SMC5450 and SMC5451 are mucoid, as described elsewhere.10 The effect of ALX-109 alone on established biofilms was strain dependent. ALX-109 alone significantly reduced cfu by ∼1–2 log units for four clinical isolates (SMC1587, SMC1596, SMC1585 and SMC5451, all P < 0.05 compared with control), but had no effect on the other two isolates (SMC1595 and SMC5450) (Figure 4). The effect of tobramycin alone on established biofilms was also strain dependent. Tobramycin alone significantly reduced cfu by ∼1–3.5 log units for four clinical isolates (SMC1587, SMC1595, SMC1585 and SMC5450, all P < 0.05 compared with control), but had no effect on SMC1596 and SMC5451 (Figure 4). As observed for ALX-109, mucoidy did not seem to play a role in the resistance of the clinical isolates to tobramycin, in agreement with previous studies.10 Interestingly, the strains that were not responding to ALX-109 were different from the strains that were resistant to tobramycin.
Figure 4.
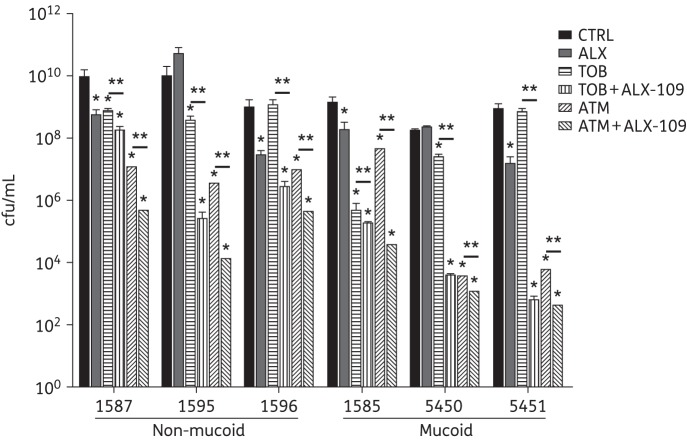
Combined treatment with ALX-109 and tobramycin or aztreonam is efficacious against P. aeruginosa clinical isolates. Mucoid and non-mucoid P. aeruginosa clinical isolates were collected from the sputum of CF patients and used in the co-culture disruption assay as described in the Materials and methods section. In the absence of antibiotics and even in the presence of ALX-109, all clinical isolates of P. aeruginosa developed biofilms that destroyed the integrity of CFBE monolayers after 16 h. However, ALX-109 (8 g/L apo-bovine lactoferrin and 100 μM OSCN−) and tobramycin (5 mg/L) maintained the integrity of CFBE monolayers and were additive in disrupting biofilms formed by clinical isolates of P. aeruginosa. Mucoid isolates were the most susceptible to the combined treatment. Similar results were obtained when aztreonam (700 mg/L) was used instead of tobramycin. Experiments were performed in triplicate and results are presented as mean ± SEM. Data were log-transformed and analysed by one-way ANOVA and Bonferroni's multiple comparison test. *P < 0.05 versus the untreated control. **P < 0.05 versus the indicated comparison.
The combination of ALX-109 and tobramycin reduced the cfu by ∼2–6 log units in all six clinical isolates compared with control (P < 0.05 for all isolates). In addition, the combination of ALX-109 and tobramycin also reduced the cfu by ∼1–4 log units in all six clinical isolates compared with tobramycin alone (P < 0.05 for all isolates). Mucoid phenotype does not seem to be a factor influencing the efficacy of ALX-109 on P. aeruginosa biofilms; however, mucoid P. aeruginosa isolates were the most susceptible to the combination of ALX-109 and tobramycin.
In contrast to tobramycin, aztreonam alone reduced the cfu by ∼2–5 log units for all six clinical isolates of P. aeruginosa (Figure 4, P < 0.05 compared with control). The combination of ALX-109 and aztreonam reduced cfu by an additional ∼1–3 log units compared with aztreonam alone, indicating that the effect of ALX-109 and aztreonam was additive for all six clinical isolates tested (Figure 4). Unlike tobramycin, the effect of aztreonam was equally robust on both mucoid and non-mucoid clinical isolates (Figure 4).
ALX-109 potentiates the anti-biofilm effect of tobramycin: dose-dependent effects
Additional studies were conducted to examine the ability of ALX-109 to reduce established P. aeruginosa biofilms on CFBE cells in combination with tobramycin used at clinically relevant concentrations from 0 to 500 mg/L. The rationale for this experiment is that in the CF lung the peak concentration of tobramycin is ∼1000 mg/L immediately after administration, and rapidly declines to 100 mg/L over 2 h.32 CF patients typically inhale tobramycin twice a day, resulting in a tobramycin concentration that fluctuates in the CF lung. For these reasons, we selected a range of tobramycin concentrations representing those measured in the lung environment of CF patients between inhalations. Tobramycin reduced the cfu of established biofilms in a dose-dependent manner for all six clinical isolates of P. aeruginosa studied (Figure 5). Moreover, tobramycin alone completely eliminated biofilms formed by four of the six clinical isolates of P. aeruginosa studied; however, the highest dose of tobramycin tested did not eliminate biofilms formed by SMC1587 and SMC5451 (Figure 5).
Figure 5.
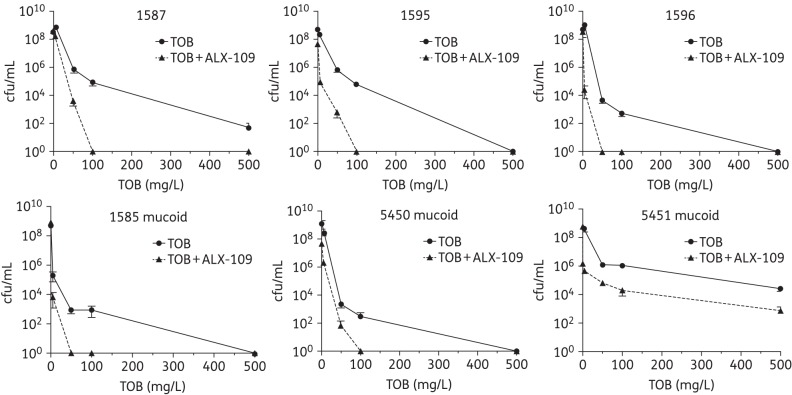
Dose-dependent effect of tobramycin on disrupting P. aeruginosa established biofilms treated with ALX-109. Mature biofilms formed by clinical isolates of P. aeruginosa on CF airway cells were exposed to a range of clinically relevant tobramycin concentrations (5, 50, 100 and 500 mg/L) alone (continuous lines) or in combination with ALX-109 (8 g/L apo-bovine lactoferrin and 100 μM OSCN−; broken lines). This range of tobramycin concentrations represents the tobramycin concentrations measured in the lung environment of CF patients between inhalations. In the absence of tobramycin, all clinical isolates of P. aeruginosa developed biofilms that destroyed the integrity of CFBE monolayers overnight. However, all doses of tobramycin tested (alone and in combination with ALX-109) maintained the integrity of CFBE monolayers. For five of the six clinical isolates of P. aeruginosa tested, ALX-109 reduced the concentration of tobramycin required to eradicate established biofilms formed by clinical isolates of P. aeruginosa ∼5-fold. For one clinical isolate (5451) the combination of ALX-109 and tobramycin reduced the cfu by 2 log units compared with tobramycin alone. Experiments were performed in triplicate and results are presented as mean ± SEM.
In the last series of studies we examined the ability of ALX-109 to enhance the ability of tobramycin to eliminate established biofilms formed by six clinical isolates of P. aeruginosa. Importantly, ALX-109 dramatically enhanced the ability of tobramycin to eliminate established P. aeruginosa biofilms on CFBE cells. In five of the six clinical isolates of P. aeruginosa, ALX-109 reduced the concentration of tobramycin required to eliminate biofilms at least 5-fold (Figure 5). Thus, taken together, studies presented in Figure 5 demonstrate that ALX-109 will enhance the ability of tobramycin to eliminate drug-resistant biofilms in the CF airway.
Conclusions
Here, we showed that ALX-109, a defined combination of an investigational drug composed of lactoferrin and OSCN−, potentiates the ability of tobramycin and aztreonam to reduce biofilm formation and to disrupt P. aeruginosa biofilms established on CF airway epithelial cells. ALX-109 reduced the concentration of tobramycin required to eradicate P. aeruginosa biofilms 5-fold. This observation is clinically significant because current treatments with tobramycin and/or aztreonam do not alleviate the chronic respiratory infection with P. aeruginosa in CF patients. P. aeruginosa is naturally resistant to many antibiotics40,41 and subinhibitory concentrations of aminoglycosides, such as tobramycin, have been shown to enhance biofilm formation by P. aeruginosa.42 As such, antimicrobial agents are often used in combination in order to achieve synergistic effects against P. aeruginosa CF isolates. Several classes of compounds have been tested in combination with tobramycin43–45 and have resulted in mixed effects against P. aeruginosa. The high-sodium environment of the CF lung has also been shown to potentially play a role in interfering with antibiotic efficacy.45 Here, the combination of tobramycin with lactoferrin and OSCN− resulted in an additive effect against P. aeruginosa clinical isolates. In conclusion, these data suggest that the association of lactoferrin and OSCN− in combination with inhaled antibiotics used in CF (tobramycin and aztreonam) has the potential to become an inhalation therapy beneficial to CF patients by decreasing the airway bacterial burden of P. aeruginosa. Additional studies are required to identify the optimal concentrations of lactoferrin and OSCN− (i.e. ALX-009, the formulation in development) that in combination with tobramycin or aztreonam will maximally both prevent P. aeruginosa biofilm formation and reduce established P. aeruginosa biofilms.
Funding
The National Institutes of Health R01-HL074175 (B. A. S.) and P20-RR018787/GM103413 (B. A. S.) and the Cystic Fibrosis Foundation (STANTO14IO, STANTO11R0) supported this work.
Transparency declarations
None to declare.
Author contributions
S. M.-M. and B. A. S. conceived and designed the experiments, S. M.-M. and B. C. performed the experiments, S. M.-M., B. C. and B. A. S. analysed the data, and S. M.-M., B. C. and B. A. S. wrote the paper.
Acknowledgements
Preliminary results of this paper were presented at the Twenty-sixth Annual North American Cystic Fibrosis Conference, Orlando, FL, USA, 2011 (abstracts 268 and 269).
ALX-109 was a generous gift of Alaxia (Lyon, France). We thank Phillippe Bordeau, Annie-Claude Benichou, Pascale Gaillard and Victor Juarez for their comments on the manuscript prior to submission.
References
- 1.Riordan J, Rommens J, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–73. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 2.Kerem B, Rommens J, Buchanan J, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–80. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 3.Heijerman H. Infection and inflammation in cystic fibrosis: a short review. J Cystic Fibrosis. 2005;4:3–5. doi: 10.1016/j.jcf.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Høiby N. Recent advances in the treatment of Pseudomonas aeruginosa infections in cystic fibrosis. BMC Med. 2011;9 doi: 10.1186/1741-7015-9-32. doi:10.1186/741-7015-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramsey BW. Management of pulmonary disease in patients with cystic fibrosis. New Engl J Med. 1996;335:179–88. doi: 10.1056/NEJM199607183350307. [DOI] [PubMed] [Google Scholar]
- 6.Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev. 2002;15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh PK, Schaefer AL, Parsek MR, et al. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407:762–4. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- 8.Worlitzsch D, Tarran R, Ulrich M, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109:317–25. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreau-Marquis S, Bomberger JM, Anderson GG, et al. The ΔF508-CFTR mutation results in increased biofilm formation by P. aeruginosa by increasing iron availability. Am J Physiol Lung Cell Mol Physiol. 2008;295:L25–37. doi: 10.1152/ajplung.00391.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu Q, Griffin EF, Moreau-Marquis S, et al. In vitro evaluation of tobramycin and aztreonam versus Pseudomonas aeruginosa biofilms on cystic fibrosis-derived human airway epithelial cells. J Antimicrob Chemother. 2012;67:2673–81. doi: 10.1093/jac/dks296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stites S, Walters B, O'Brien-Ladner A, et al. Increased iron and ferritin content of sputum from patients with cystic fibrosis or chronic bronchitis. Chest. 1998;114:814–9. doi: 10.1378/chest.114.3.814. [DOI] [PubMed] [Google Scholar]
- 12.Stites SW, Plautz MW, Bailey K, et al. Increased concentrations of iron and isoferritins in the lower respiratory tract of patients with stable cystic fibrosis. Am J Respir Crit Care Med. 1999;160:796–801. doi: 10.1164/ajrccm.160.3.9811018. [DOI] [PubMed] [Google Scholar]
- 13.Alexis N, Richards J, Carter JD, et al. Iron-binding and storage proteins in sputum. Inhal Toxicol. 2002;14:387–400. doi: 10.1080/08958370252871014. [DOI] [PubMed] [Google Scholar]
- 14.Moreau-Marquis S, O'Toole GA, Stanton BA. Tobramycin and FDA-approved iron chelators eliminate Pseudomonas aeruginosa biofilms on cystic fibrosis cells. Am J Respir Cell Mol Biol. 2009;41:305–13. doi: 10.1165/rcmb.2008-0299OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogan MP, Taggart CC, Greene CM, et al. Loss of microbicidal activity and increased formation of biofilm due to decreased lactoferrin activity in patients with cystic fibrosis. J Infect Dis. 2004;190:1245–53. doi: 10.1086/423821. [DOI] [PubMed] [Google Scholar]
- 16.Moskwa P, Lorentzen D, Excoffon KJDA, et al. A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med. 2007;175:174–83. doi: 10.1164/rccm.200607-1029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganz T. Antimicrobial polypeptides in host defense of the respiratory tract. J Clin Invest. 2002;109:693–7. doi: 10.1172/JCI15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh P. Iron sequestration by human lactoferrin stimulates P. aeruginosa surface motility and blocks biofilm formation. Biometals. 2004;17:267–70. doi: 10.1023/b:biom.0000027703.77456.27. [DOI] [PubMed] [Google Scholar]
- 19.Drago-Serrano ME, de la Garza-Amaya M, Luna JS, et al. Lactoferrin-lipopolysaccharide (LPS) binding as key to antibacterial and antiendotoxic effects. Int Immunopharmacol. 2012;12:1–9. doi: 10.1016/j.intimp.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Farnaud S, Spiller C, Moriarty L, et al. Interactions of lactoferricin-derived peptides with LPS and antimicrobial activity. FEMS Microbiol Lett. 2004;233:193–209. doi: 10.1016/j.femsle.2004.01.039. [DOI] [PubMed] [Google Scholar]
- 21.Britigan BE, Hayek MB, Doebbeling BN, et al. Transferrin and lactoferrin undergo proteolytic cleavage in the Pseudomonas aeruginosa-infected lungs of patients with cystic fibrosis. Infect Immun. 1993;61:5049–55. doi: 10.1128/iai.61.12.5049-5055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conner GE, Salathe M, Forteza R. Lactoperoxidase and hydrogen peroxide metabolism in the airway. Am J Respir Crit Care Med. 2002;166:S57–61. doi: 10.1164/rccm.2206018. [DOI] [PubMed] [Google Scholar]
- 23.El-Chemaly S, Salathe M, Baier S, et al. Hydrogen peroxide-scavenging properties of normal human airway secretions. Am J Respir Crit Care Med. 2003;167:425–30. doi: 10.1164/rccm.200206-531OC. [DOI] [PubMed] [Google Scholar]
- 24.Thomas E. Lactoperoxidase-catalyzed oxidation of thiocyanate: equilibria between oxidized forms of thiocyanate. Biochemistry. 1981;20:3273–80. doi: 10.1021/bi00514a045. [DOI] [PubMed] [Google Scholar]
- 25.Thomas EL, Aune TM. Lactoperoxidase, peroxide, thiocyanate antimicrobial system: correlation of sulfhydryl oxidation with antimicrobial action. Infect Immun. 1978;20:456–63. doi: 10.1128/iai.20.2.456-463.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aune T, Thomas E. Oxidation of protein sulfhydryls by products of peroxidase-catalyzed oxidation of thiocyanate ion. Biochemistry. 1978;17:1005–10. doi: 10.1021/bi00599a010. [DOI] [PubMed] [Google Scholar]
- 27.Fragoso MA, Fernandez V, Forteza R, et al. Transcellular thiocyanate transport by human airway epithelia. J Physiol. 2004;561:183–94. doi: 10.1113/jphysiol.2004.071548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wijkstrom-Frei C, El-Chemaly S, Ali-Rachedi R, et al. Lactoperoxidase and human airway host defense. Am J Respir Cell Mol Biol. 2003;29:206–12. doi: 10.1165/rcmb.2002-0152OC. [DOI] [PubMed] [Google Scholar]
- 29.Xu Y, Szep S, Lu Z. The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation-related diseases. Proc Natl Acad Sci USA. 2009;106:20515–9. doi: 10.1073/pnas.0911412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van der Vliet A, Nguyen MN, Shigenaga MK, et al. Myeloperoxidase and protein oxidation in cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2000;279:L537–46. doi: 10.1152/ajplung.2000.279.3.L537. [DOI] [PubMed] [Google Scholar]
- 31.Dias C, Aires A, Bennett R, et al. First study on antimicrobial activity and synergy between isothiocyanates and antibiotics against selected Gram-negative and Gram-positive pathogenic bacteria from clinical and animal source. Med Chem. 2012;8:474–80. doi: 10.2174/1573406411208030474. [DOI] [PubMed] [Google Scholar]
- 32.Geller DE, Pitlick WH, Nardella PA, et al. Pharmacokinetics and bioavailability of aerosolized tobramycin in cystic fibrosis. Chest. 2002;122:219–26. doi: 10.1378/chest.122.1.219. [DOI] [PubMed] [Google Scholar]
- 33.FDA Briefing Document for Anti-Infective Drugs Advisory Committee Meeting. Aztreonam for Inhalation Solution (NDA 50–814) for Improvement of Respiratory Symptoms in Cystic Fibrosis Patients. http://wwwfdagov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM193023pdf .
- 34.Bruscia E, Sangiuolo F, Sinibaldi P, et al. Isolation of CF cell lines corrected at DeltaF508-CFTR locus by SFHR-mediated targeting. Gene Ther. 2002;9:683–5. doi: 10.1038/sj.gt.3301741. [DOI] [PubMed] [Google Scholar]
- 35.Cozens A, Yezzi M, Kunzelmann K, et al. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1994;10:38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- 36.Anderson GG, Moreau-Marquis S, Stanton BA, et al. In vitro analysis of tobramycin-treated Pseudomonas aeruginosa biofilms on cystic fibrosis-derived airway epithelial cells. Infect Immun. 2008;76:1423–33. doi: 10.1128/IAI.01373-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreau-Marquis S, Redelman C, Stanton B, et al. Co-culture models of Pseudomonas aeruginosa biofilms grown on live human airway cells. J Vis Exp. 2010;44:2186. doi: 10.3791/2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Retsch-Bogart GZ, Quittner AL, Gibson RL, et al. Efficacy and safety of inhaled aztreonam lysine for airway pseudomonas in cystic fibrosis. Chest. 2009;135:1223–32. doi: 10.1378/chest.08-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guntupalli K, Dean N, Morris P, et al. A phase 2 randomized, double-blind, placebo-controlled study of the safety and efficacy of talactoferrin in patients with severe sepsis. Crit Care Med. 2013;41:706–16. doi: 10.1097/CCM.0b013e3182741551. [DOI] [PubMed] [Google Scholar]
- 40.Hancock REW, Speert DP. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and impact on treatment. Drug Resistance Updates. 2000;3:247–55. doi: 10.1054/drup.2000.0152. [DOI] [PubMed] [Google Scholar]
- 41.Poole K. Efflux-mediated antimicrobial resistance. J Antimicrob Chemother. 2005;56:20–51. doi: 10.1093/jac/dki171. [DOI] [PubMed] [Google Scholar]
- 42.Hoffman LR, D'Argenio DA, MacCoss MJ, et al. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature. 2005;436:1171–5. doi: 10.1038/nature03912. [DOI] [PubMed] [Google Scholar]
- 43.Cohn RC, Jacobs M, Aronoff SC. In vitro activity of amiloride combined with tobramycin against Pseudomonas isolates from patients with cystic fibrosis. Antimicrob Agents Chemother. 1988;32:395–6. doi: 10.1128/aac.32.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohn R, Rudzienski L, Putnam R. Verapamil-tobramycin synergy in Pseudomonas cepacia but not Pseudomonas aeruginosa in vitro. Chemotherapy. 1995;41:330–3. doi: 10.1159/000239363. [DOI] [PubMed] [Google Scholar]
- 45.Treerat P, Widmer F, Middleton PG, et al. In vitro interactions of tobramycin with various nonantibiotics against Pseudomonas aeruginosa and Burkholderia cenocepacia. FEMS Microbiol Lett. 2008;285:40–50. doi: 10.1111/j.1574-6968.2008.01219.x. [DOI] [PubMed] [Google Scholar]