Abstract
Objectives
To assess the influence of body weight and missed doses on lopinavir pharmacokinetics with standard and increased doses of lopinavir/ritonavir melt extrusion tablets during late pregnancy.
Patients and methods
Lopinavir concentration data during the third trimester of pregnancy were pooled from clinical trials in Thailand (NCT00409591) and the USA (NCT00042289). A total of 154 HIV-infected pregnant women receiving either 400/100 mg (standard) or 600/150 mg (increased) twice daily had lopinavir plasma concentration data available. Population parameters were estimated using non-linear mixed-effects regression models. Monte Carlo simulations were performed to estimate the probability of achieving target lopinavir trough concentrations (>1.0 mg/L) with standard and increased doses of lopinavir/ritonavir during pregnancy.
Results
The median (range) age, weight and gestational age were 28 years (18–43), 62 kg (45–123) and 33 weeks (29–38), respectively. Body weight influenced lopinavir oral clearance (CL/F) and volume of distribution (V/F). Population estimates of lopinavir CL/F and V/F were 6.21 L/h/70 kg and 52.6 L/70 kg, respectively. Based on simulations, the highest risk of subtherapeutic trough concentrations was for women weighing >100 kg using the standard dose (∼7%), while the risk was <2% with the 600/150 mg dose for women weighing 40–130 kg. After a missed dose, 61% of women have lopinavir concentrations below target prior to the next dose with the standard dose compared with 42% with the increased dose.
Conclusions
Standard dosing provides adequate lopinavir trough concentrations for the majority of pregnant women but increased doses may be preferable for women weighing >100 kg and with a history of lopinavir/ritonavir use and/or adherence issues.
Keywords: HIV, pharmacokinetics, Thailand, USA
Introduction
Physiological changes associated with pregnancy can impact antiretroviral drug disposition.1 Optimal antiretroviral exposure throughout pregnancy is critical to ensure maximal viral load suppression for the prevention of mother-to-child transmission of HIV and to prevent the selection of drug-resistant viruses. The HIV PI lopinavir is coformulated with low-dose ritonavir (lopinavir/ritonavir) to enhance its pharmacokinetic profile and is commonly prescribed as part of combination ART during pregnancy.
The first study assessing lopinavir/ritonavir during pregnancy found that US pregnant women receiving the standard dose of 400/100 mg twice daily (soft-gel capsule formulation) had significantly lower exposure (i.e. AUC) during the third trimester compared with non-pregnant adults.2 Subsequent studies in European pregnant women using the same dose and formulation reported that the majority of women achieved efficacious lopinavir trough concentrations (Ctrough) during the third trimester.3,4 Using a higher lopinavir/ritonavir dose of 533/133 mg twice daily (soft-gel capsules) in US pregnant women during the third trimester achieved similar drug exposures to non-pregnant adults.5 In 2005, a new lopinavir/ritonavir tablet formulation (200 mg of lopinavir and 50 mg of ritonavir), with improved bioavailability, received US FDA approval. A higher lopinavir/ritonavir dose of 600/150 mg (i.e. three tablets) twice daily was assessed in US pregnant women and provided comparable lopinavir exposure to non-pregnant adults.6 More recently, studies in Thailand have assessed the new lopinavir/ritonavir tablet formulation in HIV-infected pregnant women receiving the standard 400/100 mg twice-daily dose and found the reduction of lopinavir exposure was less pronounced and no dose increase was necessary.7,8
Individual patient characteristics may influence lopinavir exposure during pregnancy. Differences in body weight can explain part of the interindividual variability (IIV) of lopinavir oral clearance in non-pregnant adults.9 Substantial differences in the body weight of pregnant women exist between geographical regions and may help explain the difference in lopinavir exposures observed.
No clear consensus on the optimal dose of lopinavir/ritonavir for pregnant women has been reached. The risk of lower exposure is expected to increase during late pregnancy compared with non-pregnant women, but the clinical consequences remain unknown. The recently updated Department of Health and Human Services (DHHS) perinatal guideline recommends the standard lopinavir/ritonavir dose, but adds that some experts advise the higher dose during the second and third trimesters.10 A higher lopinavir/ritonavir dose may be preferable for women with high body weight. Moreover, a possible advantage of a higher lopinavir/ritonavir dose during pregnancy may be that it is more ‘forgiving’ than the standard dose, i.e. the risk of subtherapeutic concentrations following a missed dose may be lower, thereby reducing the impact of a missed dose at a time when maximal viral load suppression is vital to prevent vertical HIV transmission.
Using pooled data from pharmacokinetic studies performed in pregnant women from Thailand and the USA, our objectives were to develop a population pharmacokinetic model to describe lopinavir concentrations during the third trimester of pregnancy and investigate the impact of covariates such as body weight on maintaining therapeutic concentrations. The impact of a missed dose of lopinavir/ritonavir on subsequent lopinavir concentrations following standard and increased doses during late pregnancy was also assessed.
Patients and methods
Study population
Lopinavir plasma concentration data collected within two prospective clinical trials were pooled for this analysis.
Study 1
PHPT-5 was a multicentre, Phase III, three-arm, randomized trial investigating the efficacy of three different maternal and infant treatment strategies for the prevention of mother-to-child transmission of HIV-1 in non-immunocompromised women (CD4 <350 cells/mm3) in Thailand (ClinicalTrials.gov identifier: NCT00409591). In one of the treatment arms, women were randomized to receive 300 mg of zidovudine twice daily and 400/100 mg of lopinavir/ritonavir (Kaletra®/Aluvia®, melt extrusion tablets) twice daily from 28 weeks' gestation until delivery. Both full lopinavir pharmacokinetic curves and sparse evaluations were available. Thirty-eight women were enrolled in a pharmacokinetic substudy and had steady-state 12 h blood sampling performed during the third trimester.7 A pre-dose blood sample was drawn prior to the scheduled lopinavir/ritonavir dose, after which lopinavir/ritonavir was administered with a standard low-fat meal (rice soup with pork) and blood samples were collected at 1, 2, 4, 6, 8 and 12 h after dosing. An additional 85 women had at least one random blood sample available. These women had a single random blood sample drawn (i.e. at a non-predefined time) at each study visit during the third trimester. Food intake with lopinavir/ritonavir administered was not controlled for these women. The exact time of last drug intake and blood draw were recorded and the plasma frozen at −20°C. Patients with concomitant treatments that could interfere with the pharmacokinetics of lopinavir/ritonavir, such as rifampicin, were excluded. Drug adherence was assessed by exact pill count. The PHPT-5 study was approved by the Ethics Committee at the Ministry of Public Health, Thailand and the local hospital ethics committees. Signed informed consent was obtained from all subjects prior to participation.
Study 2
P1026s—the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network Protocol 1026s is an ongoing, multicentre, non-blinded, prospective study to evaluate the pharmacokinetics of antiretrovirals among pregnant HIV-infected women (NCT00042289). The P1026s study design involves performing intensive steady-state pharmacokinetic blood sampling during the second trimester (optional), third trimester and early post-partum period as previously described.2 Several formulations and doses of lopinavir/ritonavir have been studied within P1026s. For this analysis, 31 pregnant women receiving 600/150 mg of lopinavir/ritonavir (Kaletra®/Aluvia®, melt extrusion tablets) twice daily during the third trimester were included.6 Pharmacokinetic sampling was performed between 30 and 36 weeks' gestation. Subjects must have been stable on their antiretroviral regimen for ≥2 weeks prior to pharmacokinetic sampling. Blood samples were collected at pre-dose and 1, 2, 4, 6, 8 and 12 h after an observed dose. Lopinavir was given as an observed dose after a standardized meal of ∼850 kilocalories, with ∼55% of calories from fat. Concomitant antiretroviral drugs included zidovudine/lamivudine (n = 19) and zidovudine/lamivudine/abacavir (n = 9) and 7 women received other medications including stavudine, didanosine, emtricitabine, tenofovir and nevirapine. Local institutional review boards approved the protocol at all participating sites and signed informed consent was obtained from all subjects prior to participation.
Antiretroviral drug concentration measurement
Lopinavir and ritonavir plasma drug concentrations were measured using validated reversed-phase HPLC methods. Plasma samples collected from women enrolled in PHPT-5 were assayed at the PHPT laboratory at the Faculty of Associated Medical Sciences, Chiang Mai University, Thailand. The average accuracy was 103%–112% and precision (interassay and intra-assay) was <4% of the coefficient of variation (CV). The lower limit of assay quantification (LLOQ) was 0.078 mg/L for lopinavir and 0.048 mg/L for ritonavir. Samples collected from women enrolled in P1026s in the USA were assayed at the Paediatric Clinical Pharmacology Laboratory at the University of California, San Diego, USA. The LLOQ was 0.091 mg/L for lopinavir and 0.094 mg/L for ritonavir. The interassay CV was 11% at the LLOQ for both drugs and <10% CV for low, middle and high controls. Both pharmacology laboratories participate in the AIDS Clinical Trial Group (USA) Pharmacology Quality Control (Precision Testing) programme, which performs standardized interlaboratory testing twice a year.11
Population pharmacokinetic analysis
To estimate the population means and variances of lopinavir pharmacokinetic parameters, the plasma concentration data were analysed using non-linear mixed-effects regression. Using the software program Monolix version 4.20 (http://www.lixoft.eu),12,13 concentration–time data were fitted by computing the maximum likelihood estimator of the parameters without any approximation of the model (no linearization) using the stochastic approximation expectation maximization algorithm combined with a Markov chain Monte Carlo procedure. Drug concentration data below the LLOQ of the assay are set to LLOQ/2 values. Pharmacokinetic models were assessed using both statistical and graphical methods. The objective function value (OFV) (i.e. a likelihood ratio test including the −2x log-likelihood) was used to test different hypotheses regarding the final model, covariate effect(s) on pharmacokinetic parameter(s), residual variability model and structure of the variance–covariance matrix for the IIV parameters. Diagnostic graphics were generated using the R program (version 2.11.1).
One- and two-compartment pharmacokinetic models with linear and non-linear elimination were fitted to the data. Zero- and first-order absorption models, with and without a lag time, were tested. Using transit compartments to describe the absorption process was also assessed.14 An exponential error model was used to describe IIV in the pharmacokinetic parameters, i.e. CL/Fi = TVCL × exp (ηi), where CL/Fi represents the oral clearance of the ith individual, TVCL is the population CL/F value and ηi is the interindividual random effect with mean 0 and variance ω2. Covariances between the individual random effects of the pharmacokinetic parameters were assessed graphically. The shrinkage of the empirical Bayesian estimates are taken into account when assessing diagnostic plots.15 Interoccasion variability (IOV) in the pharmacokinetic parameters was also tested. Proportional, constant and combined error models were investigated to describe the residual variability (ε). Individual patient characteristics that could potentially influence pharmacokinetic parameters were evaluated for their inclusion in the model. The patient characteristics assessed included age, weight, BMI, gestational age (GA) and fat-free mass. All continuous covariates were modelled using a power relationship, e.g. CL/Fi = TVCL × (WTi/MWT)θBW, where WT represents the weight of the ith individual, MWT is the median weight of the study population and θBW is the factor associated with body weight that influences lopinavir oral clearance. A ‘Study’ effect was also tested as a binary covariate using the following equation: CL/Fi = TVCL × θ(Study−1), where θ is the factor associated with Study that influences lopinavir oral clearance using Study equals 1 for women enrolled in PHPT-5 and 2 for women enrolled in P1026s. The inclusion of observed ritonavir concentrations to predict lopinavir concentrations was also assessed using a maximum-effects (Emax) model with observed ritonavir concentrations inhibiting lopinavir CL/F. Covariates were tested using a stepwise forward inclusion and backward elimination model building procedure.
The final population model was evaluated using a visual predictive check.16 Lopinavir concentration profiles were simulated and compared with the observed data to evaluate the predictive performance of the model.
Bayesian estimates of the pharmacokinetic parameters were used to calculate the individual lopinavir Ctrough, i.e. 12 h post-dose.
Model simulations
The impact of body weight on lopinavir concentrations during pregnancy was assessed using the final model. Lopinavir Ctrough were simulated for pregnant women weighing 40–130 kg (at 5.0 kg increments) receiving the standard (400/100 mg, twice daily) and increased (600/150 mg, twice daily) doses. One-thousand Monte Carlo simulations were performed at each body weight. The proportions of women achieving lopinavir target Ctrough (>1.0 mg/L for treatment-naive patients) at different weights were calculated by dividing the number of simulated concentrations above the target by the total number of simulated troughs.
Lopinavir concentrations profiles were also simulated for a theoretical population of pregnant women weighing 40–130 kg following a single ‘missed dose’ while receiving standard and increased lopinavir/ritonavir doses. The proportion of women having a lopinavir Ctrough >1.0 mg/L following a missed dose (i.e. 12 h after a missed dose) was determined for each dose. The 95% CI was calculated using the study sample size (n = 154) rather than the number of simulations performed.
Results
Lopinavir and ritonavir concentration data from 154 HIV-infected pregnant women (123 Thai and 31 American) were included in the analysis. A total of 634 lopinavir and 634 ritonavir plasma concentrations were available (note: no samples were below the LLOQ). Sixty-nine women had full pharmacokinetic curves and 85 women had sparse evaluations. The characteristics of the women in the PHPT-5 and P1026s studies are shown in Table 1. Overall, the median (range) age, weight and GA were 28 years (18–43), 62 kg (45–123) and 33 weeks (29–38), respectively.
Table 1.
Characteristics of HIV-infected pregnant women
| PHPT-5 study | P1026s study | Total | |
|---|---|---|---|
| Number of women | 123 | 31 | 154 |
| Number of lopinavir concentrations | 430 | 204 | 634 |
| Number of ritonavir concentrations | 430 | 204 | 634 |
| Age (years) | 27 (18–43) | 30 (22–39) | 28 (18–43) |
| GA (weeks) | 32 (29–38) | 35 (30–37) | 33 (29–38) |
| Weight (kg) | 61 (45–100) | 79 (58–123) | 62 (45–123) |
| BMI (kg/m2) | 25 (19–41) | 31 (23–44) | 26 (19–44) |
| Race/ethnicity | Thai | 6 black, 19 Hispanic and 6 white |
Values: median (range), unless otherwise stated.
Lopinavir population pharmacokinetic model
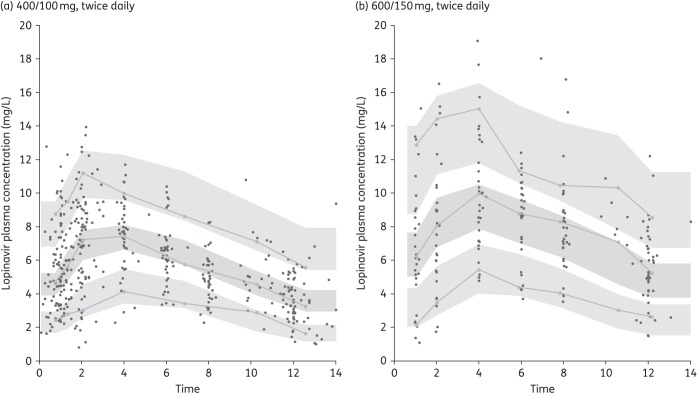
Lopinavir concentrations were best described by a one-compartment pharmacokinetic model with first-order absorption and linear elimination. Study-specific residual variability was evaluated, but a single combined additive and proportional error model was found to provide the best model fit. The addition of an absorption lag time improved the model fit (OFV ↓73). Estimating IIV for CL/F and IOV on lag time, Ka and bioavailability (F) further improved the model fit (OFV ↓70). Using transit absorption compartments rather that a lag time did not improve the model fit. Among the covariates tested, only body weight influenced lopinavir pharmacokinetic parameters. In a univariate analysis, body weight significantly influenced both lopinavir CL/F (OFV ↓10) and V/F (OFV ↓8). A model estimating both the power exponents for CL/F and V/F (i.e. θBW) significantly reduced the objective function (OFV ↓27) and corresponding interindividual CL/F variability by 5%. The power exponents were 0.4 for CL/F and 0.7 for V/F. Allometrically scaling CL/F and V/F to a 70 kg adult provided a similar fit, i.e. CL/F (L/h/70 kg) = TVCL × (WT/70)0.75 and V/F (L/70 kg) = TVV × (WT/70) and was selected in the final model. A model with lopinavir CL/F inhibited by observed ritonavir concentrations following a maximum-effects model significantly improved the fit (OFV ↓724). The maximum inhibition effect of ritonavir on lopinavir (Emax) was estimated to be 0.90 and the ritonavir concentration required to reach half of Emax (EC50) was 0.053 mg/L. The theoretical CL/F of lopinavir in the absence of ritonavir was estimated to be 25.7 L/h/70 kg and V/F was 111 L/70 kg; however, the Ka estimate was small, 0.1 h−1, less than the elimination rate constant indicating a flip-flop effect. At the same time, it was thought that adding this level of complexity may restrict the application of the model in clinical settings, especially as ritonavir concentrations are not always available. The lopinavir model without including ritonavir concentrations provided accurate estimates of the pharmacokinetic parameters and the visual predictive check provide a good fit to the observed concentration data for both lopinavir/ritonavir doses (Figure 1). Thus, to facilitate the interpretation and application of the pharmacokinetic model developed, the simpler model without ritonavir was selected. The final population pharmacokinetic parameters for lopinavir are presented in Table 2.
Figure 1.
Visual predictive check for lopinavir population pharmacokinetic models following (a) 400/100 mg of lopinavir/ritonavir twice daily and (b) 600/150 mg of lopinavir/ritonavir twice daily. Lines represent the predicted population 5%, 50% and 95% percentiles and shaded areas are the 90% CIs. Observed concentrations from the patients are superimposed.
Table 2.
Population pharmacokinetic parameter estimates for lopinavir in HIV-infected women during the third trimester of pregnancy
| Estimate | RSE (%) | |
|---|---|---|
| Lopinavir parameters | ||
| Tlag (h) | 0.76 | 13 |
| Ka (h−1) | 0.67 | 11 |
| CL/F (L/h/70 kg) | 6.21 | 3 |
| V/F (L/70 kg) | 52.6 | 4 |
| IIV | ||
| CL | 24 | 8 |
| IOV | ||
| Tlag | 114 | 9 |
| Ka | 74 | 11 |
| F | 14 | 16 |
| Residual error | ||
| additive (mg/L) | 0.44 | 19 |
| proportional (%) | 0.05 | 27 |
CL/F, oral clearance; V/F, apparent volume of distribution; Ka, absorption rate constant; Tlag, lag time; F, bioavailability; RSE, relative standard error (standard error of estimate/estimate × 100).
CL/F = 6.21 × (WT/70)0.75; V/F = 52.6 × (WT/70).
All variability parameters are expressed as % CV.
Impact of body weight on lopinavir Ctrough
Lopinavir Ctrough were simulated for pregnant women weighing 40–130 kg receiving either 400/100 or 600/150 mg of lopinavir/ritonavir twice daily. Only 1% of pregnant women weighing 40 kg would have lopinavir Ctrough <1.0 mg/L using the standard 400/100 mg dose; however, this percentage increases to ∼7% for women weighing 130 kg (Figure 2). At the higher 600/150 mg dose, it was predicted that <2% of women would have a lopinavir Ctrough below target across the entire weight range.
Figure 2.
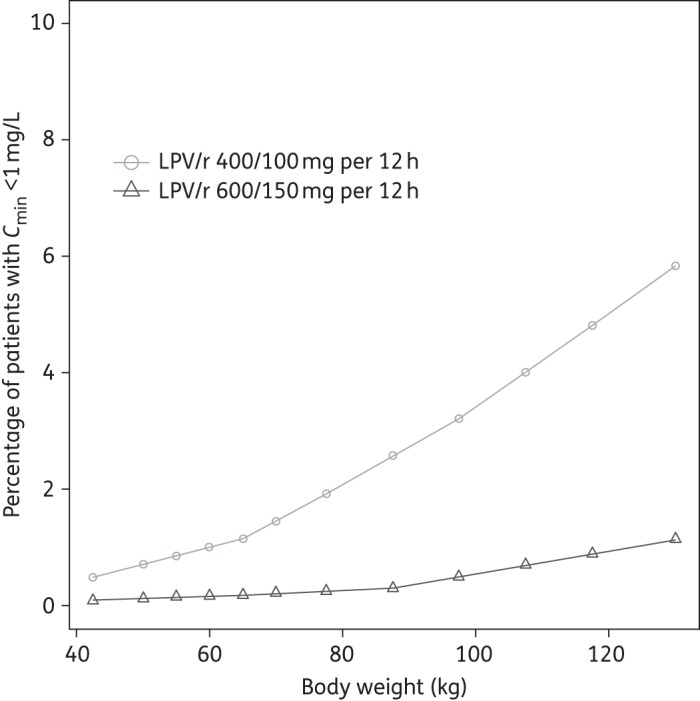
Percentage of women failing to achieve lopinavir trough >1.0 mg/L with 400/100 and 600/150 mg of lopinavir/ritonavir twice daily, for pregnant women with body weights between 40 and 130 kg based on 500 Monte Carlo simulations. LPV/r, lopinavir/ritonavir.
Impact of missed dose of lopinavir/ritonavir on lopinavir concentrations following standard and increased doses during last pregnancy
With standard and increased lopinavir/ritonavir doses, the lopinavir Ctrough at steady-state was predicted prior to a missed dose (i.e. 12 h post-dose), after a missed dose (i.e. 24 h post-dose) and following the next dose (Figure 3). Among pregnant women weighing between 40 and 130 kg, it was estimated that 3% (95% CI: 1%–7%) would have a lopinavir Ctrough below target at the standard 400/100 mg twice-daily dose during the third trimester of pregnancy; however, if the next 12 hourly dose was missed, 61% (95% CI: 53%–69%) of women would have a Ctrough below target prior to the next scheduled dose (Figure 3a). If a lopinavir/ritonavir dose was taken as scheduled after this missed dose, only 6% of women would have a trough below target 12 h later. The increased 600/150 mg dose is more forgiving following a missed dose. Prior to a missed dose, <1% (95% CI: 0.1%–5%) of women have a Ctrough below target, while following a missed dose 42% (95% CI: 34%–50%) of women would have a trough below target 12 h later (Figure 3b).
Figure 3.
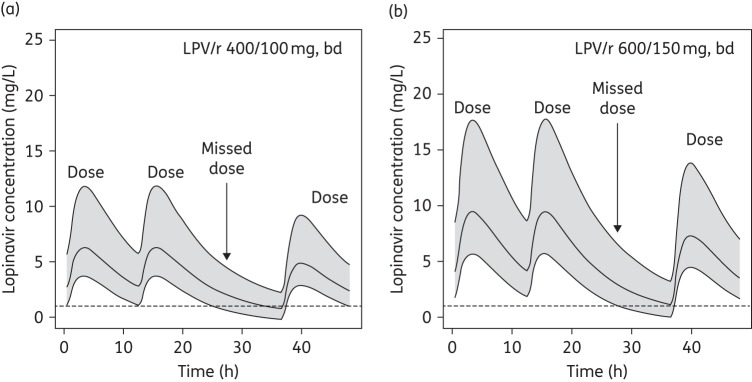
Simulated lopinavir concentration–time curves following a missed dose with (a) 400/100 mg of lopinavir/ritonavir twice daily and (b) 600/150 mg of lopinavir/ritonavir twice daily. Lines represent the 5%, 50% and 95% percentiles and shaded areas are the 90% CIs. The broken line represents the target lopinavir Ctrough of 1.0 mg/L for treatment-naive patients. LPV/r, lopinavir/ritonavir; bd, twice daily.
Discussion
Combination ART is now recommended for all HIV-infected pregnant women.10,17,18 Lopinavir/ritonavir-based ART remains one of the favoured regimens during pregnancy due to its potency and safety profile. Lopinavir exposure is reduced during pregnancy and there is debate whether a lopinavir/ritonavir dose increase is required during late pregnancy. It has also been speculated that body weight may contribute to the impact of pregnancy on lopinavir exposure.7
We developed a population pharmacokinetic model to describe lopinavir concentrations during the third trimester of pregnancy using data from studies in Thailand and the USA, focusing on assessing the influence of body weight on lopinavir pharmacokinetics and, in the context of reduced exposure, the possible advantage of a higher dose in terms of maintaining target concentrations following a missed dose. A robust lopinavir pharmacokinetic model was developed and body weight was found to influence lopinavir oral CL/F and V/F. Among women weighing 40–130 kg, the predicted risk of subtherapeutic lopinavir Ctrough was relatively low with both standard and increased dosing. The highest risk was for women weighing >100 kg using the standard dose, with ∼7% of women having a Ctrough <1.0 mg/L. The risk of subtherapeutic Ctrough with the higher 600/150 mg dose was minimal across the entire weight range (<2%). As expected, the higher lopinavir/ritonavir dose was more ‘forgiving’ than the standard dose. At the standard dose, >60% of the women are expected to have a Ctrough below target prior to the next scheduled dose, compared with ∼40% of women at the higher dose.
Several population pharmacokinetic models of lopinavir in HIV-infected adults have been published. The mean population estimate of lopinavir CL/F in our model was 6.21 L/h/70 kg. This mean CL/F during pregnancy produces lopinavir AUCs of 96.6 and 64.4 mg · h/L/70 kg following increased and standard doses, respectively. A population model developed in non-pregnant adults also found that body weight influenced lopinavir CL/F and the mean population estimate of lopinavir CL/F was 4.74 L/h/70 kg.9 A study assessing pregnancy-related modifications on lopinavir pharmacokinetics included pregnancy status as a binary covariate and reported that GA >15 weeks increased lopinavir CL/F by 39% to 6.1 L/h.19
Some models have incorporated ritonavir exposure or concentrations into the model to estimate lopinavir clearance, either sequentially20 or simultaneously.21 Similar to Molto et al.,22 we assessed a model that simultaneously modelled lopinavir and ritonavir plasma concentrations, wherein lopinavir CL/F was inhibited by ritonavir concentrations following a maximum-effects model. Including the observed ritonavir concentrations significantly improved the model fit and reduced the IIV of CL/F. The estimates of the Emax (0.9) and EC50 (0.05 mg/L) parameters were comparable to those previously reported.22 However, it was thought that adding the necessity of ritonavir concentrations to the model may restrict its application in clinical settings. Clearly, the inclusion of the ritonavir concentrations will yield a more pharmacologically accurate model, but given that the model without including ritonavir concentrations provided accurate estimates, we opted for the simpler model. Indeed, several lopinavir pharmacokinetic models have been published in children23 and adults24 without including ritonavir concentrations.
Body weight and ritonavir concentrations were found to explain part of the interpatient variability observed. Nevertheless, there are clearly other factors involved in explaining interpatient variability. Lopinavir is primarily metabolized by the hepatic cytochrome 3A4 enzyme (CYP3A4) and host genetic polymorphisms could play a role in lopinavir pharmacokinetics. Evidence suggests that polymorphisms within the CYP3A and SLCO1B1 (a member of the organic anion transporting polypeptides family) genes contribute towards variability in lopinavir pharmacokinetics.25,26 Including individual pharmacogenetic information on metabolic enzyme or drug transporters in the model may improve the predictions.
The recommended Ctrough target for patients receiving lopinavir is 1.0 mg/L.27 This target is ∼15 times the IC50 and has been reported to correlate with an HIV RNA viral load of <400 copies/mL.28 Although the clinical consequences of reduced exposure during pregnancy remain unclear, it is rational to target equivalent drug exposures during pregnancy to those seen in non-pregnant adults. The improved bioavailability of the new melt extrusion tablet formulation of lopinavir/ritonavir has been shown to reduce the impact of pregnancy on lopinavir exposure.29 The intensive pharmacokinetic data collected within PHPT-5 on 400/100 mg of lopinavir/ritonavir (tablets) showed a 20% reduction in AUC during pregnancy but only 3% of pregnant women had a Ctrough <1.0 mg/L (median body weight 61 kg). The higher bioavailability of the tablet coupled with the lower body weight in Thai women and unknown host genetic polymorphisms probably explains the more modest reduction in exposure observed during the third trimester in the PHPT-5 study compared with US women in P1026s.7 In our model of tablet dosing, the risk of suboptimal concentrations at higher body weights is not negligible, especially for women who weigh >100 kg, and higher lopinavir/ritonavir doses may be preferred for these women to limit this risk. An argument supporting the use of the higher lopinavir/ritonavir dose can also be made in terms of forgiveness for women who may have potential adherence issues, especially in treatment-experienced women with partial viral resistance to lopinavir. A recent studying assessing the standard and increased dose of lopinavir/ritonavir during pregnancy found that the percentage of women who discontinued lopinavir/ritonavir treatment because of adverse events was not significantly different between doses, while the increased dose was preferred for women with a viral load >50 copies/mL at baseline.30
Overall, using the standard dose of lopinavir/ritonavir during pregnancy provides therapeutic Ctrough for the majority of treatment-naive women up to 130 kg; however, higher doses could be considered in women weighing >100 kg and/or women who may be suspected of poor drug adherence and women with a history of lopinavir use prior to pregnancy.
PHPT-5 team/site investigators
Phayao Provincial Hospital: Pornnapa Suriyachai. Chiangrai Prachanukroh Hospital: Jullapong Achalapong. Mae Chan Hospital: Sudanee Buranabanjasatean. Prapokklao Hospital: Prapap Yuthavisuthi. Banglamung Hospital: Kamol Boonrod. Chonburi Hospital: Nantasak Chotivanich. Rayong Hospital: Weerapong Suwankornsakul. Nakornping Hospital: Aram Limtrakul. Nopparat Rajathanee Hospital: Boonsong Rawangban. Bhumibol Adulyadej Hospital: Sinart Prommas. Hat Yai Hospital: Tapnarong Jarupanich. Nong Khai Hospital: Noossara Puarattana.aroonkorn. Samutsakhon Hospital: Supang Varadisai. Nakhonpathom Hospital: Rucha Kongpanichkul. Samutprakarn Hospital: Prapan Sabsanong. Lampang Hospital: Prateung Lianpongsabuddhi. Vachira Phuket Hospital: Somnuk Chirayus. Pathumthani Hospital: Boonrak Wiriyachoke. Panasnikom Hospital: Manoch Chakorngowit. Ministry of Public Health: Nipunporn Voramongkol. PHPT-IRD174: Sophie Le Coeur, Ken McIntosh, Pra-ornsuda Sukrakanchana, Suwalai Chalermpantmetagul, Kanchana Than-in-at, Yardpiron Taworn, Pimpinun Punyati, Luc Decker and Dujrudee Chinwong.
P1026s team/site investigators
D. Heather Watts, David Shapiro, Elizabeth Smith, Sandra K. Burchett, Francesca Aweeka, Emily Barr, Steve Rossi, Michael Basar, Kenneth D. Braun, Jennifer Bryant, Elizabeth Hawkins, Kathleen A. Medvik, Amy Gonzalez and Beth Sheeran.
Study teams at enrolling sites
Los Angeles County + University of Southern California Medical Center: Françoise Kramer, LaShonda Spencer, James Homans and Andrea Kovacs. University of California San Diego Maternal, Child and Adolescent HIV CRS: Andrew Hull, Mary Caffery, Jeanne Manning and Stephen A. Spector. Universities of Medicine and Dentistry of New Jersey, New Jersey Medical School: Arlene Bardeguez, Charmane Calilap-Bernardo, Linda Bettica and Julliette Johnson. Cook County Medical Center: Helen Cetjin, Julie Schmidt, Maureen Haak and James McAuley. University of Colorado Denver: Emily Barr, Jill Davies, Suzanne Paul and Carol Salbenblatt. Texas Children's Hospital: Shelly Buschur, Chivon McMullen-Jackson, Mary E. Paul and William T. Shearer. Columbia IMPAACT CRS: Sree Gaddipati, Marc Foca, Seydi Vazquez and Alice Higgins. University of Miami Pediatrics Perinatal HIV/AIDS CRS: Amanda Cotter, Liset Taybo, Patricia Bryan and Erika Lopez.
Funding
The PHPT-5 study was supported by the National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), USA. Grant numbers: R01-HD052461 and R01-HD056953. Pharmaceutical support for the PHPT-5 study drugs was provided by GlaxoSmithKline and Boehringer Ingelheim. Lopinavir and ritonavir powders for the antiretroviral drug assay were obtained through the NIH AIDS Research and Reference Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), NIH. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the NIAID of the NIH under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH).
Transparency declarations
None to declare.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Acknowledgements
We wish to thank the women that participated in the protocol and the staff of the participating PHPT-5 and IMPAACT centres.
References
- 1.Buckoreelall K, Cressey TR, King JR. Pharmacokinetic optimization of antiretroviral therapy in pregnancy. Clin Pharmacokinet. 2012;51:639–59. doi: 10.1007/s40262-012-0002-0. [DOI] [PubMed] [Google Scholar]
- 2.Stek AM, Mirochnick M, Capparelli E, et al. Reduced lopinavir exposure during pregnancy. AIDS. 2006;20:1931–9. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- 3.Lyons F, Lechelt M, De Ruiter A. Steady-state lopinavir levels in third trimester of pregnancy. AIDS. 2007;21:1053–4. doi: 10.1097/QAD.0b013e3281053a1e. [DOI] [PubMed] [Google Scholar]
- 4.Manavi K, McDonald A, Al-Sharqui A. Plasma lopinavir trough levels in a group of pregnant women on lopinavir, ritonavir, zidovudine, and lamivudine. AIDS. 2007;21:643–5. doi: 10.1097/QAD.0b013e328031f42e. [DOI] [PubMed] [Google Scholar]
- 5.Mirochnick M, Best BM, Stek AM, et al. Lopinavir exposure with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2008;49:485–91. doi: 10.1097/QAI.0b013e318186edd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Best BM, Stek AM, Mirochnick M, et al. Lopinavir tablet pharmacokinetics with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2010;54:381–8. doi: 10.1097/qai.0b013e3181d6c9ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cressey TR, Jourdain G, Rawangban B, et al. Pharmacokinetics and virologic response of zidovudine/lopinavir/ritonavir initiated during the third trimester of pregnancy. AIDS. 2010;24:2193–200. doi: 10.1097/QAD.0b013e32833ce57d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramautarsing RA, van der Lugt J, Gorowara M, et al. Thai HIV-1-infected women do not require a dose increase of lopinavir/ritonavir during the third trimester of pregnancy. AIDS. 2011;25:1299–303. doi: 10.1097/QAD.0b013e328347f7e9. [DOI] [PubMed] [Google Scholar]
- 9.Bouillon-Pichault M, Jullien V, Piketty C, et al. A population analysis of weight-related differences in lopinavir pharmacokinetics and possible consequences for protease inhibitor-naive and -experienced patients. Antivir Ther. 2009;14:923–9. doi: 10.3851/IMP1414. [DOI] [PubMed] [Google Scholar]
- 10.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. Last updated: 28/3/2014; last reviewed: 28/3/2014. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf .
- 11.Difrancesco R, Rosenkranz SL, Taylor CR, et al. Clinical pharmacology quality assurance program: models for longitudinal analysis of antiretroviral proficiency testing for international laboratories. Ther Drug Monit. 2013;35:631–42. doi: 10.1097/FTD.0b013e31828f5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhn E, Lavielle M. Maximum likelihood estimation in nonlinear mixed effects models. Comput Statist Data Anal, 2005;49:1020–38. [Google Scholar]
- 13.Lavielle M, Mentre F. Estimation of population pharmacokinetic parameters of saquinavir in HIV patients with the MONOLIX software. J Pharmacokinet Pharmacodyn. 2007;34:229–49. doi: 10.1007/s10928-006-9043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savic RM, Jonker DM, Kerbusch T, et al. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn. 2007;34:711–26. doi: 10.1007/s10928-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 15.Savic RM, Karlsson MO. Importance of shrinkage in empirical Bayes estimates for diagnostics: problems and solutions. AAPS J. 2009;11:558–69. doi: 10.1208/s12248-009-9133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergstrand M, Hooker AC, Wallin JE, et al. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–51. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor GP, Clayden P, Dhar J, et al. British HIV Association guidelines for the management of HIV infection in pregnant women 2012. HIV Med. 2012;13(Suppl 2):87–157. doi: 10.1111/j.1468-1293.2012.01030_2.x. [DOI] [PubMed] [Google Scholar]
- 18.WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. June 2013. http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf . [PubMed]
- 19.Bouillon-Pichault M, Jullien V, Azria E, et al. Population analysis of the pregnancy-related modifications in lopinavir pharmacokinetics and their possible consequences for dose adjustment. J Antimicrob Chemother. 2009;63:1223–32. doi: 10.1093/jac/dkp123. [DOI] [PubMed] [Google Scholar]
- 20.Dickinson L, Boffito M, Back D, et al. Sequential population pharmacokinetic modeling of lopinavir and ritonavir in healthy volunteers and assessment of different dosing strategies. Antimicrob Agents Chemother. 2011;55:2775–82. doi: 10.1128/AAC.00887-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crommentuyn KM, Kappelhoff BS, Mulder JW, et al. Population pharmacokinetics of lopinavir in combination with ritonavir in HIV-1-infected patients. Br J Clin Pharmacol. 2005;60:378–89. doi: 10.1111/j.1365-2125.2005.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molto J, Barbanoj MJ, Miranda C, et al. Simultaneous population pharmacokinetic model for lopinavir and ritonavir in HIV-infected adults. Clin Pharmacokinet. 2008;47:681–92. doi: 10.2165/00003088-200847100-00005. [DOI] [PubMed] [Google Scholar]
- 23.Nikanjam M, Chadwick EG, Robbins B, et al. Assessment of lopinavir pharmacokinetics with respect to developmental changes in infants and the impact on weight band-based dosing. Clin Pharmacol Ther. 2012;91:243–9. doi: 10.1038/clpt.2011.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez Aspiroz E, Santos Buelga D, Cabrera Figueroa S, et al. Population pharmacokinetics of lopinavir/ritonavir (Kaletra) in HIV-infected patients. Ther Drug Monit. 2011;33:573–82. doi: 10.1097/FTD.0b013e31822d578b. [DOI] [PubMed] [Google Scholar]
- 25.Hartkoorn RC, Kwan WS, Shallcross V, et al. HIV protease inhibitors are substrates for OATP1A2, OATP1B1 and OATP1B3 and lopinavir plasma concentrations are influenced by SLCO1B1 polymorphisms. Pharmacogenet Genomics. 2010;20:112–20. doi: 10.1097/FPC.0b013e328335b02d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lubomirov R, di Iulio J, Fayet A, et al. ADME pharmacogenetics: investigation of the pharmacokinetics of the antiretroviral agent lopinavir coformulated with ritonavir. Pharmacogenet Genomics. 2010;20:217–30. doi: 10.1097/FPC.0b013e328336eee4. [DOI] [PubMed] [Google Scholar]
- 27.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services; http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf . [Google Scholar]
- 28.Ananworanich J, Kosalaraksa P, Hill A, et al. Pharmacokinetics and 24-week efficacy/safety of dual boosted saquinavir/lopinavir/ritonavir in nucleoside-pretreated children. Pediatr Infect Dis J. 2005;24:874–9. doi: 10.1097/01.inf.0000180578.38584.da. [DOI] [PubMed] [Google Scholar]
- 29.Else LJ, Douglas M, Dickinson L, et al. Improved oral bioavailability of lopinavir in melt-extruded tablet formulation reduces impact of third trimester on lopinavir plasma concentrations. Antimicrob Agents Chemother. 2012;56:816–24. doi: 10.1128/AAC.05186-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonafe SM, Costa DA, Vaz MJ, et al. A randomized controlled trial to assess safety, tolerability, and antepartum viral load with increased lopinavir/ritonavir dosage in pregnancy. AIDS Patient Care STDs. 2013;27:589–95. doi: 10.1089/apc.2013.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]