Abstract
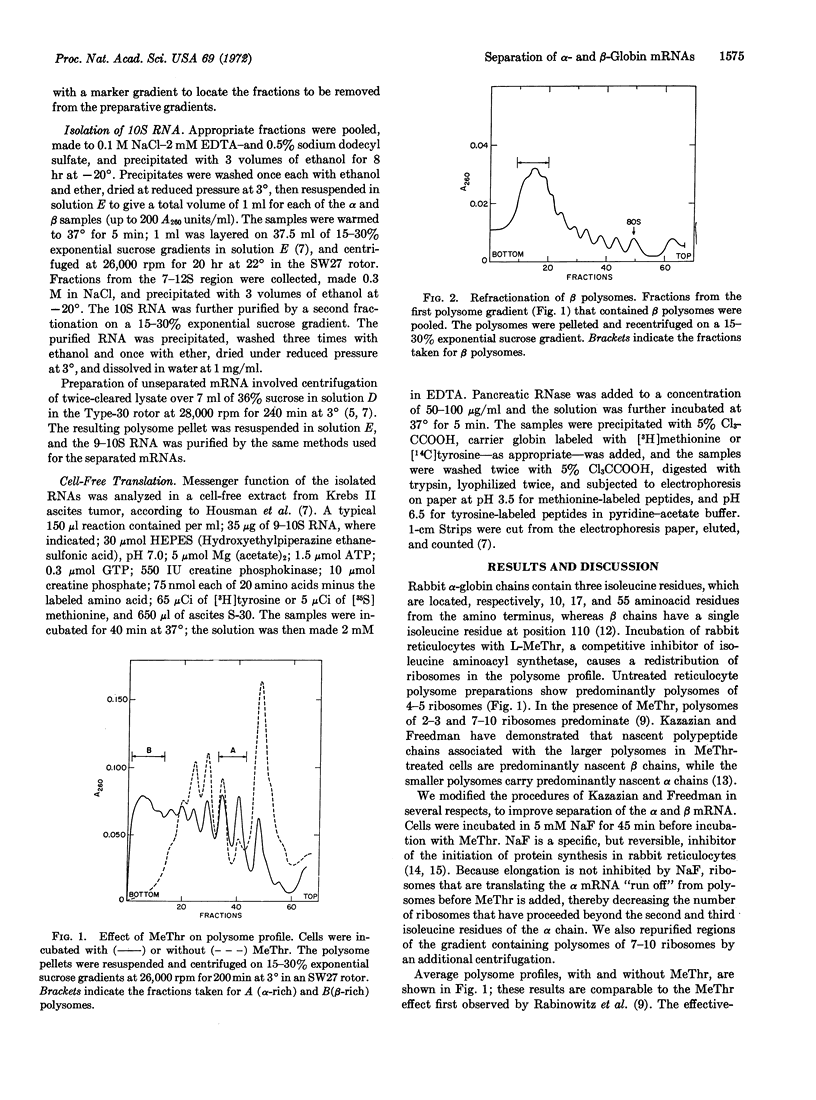
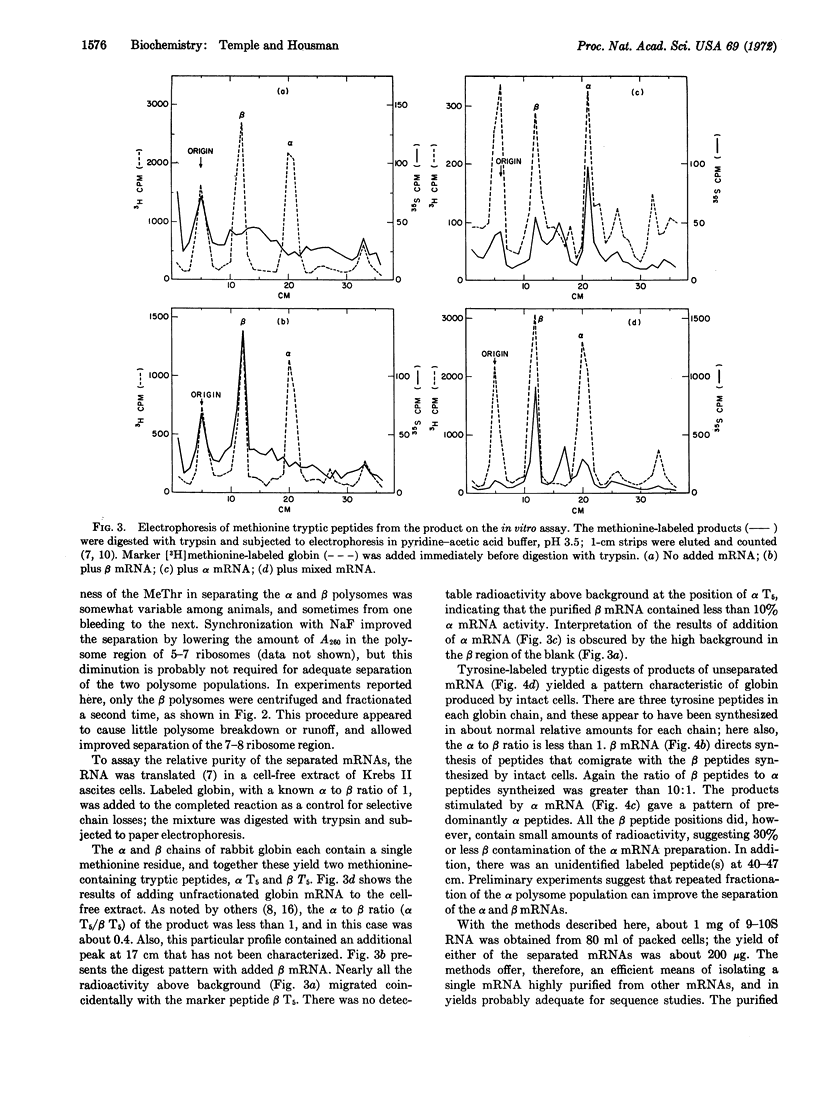
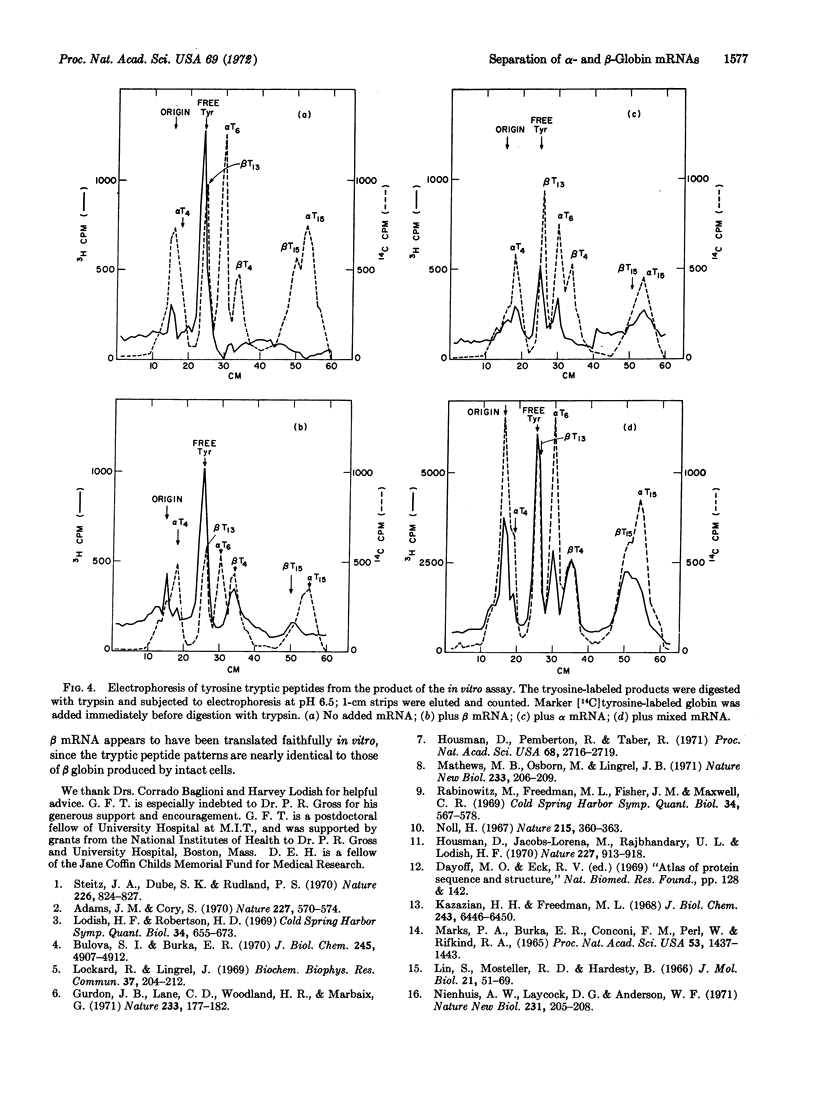
A method is presented to separate the mRNAs coding for α and β globins of rabbit reticulocytes. 10S RNA was extracted from the light and heavy polysomes created by incubation of reticulocytes with L-O-methylthreonine, and assayed for mRNA activity in an ascites cell-free extract. Tryptic digests of the in vitro products demonstrated that the heavy polysomes yielded β globin mRNA at least 90% free of α mRNA activity, and that the light polysomes yielded α mRNA at least 70% free of β mRNA activity.
Keywords: L-O-methylthreonine, in vitro protein synthesis, tryptic peptides
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Cory S. Untranslated nucleotide sequence at the 5'-end of R17 bacteriophage RNA. Nature. 1970 Aug 8;227(5258):570–574. doi: 10.1038/227570a0. [DOI] [PubMed] [Google Scholar]
- Bulova S. I., Burka E. R. Biosynthesis of nonglobin protein by membrane-bound ribosomes in reticulocytes. J Biol Chem. 1970 Oct 10;245(19):4907–4912. [PubMed] [Google Scholar]
- Gurdon J. B., Lane C. D., Woodland H. R., Marbaix G. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature. 1971 Sep 17;233(5316):177–182. doi: 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- Housman D., Jacobs-Lorena M., Rajbhandary U. L., Lodish H. F. Initiation of haemoglobin synthesis by methionyl-tRNA. Nature. 1970 Aug 29;227(5261):913–918. doi: 10.1038/227913a0. [DOI] [PubMed] [Google Scholar]
- Housman D., Pemberton R., Taber R. Synthesis of and chains of rabbit hemoglobin in a cell-free extract from Krebs II ascites cells. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2716–2719. doi: 10.1073/pnas.68.11.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Freedman M. L. The characterization of separated alpha and beta-chain polyribosomes in rabbit reticulocytes. J Biol Chem. 1968 Dec 25;243(24):6446–6450. [PubMed] [Google Scholar]
- Lin S. Y., Mosteller R. D., Hardesty B. The mechanism of sodium fluoride and cycloheximide inhibition of hemoglobin biosynthesis in the cell-free reticulocyte system. J Mol Biol. 1966 Oct 28;21(1):51–69. doi: 10.1016/0022-2836(66)90079-9. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Lingrel J. B. The synthesis of mouse hemoglobin beta-chains in a rabbit reticulocyte cell-free system programmed with mouse reticulocyte 9S RNA. Biochem Biophys Res Commun. 1969 Oct 8;37(2):204–212. doi: 10.1016/0006-291x(69)90720-7. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Robertson H. D. Regulation of in vitro translation of bacteriophage f2 RNA. Cold Spring Harb Symp Quant Biol. 1969;34:655–673. doi: 10.1101/sqb.1969.034.01.076. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Burka E. R., Conconi F. M., Perl W., Rifkind R. A. Polyribosome dissociation and formation in intact reticulocytes with conservation of messenger ribonucleic acid. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1437–1443. doi: 10.1073/pnas.53.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B., Osborn M., Lingrel J. B. Translation of globin messenger RNA in a heterologous cell-free system. Nature. 1971 Oct 13;233(5320):206–209. [PubMed] [Google Scholar]
- Nienhuis A. W., Laycock D. G., Anderson W. F. Translation of rabbit haemoglobin messenger RNA by thalassaemic and non-thalassaemic ribosomes. Nat New Biol. 1971 Jun 16;231(24):205–208. doi: 10.1038/newbio231205a0. [DOI] [PubMed] [Google Scholar]
- Noll H. Characterization of macromolecules by constant velocity sedimentation. Nature. 1967 Jul 22;215(5099):360–363. doi: 10.1038/215360a0. [DOI] [PubMed] [Google Scholar]
- Rabinovitz M., Freedman M. L., Fisher J. M., Maxwell C. R. Translational control in hemoglobin syntheskis. Cold Spring Harb Symp Quant Biol. 1969;34:567–578. doi: 10.1101/sqb.1969.034.01.064. [DOI] [PubMed] [Google Scholar]
- Steitz J. A., Dube S. K., Rudland P. S. Control of translation of T4 phage: altered ribosome binding at R17 initiation sites. Nature. 1970 May 30;226(5248):824–827. doi: 10.1038/226824a0. [DOI] [PubMed] [Google Scholar]



