Abstract
Purpose of the study
Cognitive stimulation therapy (CST) is a widely used, evidence-based intervention for people with dementia (PwD). Although designed as a 14 session, twice weekly intervention, many services in the UK deliver CST once a week for 14 weeks. However, this method of delivery has yet to be evaluated. In addition, CST does not include any formal carer training. This study aimed to evaluate the effectiveness of once weekly CST and determine any additional impact when enhanced with a carer training program.
Design and methods
A single blind, randomized controlled trial was conducted. Sixty eight PwD and their carers were recruited through three community Memory Assessment Services. PwD and their carers were randomized to one of three conditions: CST plus carer training, CST only, or a wait list control. PwD were administered standardized measures of cognition, quality of life, and quality of relationship with carer at baseline and the 15 week follow-up.
Results
There were no baseline differences across the three groups. At follow-up, there were no significant differences between PwD in the three groups on any outcomes.
Implications
Weekly CST with or without carer training may not be an effective form of delivery. Several possible explanations for the outcomes are proposed. Weekly CST may not offer the necessary “dose” required to combat decline, and equally the carer training may have been too brief to have made a difference. Services currently offering weekly CST should collect routine outcome data to support its use and provide practice-based evidence.
Keywords: Alzheimer’s disease, cognition, intervention, caregiver
Introduction
Dementia is a progressive mental health disorder characterized by pervasive impairment of mental function. Currently in the UK there are an estimated 800,000 people living with dementia, and it is predicted that by 2021 this figure will rise to over one million.1 The need for effective and accessible treatments is paramount. Cognitive stimulation is a non-pharmacological treatment with consistent evidence of positive outcomes for people with dementia (PwD) with respect to their cognitive functioning, social interaction, communication and quality of life.2
One example of cognitive stimulation is Cognitive Stimulation Therapy (CST), a brief, evidence-based, 14 session, twice-weekly group therapy for people with mild to moderate dementia. CST leads to improved cognition and quality of life when compared to treatment as usual3 and has positive effects on the behavior of PwD.2 CST is also cost effective,4 shows comparable benefits to acetylcholinesterase inhibitor drugs2 and is effective regardless of whether participants are also taking medication for their dementia.5
Dementia can have significant negative impacts on caregivers including poorer physical and mental health, quality of life, self-efficacy and social support.6 These negative carer outcomes are also associated with poorer outcomes for the PwD, including decreased quality of life and early admission to care homes.6 Involving carers in cognitive stimulation interventions for the person they care for can have positive benefits for both carers and the PwD.7,8 These studies found that involving carers in cognitive stimulation resulted in improved memory, problem solving and verbal fluency for PwD, whilst carers experienced enhanced communication and interaction with the person they care for, as well as maintenance of their quality of life and psychological well-being. To date, formal carer involvement in CST has not, to our knowledge, been evaluated. At present, CST does not include any formal carer involvement. However, involving carers in CST may allow PwD to receive a higher “dose” of CST if carers apply its principles and use CST activities between sessions. This may produce additional benefits for PwD in line with previous research.
Limited Phase IV implementation work9 has been conducted to evaluate the effectiveness of CST in clinical practice. Although CST was designed and evaluated as a 7 week, 14 session program, in practice many National Health Service services deliver the program once a week over 14 weeks, due to time constraints and resource limitations. However, this format is yet to be evaluated.
Consequently, the aims of this study were to establish the effectiveness of CST when delivered once a week for 14 weeks, and to evaluate whether additional carer training led to any benefits above and beyond weekly CST.
Design and methods
Design
A single blind, randomized control design was used with three independent conditions. In treatment condition one (N=21), PwD received 14 sessions of weekly CST and their carers received CST training (CST plus carer training). In treatment condition two (N=24), PwD received 14 sessions of weekly CST (CST only). The control condition (N=23) was a waiting list group (no CST, no carer training). On completion of the study, PwD in the control group were offered CST and carers in the CST only and control groups were offered session one of the carer training program. Assessors were blinded to treatment allocation. In an attempt to facilitate this, participants were reminded not to reveal the group they were in before each assessment.
Randomization
Participants were randomized using the block method10 to achieve equal group sizes and using Random Allocation Software version 1.11 Randomization was conducted separately for each site (to minimize travel time), by the clinician who would be running the CST groups, but not undertaking assessments.
Setting
Data collection took place across three sites within South Essex Partnership Trust in Bedfordshire, UK. All CST groups and carer training sessions were conducted in community settings, with transport available for those who needed it. Care for the person with dementia was provided when needed, to facilitate carers’ attendance.
Participants
Inclusion criteria
Eligibility criteria for PwD were adapted from previous research.3 Subjects were eligible for participation if they:
Met the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria for dementia of any type.12
Scored 18 to 30 on the Mini-Mental State Examination (MMSE)13 indicating mild to moderate dementia.
Could speak English and had some ability to communicate and understand communication – a score of 1 or 0 on questions 12 and 13 of the Clifton Assessment Procedures for the Elderly – Behaviour Rating Scale (CAPE–BRS).14
Lived in the community (ie, not in a residential setting).
Were able to see and hear well enough to participate in the group and make use of most of the material in the program.
Could engage in group activity for at least 45 minutes.
Did not have major physical illness or disability which could affect participation.
Did not have a diagnosis of a learning disability.
Had a carer who was willing to take part in the study (and met inclusion criteria – see carer criteria to follow).
The research team developed a set of inclusion criteria for carers to ensure that they could participate fully in the research. Carers were considered eligible for participation if they:
Had a minimum of three contacts per week with the person they cared for, and were able to continue this for the period of the research study.
Were aged 18 years or above.
Could speak English.
Did not have major physical illness or disability that could affect participation.
All PwD who had a) been through one of the South Essex Partnership Trust’s Memory Assessment Clinics during the previous 2 years, or who were on the waiting list for CST; b) met the inclusion criteria; and c) had a carer who met the inclusion criteria, were invited to take part in the study, along with their carer.
Power analysis
As no previous research was found examining the effect of once weekly CST or carer involvement, it was not possible to calculate an a priori sample size. However, using G*Power 3.115 it was determined that a sample size of 144 participants (72 PwD and their carers; dyads), with power set at 0.80 and 5% significance would be adequate to detect an effect size of 0.34 or above.
Procedure
Recruitment
Dyads who met inclusion criteria were contacted to discuss the study. Separate information sheets for PwD and carers were sent to those who expressed an interest in participating. For those who agreed to participate, written informed consent was obtained, in accordance with the provisions of the Mental Capacity Act.16
Intervention: CST
The study followed the standardized CST manual.17 Groups were held weekly for 14 weeks, with individually themed sessions lasting approximately 45 minutes. A reality orientation board was used and sessions opened with the group song, followed by a warm up exercise and discussion of a recent news article. The main activity then followed, based on that week’s theme. Sessions were designed to be as inclusive as possible and activities were tailored to the groups’ abilities.
Intervention: carer CST training program
This was adapted from the current CST training program and training manual.18 An initial version of session one was field-tested prior to the start of the research with a group of nine carers of PwD who had recently attended a CST group. Adaptations were made based on feedback received. Carers were asked to attend two sessions, with an optional workshop offered between the sessions. Session one lasted 3 hours and was delivered to coincide with the first CST group. Carers were given an overview of dementia and of the development of/rationale for CST. The CST program was outlined and details of the individual sessions were presented. The 18 guiding principles of CST were described and ways of engaging the person at home according to these principles were suggested. Carers were given a workbook outlining activities that related to each theme undertaken in the CST program, which they could try with the PwD between CST sessions. The workbook contained a diary to record and rate the success of activities tried at home.
Session two was delivered during the final week of the CST program and lasted approximately 1 hour. The focus was on maintaining the skills acquired into the future. An overview of CST sessions and underlying principles was presented and time was given for addressing concerns and sharing ideas. An optional 1 hour question and answer session was offered at week 7.
The aim of the program was not to train carers to deliver CST, but to provide them with training about the nature and rationale of CST, introduce essential skills around interacting with the person they care for, and on implementing activities at home using the guiding principles of CST. The objective was to enhance the interactions between the carer and the person they care for in their home environment in such a way that carers felt empowered and could support the experience of the CST group for the person cared for.
Assessments
All participants were assessed at baseline (the 2 week period before the intervention) and at follow-up (the 2 week period following the intervention). Carers were asked to provide demographic details for themselves and the PwD.
Outcome measures
Measures of cognition and quality of life that had showed sensitivity to change in previous CST research were selected.3 In addition, the quality of the PwD-carer relationship was assessed. This has not been previously assessed in CST trials, as they have not included carer input.
Cognition
Two measures were used – the widely used MMSE,13 a brief 30-item test; and the Alzheimer’s Disease Assessment Scale–Cognition (ADAS–Cog),19 a more comprehensive and extensive measure of cognitive function than the MMSE, with good reliability and validity in dementia.
Quality of life
Quality of life was assessed using the Quality of Life– Alzheimer’s Disease scale (QoL-AD)20 a brief 13-item questionnaire delivered in interview format. The QoL-AD has good internal consistency, validity, and reliability.21
Quality of the caregiving relationship
The quality of the caregiving relationship was assessed using the Quality of Caregiver and Patient Relationship (QCPR).22 This scale is a 14-item measure assessing relationship quality. Good reliability and validity have been demonstrated.22
Ethics
Ethical approval for this study was granted by London South East National Research Ethics Service (NRES) Committee.
Data analysis
SPSS version 17 was used to analyze data. Intention-to-treat analysis was applied using the last observation carried forward method for data missing at follow-up. One-way analysis of variance and χ2 tests were used to check for differences in demographics between participants in the three conditions at baseline. Outcomes were analyzed using mixed-method analysis of covariance to allow for variability in baseline characteristics (covariates) to be controlled. The age and sex of the PwD were entered as covariates.
Results
Recruitment and attrition
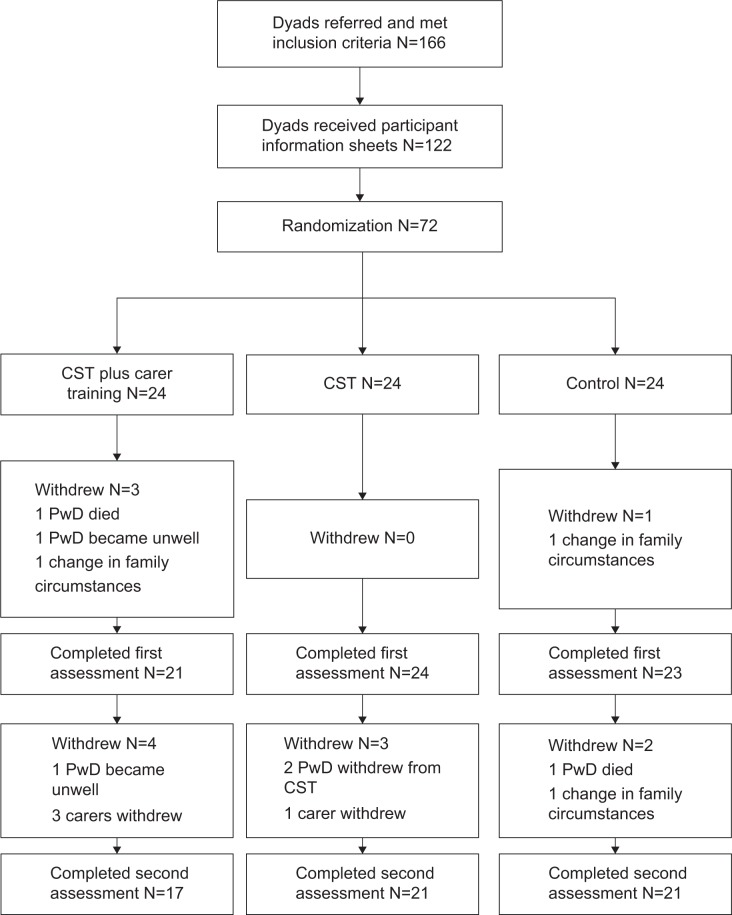
One hundred and sixty six dyads were identified as suitable for inclusion. Of these, 122 dyads consented to receive information packs, and of these 72 dyads consented to participate and were randomized into one of the three treatment conditions. Four dyads dropped out before the first assessment, therefore no data was available for these participants, and they were not included in the final analyses. Nine dyads dropped out between the first assessment and follow-up. Figure 1 displays details of the flow of participants through the research. There were no significant differences in the proportion of completers and non-completers across the three conditions, χ2(2)=1.042, P=0.594, and groups were well matched in baseline characteristics (age, sex, diagnosis, carer age, carer sex, scores on outcome measures).
Figure 1.
Participant flow through the study.
Abbreviations: CST, cognitive stimulation therapy; PwD, people with dementia.
Participant characteristics
A description of the characteristics of PwD across the three conditions can be found in Table 1.
Table 1.
Baseline characteristics of participants with dementia
| CST plus carer training | CST | Control | |
|---|---|---|---|
| Age in years, mean (sd) | 75.4 (5.56) | 76.8 (6.62) | 77.8 (7.47) |
| Sex | |||
| Male (%) | 11 (52.4) | 15 (62.5) | 10 (43.5) |
| Female (%) | 10 (47.6) | 9 (37.5) | 13 (56.5) |
| Ethnicity | |||
| White British (%) | 17 (81.0) | 23 (95.8) | 19 (82.6) |
| White Irish (%) | 0 (0) | 0 (0) | 2 (8.7) |
| White other (%) | 2 (9.5) | 0 (0) | 0 (0) |
| Black Caribbean (%) | 2 (9.5) | 0 (0) | 2 (8.7) |
| Indian (%) | 0 (0) | 1 (4.2) | 0 (0) |
| Living situation | |||
| Private accommodation (%) | 20 (95.2) | 20 (83.3) | 23 (100) |
| Sheltered housing (%) | 0 (0) | 1 (4.2) | 0 (0) |
| Supported living (%) | 1 (4.8) | 3 (12.5) | 0 (0) |
| Dementia diagnosis sub-type | |||
| Alzheimer’s disease (early onset) (%) | 0 (0) | 0 (0) | 1 (4.3) |
| Alzheimer’s disease (late onset) (%) | 15 (71.4) | 11 (47.8) | 10 (43.5) |
| Alzheimer’s disease (atypical/mixed) (%) | 1 (4.8) | 2 (8.7) | 6 (26.1) |
| Vascular dementia (%) | 0 (0) | 3 (13.0) | 1 (4.3) |
| Subcortical vascular dementia (%) | 2 (9.5) | 1 (4.3) | 2 (8.7) |
| Dementia in Parkinson’s disease (%) | 0 (0) | 4 (17.4) | 1 (4.3) |
| Unspecified dementia (%) | 3 (14.3) | 2 (8.7) | 2 (8.7) |
| Dementia severity | |||
| Mild (%) | 15 (71.4) | 18 (75.0) | 17 (73.9) |
| Moderate (%) | 6 (28.6) | 6 (25.0) | 6 (26.1) |
| Living with carer | |||
| Yes (%) | 18 (85.7) | 19 (79.2) | 19 (82.6) |
| No (%) | 3 (14.3) | 5 (20.8) | 4 (17.4) |
| Relationship to carer | |||
| Spouse (%) | 17 (81.0) | 17 (70.8) | 17 (73.9) |
| Partner (%) | 0 (0) | 0 (0) | 1 (4.3) |
| Mother/father (%) | 4 (19.0) | 5 (20.8) | 4 (17.4) |
| Mother/father-in-law (%) | 0 (0) | 2 (8.3) | 0 (0) |
| Aunt/uncle (%) | 0 (0) | 0 (0) | 1 (4.3) |
| Age of carer, mean (sd) | 68.81 (10.39) | 67.13 (11.26) | 70.43 (11.12) |
| Number of medications, mean (sd) | 5.19 (4.14) | 3.88 (2.62) | 5.70 (4.16) |
| Dementia medication | |||
| Yes (%) | 10 (47.6) | 16 (66.7) | 13 (56.5) |
| No (%) | 11 (52.4) | 8 (33.3) | 10 (43.5) |
| Attended previous dementia intervention | |||
| Yes (%) | 1 (4.8) | 3 (12.5) | 3 (13.0) |
| No (%) | 20 (95.2) | 21 (87.5) | 20 (87.0) |
| Number of CST sessions attended, mean (sd) | 10.95 (3.64) | 10.50 (4.53) | N/A |
Abbreviations: CST, cognitive stimulation therapy; sd, standard deviation; N/A, not applicable.
Carer attendance at the training program
Of the 21 carers in the CST plus carer training condition, 14 attended all sessions. Two attended sessions one and two, but not the optional workshop; four attended session one only; and one did not attend any sessions. Although carers were asked to record all CST activity they used at home, the majority of carers did not, meaning very little quantitative data about level of use of CST activities or principles were available.
Analysis of outcomes
There were no significant baseline differences across the three conditions on any of the outcome measures: MMSE (F[2, 67]=0.16, P=0.85); ADAS–Cog (F[2, 65]=0.05, P=0.96); QoL-AD (F[2, 67]=0.69, P=0.51); QCPR (F[2, 66]=0.22, P=0.81). Mean scores at baseline and follow-up for each outcome measure for each condition are displayed in Table 2, which also displays between-group effects and effect sizes from the analysis of covariance.
Table 2.
Mean scores at baseline and follow-up for each outcome measure
| Baseline
|
Follow-up
|
ANCOVA between group difference | Effect size (ηp2) | |||||
|---|---|---|---|---|---|---|---|---|
| CST plus carer training | CST | Control | CST plus carer training | CST | Control | |||
| Mini-Mental State Examination | 22.33 (3.54) | 22.71 (3.76) | 22.91 (3.01) | 22.19 (4.48) | 22.38 (4.75) | 22.13 (3.40) | F=0.84, P=0.92 | 0.003 |
| Alzheimer’s Disease Assessment scale–Cognition | 18.35 (7.1) | 18.13 (8.24) | 17.68 (6.51) | 20.10 (7.6) | 19.04 (8.13) | 20.09 (7.2) | F=0.02, P=0.98 | 0.001 |
| Quality of Life–Alzheimer’s Disease scale | 36.43 (6.06) | 36.42 (5.44) | 34.78 (5.43) | 36.45 (5.6) | 35.65 (5.83) | 35.32 (5.51) | F=0.82, P=0.44 | 0.03 |
| Scale for the Quality of the Current Relationship in Caregiving | 57.38 (6.49) | 57.09 (6.91) | 56.13 (6.53) | 57.90 (6.61) | 55.65 (6.83) | 56.41 (6.53) | F=0.97, P=0.39 | 0.03 |
Note: Standard deviations shown in brackets.
Abbreviations: ANCOVA, analysis of covariance; CST, cognitive stimulation therapy.
There were no changes in cognition as assessed by the MMSE over time (F[1, 63]=0.81, P=0.37 [ηp2=p =0.01]) and no significant differences between the three groups at follow-up (F[1, 63]=0.84, P=0.92 [ηp2=0.003]). Although there was a significant decline in cognition between baseline and follow-up across the whole group as assessed by the ADAS–Cog (F[1, 61]=4.38, P=0.04) this effect was very small (ηp2=0.07), and there were no between-group differences on this measure at follow-up (F[1, 61]=0.02, P=0.98 [ηp2=0.001]). There were no between-group differences on any of the 12 subscales of the ADAS–Cog. There were no changes in QoL-AD over time (F[1, 61]=0.003, P=0.96 [ηp2=0.0001]), and no differences between the three groups at follow-up (F[1, 63]=0.82, P=0.44 [ηp2=0.03]). Similarly, there were no changes in the QCPR over time (F[1, 62]=1.68, P=0.20 [ηp2=0.03]), and no between-group differences at follow-up (F[1, 62]=0.97, P=0.39 [ηp2=0.03]).
Discussion
No improvements in cognition, quality of life or the quality of the caregiving relationship were observed in PwD receiving once weekly CST, with or without carer training. These results suggest that delivering manualized CST weekly may not be enough to make a difference, and that this cannot be enhanced through provision of carer training. Twice weekly CST may be necessary to provide the required “dose” to combat the natural deterioration in dementia and have a positive effect. However, it is important to consider several other possible explanations of the results observed.
Firstly, cognitive functioning as assessed by the MMSE and the ADAS–Cog was higher in the present study than in previous trials.2,3 It may be that CST is less effective for this higher functioning group or that these measures are less sensitive to change as they approach ceiling effects. This latter conclusion is supported by one study23 which included participants with high baseline MMSE scores. Following CST, they observed no changes in cognition as assessed by the MMSE, yet found significant changes in other more sensitive neuropsychological tests.
Secondly, outcomes were selected based on those which had shown improvements in previous research.3 However, it is possible that higher functioning PwD benefit in a different way from CST and there could have been positive outcomes in other unmeasured domains, such as wider social benefits or self-esteem. It may also be that once weekly CST reduces health and social care costs, eg, through providing an ongoing support network, and reducing other use of services such as general practitioner (GP) visits and hospital admissions.
The lack of effect of carer training may be due to failure to achieve its aim of providing a higher “dose” of CST. The maximum number of hours training received was five, with many people receiving fewer. This may simply not have been enough to achieve changes in interactions or activities undertaken at home. Furthermore, almost no quantitative data were available to show the extent to which carers used any of the recommended activities, or adapted their interactions according to the CST principle. It is therefore possible that carers were not using the CST at home, meaning that PwD in the CST plus carer training group did not receive a higher “dose”’ of the intervention than those in the CST group as planned.
Limitations
Attempts were made to ensure that assessors were blind to participants’ group allocation. However, no formal measure of the integrity of the blinding process was included. Therefore, it was not possible to determine the extent of observer bias. Secondly, no monitoring of treatment fidelity was undertaken, hence the extent to which CST sessions were implemented as planned is unknown. Adherence checks would have increased confidence in treatment fidelity. Finally, although the study was powered to detect large effect sizes, the relatively small sample size meant that it was underpowered to detect smaller effects. A larger sample size would be preferable in order to draw conclusions with acceptable statistical significance.
Implications
The current study does not provide support for weekly CST and the addition of a carer training program. Services should consider carefully whether to run CST groups once or twice weekly, balancing the evidence-based and the National Institute for Health and Care Excellence (NICE) guidelines alongside practical issues, such as time, resources, and participant availability. Where a once weekly format is used, service-level monitoring of outcomes is essential to ensure that those who participate are benefiting, and to provide service-level data that can be used to further explore the effectiveness, or not, of weekly CST. Any benefit for PwD of including carer training should similarly be monitored and assessed using relevant outcome measures.
It is recommended that the current study be replicated using a larger sample to enhance power, and use a wider range of outcome measures to capture possible benefits missed, eg, mood, communication and behavior, and longer term costs. Including a cost-effective analysis in future research would facilitate a comparison with other services to determine whether CST offers a lower cost alternative to such services.
Further development of the carer training program will also be of benefit. We developed the carer training program to be a brief, low intensity intervention, however a more intensive program may be necessary to achieve the desired effects. Furthermore, future studies should ensure carers adequately use CST activities and principles at home.
Conclusion
The current project does not provide evidence that weekly CST is effective, or that enhancing CST with carer training offers additional benefits to PwD. While service providers should 1) exercise caution if offering CST in a weekly program; and 2) consider changing to offer a twice weekly program, continued collection of outcome data will allow for on-going monitoring of the progress of participants to establish whether self-reported benefits from participants of weekly CST are being achieved.
Footnotes
Disclosure
Dr Helen Donovan and Nicola Jacobi are both employees of South Essex Partnership Trust, which received a grant from Anglia Ruskin University toward the funding of this research. Dr Aimee Spector, Dr Helen Donovan and Dr Josh Stott offer CST training on a commercial basis. The authors report no other conflicts of interest in this work.
References
- 1.Alzheimer’s Society . Dementia 2012: A national challenge. London: Alzheimer’s Society; 2012. [Accessed November 5, 2014]. Available from: http://www.alzheimers.org.uk/site/scripts/download_info.php?fileID=1389. [Google Scholar]
- 2.Aguirre E, Woods B, Spector A, Orrell M. Cognitive stimulation for dementia: a systematic review of the evidence of effectiveness from randomised controlled trails. Ageing Res Rev. 2013;12:253–262. doi: 10.1016/j.arr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Spector A, Thorgrimsen L, Woods B, et al. Efficacy of an evidence-based cognitive stimulation therapy programme for people with dementia: randomised controlled trial. Br J Psychiatry. 2003;183:248–254. doi: 10.1192/bjp.183.3.248. [DOI] [PubMed] [Google Scholar]
- 4.Knapp M, Thorgrimsen L, Patel A, et al. Cognitive stimulation therapy for people with dementia: cost effectiveness analysis. Br J Psychiatry. 2006;188:574–580. doi: 10.1192/bjp.bp.105.010561. [DOI] [PubMed] [Google Scholar]
- 5.Aguirre E, Hoare Z, Streater A, et al. Cognitive stimulation therapy (CST) for dementia – who benefits most? Int J Geriatr Psychiatry. 2013;28:284–290. doi: 10.1002/gps.3823. [DOI] [PubMed] [Google Scholar]
- 6.Etters L, Goodall D, Harrison BE. Caregiver burden amongst dementia carergivers: a review of the literature. J Am Acad Nurse Prac. 2008;20:423–428. doi: 10.1111/j.1745-7599.2008.00342.x. [DOI] [PubMed] [Google Scholar]
- 7.Onder G, Zanetti O, Giacobini E, et al. Reality orientation therapy combined with cholinesterase inhibitors in Alzheimer’s disease: randomised control trial. B J Psychiatry. 2005;187:450–455. doi: 10.1192/bjp.187.5.450. [DOI] [PubMed] [Google Scholar]
- 8.Quayhagen M, Quayhagen M. Testing of a cognitive stimulation intervention for dementia caregiving dyads. Neuropsychol Rehab. 2001;11:319–332. [Google Scholar]
- 9.Medical Research Council . Developing and Evaluating Complex Interventions: New Guidance. London: MRC Health Services and Public Health Research Board; 2008. [Accessed November 5, 2014]. Available from: http://www.mrc.ac.uk/documents/pdf/complex-interventions-guidance/ [Google Scholar]
- 10.Schulz KF, Grimes DA. Generation of allocation sequences in randomised trials: chance, not choice. Lancet. 2002;359:515–519. doi: 10.1016/S0140-6736(02)07683-3. [DOI] [PubMed] [Google Scholar]
- 11.Saghaei M. Random allocation software for parallel group randomized trials. BMC Med Res Methodol. 2004;4:26. doi: 10.1186/1471-2288-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Pattie AH, Gilleard CJ. Clifton Assessment Procedures for the Elderly (CAPE) Sevenoaks: Hodder & Stoughton; 1979. [Google Scholar]
- 15.Faul F, Erdfelder E, Lang A, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Beh Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 16.Department for Constitutional Affairs . Mental Capacity Act 2005. London: The Stationery Office; 2007. [Accessed November 5, 2014]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/224660/Mental_Capacity_Act_code_of_practice.pdf. [Google Scholar]
- 17.Spector A, Thorgrimsen L, Woods B, Orrell M. Our Time: An Evidence-Based Group Program to Offer Cognitive Stimulation to People with Dementia – Manual for Group Leaders. Cedar Falls, USA: Freiberg Press; 2005. [Google Scholar]
- 18.Aguirre E, Spector A, Streater A, Hoe J, Woods B, Orrell M. Making a Difference 2. Hawker Publications; UK: 2011. [Google Scholar]
- 19.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 20.Logsdon R, Gibbons LE, McCurry SM, Teri L. Quality of life in Alzheimer’s disease: patient and carer reports. Journal of Mental Health and Aging. 1999;5:21–32. [Google Scholar]
- 21.Thorgrimsen L, Selwood A, Spector A, et al. Whose quality of life is it anyway? The validity and reliability of the Quality of Life – Alzheimer’s Disease (QOL-AD) scale. Alzheimer’s Dis Assoc Disord. 2003;17:201–208. doi: 10.1097/00002093-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Spruytte N, Van Audenhove C, Lammertyn F, Storms G. The quality of the caregiving relationship in informal care for older adults with dementia and chronic psychiatric patients. Psychol Psychother. 2002;75:295–311. doi: 10.1348/147608302320365208. [DOI] [PubMed] [Google Scholar]
- 23.Hall L, Orrell M, Stott J, Spector A. Cognitive stimulation therapy (CST): neuropsychological mechanisms of change. Int Psychogeriatr. 2013;25:479–489. doi: 10.1017/S1041610212001822. [DOI] [PubMed] [Google Scholar]