Abstract
Cancer may be a disease of geometry: a misregulation of the field of information that orchestrates individual cells’ activities towards normal anatomy. Recent work identified molecular mechanisms underlying a novel system of developmental control: bioelectric gradients. Endogenous spatio-temporal differences in resting potential of non-neural cells provide instructive cues for cell regulation and complex patterning during embryogenesis and regeneration. It is now appreciated that these cues are an important layer of the dysregulation of cell: cell interactions that leads to cancer. Abnormal depolarization of resting potential (Vmem) is a convenient marker for neoplasia and activates a metastatic phenotype in genetically-normal cells in vivo. Moreover, oncogene expression depolarizes cells that form tumor-like structures, but is unable to form tumors if this depolarization is artificially prevented by misexpression of hyperpolarizing ion channels. Vmem triggers metastatic behaviors at considerable distance, mediated by transcriptional and epigenetic effects of electrically-modulated flows of serotonin and butyrate. While in vivo data on voltages in carcinogenesis comes mainly from the amphibian model, unbiased genetic screens and network profiling in rodents and human tissues reveal several ion channel proteins as bona fide oncogene and promising targets for cancer drug development. However, we propose that a focus on specific channel genes is just the tip of the iceberg. Bioelectric state is determined by post-translational gating of ion channels, not only from genetically-specified complements of ion translocators. A better model is a statistical dynamics view of spatial Vmem gradients. Cancer may not originate at the single cell level, since gap junctional coupling results in multi-cellular physiological networks with multiple stable attractors in bioelectrical state space. New medical applications await a detailed understanding of the mechanisms by which organ target morphology stored in real-time patterns of ion flows is perceived or mis-perceived by cells. Mastery of somatic voltage gradients will lead to cancer normalization or rebooting strategies, such as those that occur in regenerating and embryonic organs, resulting in transformative advances in basic biology and oncology.
Keywords: Bioelectricity, Resting potential, Voltage gradient, Normalization, Reprogramming, Microenvironment
Introduction
“Cancer is no more a disease of cells than a traffic jam is a disease of cars. A lifetime of study of the internal-combustion engine would not help anyone understand our traffic problems--
D. W. Smithers”
Ideas in cancer biology comprise two complementary paradigms. The mainstream view is that cancer cells are irreversibly damaged: they have accumulated genetic or epigenetic damage and are fundamentally a different kind of cell. On this view (the somatic mutation model), cancer cells have acquired cell-autonomous properties that underlie unlimited proliferation and metastasis [1,2]. Contrasting with this view is the idea that cells exhibit neoplastic behavior and form tumors due to a change of interactions with their environment. This view of cancer is focused on context-non-cell-autonomous signaling that activates cellular misbehavior in the host [3–8]. The latter class of models ranges from simple growth suppressive molecules (morphostats) secreted by healthy tissue [9,10] and averaging effects of cell neighbors that stabilize stochastic gene expression [11] to suppression of tumorigenesis by tissue-level organization [12,13] to global models of whole-body morphogenetic information fields [14–19].
While the mutation-centered paradigm has dominated work in this field for decades, increased attention is now focused on cancer as a progressive loss of the organization capacity of the environment over the heterogeneous behavior of isolated cells [18–24]. Interestingly, “no cancer exhibits any trait which cannot be found in some normal tissue as the expression of normal genomic activity; no cancer grows faster than an embryo nor is any cancer cell more invasive than a macrophage nor are cancer cell lines more immortal than are germ lines. The only distinction is that, in the cancer, the expression or lack of expression of many traits may be inappropriate for the tissue in which the cancer occurs” [25]. This is a view of cancer as fundamentally a developmental disorder of cell regulation.
Large-scale shape, or the correct geometric arrangement of organs and tissues in an organism, is a key concept in biological growth and development. To achieve optimal health, organisms strive to maintain shape at all levels, from the single cell to the whole organism. Cancer can be seen as an error of geometry, because tumor cells grow, migrate, and function without regard for the orderly structure within which they occur [26]. This is seen most acutely in teratomas - embryonic tumors that display extensive differentiation of a number of tissues combined with a complete absence of orderly organization of the whole. The idea that cancer is a developmental disease is an old one [9,10,26–29]. Needham and Waddington speculated that cancers represented an escape from the control of the morphogenetic field [30–32]. On this view, tumors form when cells stop obeying the normal patterning cues of the body: “cancer as part of an inexorable process in which the organism falls behind in its ceaseless effort to maintain order” [28].
Understanding cancer as a reversible physiological state of a multi-cellular dynamical system (as opposed to damage within single cancer stem cells) has significant medical implications because it suggests specific prevention and detection strategies focused on modulating the physiological interrelationships among many cells instead of looking for DNA markers in single cancer stem cells. A mechanistic dissection of these pathways may give rise to strategies that reboot [23] or normalize cancer, in contrast to current approaches that all seek to kill tumors and thus risk a compensatory proliferation response by rogue cells that still remain [33]. Thus, biologists are beginning to explore the idea that cancer is not a genetic disease of specific loci but rather a kind of attractor in a multi-dimensional transcriptional space describing cell states [17]: “The topology of the attractor is the ‘invisible hand’ driving the system functions into coherent behavioral states: they are self-organizing structures and can capture the gene expression profiles associated with cell fates” [34]. Huang et al. also point out an interesting paradox: while many studies seek to “determine which gene is mutated to explain an incremental malignant trait, no one doubts that normal cells as distinct as a mature neuron vs. a blood or epithelial stem cell share the exact same genome! No mutations are invoked to explain the remarkable phenotypes during cell lineages in development” [34], and indeed aneuploidy is routinely present in normal brain, testes, and liver, but does not usually result in cancer (reviewed in 8).
A complete picture of cancer no doubt involves both an understanding of DNA damage and cell signaling dynamics, although considerable controversy exists as to the most appropriate level of organization at which to search for the origin and cure of cancer (ranging from genes, to stem cells, to tissues, to entire body plan organizing fields). Here we focus on bioelectrical information-exchanging processes occurring within and among cell groups in the suppression and progression of cancer, followed by an in-depth discussion of the importance of context and cell: cell signaling to the cancer problem in general. Our hypothesis is that while ion channels are increasingly revealed as important oncogenes, a focus on specific channel genes is just the tip of the iceberg because bioelectric cell states result from post-translational gating of ion channels and pumps, not only from genetically-specified complements of ion translocators. A more fruitful model of cancer and its reprogramming may be a statistical dynamics view of spatial gradients of resting potential as a systems-level property of multi-cellular physiological networks of cells linked by gap junctions.
Bioelectricity as an instructive component of microenvironment
Voltage gradients in non-neural cells control cell behavior
It has long been known that bioelectrical signals, or spatio-temporally patterned ion flows in non-excitable cells, are important determinants of cell behavior [35,36]. Steady-state endogenous ion currents, resting potentials (voltage gradients), and electric fields are produced by the activity of ion channel and pump proteins across cell membranes and their slow dynamics are distinct from the rapid action potentials of nerve and muscle (Figure 1). While related biophysical phenomena include transepithelial electric fields [37,38], ultraweak photon emission [39,40], and coherent AC electromagnetic fields [41,42], here we focus on distributions of Vmem or membrane potential [43]. The many interesting studies of applied field effects are discussed in several excellent recent reviews [44–47].
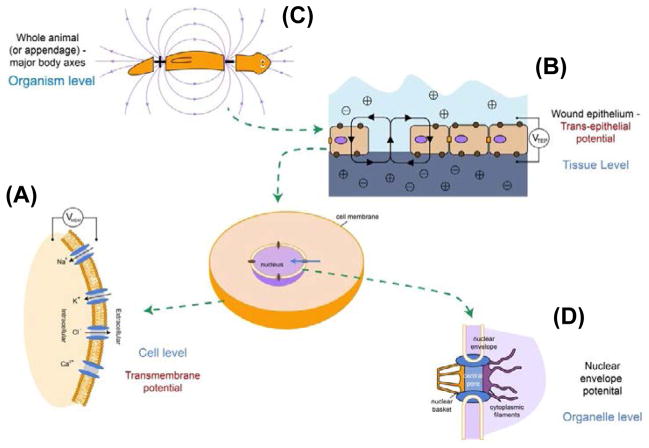
Figure 1. Bioelectric cues in vivo.
(A) Resting potential gradients in all cells result from the imbalance of ion movement across cell membranes. The resulting Vmem is a function of the internal and external concentrations of the major ion species, as well as the conductivity to each ion (open/closed states of specific ion channel proteins).
(B) At the level of tissues, trans-epithelial potentials (TEPs) result from ion flows across sheets of cells connected by tight junctions [493–494]. Breaks in epithelia provide electric fields that serve as migratory cues for galvanotaxis of cells involved in wound healing and metastasis.
(C) At a yet greater level of biological organization, spatial gradients of Vmem and TEP correlate with whole body organs or primary anatomical axes[113,495,496], providing patterning cues such as positional information that guide growth and form.
(D) Still mysterious with respect to their function in patterning are the gradients across intracellular membranes, such as the nuclear envelope potential [497–498]. The role of such gradients for active targeting and distribution of intracellular components or conformation/transcriptional status of components of the DNA in the nucleus remain to be analyzed.
Bioelectric properties of cells and the electrical states of cells in the microenvironment are known to control several key behaviors of relevance to cancer [38,48–54]. For example, electric fields generated by ion pumping across epithelia serve as migration cues for cellular galvanotaxis [55–59] - an important guidance modality for cell movement within the host. Cell shape changes, such as increased arborization, are also driven by endogenous electric fields and changes in Vmem [60–64]. Together, migration and shape properties are key elements of successful metastasis.
Moreover, resting potential established by ion channel and pump proteins is important for determination of differentiation state and proliferation; generally, a depolarized state is indicative of plastic, undifferentiated cells (e.g., stem cells), while differentiation is caused by increase of negative Vmem. Functional control of cell state by changes in Vmem has been observed in many kinds of stem and progenitor cells [65–75], including adult human mesenchymal stem cell [71,76] which can be kept stem-like despite the presence of chemical differentiation factors by forced depolarization, and in induced pluripotentent stem cells [77]. Even mature CNS neurons can be made to re-enter mitosis by sustained depolarization [78,79] revealing the power of transmembrane potential to regulate proliferative potential in adult somatic cells.
Importantly, the molecular mechanisms by which cell-autonomous [80] and non-autonomous [49,81] bioelectric events control downstream processes are now beginning to be fleshed out. Electric fields are transduced to cell migration machinery via Ca2+-dependent mechanisms. A cell with a negative membrane potential, when exposed to an electric field, becomes more hyperpolarized near the anode, allowing the passive inflow of Ca2+ through voltage-gated calcium channels. An increase in Ca2+ in the anodal side of the membrane results in increased polymerization/depolymerization of actin, contraction of actomyosin, and decreased adhesion; collectively, the anodal side contracts and is propelled towards the cathode [82]. Inositol-phospholipid signaling, PI(3)Kγ, and cdc42/rho have been especially implicated in setting the directionality of cytoskeleton-mediated migration polarity in several cell types [83,84].
Changes in Vmem of cells, such as cancer-associated depolarization, can trigger transcriptional changes by 1) regulating the movement of morphogens such as serotonin, calcium, and inositol triphosphate through gap junctions [85–91], 2) controlling the import/export of small signaling molecules such as serotonin and butyrate across membrane exchangers [60,91–94], and 3) modulating the activity level of phosphatases such as PTEN [95–98]. Together, these transduction mechanisms convert an essentially biophysical state change into secondary messenger events that impact on transcriptional and epigenetic regulation of loci such as NODAL which are important for the cancer phenotype [94,99]. While bioelectric cues feed into the same molecular-genetic pathways that are known to regulate normal and neoplastic cell behaviors, they form a pathway that functions alongside biochemical gradients, but with significantly different spatial dynamics, to coordinate normal tissue morphology.
Spatio-temporal gradients of Vmem are instructive patterning cues
Disruption of the electrical gradients, or the mechanisms by which they are perceived by cells, are one way that complex anatomical order is subverted during carcinogenesis. Recent development of state-of-the-art tools for the detection and experimental manipulation of biophysical signals in multicellular patterning contexts [80,100–102] has revealed how distributions of voltage gradients mediate positional information, organ identity of large cell groups, and initiation signals for complex developmental modules such as tail or limb regeneration. Using a combination of fluorescent voltage-reporter dyes to characterize spatial Vmem distributions and functional studies using targeted misexpression of a panel of well-characterized ion transporters to specifically modify those gradients in vivo, instructive signaling roles of transmembrane voltage gradients have been identified in embryogenesis and regeneration, adding to the list of such roles identified in earlier work using functional physiology [103,104].
During early frog development, the redistribution of maternal potassium channels and proton pumps in early blastomeres results in a Vmem difference across gap junction-coupled cells [89,90,105–108]. The resulting voltage gradient redistributes pre-nervous serotonin to the right side, which then interacts with a cytoplasmic receptor that binds histone deacetylase [1] and shuts off Nodal expression on the right side of the embryo [99,106,109]. Later, during craniofacial patterning, the position of the eyes [110] and other elements of the face [111] is determined by a regionalization of naïve ectoderm into distinct domains of hyperpolarized cells. These voltage gradients regulate the expression of genes like Frizzled, and artificially altering this pattern by misexpression of specific ion channels and pumps is sufficient to perturb normal craniofacial anatomy and to reprogram tissues far away from the head to form properly-patterned eyes [110]. Importantly, in such cases, as in the cancer phenotypes discussed below, it is really the Vmem that is the necessary and sufficient factor for inducing specific shape change – it does not matter which ion translocator protein is involved, or what ion species is used: a given voltage change, no matter how it is produced, activates specific downstream events.
During regeneration of flatworms, the patterning activity of adult stem cells (neoblasts) is regulated by gap junctional connectivity and a set of proton and potassium flows [112–114]. By regulation of apoptotic remodeling and downstream activity of genes such as Wnt11 [115], the physiological gradient determines the anatomy of the organs built after injury. In vertebrates, where electric fields were long ago implicated in limb regeneration [116–123], recent experiments showed that driving proton and sodium fluxes can initiate complete tail [124,125] or limb [126] regeneration in a range of non-regenerative conditions. The mechanisms involve guidance of innervation into the stump, activation of blastema genes such as MSX1, Notch, Delta, BMP2, and BMP4, and induction of cell proliferation in the wound mesenchyme.
Indeed, a number of recent molecular studies using unbiased approaches have identified a range of ion channels, gap junctions, and ion pumps in: morphogenesis of the trachea [127], development of skin pigmentation pattern [128,129], regeneration of the zebrafish fin [130], development of mammalian face [131–139], growth of the cerebellum [140–143], and formation of the skeletal [144], cardiac [145,146], and urogenital [147,148] systems. Thus in addition to experiments directly studying bioelectricity in amphibian, avian, and planarian systems, data from genetic models such as Drosophila also identifies channels such as Kir2.1 as important regulators of Dpp signaling and wing patterning [137].
With respect to wound healing, inhibition of which is known to be a tumor promoting agent [149–151], elegant molecular genetics experiments have now revealed some of the elements underlying endogenous electric field-mediated cell migrations. Epithelial wound closure involves Integrin Beta-4 (ITGB4), Cyclic AMP, betaphosphatidylinositol-3-OH kinase-γ (PI(3)Kγ) and phosphatase and tensin homolog (PTEN) [49,81,83,84,152]. Having seen that endogenous electric fields and Vmem gradients play an instructive role in normal patterning, what is the evidence that dysregulation of bioelectrical communication can underlie the cancer phenotype?
Bioelectric gradients in cancer at the cell level
Ion channels are oncogenes and important drug targets
The view that cancer is a developmental disorder predicts that molecular mechanisms known to be important mediators of the morphogenetic field would be involved in tumorigenesis. Indeed, there is mounting evidence (Figure 2) that the bioelectric cues that establish normal pattern can go awry and result in cancerous growth [51,153,154]. The function of ion channels is involved in the self-sufficiency in growth signals, insensitivity to anti-growth signals, evasion of programmed cell death, limitless replicative potential, sustained angiogenesis, and tissue invasion and metastasis [52]. Ion channels, pumps, and gap junctions are now recognized as oncogenes [51], predictive markers [52], and an important set of targets for new cancer drugs that’s seek to modulate cell behavior by tweaking electrical controls of proliferation or metastatic behavior [155]. Importantly however, oncochannel misregulation occurs not only through mutations in channel genes but also by changes in the rich network of events that implement post-translational gating of wild-type ion channels.
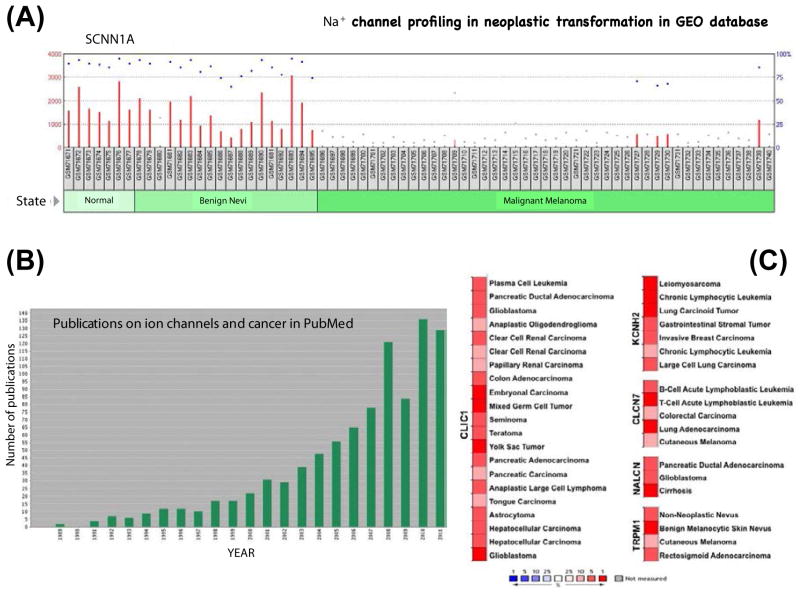
Figure 2. Molecular genetics implicates ion translocators in cancer.
(A) The Gene Expression Omnibus (GEO) database consists of high-throughput functional genomic data on collections of biologically and statistically comparable samples. A profile generated from the cutaneous malignant melanoma dataset readily reveals changes of transcription of many ion channel genes during neoplastic progression; here is shown the down-regulation of a specific sodium channel at the transition from benign nevi to malignant melanoma.
(B) The amount of published data on ion channels is growing more voluminous every year. Importantly, the current level of interest in oncochannels as genetic and pharmacological targets is a significant underestimate of their true importance, since current studies take place almost exclusively at the levels of mRNA or protein profiling. Since channels do most of their regulation post-translationally (being opened or closed by a range of local and non-cell-autonomous signals), analyses such as profiling, microarray, deep sequencing, knockout screens, etc. inevitably miss all of the regulation that takes place at the level of physiology.
(C) Oncomine is a cancer profiling database consisting of genes, pathways, and networks deregulated in more than 50,000 cancer gene expression profiles. Analysis of genes with highly altered expression levels (fold change compared to relevant normal tissue in the top 10 percentile) in multiple cancer types implicates several chloride, potassium, and sodium channels as oncogenes.
Tumor cells differ from untransformed cells in terms of the type of ion channels and pumps they express and in the resulting membrane potential of the cells [156–165]. Some channel levels are thus used as markers, such as the K2P channel TREK-1 and the sodium channel NaV in prostate cancer [166,167], and the TRPM1 channel in melanoma [168,169]. Tumor cells’ membrane voltage is often determined by a different transporter than that of normal cells and it has been suggested that this gives the cells a selective advantage [160]. For example, hepatocellular carcinoma up-regulates the V-ATPase, which is then localized to the plasma membrane [170].
The function of ion translocators, (Table 1) such as voltage-gated K+ channels [171,172] and Cl− channels [173], controls the proliferation rate of a number of cells that often form tumors [174–185] or leukemia [186]. ERG is particularly involved in cell growth signals [160,187–191], and is implicated in transformation of prostate epithelium [192]. Also implicated are 2-pore channels such as KCNK9 [193,194], and voltage-gated sodium channels, being definitive oncogenes – necessary and sufficient for a transformed phenotype [195]. Transfection of the EAG K+ channel confers a transformed phenotype in mammalian cells [196], and hEAG1 channel expression is regulated by p53 (via miR-34 and E2F1) [197]. In human breast cancer cells, K+ current controls progression through the cell cycle [198]; activation of an ATP-sensitive potassium channel is required for breast cancer cells to undergo the G1/G0-S transition [199]. Metastatic potential correlates with voltage-gated inward sodium current and it has been suggested that some sodium channels may be oncofetal genes, encoding signals that are active during the rapid and autonomous growth of tumors and embryos [167,200–203].
Table 1.
Ion translocators implicated in cancer.
| Ion channel/pump Protein | Species | Reference |
|---|---|---|
| NaV1.5 sodium channel | Human | 253,499 |
| EAG-1 potassium channel | Human | 196 |
| KCNK9 potassium channel | Mouse | 194 |
| Ductin (proton V-ATPase subunit) | Mouse | 495 |
| SLC5A8 sodium/butyrate transporter | Human | 496 |
| KCNE2 potassium channel | Mouse | 497 |
| KCNQ1 potassium channel | Human | 214,215 |
| SCN5A sodium channel | Human | 498 |
| Metabotropic glutamate receptor | Mouse, Human | 236,485,499 |
Migration of cells including B-16 melanoma is dependent on K+ channels [204]. The voltage-gated sodium channels (VGSCs) potentiate breast cancer metastasis [203], and indeed the involvement of NaV in the galvanotaxis that allows prostate and breast cancer cells to move across vessel lumens [82,195,205–211] is one of the leading stories on ion channels in cancer. Highly up-regulated activities of NaV confers on cancer cells directional motility and invasive characteristics via Ca2+ and pH-sensitive cytoskeletal remodeling processes which facilitate metastasis [195,203]. Certain channelopathies result in syndromes associated with cancer such as the lung cancer seen in Lambert-Eaton syndrome [212], and the tumors present in Beckwith-Wiedemann syndrome, which is caused by abnormal imprinting of a voltage-gated potassium channel [213–215]. As will be discussed below, Vmem induces cancer-like cell states; while a complete picture of the process remains to be worked out in mammalian cells, a few entry-points have been identified. For example, KID-1, a kinase induced by depolarization [216,217], is a member of the Pim family of proto-oncogenes [218].
Voltage control functions together with the more commonly-studied signals such as growth factors and adhesion molecules. For example, adhesion to specific substrate molecules (mediated by integrin) causes a 20 mV hyperpolarization of resting potential in murine neuroblastoma cells; the hyperpolarization is due to Kir channels and works through a G protein; this hyperpolarization is gone after 1 hour and is necessary for neurite outgrowth [219,220]. Proton pump blockers such as concanamycin and bafilomycin are known anti-tumor agents [221–228] and repression of pancreatic tumor cells occurs after selective blockade of IK-type channels [229]. The involvement of neurotransmitters in cancer [230,231] could also be explained by a voltage-dependent mechanism. For example, GABA is a tumor suppressor [232–234] and GABAA and nAChR are ligand-gated ion channels often expressed in tumors [51].
The involvement of ion channels in transformation, growth control, and metastasis has led to efforts to develop potassium, chloride, and sodium channel and pump modulators as clinical agents for ovarian [235], breast [236], and prostate [237] cancer [155,238]. Unbiased drug screens for inhibitors of cancer stem cells have identified salinomycin (a potassium ionophore) [239] and tetraethylammonium (TEA, a potassium channel blocker) as anticancer drugs that target tumor initiating cells. For example, TEA was found to suppress colony formation in endometrial cancer cells while its withdrawal resulted in a significant enhancement of tumorigenesis [240].
Which ion channels should be targeted by therapeutic drugs? In an important sense, focusing on the channel gene or protein may be missing the bigger picture. In the current literature, ion translocators are usually treated as single genes or proteins responsible for a specific cell behavior (metastasis, hyperproliferation, etc.) – a cell-level view that neglects the fact that numerous channels and pumps contribute to the Vmem gradients that mediate large-scale patterning cues [54,242]. The true impact of bioelectricity in cancer will only occur when we understand and target the storage of patterning information in physiological networks that is misprocessed in cancer [243,244]; such networks are dynamical systems with complex feedback between the post-translational gating of many different channel and pump proteins.
Resting potential: a statistical dynamics view
“For those who believe in the simplification and rationalization of the cancer process, the actual course of research on the molecular basis of cancer has been largely disappointing. Rather than revealing a small number of genetic and biochemical determinants operating within cancer cells, molecular analyses of human cancers have revealed a bewilderingly complex array of such factors.” [245]. It is now appreciated that the essence of cancer may not be in specific driver genes but in the dynamics of cells traversing state spaces and shifting between different attractors [34,246]. While these state spaces are commonly thought of in terms of transcription (gene-regulatory networks), the data on bioelectricity in cancer suggests that another important concept may be the physiological state space.
It has long been realized that cancer differs from normal cells by the relatively depolarized state of its cells [247–250]. As far back as the 1930’s, Burr was able to detect tumors based on voltmeter readings [251,252]. What these classical data (and the molecular data summarized below) had in common is a focus on bioelectrical state of the cell rather than its genetics: a given resting potential level is contributed to by all of the ion channels and pumps in the cell. Thus, while Vmem relies on gene products, it is a complex function of all of them and cannot even in principle be reduced to genetics or transcriptional profiles because all of these translocator proteins are gated at the post-translational level. The dependence of voltage upon the activities of a myriad channels which are regulated by each other and additional physiological events (e.g., phosphorylation), and the ability of voltage change to induce specific outcomes (regardless of which ion channels are used to alter the Vmem of this cell), suggest a powerful paradigm borrowed from physics: statistical mechanics.
We propose that the right concept to describe the role of Vmem in cellular control is akin to “pressure” in physics. Pressure is a systems property – it is created by the contribution of individual molecules’ motions, but tracking any individual molecule in an attempt to understand or manipulate what the system will do would be missing the point entirely. There is no “driver particle” in a gas under pressure any more than there is anything special about a particle that happens to be at the “center of gravity” of a complex object. Pressure is a concept that exists at a higher level of organization than individual molecules, but is causal in the sense that appropriate measurement and control of pressure as such is sufficient to efficiently predict and rationally alter the behavior of systems. We propose that a statistical mechanics view of Vmem is the right level to understand its involvement in cancer. Focusing on the details of specific channel genes obscures the “necessary and sufficient causal state” for inducing or suppressing cancer (e.g., depolarized Vmem, see below). Specific channels may dominate the Vmem in specific cell types, and in those cell types serve as convenient and simple genes to be targeted. However, the general situation is that one can often use any well-characterized channel or pump to make the necessary (and functionally sufficient) changes in Vmem. True, databases like Gene Expression Omnibus and Oncomine are revealing many associations between ion channels and cancer, and specific network analyses do implicate ion channels [253] in processes such as invasiveness. We argue that this is only the tip of the iceberg – an immense under-estimate of the true importance of voltage in oncology, because most of the physiological changes are occurring at the post-translational gating level, and thus are utterly invisible to the mRNA or protein profiling that is so extensively used today.
This was seen for example in the regeneration and left-right asymmetry field, where any number of appropriate gain-of-function channel and pump constructs could be used to induce specific anatomical outcomes such as randomization of the asymmetric organs’ laterality or the regrowth of a tail [80,100,126]. Focusing on individual channels as “genes for regeneration” is missing the point that the necessary and sufficient factor is often a voltage state, such as the narrow range of Vmem that induces eye formation in any region of the Xenopus larva [110]. With increasingly detailed profiling data, the picture is going to only become more complicated unless we define the distinct physiological states that are responsible for inducing specific cell behaviors such as transformation or metastasis and formulate models at that level of biological organization. This in turn will enable us to rationally design reagents (e.g., select specific gain-of-function channels or pharmacological cocktails) to control Vmem appropriately in vivo.
But, if distinct Vmem levels are transduced by various second-messenger mechanisms into transcriptional and epigenetic responses, why not simply focus on those downstream endpoints directly? This is the situation with every complex regulatory network – any node event (transcriptional or biophysical) has an upstream cause and a downstream effect. The trick is to find “key nodes” – components of the functional network that are convenient to manipulate because they offer optimal control over complex downstream events. This is seen for example in the regeneration field, where a single hour’s treatment of a non-regenerative tail blastema is sufficient to induce the entire 8-day regeneration program of this complex appendage (containing spinal cord) [125]. Bioelectric states appear to be powerful master regulators that trigger complex downstream cascades (self-limiting and self-organizing patterning modules) without the need to micromanage the process. Importantly, recent data reveal that Vmem is a similarly potent control node in the genetic and biophysical networks that underlie cancer.
Bioelectrical regulation of cancer in vivo
Vmem signature detects cancer
A variety of bioelectric properties have been used as detection modalities for tumors; these capitalize on cancer cells’ distinct electrical impedance [254–268] or ion content [269,270]. Zeta potential is also associated with cancer; for example asbestos fibers and sheets of positively-charged materials (but not powders of the same material) induce tumors, probably by acting as a capacitor for bioelectric potential, the positive side corresponding to the electron sink existing at a wound [271]. In this section, we focus on depolarized Vmem, which has been suggested to correspond to the cancer state [247–250].
One way to probe the physiology of the effects of canonical mammalian oncogenes (Gli1, Xrel3 and KRASG12D) and a mutant tumor suppressors (p53Trp248) in vivo is to misexpress them in Xenopus and zebrafish embryos [272–275], which induces tumor like structures (ITLS, Figure 3A,A′). ITLS’s thus form as a result of genetic interference with signaling pathways altered in several cancer types including basal cell carcinoma, lung cancer, leukemia, melanoma, and rhabdomysarcoma [276–279]. Examination of injected animals using fluorescence reporters of Vmem [280] revealed unique depolarization of tumors (and increased sodium content) compared to healthy surrounding tissues (Figure 3B) [93,281]. Moreover, depolarization foci are present in oncogene-expressing, preneoplastic cells that are yet to undergo transformation or show any morphological phenotype. Such depolarized foci, while present in only 19–30% of oncogene-injected embryos (depending on oncogene used), predict tumor formation with 50–56% success rate (15–21% false negatives). For comparison, prostate specific antigen (PSA) level in the serum, when used as a biomarker for prostate cancer, has ~29% predictive value [282,283]. An added advantage of Vmem as a biomarker is that it is associated with tumors of diverse molecular origin, suggesting a general role for Vmem change as an early indicator of tumorigenesis.
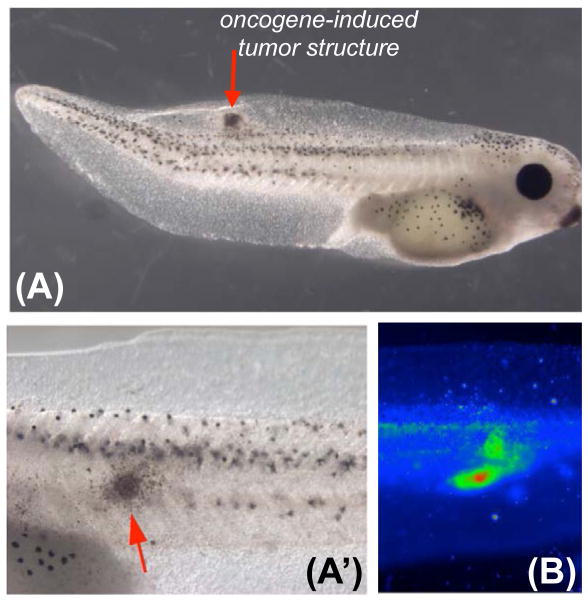
Figure 3. Transmembrane potential as a diagnostic modality for tumor detection.
(A, closeup in A′) Tumors (red arrow) can be induced in vivo in tractable model systems such as Xenopus larvae using targeted misexpression of mammalian oncogenes such as dnP53, Rel3, Gli1, RAS, etc.).
(B) Using voltage-sensitive fluorescent dyes, areas of depolarization (green, red) are detected non-invasively [93,281]. While a tightly-defined physiological signature remains to be developed (likely necessitating concomitant use of several different physiological dyes, such as those reporting voltage, sodium content, and pH), the scanning of bioelectric properties with light-emitting dyes in vivo is a promising modality for early detection of pre-cancerous tissue and tumor margins during surgery.
The next major areas of opportunity for bioelectric detection of cancer are four-fold. First, a more specific physiological signature needs to be developed (to distinguish tumor cells from adult stem cells – another depolarized population) and appropriate voltage-sensitive dye technology implemented as a diagnostic tool to visualize areas of pre-cancer on patients as well as observe tumor margins during surgery. In addition to visualization, a better characterization of bioelectric state could be used to guide drug delivery vehicles such as nanoparticles [284,285]. Second, this strategy needs to be validated in a mammalian model system, and in a range of well-characterized human tumor cell lines. Third, it is critical to begin to tackle the long-range aspects of biological disturbance introduced by cancer. While body-wide morphogenetic fields and the role that Vmem distributions play in these are only beginning to be understood [14,15], it is imperative to establish molecular models in which to investigate the fact that transplanted or chemically-induced tumors can be detected by aberrant voltmeter readings taken at locations far away from the tumor [251,252,286–290]. Lastly, modern work on bioelectricity in non-excitable cells has not yet addressed the information encoded in time-dependent changes in Vmem. For example, fibroblasts expressing Ras-oncogene respond to the drug bradykinin with Vmem oscillations, while control cells exhibit a single transient hyperpolarization. In human carcinoma cells, fluctuations of membrane potential are triggered by EGF and persist for long periods of time after EGF application [291]. The mechanisms and significance of the temporal Vmem changes for cancer initiation and progression remain to be discovered.
Depolarization of specific cells induces metastatic phenotype at a distance
Given that a depolarized Vmem is an indicator of tumorigenic potential, is it merely a side-effect of cancer, or is it functionally instructive? This question was addressed for the first time in vivo in a frog model [60], by the selective depolarization of a sparse set of cells expressing the glycine-gated chloride channel (Figure 4). Using a pharmacological strategy designed to depolarize this subpopulation, a remarkable phenotype was observed: hyperpigmentation of the animals due to over-proliferation, increased migration, and drastic arborization of melanocytes (pigment cells). By transiently depolarizing cells in the body, a different cell type underwent a metastatic-like conversion, turning on expression of genes such as Sox10 and SLUG 61. The melanocytes acquired a dendritic morphology, upregulated mitotic activity, and invaded blood vessels and soft tissues like the neural tube lumen and brain (Figure 5). In addition to melanocytes, disorganization and ectopic growth of blood vessels was also observed [281], but otherwise the tadpoles were remarkably normal in terms of overall growth and development. Importantly, the same exact effect was induced by any method of depolarization, including by the movement of chloride, sodium, potassium, or hydrogen ions – truly an effect initiated by Vmem depolarization, not any specific gene product or chemical ion species. This metastatic-like conversion occurred by a bioelectrical signal alone, without any oncogene, DNA damage, or cancer-causing chemical being applied. Also of note here is that, like in some blood cancers, there was no primary tumor site but a direct metastatic behavior of a normal embryonic stem cell (neural crest) derivative. Thus, while this is an example of non-genotoxic cell-cell communication effect, it differs from the epithelium:stroma interaction at a primary tumor site described by others [292].
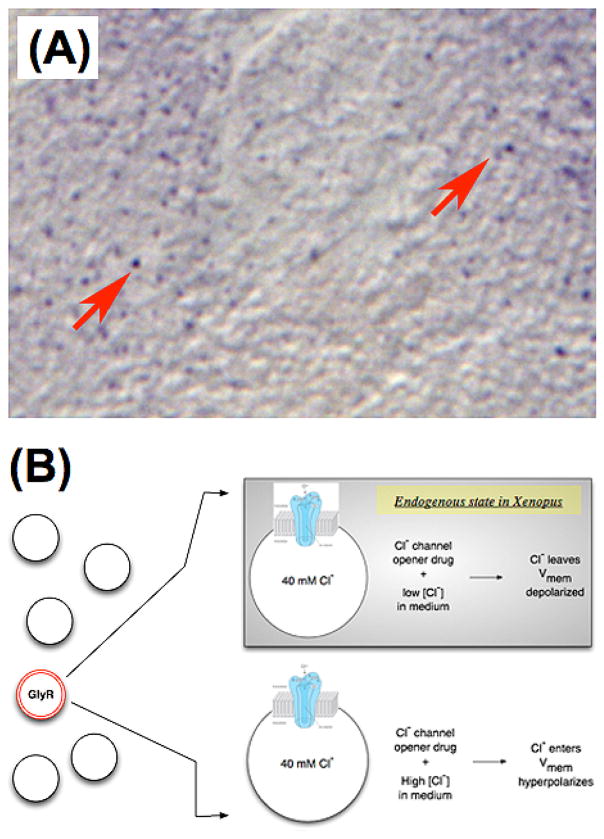
Figure 4. Experimental control of Vmem in a defined cell subpopulation.
(A) In Xenopus, a sparse but ubiquitous population of cells expresses the Glycine-gated chloride channel (GlyR or GlyCl) [60]. Here a section of a frog larva has been subjected to immunohistochemistry revealing these cells as purple dots (red arrows).
(B) A strategy for selective depolarization of these cells in vivo uses a specific channel opener, ivermectin, to render these cells permeable to chloride. Then the level of chloride in surrounding medium is varied, to induce depolarization (efflux of negative Cl− ions) or hyperpolarization (influx of negative chloride ions) at will. This technique allows the experimenter to study the effects of Vmem change in a specific cell population within a living organism.
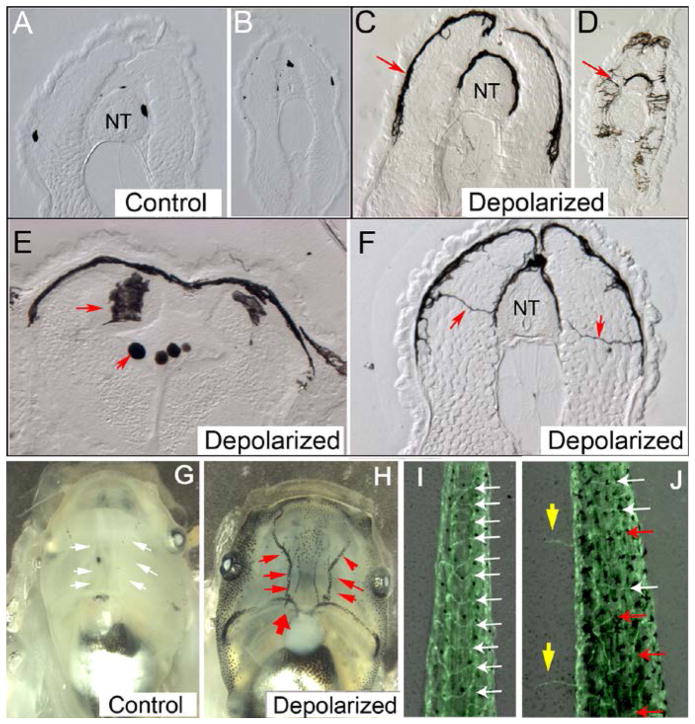
Figure 5. Instructor cell depolarization induces metastatic phenotype.
When the GlyR-expressing cells are depolarized, a remarkable phenotype is observed among melanocytes – pigment cell derivatives of the neural crest [60–61]. Panels A–F shows cross sections of tadpoles.
(A, B) in control sections across the anterior and posterior trunk, small numbers of normal round melanocytes are observed (nt = neural tube). In contrast (C,D), animals in which the instructor cells have been depolarized show high numbers of highly arborized melanocytes. In fact these melanocytes not only become much more dendritic and overproliferate, but also thoroughly invade soft body tissues such as the neural tube and its lumen (E, red arrows) and form long nerve-like projections across the entire somatic mesoderm (F). These cells preferentially target the blood vessels (H, red arrows, compares to G).
Not only melanocytes are affected: blood vessels (visualized in a transgenic animal in which all flk1-positive cells express GFP) also lose their normal patterned organization and grow ectopically (blue arrows, compare J to I) [281]. When depolarized using any method (whether relying on chloride or another ion), the instructor cells activate a metastatic-like program of behavior in several target cell types, without the involvement of genetic damage or the presence of canonical oncogenes.
Most interesting was the non-cell-autonomous nature of the effect: the cells that acquired a melanoma-like phenotype were not the cells whose resting potential was changed (the GlyR-bearing cells were thus called “instructor cells”) [60,61]. Indeed, only a small number of instructor cells had to be depolarized in order to induce the hyperpigmentation phenotype (which is all-or-none within any individual animal). How was the communication between these two cell types mediated? A suppression screen testing the several known methods of transducing voltage change into transcriptional cascades revealed (Figure 6) that the serotonin transporter SERT, which powers uptake or efflux of serotonin depending on resting potential, was involved (much like in the bioelectric regulation of left-right patterning); blockade of SERT could rescue the hyper pigmentation effect, and direct application of serotonin could trigger a similar phenotype.
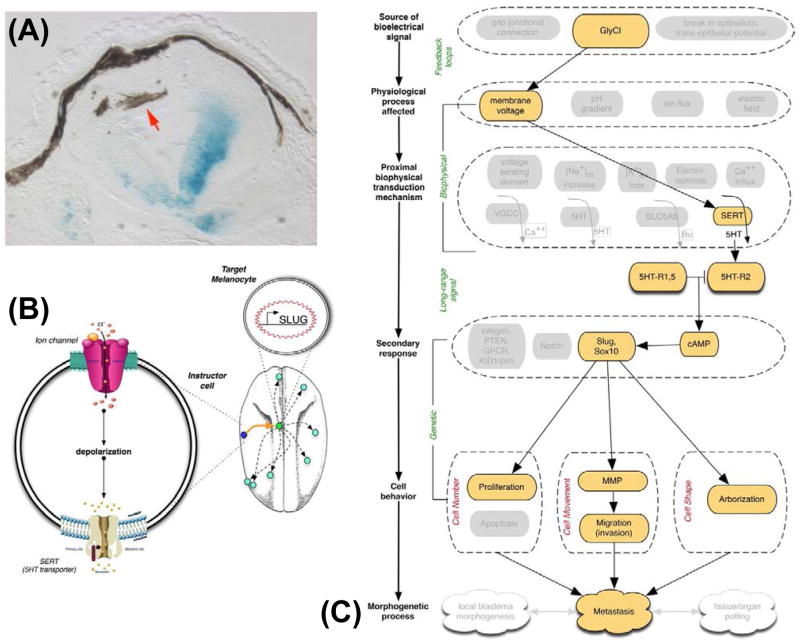
Figure 6. Instructor cells manipulate cell behavior using serotonin.
(A) The cells that overproliferate, change shape, and migrate inappropriately (brown melanocytes, red arrow) are not the same cells that are depolarized (blue lineage label, showing the location of a depolarizing channel’s misexpression in this section of a tadpole) [60,61]. Indeed, the effect takes place at considerable distance and recent studies showed that a very few depolarized cells at one end of a Xenopus larva is sufficient to induce the metastatic behavior of melanocytes at the far end of the animal [281].
(B) The non-cell-autonomous transformation of melanocyte behavior is mediated by a non-neural function of the neurotransmitter serotonin, and can be completely rescued by fluoxetine – the blocker of SERT (the 5HT transporter). A current model of these events is that an instructor cell, when depolarized (causing SERT to run backwards instead of performing serotonin reuptake), begins to secrete serotonin. Serotonin itself can induce the same hyperpigmented phenotype, turning on genes like Slug and Sox10.
(C) The induction of metastatic behavior by voltage change forms a paradigm case for understanding bioelectric events in cancer. In this case, all of the key points are known: the endogenous channel regulating Vmem, the physiological parameter that is necessary and sufficient for activating the effect (depolarization, no matter which ion species is used), the transduction mechanism that converts biophysical property into movement of a small molecule chemical signal (SERT), the receptor machinery (5HT-R1,2,5 and cAMP) and key transcriptional downstream responses, and the cell behaviors regulated by these downstream events.
A number of key questions remain. First, although it is clear that serotonin signaling is involved in the imposition of a metastatic phenotype by depolarization, serotonin is too small to be fluorescently tagged without drastically altering its transport properties. Thus, the movement of serotonin across long distances has not been imaged directly in this model system. Second, it remains to be understood how hyperpigmentation occurs in an all-or-none manner: treatments that inhibit specific serotonin receptors for example partially rescue the effect, but they do not inhibit metastatic phenotype in some melanocytes, but instead completely rescue only some animals in a test cohort. The current model of this effect [281] relies on a model of amplification and antagonistic function among three different subtypes of serotonin receptors, leading to stochastic effects in the downstream activation of cAMP signaling. However this model remains to be tested in detail. Finally, how does this pathway relate to mammalian cells? It is known that ivermectin, a specific opener of the glycine-gated chloride channel, can regulate the growth of neuroblastoma [293] and leukemia cells [294], although neither of these studies looked at the Vmem changes that would have been induced by ivermectin in mammalian tissue culture medium. The findings in the Xenopus model make a number of predictions for human medicine that could be tested. First, the GlyR channel opener Ivermectin, which was used to induce the melanoma-like conversion, could have increased the rate of melanoma and other cancer in human patients. While this drug is no longer widely prescribed as an antiparasitic agent, this class of molecules was used in human medicine [295] and is known to cause cancer in the parasites that it (usually) kills by muscle depolarization and paralysis [296]. Conversely, we predict that patients taking Prozac (the SERT blocker fluoxetine) may have lower incidence of melanoma. These predictions await epidemiological testing.
Hyperpolarization inhibits oncogene-induced tumorigenesis
he above data show that depolarization of Vmem can, itself, activate a metastatic-like phenotype. What role could Vmem play in carcinogenesis induced by genetic perturbation? Well-characterized channels such as inward rectifying potassium channel (Kir 4.1) and constitutively open glycine-gated chloride channel mutant (GlyRF99A) can be used to generate strong hyperpolarizing currents [297,298]. When GlyRF99A is co-injected with the Xrel3 oncogene in Xenopus larvae [93], it significantly suppresses the incidence of ITLS formation compared to oncogene alone (Figure 7). Florescent tags on the oncogene protein revealed that hyperpolarization could prevent the formation of tumor-like structures despite very robust expression of oncogene in cells. The use of several different hyperpolarizing channels based on Cl− and K+ confirmed that suppression of neoplastic transformation was due to Vmem hyperpolarization per se, as opposed to ion-specific or scaffolding functions of the ion channel proteins. Consistent with this, data in rats showed that the ion channel modulator drug ivermectin can likewise modulate the effectiveness of carcinogenic compounds [299].
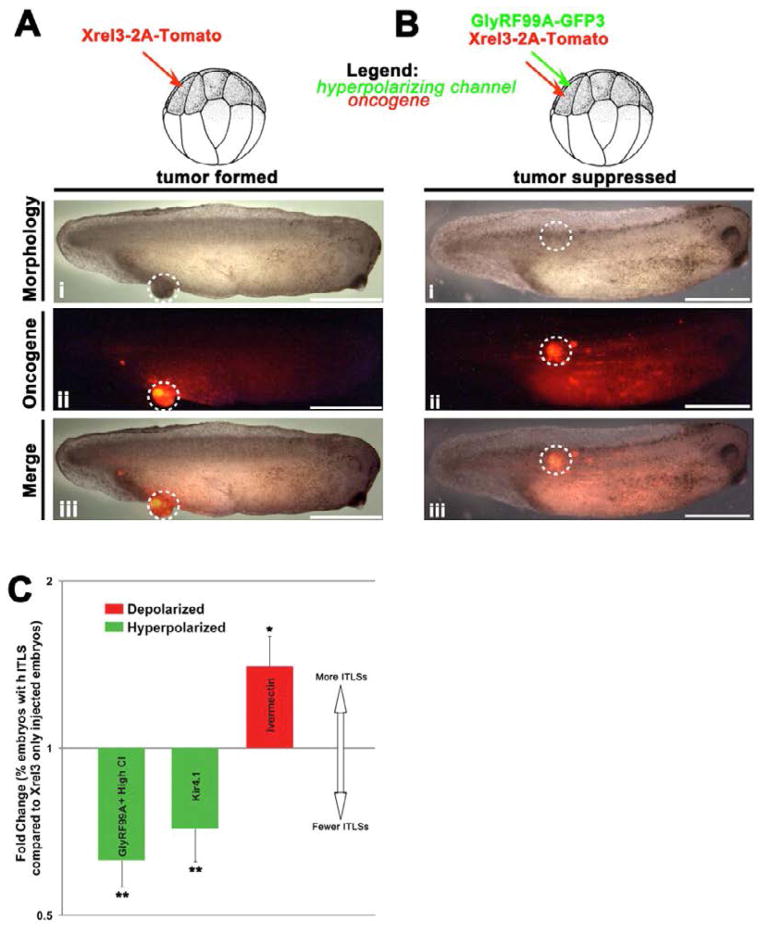
Figure 7. Vmem as a regulator of oncogene-mediated tumorigenesis.
(A) When an oncogene (Xrel3) fused with a red fluorescent tag (tdTomato) is injected into a frog embryo, the larvae develop fluorescently-labeled tumors. Remarkably, when a hyperpolarizing channel mRNA is co-injected with the oncogene (B), the incidence of tumors is significantly reduced (C). The ability of a hyperpolarized state to suppress tumorigenesis despite the strong presence of oncogene protein (dashed circle in panel B-ii) reveals the functional importance of the depolarized state acquired by prospective tumor cells, and shows that at least in some contexts, cancer can be suppressed by physiological signals despite the presence of a genetic component normally sufficient to induce a tumor [93].
How do changes in Vmem transduce into suppression of oncogene-mediated tumorigenesis? A pharmacological suppression screen of several candidate mechanisms, followed by molecular-genetic loss-of-function validation [93], implicated the sodium-coupled monocarboxylate transporter (SLC5A8). SLC5A8 has previously been identified as a tumor-suppressor whose transport of butyrate or other short chain fatty acids (HDAC inhibitors) is essential in maintaining a healthy colon and/or control colon cancer invasion [300–306]. A model of the bioelectrical regulation of oncogene activity is shown in Figure 8: oncogene expression causes the observed depolarization, which limits the intake of Na+ through SLC5A8, also limiting butyrate intake (co-transport, Figure 8A). Lack of HDAC regulation due to reduced butyrate presence leads to hyperpolarization and tumor progression (Figure 8B). However, forced hyperpolarization within oncogene-expressing cells facilitates the uptake of the positive Na+. This powers the import of butyrate through SLC5A8, resulting in continuous suppression of HDAC and thus reduced proliferation and ITLS suppression (Figure 8C). The specific genetic targets of hyperpolarization in this context remain to be thoroughly explored, however p21 is a likely mediator. Hyperacetylation of histones – as a result of butyrate-induced HDAC inhibition – has been shown to up-regulate p21 at both at the mRNA and protein level [307,308]. p21 inhibits cyclins/cdk’s activities, thus inhibiting downstream substrate phosphorylation and causing cell cycle arrest (reduced proliferation) at the G1/S transition and subsequent suppression of ITLSs (Figure 8D). It should be noted however that many other (physiological, non-genetic) events can induce a similar depolarization as that initiated by oncogene function, thus inducing the rest of the downstream steps in this tumorigenesis pathway without necessitating oncogenic mutation.
Figure 8. SLC5A8, Butyrate, and HDAC mediate tumor suppression by hyperpolarization.
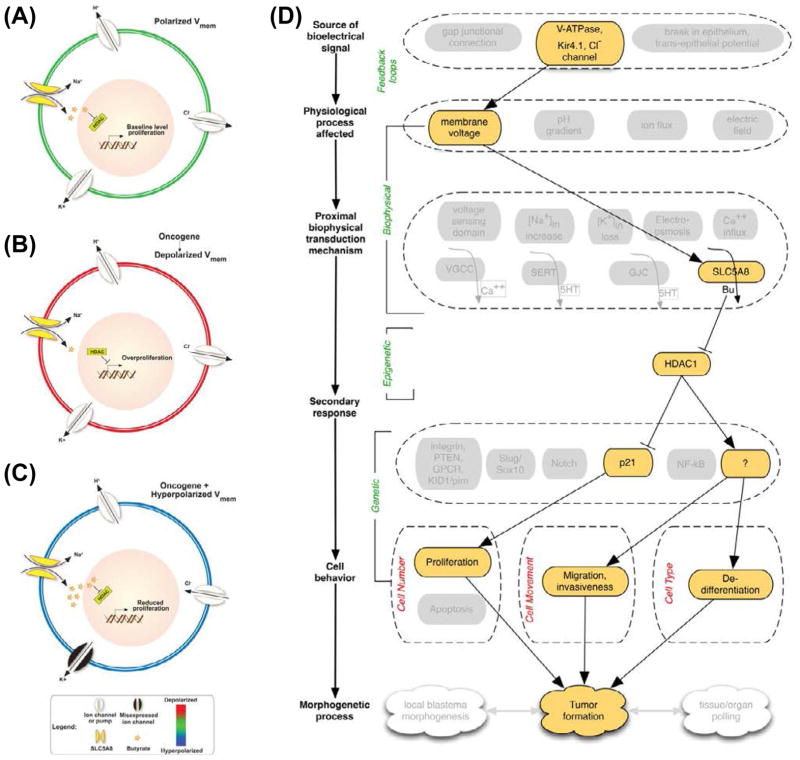
The current model of how Vmem regulates ability of cells to form tumors is as follows [93].
(A) In unperturbed embryos, polarized Vmem is generated and maintained by several ion channels and pumps present in the plasma membrane; this condition allows moderate amounts of butyrate to influx through SLC5A8 and inhibits histone deacetylases (HDACs). This epigenetically regulates transcription machinery thereby maintaining baseline level proliferation and differentiation compatible with normal somatic morphostasis.
(B) Expression of oncogenes, or other physiological events (e.g., non-genetically induced depolarization, as described in [60–61]), results in the inability of SLC5A8 to import butyrate. Higher HDAC activity then leads to overproliferation and other neoplastic changes leading to appearance of tumor structures.
(C) The effect can be blocked by forced hyperpolarization via molecular and/or pharmacological targeting of H+, K+, or Cl− ion translocators. Forced hyperpolarization of the overall transmembrane potential efficiently powers the uptake of Na+ through SLC5A8. This energetically favorable intake of Na+ drives the inward flux of butyrate through SLC5A8. High levels of butyrate continually block HDAC, which leads to hyperacetylation of important genes resulting in cell cycle arrest and suppression of tumor formation.
(D) The bioelectric pathway is similar to that of the depolarization-induced metastasis (Figure 7C), except that the transduction mechanism involves SLC5A8 and butyrate (instead of SERT and serotonin), and initially regulates a chromatin modification enzyme (HDAC) upstream of transcriptional changes that lead to tumor formation.
Cancer: a disease of geometry?
Given the data implicating physiological cell properties and bioelectric cell:cell communication in cancer and its normalization, it is important to place this body of work in the context of developmental signaling. Bioelectric gradients are one component of morphogenetic cues mediating positional information, tissue specification, and intercellular coordination [14,43].
Morphogenetic Field as tumor suppressor: importance of community
The sum total of the instructive patterning signals that impinge upon cells in the organism (Figure 9A) is referred to as the Morphogenetic Field [14,18,309,310]. This instructive information is mediated by a range of dynamically-varying spatio-temporal gradients of secreted biochemical factors, extracellular matrix properties, stresses/strains/stiffness values, and electric properties. Disturbances of the normal interactions between cells and the signals that normally orchestrate individual cell activities into maintenance of host tissues and organs can manifest as cancer. The hypothesis that cancer is fundamentally a phenomenon at the level of multicellular organization makes a number of unique predictions confirmed by experimental data (that are not predicted by the somatic mutation model). For example, one way to perturb field structure is to introduce ectopic organizer nodes. Indeed, implantation of early embryos (which organize their own field of signals) under the kidney capsule of an adult makes transplantable malignant teratomas despite a lack of any infective, chemical, or radiation initiator to cause genetic damage [8], while normal adult Xenopus kidney implanted in the non-amputated forelimbs of recently-etamorphosed larvae will make lymphosarcomas as well as accessory limb structures [311]. Implantation of mouse embryos into adults causes teratocarcinomas [312], possibly due to an interference between the host and implanted embryo’s morphogenetic field signals.
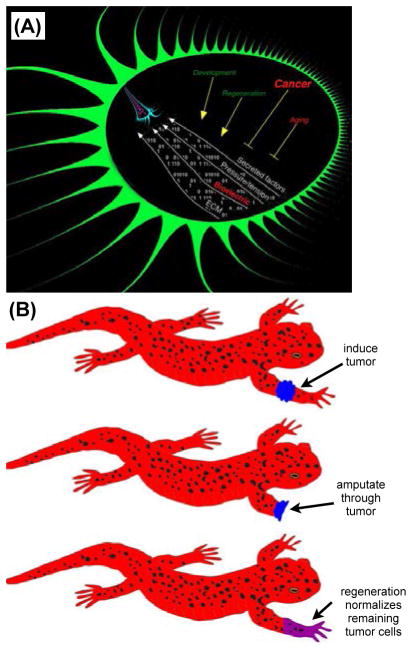
Figure 9. Cancer as a disease of geometry.
(A) Within any organism, substructures are provided with a field of instructive information that continually orchestrates individual cell activities into large-scale anatomical target morphologies [14,15]. These signals are mediated by gradients of chemical factors, pressures/tensions, extracellular matrix components, and bioelectrical events. This morphogenetic field operates during embryogenesis and regenerative repair, as well as maintains the organism for decades against aging, and disorders of cell: field interaction manifest as cancer.
(B) When half of a tumor is removed during a limb amputation in regenerative organisms such as salamanders, remaining tumor tissue is normalized and participates in the formation of a healthy limb [397,398,400]. The ability of embryonic and regenerative contexts to reprogram cancer cells towards correct anatomy reinforces the idea that cancer is a disease of organization and cell:cell communication, and suggests normalization strategies as alternatives to biomedical paradigm of mandatory killing of permanently damaged (malignant) cells.
Cancerous failure of morphostasis can occur because a morphogenetic field is missing, altered, or not successfully perceived (all three of which can occur due to genetic or physiological state change). Cells in dispersed monolayer culture are several orders of magnitude more sensitive to chemical carcinogenesis than are organized tissues within an intact organism [313], and placing normal primary mammalian cells in culture results in the appearance of cells with malignant potential [313–316]. Chick embryos infected with the v-Src virus exhibit no malignant phenotype, but the same cells in culture undergo massive transformation [317]. A number of recent papers stress the suppressive nature of signals from neighboring tissues [4,9,10,29]. Consistently, re-establishing appropriate interactions of human cancer cells with the microenvironment and normal neighbors underlies the observed reversion of malignant phenotype in a number of cell lines [318–320].
Consistent with the need for cell: cell interactions in suppressing cancer are data showing that tumorigenesis is promoted when cells are isolated from their neighbors (and thus from the morphogenetic guidance they would otherwise receive) by physical barriers. Implanting into connective tissue of the rat rectangles of inert plastic, metal foil, or glass cover slips induces sarcomas when the material is >1cm2. If the material is perforated, the incidence is reduced, and the effect does not occur with powders of the same material (which actually increases surface area, ruling out chemical induction or genetic damage mechanisms) [321–324].
More recent data has focused attention on interruption of cell:cell communication via ions and other small molecules through gap junctions (GJs) – aqueous channels made of connexin proteins that allow physiological signaling directly between the cytoplasmic interiors of docking adjacent cells [325–330]. For example, Connexin32-deficient mice have a 25-fold increased incidence of spontaneous liver tumors [331]. Gap junctional isolation is known to be a tumor-promoting agent [330,332–337], although there are counter-examples [338]. Active GJ communication allows cells to make sophisticated decisions comparing relative levels of specific compounds between themselves and their neighbors [339] and thus can underlie the transmission of physiological patterning signals [89,90,114,340–347].
Another mechanism of coordination across large cell fields that was recently implicated in cancer is the planar cell polarity (PCP) pathway – a set of protein components designed to coordinate orientation and function of cells over long distances [348]. PCP has now been shown to function as a non-canonical tumor suppressor [349,350]. While the direct causal relationship between loss of PCP and tumor initiation in humans is not yet proven, it is clear that loss of polarity can be an initiating event in tumor formation in Drosophila [351]. Consistent with conserved mechanisms underlying coordination and maintenance of long-range order in cancer and normal development, PCP is also involved in dynamic morphostasis: grafts of embryonic skin (after the planar polarity of hair becomes evident), when implanted into adults, realign their hair polarity to match that of the hosts [352] – this dynamic readjustment to local conditions is a factor that distinguishes cancerous tissue from its normal counterpart (see below). PCP allows cells to align axes orthogonal to their apical-basal polarity with each other, and with major anatomical axes of the organism, linking large-scale order with regulation of single-cell behavior.
Another mechanism used to coordinate cell activity away from cancer is communication via the nervous system. Tumors are readily induced by denervation in salivary organ and alimentary canal in cockroach [353,354] and in mammalian skin [355]. Similarly, tumors are chemically induced more easily in denervated rabbit ears as compared with contralateral controls bearing normal innervation [355]; the same has been observed in sarcomas implanted into normal or denervated frog limbs [356]. These remarkable results are predicted by models in which nervous system components transmit long-range morphogenetic field cues [357–360], but have been unfortunately neglected in the modern literature focused on DNA. Much works remains to characterize the role of the nervous system in providing information and cancer-suppressive cues to existing adult tissue, and to dissect which signals are broadcast via the long-range communication systems provided by planar cell polarity, nerves, and gap-junctional networks.
Positional information and cancer
Models of suppression of cancer by long-range morphogenetic cues, as opposed to simpler models of growth-inhibitory signals from any normal neighbors, predict that tumorigenesis would be modulated by global position within the host, as are events operating during embryonic development and regeneration. Microarray analysis reveals quite different profiles of human glioma cell lines grown in leg vs. brain [361]. Moreover, tumors grow on posterior regions of Triturus less readily than they do on anterior regions [362], and numerous such differences are observed in human tumors as well [363–368]. These studies suggest a link between large-scale axial patterning in adult organisms and the potential for failed perception of morphogenetic cues by cells.
A most impressive example of the importance of location and environment in cancer growth is provided by patients with peritoneo-venous shunts, who have a steady infusion of peritoneal fluid that carries billions of desquamated cancer cells into the systemic circulation, for months or years [7]. Known metastases in some organs before insertion of the shunt exhibited additional deposits in the same organs but not anywhere else, despite millions of viable cancer cells being distributed to every organ. This work (which ruled out immune clearance of cancer cells) revealed that the disseminating cancer cells were capable of establishing secondary tumor colonies in some anatomical sites in a given patient but could not do so in other organs, raising the question of whether some body regions have more active morphostasis pathways.
Even more interestingly, surgical disruption of normal topographical tissue relationships tends to induce cancer, which suggests a feedback model where the morphogenetic field can be altered by scrambled anatomy, or perhaps difficulty in cells’ reading instructions at the borders of fields that are not supposed to be geometrically adjacent. For example, despite lack of DNA damage or cytotoxic chemical stressor, transplantation of rat testis to the spleen induces formation of interstitial cell tumors [369], while normal rat ovary tissue put into normal rat spleen results in malignant neoplasm [370]. Cancer thus is not only a disruption of normal patterning within the tumor but also reveals an interplay between its activity and the context of the large-scale spatial organization of the host.
Normalization of cancer by developmental and regenerative patterning
The morphogenetic field ought to be the most active and accessible during embryogenesis. It is thus not surprising that despite considerable malignancy and aneuploidy, tumor cells introduced into wild-type embryos become integrated as normal tissue [371–381]. Human metastatic melanoma cells injected into zebrafish embryos acquire a non-neoplastic phenotype, but form tumors when injected into zebrafish after organogenesis [382,383]. Likewise, implanted sarcoma progressed in 80% of adult rats but only in 6.4% of rat embryos. Similar data have been recently shown for chick and other kinds of embryos that are able to tame aggressive cancer cells when these are implanted [383–386]. Cancer normalization can occur cell-autonomously [387], or induced by communication from other cells, such as the mammary stroma [371,388–393]. Indeed the embryonic field present in the blastocyst can normalize several types of cancer cells including those isolated from embryonic carcinoma, leukemia and neuroblastoma [381], although the limits of this normalization process (with respect to large-scale chromosome aberrations found in some tumors) remain to be probed fully. Thus, active patterning signals can normalize cancer (over-ride genetic defects and reboot cell behavior programs); this is a finding that is not predicted by the cell-level view of cancer as embryos have high levels of many growth factors that could be expected to potentiate tumor growth (and do, in experimental contexts such as cell culture which is devoid of large-scale patterning structure).
Tumors have also been described as wounds that do not heal – areas of disruption and cell growth without an appropriate patterning program that reaches a terminal goal state [149,394]. This analogy is supported by profiling data showing the molecular similarity of repair vs. carcinoma in renal tissue [149]. Successful tumors have developed the capacity to preempt and subvert the wound-healing response of the host [395]. What about wounds that not only heal but successfully rebuild a missing structure? Some animals, such as salamanders, can regenerate entire limbs, eyes, hearts, and jaws. Even mammals regenerate some organs (e.g., liver in humans, and antlers – meters of innervation, bone, and skin – in deer).
It has been long known that regeneration and cancer are closely related [396–401]. Highly-regenerative organisms are resistant to carcinogenesis and indeed activating regenerative response can normalize existing tumors [396–399,401–405], although this does not always occur [406]. The inverse relationship between regeneration and cancer susceptibility [407,408] is more compatible with the importance of morphogenetic field guidance than with cancer risk associated with the presence of highly-active, undifferentiated cells [18]. Mammalian liver regeneration can overcome cancer - early nodules initiated by carcinogens are remodeled to normal-appearing liver [409,410], hepatocarcinoma cells can be normalized by injection into wild-type liver [411,412], and over 95% of nascent tumor sites remodel into normal tissue by the highly-regenerative liver [413–415]. In zebrafish brain regeneration, a remarkable degree of aneuploidy does not lead to cancer -an active patterning program trumps chromosomal damage [416], and amphibian limb regeneration can likewise normalize tumors [31,32,400]. Thus, tumors may be wounds that do not pattern.
Modern molecular model systems are now available for the study of these still poorly-understood mechanisms: regeneration of the zebrafish tail prevented tumor formation from BRAFV600E mutation + p53 knockout [417], offering the opportunity to use the numerous available zebrafish reporter lines and functional morpholino strategies to investigate the relationship between regeneration and tumorigenesis. Remarkably, such influence is not necessarily local. Induction of anterior regeneration in planaria turns posterior infiltrating tumors into differentiated accessory organs such as the pharynx [362], which suggests the presence of regulatory long-range signals that are initiated by large-scale regeneration. It is likely that the normalization of tumors by active remodeling represents one of the most profound and exciting areas for future work in understanding morphogenetic fields and their interpretation by growing tissue.
Interestingly, the interplay between proper patterning and cancer suppression is retained throughout life; for example, if the endocrine gland is removed in Dixippus, regenerative capacity is lost, and spontaneous tumors begin to appear [418–421]. Work in highly-regenerative model species such as amphibia and some invertebrates is likely to be the fastest route to understanding this fascinating phenomenon of tumor and cancer cell reprogramming but it is important to note that these signaling pathways are likely to be of relevance to human patients. For example, childhood neuroblastoma has a high rate of spontaneous regression [422,423], and a number of other cancers often regress spontaneously [7]. The efforts of regenerative medicine to improve regeneration prospects in man may thus have a significant side benefit of impacting cancer treatment.
Explanations at above the single-cell level
Is cancer fundamentally a cell-level property or a multicellular phenomenon that, like the wetness of water, which is not applicable to individual H2O molecules, applies only to collections of cells and characterizes the interactions between them? The current paradigm focuses on cell-level activity (proliferation, differentiation, migration), but tissue- or organ-level systems properties might be the right basal concepts with which to formulate models and intervention modalities [424–427].
The difference between these approaches is not mere philosophy – it has testable implications that allow data to distinguish between the two classes of models. For example, a focus on cell cycle checkpoints and TGF-β molecules (a view at the cell level) leads to the prediction that cancer and regenerative potential should go together: animals with ready access to plastic, highly proliferative cells should be prone to neoplasia, and long-lived humans would be forever barred from powerful regenerative pathways because of the evolutionary pressure to suppress cancer over decades. Conversely, a morphogenetic field model (cancer as a failure to transmit or receive anatomical cues) suggests that regeneration and cancer should be inversely related, as robust patterning pathways necessary for regenerating complex organs from new cell growth would also keep cells within a coherent morphological plan and away from tumorigenesis during normal lifespan.
In fact, the most highly regenerative animals tend to have the lowest incidence of cancer [396,399–401]. Moreover, if a tumor is induced on the limb of a salamander and the limb is amputated through the tumor (Figure 9B), the remaining cancer tissue becomes part of the newly regenerating limb [396–401]! This readily illustrates the profound relationship between cancer and regeneration and the importance of studying systems-level concepts (the mechanistic details of “exerting strong patterning control at the level of a whole appendage”) for what is often thought of as a cellular- or gene-level process. It also suggests a highly optimistic view of the potential for regenerative pattern control in human cancer medicine.
As in regenerative medicine, the answer to this question impacts treatment strategies: do we micromanage individual gene products, or attempt to initiate complex patterning cascades? Up-regulating embryonic genes in adults results in cancer [428–430], while, as discussed above, inducing embryonic genetic programs leads to cancer normalization [18]. Moreover, drugs that target upstream functions in signaling networks have less general toxicity than those that interact with targets further downstream [431]; this is consistent with the view that the right level of intervention to optimize effectiveness and compatibility with overall health (lack of toxicity) is by activating large-scale physiological modules that have been evolved to implement mutually compatible (healthful) downstream events, instead of specifically impacting individual downstream players which may induce unwanted interference with other functions in the organism.
A closely related question to the scale and level of organization of cancer state is the spatial distance over which the disturbance acts [432–434]. In mammalian breast cancer [292] and frog melanoma-like transformation [60,61], clear roles for non-local (long-range) influence over carcinogenesis have been found and can now be dissected. This is clinically relevant, as seen in field effects in many different kinds of cancer in which surrogate sites are not necessarily adjacent to the main tumor [435,436]. The characteristic size scale of the “cancer field” is still not mechanistically understood and is relevant not only for understanding the basic biology of signaling but for designing detection and treatment modalities intending to impact specific tumor sites. Although most work on cell:cell interactions today deals with chemical gradients as a signaling modality, bioelectricity is an ideal medium for long-range coordination and information exchange among cells during pattern maintenance and repair.
Future Prospects/Speculations
Tumor boundaries and selves
The fundamental fact of cancer is that cells cease to work towards the anatomical needs of a host organism and narrow their dynamic goal-seeking behavior to the level of single cells – an increase in “selfish” behavior away from the normal cooperativity of multicellular life. Cancer could result from a failure of the host to impose or transmit necessary patterning information within a particular region; it is also possible that tumor cells are those that stopped attending to the morphogenetic field cues [27,349,398]. Anticipating recent discoveries of the importance of gap-junction cell:cell communication for planarian regenerative patterning [112,114], in 1965 Seilern-Aspang described planarian experiments in which a carcinogen led to formation of many head teratomas with irregular nerves and un-oriented eyes concluding that “the cell-isolating action of the carcinogen prevents formation of a single morphogenetic field and leads to the establishment of several separated fields of reduced dimensions” [362]. Thus, tumors could also represent establishment of a local “subfield” – a fragmentation of the host’s morphogenetic field such that integration with the host body plan is lost. Unlike normal somatic tissues, which remodel when transplanted into foreign locations [437–439], the histopathological structure of metastasis reflects the tissue of origin, not of their destination [8], confirming an inability to respond to neighboring signals such as positional information and remodeling cues.
Interestingly, cancer is not only a loss of patterning, but also a coherent, goal-seeking subsystem: tumors are not just aggregates of replicating neoplastic cells but complex living entities composed of numerous cell types that work together to acquire nutrients, survive, and evade the efforts of an environment that is trying to kill them [5,440]. One way to model such changes in dynamics is as a reduced scope of “self” – the view that a tumor is, in some practical sense, an independent organism [441] with its own (primitive) morphogenetic field. In a tumor, the boundary of self has been reduced from the whole body to that of a much smaller structure, making the rest of the body just part of the pseudo-organism’s outside environment. Such a view is suggested by a number of findings. First, histological analysis indicates that tumors can indeed be regarded as complex tissues with a distinct internal organization [314,442]. Tumors reproduce themselves via metastasis, and execute many adaptive strategies (such as up-regulating multi-drug resistance proteins in the face of chemotherapy) to preserve their homeostasis and existence – just as organisms within an ecological niche do [443–445]. Much like organisms maintaining morphostasis, tumors maintain their identity during massive cell turnover during selection for founder cells resistant to chemotherapy drugs [446]. Recent work describes the highly malignant brain tumor as an “opportunistic, self-organizing, and adaptive complex dynamic biosystem” [447]; proper characterization of the essential principles predictive of the properties of tumor invasion makes uses of concepts such as least resistance, most permission, and highest attraction – these are systems-level, goal-directed elements that are very compatible with the conceptual modeling techniques suggested for understanding embryogenesis and regeneration of whole organisms.
The defection of cells from the goal states of the body to those of a much smaller entity (a tumor, or perhaps individual cells) implements a contraction of the functional boundaries of the self-organizing system [448,449]. Tumors of course pursue goals quite at odds with those of their host. “Glioma cells are ill-equipped to participate in ion and amino acid homeostasis, those important altruistic tasks performed by their nonmalignant counterparts. Instead, gliomas are more concerned about their relentless growth and invasive migration” [450]. Interestingly, cooperation occurs among the tumor cells that can be analyzed via the same mathematical tools that explain cooperation and competition among somatic cells and members of societal groups [339,451,452]. While tumors typically lose heterologous gap-junctional communication to surrounding stroma, they often maintain good gap junctional connections among their own cells. Interestingly, gap-junctional connections have been proposed as a mechanism by which cells can recognize “self” [453,454].
The questions of size control and field boundaries are central to developmental biology as well as cancer. During planarian regeneration, a regenerating head will inhibit the formation of heads elsewhere, but parts of the regenerating head do not inhibit the rest of that same head from forming. A specific voltage range causes tissues to reorganize into eyes [110], but these eyes are of normal (limited) size, and at the same time contain numerous distinct tissues that clearly result from a process more complex than simple control of cell fate and differentiation by a resting potential value. To really understand the fascinating ability of active morphogenetic fields during regeneration to normalize or prevent tumors will require new, molecularly-tractable models of tumor normalization. Axolotls are a powerful system in which this could readily be dissected [455,456]. Future work must uncover the mechanisms that establish size and scope of morphogenetic fields, to understand how boundaries are established and altered during pattern formation. Cybernetic models of goal-seeking behavior among dynamical systems such as embryos and tumors are needed to understand the kinds of signals that can be manipulated for desired outcomes in regenerative biomedicine and oncology contexts [457–459].
Genetics and physiology
One of the most important lessons to come from the recent work on bioelectrical controls of morphology is the fact that significant patterning information can be generated and maintained at the level of physiology and de-coupled from changes in transcription and translation (Figure 10). While biophysical events are certainly transduced into genetic cascades, the laws of physics and the post-translational gating of channel and pump proteins guarantee that cells expressing precisely the same complement of proteins can be in very different bioelectrical states. Of course this is akin to familiar phenomena such as action potentials propagating down an axon and calcium fertilization waves moving over an oocyte, neither of which requires changes in mRNA or protein levels for the dynamics of bioelectrical cell state to evolve. On longer time scales, networks of voltage-gated ion channels and voltage-regulated gap junctional current paths can form cell fields with rich feedback dynamics in the distribution of voltage levels that are not at all captured by analysis of protein content. Protein profile does not determine physiological state: not only can cells with the same protein be in different physiological states, cells in very similar physiological states could reach them by making use of very different ion channels. The ability of many different channels to be combined towards the same physiological end-goal results in a huge degree of compensation and redundancy [460]; this is a benefit for ensuring physiological robustness of bioelectrical control systems, but also means that the popular single-channel knockout experiments will rarely reveal phenotypes indicative of the patterning roles of transmembrane voltage potentials.
Figure 10. Bioelectric control of growth and form overrides genetic information.
The interplay of physiological and genetic information represents a key area for future research in the cancer field, as well as developmental and regenerative biology. Recent work on the bioelectric control of stem cell-mediated regeneration in planaria illustrates one example in which information stored in bioelectrical states dominates the genetically-encoded tissue pattern [113,115].
(A) An intact worm is cut into 3 pieces, and the middle fragment is subjected to transient pharmacological gap junctional inhibition [112,114]. This isolation of the wound cells from long-range signals throughout the fragment result in a 2-headed worm (B). Remarkably, when these worms are cut again (C) and again (D), without any further manipulation, the 2-headed phenotype persists (indefinitely). The ability of a brief perturbation of physiological signaling to permanently change the target morphology (the shape to which the animal regenerates upon damage) despite an undamaged genome hints at the power of bioelectrically-encoded signals to regulate cell behavior toward specific tissue outcomes. Note that this target morphology is specified non-locally and cannot be explained by a simple epigenetic modification of wound tissue, because the tail cells that would have been epigenetically reprogrammed into head identity are removed on each round of cutting, and it is the normally-differentiated central trunk cells that must know to generate an ectopic head at each wound site. Such distributed storage of target morphology in the real-time bioelectrical network present throughout the organism may explain the classical data on detection of tumors by electrical readings taken far away from the actual site of the cancer, and serves to focus attention on events outside of the immediate microenvironment of a neoplastic lesion.
As seen in the several examples discussed in detail above, changes in bioelectric state can alter patterning and induce/suppress tumorigenesis without DNA damage, and by the modulation of any number of channels/pumps at the post-translational level. Cracking the bioelectrical code to allow prevention and normalization of cancer will require fleshing out the interaction between information stored in truly epigenetic (in the original sense of the word) physiological networks, transcriptional responses, and the goal-seeking control algorithms of single cells and multicellular host organisms. This in turn will require new technique development - establishment of model systems in which voltage can be controlled directly in any cell/tissue of interest; one exciting candidate is the extension of optogenetics to non-neural, non-excitable tissues [461,462], and conceptual apparatus for modeling information processing and autonomous dynamical system properties in silico that can be applied to the initiation and reprogramming of cancer in vivo.
Speculations: cancer as a failure of morphogenetic field memory
Taken together, recent and classical data suggest that morphogenesis and morphostasis are core concepts unifying three major areas of study – development, regeneration, and cancer. Understanding the ability of systems to self-assemble complex anatomy, to repair damage, and maintain shape against aging and cancer is paramount to progress in all three fields, and will drive radical advances in biomedicine [463]. It is imperative that we identify and quantitatively model the information-processing and computational activities of patterning systems to gain control of molecular mechanisms by which morphogenetic information orchestrates low-level (cell) behaviors towards the patterning needs of the host. Cancer biology may be an ideal context in which to consider predictive, quantitative models of top-down causation and control as an alternative to the current paradigm focused on molecular pathways and emergence [464–468]. What criteria (degree of predictive control in functional experiments? parsimony of model? conformance with reductionism?) are to be used to decide among top-down and bottom-up models? This has important implications beyond philosophy and basic developmental biology. Our choice of strategies for regenerative and cancer medicine depends crucially on finding the easiest path towards gaining rational control over complex biological shapes and understanding the still mysterious link between the equally rapid growth of cancer vs. regenerative repair.
Bioelectric events have properties that make them ideal components for implementing the morphogenetic field, and indeed recent data has shown that their manipulation is a good entry-point into a molecular-level understanding of these mechanisms [80,469]. Bioelectricity is central to development, regeneration, and cancer. But the transformative impact of integrating biophysics into our genetic paradigm will be the ability to move beyond Vmem in single cells and understand the dynamics of multicellular bioelectric networks and how they store and specify shape. A key next step is the construction of specific dynamical systems models of patterning information stored in real-time physiological networks. Multidimensional spaces of many different bioelectric measurements will require concerted physiomics profiling efforts; such data may turn out to contain attractors that map to anatomical states, and may implement the “dynamically preformed morph” envisioned by Gurwitsch [470].
The data of modern bioelectricity reveal the instructive control of shape (and its derangement during cancer) by endogenous voltage gradients and ion currents. Dynamic control of morphogenesis requires processing large amounts of information about tissue and organ structure, as well as a mechanism for ceasing growth when particular target morphology has been reached (a step that is likely to be completely short-circuited in tumors). Fortunately, we have two solid precedents for storing information in dynamic patterns of ion flow. The first is storage of bits in a magnetic core memory – the information is literally encoded in the direction of ion flows and their resulting magnetic fields. Much as the ion flows among electrically active cells are invisible to techniques focused on the material structure of cells and the mRNA/proteins expressed in them, the information content of electronic storage media is invisible to a description of the material components of a computer memory system - energy flow patterns can store distinct bits among identical bi-stable units, whether they are implemented in cells [471–473] or transistor flip-flop circuits.
Even more simply, the conservation of basic molecular elements in the central nervous system and in non-neural embryonic cells reminds us that cognitive science has a mature and well-developed history of investigating spatial maps encoded in the dynamics of electrically-active cells - navigational memory in the brain [474,475]. The neurobehavioral community is quite comfortable with the storage of memory in neural networks, and techniques and results in this field should be combined with modern understanding of pattern formation and disorders. After all, both study information – spatial information processed in reorganization of geometry (morphogenesis), and temporal information remembered as patterns from the environment (learning and memory); the parallels in information-processing algorithms of neural networks and developmental dynamics was pointed out by Grossberg decades ago [476], but not seriously investigated yet. Not surprisingly, ion translocators are involved in learning and memory storage [477–479], placing these molecules at an important focal point at the intersection of morphogenesis and cognition. Likewise, heart cells have been modeled as a neural-like network to explain memory effects relevant to remodeling [471,480]. While most somatic cells process voltage change signals much more slowly than do rapidly spiking neurons, it is tempting to speculate that the analogy may indicate a real, mechanistic relationship. Such computational tissues would be ideal media in which to store and manipulate the information used by morphogenetic fields. Given that many cell types are communicating electrically via membrane potential and highly-tunable electric synapses, gap junctions [88,481–483], there may not be any fundamental difference between the information-processing functions of neural networks and similar electrical dynamics in non-neural cells. Thus, cancer could be a regenerative response that cannot remember what target morphology is to be recognized as the “end of growth signal”, and a reprogramming solution could be sought in repairing the ability of cells to access the electrically-mediated memory of appropriate tissue organization and the cell behaviors that are needed to achieve that state.
If these highly speculative parallels hold, many novel, mechanistically-tractable questions are suggested with respect to how normal tissue architecture is “remembered” in cell networks and what processes of memory failure may result in neoplasm. For example, the glutamate receptor, metabotropic [1], also known as GRM1, is an ion channel and an oncogene; notably, the knockout mice exhibited problems with long-term potentiation (memory) [484–488]. Likewise, signaling via the neurotransmitter serotonin has been implicated in cancer [60,281,489–491]. The use of cognitive modulator compounds in cancer assays is being pursued in our lab, to test predictions of a hypothesis linking bioelectric mediation of morphogenetic cues, stored memory of correct organ/tissue morphology, and its disruption in cancer.
Conclusion and Summary
Much classical and recent data reveal that cancer is not simply a result of damaged genomes but rather involves a disruption of normal developmental mechanisms and cell:cell communication across large distances. It is now known that endogenous bioelectrical gradients underlie an important layer of such cell coordination, and it is thus not surprising that ion channels are increasingly revealed as not only markers of the transformed state but also bona fide oncogenes and thus important drug targets. Work in the amphibian model demonstrated that depolarization of resting potential by voltage-sensitive fluorescent dyes is a promising modality for detecting cancer in vivo. Moreover, depolarization of key cell groups in the body is sufficient to activate metastatic behavior in other cell types through a serotonergic signaling mechanism. Finally, oncogene-induced tumor structure formation requires depolarization, and artificial hyperpolarization is able to suppress this effect, despite high levels of oncogene protein, through a butyrate and histone deacetylate mechanism. Thus, the tumor microenvironment is not only chemical but also bioelectrical [492]; indeed, it may function over considerable distances. Transformative applications in cancer medicine and beyond await our molecular dissection of the bioelectrical and other mechanisms by which active morphogenesis and nervous system activity suppresses tumorigenesis, and regenerative and embryonic environments actively reprogram tumors.
Acknowledgments
This paper is dedicated H. S. Burr, an early pioneer of non-local bioelectric field influences in cancer, and to the non-traditional model species – animals rarely used in modern molecular approaches, which have facilitated the discovery of remarkable and fundamental relationships between cancer and normal patterning processes. We thank Jessica Mustard and Jean-Francois Pare for helpful comments on a draft of this manuscript, and Vaibhav Pai for assistance with Oncomine data searches. This work was supported by the National Institutes of Health [grant numbers AR061988 and AR055993] and the G. Harold and Leila Y. Mathers Charitable Foundation.
References
- 1.Vaux DL. In defense of the somatic mutation theory of cancer. Bioessays. 2011;33:341–343. doi: 10.1002/bies.201100022. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Baker SG. Paradox-driven cancer research. Disruptive science and technology. 2013;1:143–148. [Google Scholar]
- 4.Soto AM, Sonnenschein C. The somatic mutation theory of cancer: growing problems with the paradigm? Bioessays. 2004;26:1097–1107. doi: 10.1002/bies.20087. [DOI] [PubMed] [Google Scholar]
- 5.Tarin D. Role of the host stroma in cancer and its therapeutic significance. Cancer Metastasis Rev. 2013 doi: 10.1007/s10555-013-9438-4. [DOI] [PubMed] [Google Scholar]
- 6.Tarin D. Inappropriate gene expression in human cancer and its far-reaching biological and clinical significance. Cancer Metastasis Rev. 2012;31:21–39. doi: 10.1007/s10555-011-9326-8. [DOI] [PubMed] [Google Scholar]
- 7.Tarin D. Erratum to: Clinical and Biological Implications of the Tumor Microenvironment. Cancer Microenviron. 2012;5:113. doi: 10.1007/s12307-012-0099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarin D. Cell and tissue interactions in carcinogenesis and metastasis and their clinical significance. Semin Cancer Biol. 2011;21:72–82. doi: 10.1016/j.semcancer.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Potter JD. Morphogens, morphostats, microarchitecture and malignancy. Nat Rev Cancer. 2007;7:464–474. doi: 10.1038/nrc2146. [DOI] [PubMed] [Google Scholar]
- 10.Potter JD. Morphostats: a missing concept in cancer biology. Cancer Epidemiol Biomarkers Prev. 2001;10:161–170. [PubMed] [Google Scholar]
- 11.Capp JP. Stochastic gene expression, disruption of tissue averaging effects and cancer as a disease of development. Bioessays. 2005;27:1277–1285. doi: 10.1002/bies.20326. [DOI] [PubMed] [Google Scholar]
- 12.Soto AM, Sonnenschein C. The tissue organization field theory of cancer: a testable replacement for the somatic mutation theory. Bioessays. 2011;33:332–340. doi: 10.1002/bies.201100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soto AM, Sonnenschein C. Emergentism as a default: cancer as a problem of tissue organization. J Biosci. 2005;30:103–118. doi: 10.1007/BF02705155. [DOI] [PubMed] [Google Scholar]
- 14.Levin M. Morphogenetic fields in embryogenesis, regeneration, and cancer: non-local control of complex patterning. Biosystems. 2012;109:243–261. doi: 10.1016/j.biosystems.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burr HS, Northrop F. The electrodynamic theory of life. Quarterly Review of Biology. 1935;10:322–333. [Google Scholar]
- 16.Plankar M, Jerman I, KraÅ¡ovec R. On the origin of cancer: can we ignore coherence? Prog Biophys Mol Biol. 2011;106:380–390. doi: 10.1016/j.pbiomolbio.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Dinicola S, D’Anselmi F, Pasqualato A, Proietti S, Lisi E, et al. A systems biology approach to cancer: fractals, attractors, and nonlinear dynamics. OMICS. 2011;15:93–104. doi: 10.1089/omi.2010.0091. [DOI] [PubMed] [Google Scholar]
- 18.Bizzarri M, Cucina A, Biava PM, Proietti S, D’Anselmi F, et al. Embryonic morphogenetic field induces phenotypic reversion in cancer cells. Review article. Curr Pharm Biotechnol. 2011;12:243–253. doi: 10.2174/138920111794295701. [DOI] [PubMed] [Google Scholar]
- 19.Bizzarri M, Cucina A, Conti F, D’Anselmi F. Beyond the oncogene paradigm: understanding complexity in cancerogenesis. Acta Biotheor. 2008;56:173–196. doi: 10.1007/s10441-008-9047-8. [DOI] [PubMed] [Google Scholar]
- 20.Rubin H. Ordered heterogeneity and its decline in cancer and aging. Adv Cancer Res. 2007;98:117–147. doi: 10.1016/S0065-230X(06)98004-X. [DOI] [PubMed] [Google Scholar]
- 21.Rubin H. What keeps cells in tissues behaving normally in the face of myriad mutations? Bioessays. 2006;28:515–524. doi: 10.1002/bies.20403. [DOI] [PubMed] [Google Scholar]
- 22.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingber DE. Can cancer be reversed by engineering the tumor microenvironment? Semin Cancer Biol. 2008;18:356–364. doi: 10.1016/j.semcancer.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weaver VM, Gilbert P. Watch thy neighbor: cancer is a communal affair. J Cell Sci. 2004;117:1287–1290. doi: 10.1242/jcs.01137. [DOI] [PubMed] [Google Scholar]
- 25.Prehn RT. Cancers beget mutations versus mutations beget cancers. Cancer Res. 1994;54:5296–5300. [PubMed] [Google Scholar]
- 26.Rowlatt C. New Frontiers in Cancer Causation. Taylor & Francis; 1994. [Google Scholar]
- 27.Tsonis PA. Embryogenesis and carcinogenesis: order and disorder. Anticancer Res. 1987;7:617–623. [PubMed] [Google Scholar]
- 28.Rubin H. Cancer as a dynamic developmental disorder. Cancer Res. 1985;45:2935–2942. [PubMed] [Google Scholar]
- 29.Baker SG, Soto AM, Sonnenschein C, Cappuccio A, Potter JD, et al. Plausibility of stromal initiation of epithelial cancers without a mutation in the epithelium: a computer simulation of morphostats. BMC Cancer. 2009;9:89. doi: 10.1186/1471-2407-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Needham J. Chemical embryology. Hafner Pub Co; New York: 1963. [Google Scholar]
- 31.Needham J. New Advances in the Chemistry and Biology of Organized Growth: (Section of Pathology) Proc R Soc Med. 1936;29:1577–1626. doi: 10.1177/003591573602901209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waddington CH. Cancer and the theory of organisers. Nature. 1935;135:606–608. [Google Scholar]
- 33.Fan Y, Bergmann A. Apoptosis-induced compensatory proliferation. The Cell is dead. Long live the Cell! Trends Cell Biol. 2008;18:467–473. doi: 10.1016/j.tcb.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang S, Ernberg I, Kauffman S. Cancer attractors: a systems view of tumors from a gene network dynamics and developmental perspective. Semin Cell Dev Biol. 2009;20:869–876. doi: 10.1016/j.semcdb.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borgens R, Robinson K, Vanable J, McGinnis M. Electric Fields in Vertebrate Repair: Natural and Applied Voltages in Vertebrate Regeneration and Healing. Alan R Liss, Inc; 1989. [Google Scholar]
- 36.Lund E. Bioelectric fields and growth. The University of Texas Press; USA: 1947. [Google Scholar]
- 37.Pullar CE. The physiology of bioelectricity in development, tissue regeneration, and cancer. CRC Press; 2011. [Google Scholar]
- 38.McCaig CD, Song B, Rajnicek AM. Electrical dimensions in cell science. J Cell Sci. 2009;122:4267–4276. doi: 10.1242/jcs.023564. [DOI] [PubMed] [Google Scholar]
- 39.Popp FA. Cancer growth and its inhibition in terms of coherence. Electromagn Biol Med. 2009;28:53–60. doi: 10.1080/15368370802711805. [DOI] [PubMed] [Google Scholar]
- 40.Takeda M, Kobayashi M, Takayama M, Suzuki S, Ishida T, et al. Biophoton detection as a novel technique for cancer imaging. Cancer Sci. 2004;95:656–661. doi: 10.1111/j.1349-7006.2004.tb03325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pokorný J, Hasek J, Vanis J, Jelínek F. Biophysical aspects of cancer- -electromagnetic mechanism. Indian J Exp Biol. 2008;46:310–321. [PubMed] [Google Scholar]
- 42.Frohlich H. Coherent Electric Vibrations in Biological Systems and the Cancer Problem. Microwave Theory and Techniques, IEEE Transactions on. 1978;26:613–618. [Google Scholar]
- 43.Levin M. Molecular bioelectricity in developmental biology: new tools and recent discoveries: control of cell behavior and pattern formation by transmembrane potential gradients. Bioessays. 2012;34:205–217. doi: 10.1002/bies.201100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Funk RH, Monsees T, Ozkucur N. Electromagnetic effects - From cell biology to medicine. Prog Histochem Cytochem. 2009;43:177–264. doi: 10.1016/j.proghi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Cifra M, Fields JZ, Farhadi A. Electromagnetic cellular interactions. Prog Biophys Mol Biol. 2011;105:223–246. doi: 10.1016/j.pbiomolbio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Wang ET, Zhao M. Regulation of tissue repair and regeneration by electric fields. Chin J Traumatol. 2010;13:55–61. [PubMed] [Google Scholar]
- 47.Zhao M. Electrical fields in wound healing-An overriding signal that directs cell migration. Semin Cell Dev Biol. 2009;20:674–682. doi: 10.1016/j.semcdb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Levin M. Bioelectric mechanisms in regeneration: Unique aspects and future perspectives. Semin Cell Dev Biol. 2009;20:543–556. doi: 10.1016/j.semcdb.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajnicek AM, Foubister LE, McCaig CD. Growth cone steering by a physiological electric field requires dynamic microtubules, microfilaments and Rac-mediated filopodial asymmetry. J Cell Sci. 2006;119:1736–1745. doi: 10.1242/jcs.02897. [DOI] [PubMed] [Google Scholar]
- 50.Levin M, Stevenson CG. Regulation of cell behavior and tissue patterning by bioelectrical signals: challenges and opportunities for biomedical engineering. Annu Rev Biomed Eng. 2012;14:295–323. doi: 10.1146/annurev-bioeng-071811-150114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Becchetti A. Ion channels and transporters in cancer. 1. Ion channels and cell proliferation in cancer. Am J Physiol Cell Physiol. 2011;301:C255–265. doi: 10.1152/ajpcell.00047.2011. [DOI] [PubMed] [Google Scholar]
- 52.Prevarskaya N, Skryma R, Shuba Y. Ion channels and the hallmarks of cancer. Trends Mol Med. 2010;16:107–121. doi: 10.1016/j.molmed.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 53.Pardo LA, Contreras-Jurado C, Zientkowska M, Alves F, Stühmer W. Role of voltage-gated potassium channels in cancer. J Membr Biol. 2005;205:115–124. doi: 10.1007/s00232-005-0776-1. [DOI] [PubMed] [Google Scholar]
- 54.Kunzelmann K. Ion channels and cancer. J Membr Biol. 2005;205:159–173. doi: 10.1007/s00232-005-0781-4. [DOI] [PubMed] [Google Scholar]
- 55.Stock C, Ludwig FT, Hanley PJ, Schwab A. Roles of ion transport in control of cell motility. Compr Physiol. 2013;3:59–119. doi: 10.1002/cphy.c110056. [DOI] [PubMed] [Google Scholar]
- 56.Sun YS, Peng SW, Lin KH, Cheng JY. Electrotaxis of lung cancer cells in ordered three-dimensional scaffolds. Biomicrofluidics. 2012;6:14102–1410214. doi: 10.1063/1.3671399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan X, Han J, Zhang Z, Wang J, Cheng Q, et al. Lung cancer A549 cells migrate directionally in DC electric fields with polarized and activated EGFRs. Bioelectromagnetics. 2009;30:29–35. doi: 10.1002/bem.20436. [DOI] [PubMed] [Google Scholar]
- 58.Pu J, McCaig CD, Cao L, Zhao Z, Segall JE, et al. EGF receptor signalling is essential for electric-field-directed migration of breast cancer cells. J Cell Sci. 2007;120:3395–3403. doi: 10.1242/jcs.002774. [DOI] [PubMed] [Google Scholar]
- 59.Yao L, Pandit A, Yao S, McCaig CD. Electric field-guided neuron migration: a novel approach in neurogenesis. Tissue Eng Part B Rev. 2011;17:143–153. doi: 10.1089/ten.TEB.2010.0561. [DOI] [PubMed] [Google Scholar]
- 60.Blackiston D, Adams DS, Lemire JM, Lobikin M, Levin M. Transmembrane potential of GlyCl-expressing instructor cells induces a neoplastic-like conversion of melanocytes via a serotonergic pathway. Dis Model Mech. 2011;4:67–85. doi: 10.1242/dmm.005561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morokuma J, Blackiston D, Adams DS, Seebohm G, Trimmer B, et al. Modulation of potassium channel function confers a hyperproliferative invasive phenotype on embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:16608–16613. doi: 10.1073/pnas.0808328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pu J, Zhao M. Golgi polarization in a strong electric field. J Cell Sci. 2005;118:1117–1128. doi: 10.1242/jcs.01646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harris AK, Pryer NK, Paydarfar D. Effects of electric fields on fibroblast contractility and cytoskeleton. J Exp Zool. 1990;253:163–176. doi: 10.1002/jez.1402530206. [DOI] [PubMed] [Google Scholar]
- 64.Baumann M. Early stage shape change of human erythrocytes after application of electric field pulses. Mol Membr Biol. 2001;18:153–160. doi: 10.1080/09687680110034863. [DOI] [PubMed] [Google Scholar]
- 65.Root CM, Velázquez-Ulloa NA, Monsalve GC, Minakova E, Spitzer NC. Embryonically expressed GABA and glutamate drive electrical activity regulating neurotransmitter specification. J Neurosci. 2008;28:4777–4784. doi: 10.1523/JNEUROSCI.4873-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pineda RH, Svoboda KR, Wright MA, Taylor AD, Novak AE, et al. Knockdown of Nav1.6a Na+ channels affects zebrafish motoneuron development. Development. 2006;133:3827–3836. doi: 10.1242/dev.02559. [DOI] [PubMed] [Google Scholar]
- 67.Svoboda KR, Linares AE, Ribera AB. Activity regulates programmed cell death of zebrafish Rohon-Beard neurons. Development. 2001;128:3511–3520. doi: 10.1242/dev.128.18.3511. [DOI] [PubMed] [Google Scholar]
- 68.Messenger NJ, Warner AE. Primary neuronal differentiation in Xenopus embryos is linked to the beta (3) subunit of the sodium pump. Dev Biol. 2000;220:168–182. doi: 10.1006/dbio.2000.9646. [DOI] [PubMed] [Google Scholar]
- 69.Ng SY, Chin CH, Lau YT, Luo J, Wong CK, et al. Role of voltage-gated potassium channels in the fate determination of embryonic stem cells. J Cell Physiol. 2010;224:165–177. doi: 10.1002/jcp.22113. [DOI] [PubMed] [Google Scholar]
- 70.van Vliet P, de Boer TP, van der Heyden MA, El Tamer MK, Sluijter JP, et al. Hyperpolarization induces differentiation in human cardiomyocyte progenitor cells. Stem Cell Rev. 2010;6:178–185. doi: 10.1007/s12015-010-9142-5. [DOI] [PubMed] [Google Scholar]
- 71.Lange C, Prenninger S, Knuckles P, Taylor V, Levin M, et al. The H(+) vacuolar ATPase maintains neural stem cells in the developing mouse cortex. Stem Cells Dev. 2011;20:843–850. doi: 10.1089/scd.2010.0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu JH, Bijlenga P, Fischer-Lougheed J, Occhiodoro T, Kaelin A, et al. Role of an inward rectifier K+ current and of hyperpolarization in human myoblast fusion. J Physiol. 1998;510 :467–476. doi: 10.1111/j.1469-7793.1998.467bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Occhiodoro T, Bernheim L, Liu JH, Bijlenga P, Sinnreich M, et al. Cloning of a human ether-a-go-go potassium channel expressed in myoblasts at the onset of fusion. FEBS Lett. 1998;434:177–182. doi: 10.1016/s0014-5793(98)00973-9. [DOI] [PubMed] [Google Scholar]
- 74.Bijlenga P, Occhiodoro T, Liu JH, Bader CR, Bernheim L, et al. An ether -à-go-go K+ current, Ih-eag, contributes to the hyperpolarization of human fusion-competent myoblasts. J Physiol. 1998;512 :317–323. doi: 10.1111/j.1469-7793.1998.317be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hinard V, Belin D, Konig S, Bader CR, Bernheim L. Initiation of human myoblast differentiation via dephosphorylation of Kir2.1 K+ channels at tyrosine 242. Development. 2008;135:859–867. doi: 10.1242/dev.011387. [DOI] [PubMed] [Google Scholar]
- 76.Sundelacruz S, Levin M, Kaplan DL. Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PLoS One. 2008;3:e3737. doi: 10.1371/journal.pone.0003737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang P, Rushing SN, Kong CW, Fu J, Lieu DK, et al. Electrophysiological Properties of Human Induced Pluripotent Stem Cells. Am J Physiol Cell Physiol. 2009;298:486–495. doi: 10.1152/ajpcell.00251.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stillwell EF, Cone CM, Cone CD., Jr Stimulation of DNA synthesis in CNS neurones by sustained depolarisation. Nat New Biol. 1973;246:110–111. doi: 10.1038/newbio246110a0. [DOI] [PubMed] [Google Scholar]
- 79.Cone CD., Jr Ionically mediated induction of mitogenesis in CNS neurons. Ann N Y Acad Sci. 1980;339:115–131. doi: 10.1111/j.1749-6632.1980.tb15973.x. [DOI] [PubMed] [Google Scholar]
- 80.Levin M. Large-scale biophysics: ion flows and regeneration. Trends Cell Biol. 2007;17:261–270. doi: 10.1016/j.tcb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 81.Pullar CE, Isseroff RR. Cyclic AMP mediates keratinocyte directional migration in an electric field. J Cell Sci. 2005;118:2023–2034. doi: 10.1242/jcs.02330. [DOI] [PubMed] [Google Scholar]
- 82.Mycielska ME, Djamgoz MB. Cellular mechanisms of direct-current electric field effects: galvanotaxis and metastatic disease. J Cell Sci. 2004;117:1631–1639. doi: 10.1242/jcs.01125. [DOI] [PubMed] [Google Scholar]
- 83.Zhao M, Song B, Pu J, Wada T, Reid B, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–460. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
- 84.Rajnicek AM, Foubister LE, McCaig CD. Prioritising guidance cues: directional migration induced by substratum contours and electrical gradients is controlled by a rho/cdc42 switch. Dev Biol. 2007;312:448–460. doi: 10.1016/j.ydbio.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 85.Sáez JC, Connor JA, Spray DC, Bennett MV. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc Natl Acad Sci USA. 1989;86:2708–2712. doi: 10.1073/pnas.86.8.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, et al. Intercellular calcium signaling via gap junction in connexin-43-transfected cells. J Biol Chem. 1998;273:1519–1528. doi: 10.1074/jbc.273.3.1519. [DOI] [PubMed] [Google Scholar]
- 87.Stains JP, Civitelli R. Gap junctions regulate extracellular signal-regulated kinase signaling to affect gene transcription. Mol Biol Cell. 2005;16:64–72. doi: 10.1091/mbc.E04-04-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Palacios-Prado N, Bukauskas FF. Modulation of metabolic communication through gap junction channels by transjunctional voltage; synergistic and antagonistic effects of gating and ionophoresis. Biochim Biophys Acta. 2012;1818:1884–1894. doi: 10.1016/j.bbamem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Levin M, Mercola M. Gap junctions are involved in the early generation of left-right asymmetry. Dev Biol. 1998;203:90–105. doi: 10.1006/dbio.1998.9024. [DOI] [PubMed] [Google Scholar]
- 90.Levin M, Mercola M. Gap junction-mediated transfer of left-right patterning signals in the early chick blastoderm is upstream of Shh asymmetry in the node. Development. 1999;126:4703–4714. doi: 10.1242/dev.126.21.4703. [DOI] [PubMed] [Google Scholar]
- 91.Fukumoto T, Kema IP, Levin M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr Biol. 2005;15:794–803. doi: 10.1016/j.cub.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 92.Fukumoto T, Blakely R, Levin M. Serotonin transporter function is an early step in left-right patterning in chick and frog embryos. Dev Neurosci. 2005;27:349–363. doi: 10.1159/000088451. [DOI] [PubMed] [Google Scholar]
- 93.Chernet BT, Levin M. Transmembrane voltage potential is an essential cellular parameter for the detection and control of tumor development in a Xenopus model. Dis Model Mech. 2013;6:595–607. doi: 10.1242/dmm.010835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tseng AS, Levin M. Transducing bioelectric signals into epigenetic pathways during tadpole tail regeneration. Anat Rec (Hoboken) 2012;295:1541–1551. doi: 10.1002/ar.22495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Worby CA, Dixon JE. Phosphoinositide phosphatases: emerging roles as voltage sensors? Mol Interv. 2005;5:274–277. doi: 10.1124/mi.5.5.5. [DOI] [PubMed] [Google Scholar]
- 96.Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- 97.Okamura Y, Dixon JE. Voltage-sensing phosphatase: its molecular relationship with PTEN. Physiology (Bethesda) 2011;26:6–13. doi: 10.1152/physiol.00035.2010. [DOI] [PubMed] [Google Scholar]
- 98.Lacroix J, Halaszovich CR, Schreiber DN, Leitner MG, Bezanilla F, et al. Controlling the activity of a phosphatase and tensin homolog (PTEN) by membrane potential. J Biol Chem. 2011;286:17945–17953. doi: 10.1074/jbc.M110.201749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carneiro K, Donnet C, Rejtar T, Karger BL, Barisone GA, et al. Histone deacetylase activity is necessary for left-right patterning during vertebrate development. BMC Dev Biol. 2011;11:29. doi: 10.1186/1471-213X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adams DS, Levin M. Endogenous voltage gradients as mediators of cell-cell communication: strategies for investigating bioelectrical signals during pattern formation. Cell Tissue Res. 2013;352:95–122. doi: 10.1007/s00441-012-1329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Adams DS. A new tool for tissue engineers: ions as regulators of morphogenesis during development and regeneration. Tissue Eng Part A. 2008;14:1461–1468. doi: 10.1089/ten.tea.2008.0080. [DOI] [PubMed] [Google Scholar]
- 102.Mutoh H, Perron A, Akemann W, Iwamoto Y, Knöpfel T. Optogenetic monitoring of membrane potentials. Exp Physiol. 2011;96:13–18. doi: 10.1113/expphysiol.2010.053942. [DOI] [PubMed] [Google Scholar]
- 103.Hotary KB, Robinson KR. Evidence of a role for endogenous electrical fields in chick embryo development. Development. 1992;114:985–996. doi: 10.1242/dev.114.4.985. [DOI] [PubMed] [Google Scholar]
- 104.Shi R, Borgens RB. Three-dimensional gradients of voltage during development of the nervous system as invisible coordinates for the establishment of embryonic pattern. Dev Dyn. 1995;202:101–114. doi: 10.1002/aja.1002020202. [DOI] [PubMed] [Google Scholar]
- 105.Levin M, Thorlin T, Robinson KR, Nogi T, Mercola M. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell. 2002;111:77–89. doi: 10.1016/s0092-8674(02)00939-x. [DOI] [PubMed] [Google Scholar]
- 106.Adams DS, Robinson KR, Fukumoto T, Yuan S, Albertson RC, et al. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development. 2006;133:1657–1671. doi: 10.1242/dev.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aw S, Adams DS, Qiu D, Levin M. H,K-ATPase protein localization and Kir4.1 function reveal concordance of three axes during early determination of left-right asymmetry. Mech Dev. 2008;125:353–372. doi: 10.1016/j.mod.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morokuma J, Blackiston D, Levin M. KCNQ1 and KCNE1 K+ channel components are involved in early left-right patterning in Xenopus laevis embryos. Cell Physiol Biochem. 2008;21:357–372. doi: 10.1159/000129628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vandenberg LN, Lemire JM, Levin M. Serotonin has early, cilia-independent roles in Xenopus left-right patterning. Dis Model Mech. 2013;6:261–268. doi: 10.1242/dmm.010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pai VP, Aw S, Shomrat T, Lemire JM, Levin M. Transmembrane voltage potential controls embryonic eye patterning in Xenopus laevis. Development. 2012;139:313–323. doi: 10.1242/dev.073759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vandenberg LN, Morrie RD, Adams DS. V-ATPase-dependent ectodermal voltage and pH regionalization are required for craniofacial morphogenesis. Dev Dyn. 2011;240:1889–1904. doi: 10.1002/dvdy.22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nogi T, Levin M. Characterization of innexin gene expression and functional roles of gap-junctional communication in planarian regeneration. Dev Biol. 2005;287:314–335. doi: 10.1016/j.ydbio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 113.Beane WS, Morokuma J, Adams DS, Levin M. A chemical genetics approach reveals H,K-ATPase-mediated membrane voltage is required for planarian head regeneration. Chem Biol. 2011;18:77–89. doi: 10.1016/j.chembiol.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Oviedo NJ, Morokuma J, Walentek P, Kema IP, Gu MB, et al. Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev Biol. 2010;339:188–199. doi: 10.1016/j.ydbio.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Beane WS, Morokuma J, Lemire JM, Levin M. Bioelectric signaling regulates head and organ size during planarian regeneration. Development. 2013;140:313–322. doi: 10.1242/dev.086900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bodemer CW. Evocation Of Regrowth Phenomena In Anuran Limbs By Electrical Stimulation Of The Nerve Supply. Anat Rec. 1964;148:441–457. doi: 10.1002/ar.1091480303. [DOI] [PubMed] [Google Scholar]
- 117.Becker RO. The bioelectric factors in amphibian-limb regeneration. J Bone Joint Surg Am. 1961;43–43A:643–56. [PubMed] [Google Scholar]
- 118.Becker RO, Spadaro JA. Electrical stimulation of partial limb regeneration in mammals. Bull N Y Acad Med. 1972;48:627–641. [PMC free article] [PubMed] [Google Scholar]
- 119.Borgens RB. Are limb development and limb regeneration both initiated by an integumentary wounding? A hypothesis. Differentiation. 1984;28:87–93. doi: 10.1111/j.1432-0436.1984.tb00270.x. [DOI] [PubMed] [Google Scholar]
- 120.Borgens RB, Rouleau MF, DeLanney LE. A steady efflux of ionic current predicts hind limb development in the axolotl. J Exp Zool. 1983;228:491–503. doi: 10.1002/jez.1402280309. [DOI] [PubMed] [Google Scholar]
- 121.Borgens RB, Vanable JW, Jr, Jaffe LF. Reduction of sodium dependent stump currents disturbs urodele limb regeneration. J Exp Zool. 1979;209:377–386. doi: 10.1002/jez.1402090304. [DOI] [PubMed] [Google Scholar]
- 122.Borgens RB, Vanable JW, Jr, Jaffe LF. Bioelectricity and regeneration: large currents leave the stumps of regenerating newt limbs. Proc Natl Acad Sci USA. 1977;74:4528–4532. doi: 10.1073/pnas.74.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Borgens RB, Vanable JW, Jr, Jaffe LF. Bioelectricity and regeneration. I. Initiation of frog limb regeneration by minute currents. J Exp Zool. 1977;200:403–416. doi: 10.1002/jez.1402000310. [DOI] [PubMed] [Google Scholar]
- 124.Adams DS, Masi A, Levin M. H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development. 2007;134:1323–1335. doi: 10.1242/dev.02812. [DOI] [PubMed] [Google Scholar]
- 125.Tseng AS, Beane WS, Lemire JM, Masi A, Levin M. Induction of vertebrate regeneration by a transient sodium current. J Neurosci. 2010;30:13192–13200. doi: 10.1523/JNEUROSCI.3315-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tseng A, Levin M. Cracking the bioelectric code: Probing endogenous ionic controls of pattern formation. Commun Integr Biol. 2013;6:e22595. doi: 10.4161/cib.22595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rock JR, Futtner CR, Harfe BD. The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev Biol. 2008;321:141–149. doi: 10.1016/j.ydbio.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 128.Iwashita M, Watanabe M, Ishii M, Chen T, Johnson SL, et al. Pigment pattern in jaguar/obelix zebrafish is caused by a Kir7.1 mutation: implications for the regulation of melanosome movement. PLoS Genet. 2006;2:e197. doi: 10.1371/journal.pgen.0020197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Watanabe M, Iwashita M, Ishii M, Kurachi Y, Kawakami A, et al. Spot pattern of leopard Danio is caused by mutation in the zebrafish connexin41.8 gene. EMBO Rep. 2006;7:893–897. doi: 10.1038/sj.embor.7400757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hoptak-Solga AD, Nielsen S, Jain I, Thummel R, Hyde DR, et al. Connexin43 (GJA1) is required in the population of dividing cells during fin regeneration. Dev Biol. 2008;317:541–548. doi: 10.1016/j.ydbio.2008.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350:1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- 132.Culiat CT, Stubbs LJ, Woychik RP, Russell LB, Johnson DK, et al. Deficiency of the beta 3 subunit of the type A gamma-aminobutyric acid receptor causes cleft palate in mice. Nat Genet. 1995;11:344–346. doi: 10.1038/ng1195-344. [DOI] [PubMed] [Google Scholar]
- 133.Wee EL, Zimmerman EF. GABA uptake in embryonic palate mesenchymal cells of two mouse strains. Neurochem Res. 1985;10:1673–1688. doi: 10.1007/BF00988609. [DOI] [PubMed] [Google Scholar]
- 134.Miller RP, Becker BA. Teratogenicity of oral diazepam and diphenylhydantoin in mice. Toxicol Appl Pharmacol. 1975;32:53–61. doi: 10.1016/0041-008x(75)90194-5. [DOI] [PubMed] [Google Scholar]
- 135.Homanics GE, DeLorey TM, Firestone LL, Quinlan JJ, Handforth A, et al. Mice devoid of gamma-aminobutyrate type A receptor beta3 subunit have epilepsy, cleft palate, and hypersensitive behavior. Proc Natl Acad Sci USA. 1997;94:4143–4148. doi: 10.1073/pnas.94.8.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bendahhou S, Donaldson MR, Plaster NM, Tristani-Firouzi M, Fu YH, et al. Defective potassium channel Kir2.1 trafficking underlies Andersen-Tawil syndrome. J Biol Chem. 2003;278:51779–51785. doi: 10.1074/jbc.M310278200. [DOI] [PubMed] [Google Scholar]
- 137.Dahal GR, Rawson J, Gassaway B, Kwok B, Tong Y, et al. An inwardly rectifying K+ channel is required for patterning. Development. 2012;139:3653–3664. doi: 10.1242/dev.078592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Debeer P, Van Esch H, Huysmans C, Pijkels E, De Smet L, et al. Novel GJA1 mutations in patients with oculo-dento-digital dysplasia (ODDD) Eur J Med Genet. 2005;48:377–387. doi: 10.1016/j.ejmg.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 139.Pizzuti A, Flex E, Mingarelli R, Salpietro C, Zelante L, et al. A homozygous GJA1 gene mutation causes a Hallermann-Streiff/ODDD spectrum phenotype. Hum Mutat. 2004;23:286. doi: 10.1002/humu.9220. [DOI] [PubMed] [Google Scholar]
- 140.Rakic P, Sidman RL. Sequence of developmental abnormalities leading to granule cell deficit in cerebellar cortex of weaver mutant mice. J Comp Neurol. 1973;152:103–132. doi: 10.1002/cne.901520202. [DOI] [PubMed] [Google Scholar]
- 141.Rakic P, Sidman RL. Weaver mutant mouse cerebellum: defective neuronal migration secondary to abnormality of Bergmann glia. Proc Natl Acad Sci USA. 1973;70:240–244. doi: 10.1073/pnas.70.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hatten ME, Liem RK, Mason CA. Weaver mouse cerebellar granule neurons fail to migrate on wild-type astroglial processes in vitro. J Neurosci. 1986;6:2676–2683. doi: 10.1523/JNEUROSCI.06-09-02676.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Patil N, Cox DR, Bhat D, Faham M, Myers RM, et al. A potassium channel mutation in weaver mice implicates membrane excitability in granule cell differentiation. Nat Genet. 1995;11:126–129. doi: 10.1038/ng1095-126. [DOI] [PubMed] [Google Scholar]
- 144.Civitelli R. Cell-cell communication in the osteoblast/osteocyte lineage. Arch Biochem Biophys. 2008;473:188–192. doi: 10.1016/j.abb.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chopra SS, Stroud DM, Watanabe H, Bennett JS, Burns CG, et al. Voltage-gated sodium channels are required for heart development in zebrafish. Circ Res. 2010;106:1342–1350. doi: 10.1161/CIRCRESAHA.109.213132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Teng GQ, Zhao X, Lees-Miller JP, Quinn FR, Li P, et al. Homozygous missense N629D hERG (KCNH2) potassium channel mutation causes developmental defects in the right ventricle and its outflow tract and embryonic lethality. Circ Res. 2008;103:1483–1491. doi: 10.1161/CIRCRESAHA.108.177055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Uzun S, Gokce S, Wagner K. Cystic fibrosis transmembrane conductance regulator gene mutations in infertile males with congenital bilateral absence of the vas deferens. The Tohoku journal of experimental medicine. 2005;207:279–285. doi: 10.1620/tjem.207.279. [DOI] [PubMed] [Google Scholar]
- 148.Wilschanski M, Dupuis A, Ellis L, Jarvi K, Zielenski J, et al. Mutations in the cystic fibrosis transmembrane regulator gene and in vivo transepithelial potentials. Am J Respir Crit Care Med. 2006;174:787–794. doi: 10.1164/rccm.200509-1377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Riss J, Khanna C, Koo S, Chandramouli GV, Yang HH, et al. Cancers as wounds that do not heal: differences and similarities between renal regeneration/repair and renal cell carcinoma. Cancer Res. 2006;66:7216–7224. doi: 10.1158/0008-5472.CAN-06-0040. [DOI] [PubMed] [Google Scholar]
- 150.Kenny PA, Nelson CM, Bissell MJ. The Ecology of Tumors: By perturbing the microenvironment, wounds and infection may be key to tumor development. Scientist. 2006;20:30. [PMC free article] [PubMed] [Google Scholar]
- 151.Dolberg DS, Hollingsworth R, Hertle M, Bissell MJ. Wounding and its role in RSV-mediated tumor formation. Science. 1985;230:676–678. doi: 10.1126/science.2996144. [DOI] [PubMed] [Google Scholar]
- 152.Pullar CE, Baier BS, Kariya Y, Russell AJ, Horst BA, et al. beta4 integrin and epidermal growth factor coordinately regulate electric field-mediated directional migration via Rac1. Mol Biol Cell. 2006;17:4925–4935. doi: 10.1091/mbc.E06-05-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Huber SM. Oncochannels. Cell Calcium. 2013;53:241–255. doi: 10.1016/j.ceca.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 154.Arcangeli A. Ion channels and transporters in cancer. 3. Ion channels in the tumor cell-microenvironment cross talk. Am J Physiol Cell Physiol. 2011;301:C762–771. doi: 10.1152/ajpcell.00113.2011. [DOI] [PubMed] [Google Scholar]
- 155.Arcangeli A, Crociani O, Lastraioli E, Masi A, Pillozzi S, et al. Targeting ion channels in cancer: a novel frontier in antineoplastic therapy. Curr Med Chem. 2009;16:66–93. doi: 10.2174/092986709787002835. [DOI] [PubMed] [Google Scholar]
- 156.Farias LM, Ocaña DB, Díaz L, Larrea F, Avila-Chávez E, et al. Ether a go-go potassium channels as human cervical cancer markers. Cancer Res. 2004;64:6996–7001. doi: 10.1158/0008-5472.CAN-04-1204. [DOI] [PubMed] [Google Scholar]
- 157.Killion JJ. Electrical properties of normal and transformed mammalian cells. Biophys J. 1984;45:523–528. doi: 10.1016/S0006-3495(84)84189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Martínez-Zaguilán R, Gillies RJ. A plasma membrane V-type H(+)-ATPase may contribute to elevated intracellular pH (pHin) in some human tumor cells. Ann N Y Acad Sci. 1992;671:478–480. doi: 10.1111/j.1749-6632.1992.tb43834.x. [DOI] [PubMed] [Google Scholar]
- 159.Martinez-Zaguilan R, Lynch RM, Martinez GM, Gillies RJ. Vacuolar-type H(+)-ATPases are functionally expressed in plasma membranes of human tumor cells. Am J Physiol. 1993;265:C1015–1029. doi: 10.1152/ajpcell.1993.265.4.C1015. [DOI] [PubMed] [Google Scholar]
- 160.Bianchi L, Wible B, Arcangeli A, Taglialatela M, Morra F, et al. herg encodes a K+ current highly conserved in tumors of different histogenesis: a selective advantage for cancer cells? Cancer Res. 1998;58:815–822. [PubMed] [Google Scholar]
- 161.Arcangeli A, Bianchi L, Becchetti A, Faravelli L, Coronnello M, et al. A novel inward-rectifying K+ current with a cell-cycle dependence governs the resting potential of mammalian neuroblastoma cells. J Physiol. 1995;489 :455–471. doi: 10.1113/jphysiol.1995.sp021065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.McLean LA, Roscoe J, Jorgensen NK, Gorin FA, Cala PM. Malignant gliomas display altered pH regulation by NHE1 compared with nontransformed astrocytes. Am J Physiol Cell Physiol. 2000;278:C676–688. doi: 10.1152/ajpcell.2000.278.4.C676. [DOI] [PubMed] [Google Scholar]
- 163.Wissenbach U, Niemeyer BA, Fixemer T, Schneidewind A, Trost C, et al. Expression of CaT-like, a novel calcium-selective channel, correlates with the malignancy of prostate cancer. J Biol Chem. 2001;276:19461–19468. doi: 10.1074/jbc.M009895200. [DOI] [PubMed] [Google Scholar]
- 164.Wissenbach U, Niemeyer B, Himmerkus N, Fixemer T, Bonkhoff H, et al. TRPV6 and prostate cancer: cancer growth beyond the prostate correlates with increased TRPV6 Ca2+ channel expression. Biochem Biophys Res Commun. 2004;322:1359–1363. doi: 10.1016/j.bbrc.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 165.Marino AA, Iliev IG, Schwalke MA, Gonzalez E, Marler KC, et al. Association between cell membrane potential and breast cancer. Tumour Biol. 1994;15:82–89. doi: 10.1159/000217878. [DOI] [PubMed] [Google Scholar]
- 166.Voloshyna I, Besana A, Castillo M, Matos T, Weinstein IB, et al. TREK-1 is a novel molecular target in prostate cancer. Cancer Res. 2008;68:1197–1203. doi: 10.1158/0008-5472.CAN-07-5163. [DOI] [PubMed] [Google Scholar]
- 167.Diss JK, Stewart D, Pani F, Foster CS, Walker MM, et al. A potential novel marker for human prostate cancer: voltage-gated sodium channel expression in vivo. Prostate Cancer Prostatic Dis. 2005;8:266–273. doi: 10.1038/sj.pcan.4500796. [DOI] [PubMed] [Google Scholar]
- 168.Duncan LM, Deeds J, Cronin FE, Donovan M, Sober AJ, et al. Melastatin expression and prognosis in cutaneous malignant melanoma. J Clin Oncol. 2001;19:568–576. doi: 10.1200/JCO.2001.19.2.568. [DOI] [PubMed] [Google Scholar]
- 169.Zhiqi S, Soltani MH, Bhat KM, Sangha N, Fang D, et al. Human melastatin 1 (TRPM1) is regulated by MITF and produces multiple polypeptide isoforms in melanocytes and melanoma. Melanoma Res. 2004;14:509–516. doi: 10.1097/00008390-200412000-00011. [DOI] [PubMed] [Google Scholar]
- 170.Xu J, Xie R, Liu X, Wen G, Jin H, et al. Expression and functional role of vacuolar H(+)-ATPase in human hepatocellular carcinoma. Carcinogenesis. 2012;33:2432–2440. doi: 10.1093/carcin/bgs277. [DOI] [PubMed] [Google Scholar]
- 171.Conti M. Targeting K+ channels for cancer therapy. J Exp Ther Oncol. 2004;4:161–166. [PubMed] [Google Scholar]
- 172.Fraser SP, Grimes JA, Djamgoz MB. Effects of voltage-gated ion channel modulators on rat prostatic cancer cell proliferation: comparison of strongly and weakly metastatic cell lines. Prostate. 2000;44:61–76. doi: 10.1002/1097-0045(20000615)44:1<61::aid-pros9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 173.Schlichter LC, Sakellaropoulos G, Ballyk B, Pennefather PS, Phipps DJ. Properties of K+ and Cl− channels and their involvement in proliferation of rat microglial cells. Glia. 1996;17:225–236. doi: 10.1002/(SICI)1098-1136(199607)17:3<225::AID-GLIA5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 174.Schuldiner S, Rozengurt E. Na+/H+ antiport in Swiss 3T3 cells: mitogenic stimulation leads to cytoplasmic alkalinization. Proc Natl Acad Sci USA. 1982;79:7778–7782. doi: 10.1073/pnas.79.24.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lopez-Rivas A, Adelberg EA, Rozengurt E. Intracellular K+ and the mitogenic response of 3T3 cells to peptide factors in serum-free medium. Proc Natl Acad Sci USA. 1982;79:6275–6279. doi: 10.1073/pnas.79.20.6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Burns CP, Rozengurt E. Extracellular Na+ and initiation of DNA synthesis: role of intracellular pH and K+ J Cell Biol. 1984;98:1082–1089. doi: 10.1083/jcb.98.3.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pillozzi S, Brizzi MF, Balzi M, Crociani O, Cherubini A, et al. HERG potassium channels are constitutively expressed in primary human acute myeloid leukemias and regulate cell proliferation of normal and leukemic hemopoietic progenitors. Leukemia. 2002;16:1791–1798. doi: 10.1038/sj.leu.2402572. [DOI] [PubMed] [Google Scholar]
- 178.Wohlrab D, Wohlrab J, Markwardt F. Electrophysiological characterization of human keratinocytes using the patch-clamp technique. Exp Dermatol. 2000;9:219–223. doi: 10.1034/j.1600-0625.2000.009003219.x. [DOI] [PubMed] [Google Scholar]
- 179.Dalle-Lucca SL, Dalle-Lucca JJ, Borges AC, Ihara SS, Paiva TB. Abnormal proliferative response of the carotid artery of spontaneously hypertensive rats after angioplasty may be related to the depolarized state of its smooth muscle cells. Braz J Med Biol Res. 2000;33:919–927. doi: 10.1590/s0100-879x2000000800008. [DOI] [PubMed] [Google Scholar]
- 180.Wohlrab D, Hein W. Effect of ion channel modulators on the membrane potential of human chondrocytes. Orthopade. 2000;29:80–84. doi: 10.1007/s001320050013. [DOI] [PubMed] [Google Scholar]
- 181.MacFarlane SN, Sontheimer H. Changes in ion channel expression accompany cell cycle progression of spinal cord astrocytes. Glia. 2000;30:39–48. doi: 10.1002/(sici)1098-1136(200003)30:1<39::aid-glia5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 182.Kamleiter M, Hanemann CO, Kluwe L, Rosenbaum C, Wosch S, et al. Voltage-dependent membrane currents of cultured human neurofibromatosis type 2 Schwann cells. Glia. 1998;24:313–322. [PubMed] [Google Scholar]
- 183.Wang S, Melkoumian Z, Woodfork KA, Cather C, Davidson AG, et al. Evidence for an early G1 ionic event necessary for cell cycle progression and survival in the MCF-7 human breast carcinoma cell line. J Cell Physiol. 1998;176:456–464. doi: 10.1002/(SICI)1097-4652(199809)176:3<456::AID-JCP2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 184.Knutson P, Ghiani CA, Zhou JM, Gallo V, McBain CJ. K+ channel expression and cell proliferation are regulated by intracellular sodium and membrane depolarization in oligodendrocyte progenitor cells. J Neurosci. 1997;17:2669–2682. doi: 10.1523/JNEUROSCI.17-08-02669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Puro DG, Roberge F, Chan CC. Retinal glial cell proliferation and ion channels: a possible link. Invest Ophthalmol Vis Sci. 1989;30:521–529. [PubMed] [Google Scholar]
- 186.Banderali U, Belke D, Singh A, Jayanthan A, Giles WR, et al. Curcumin blocks Kv11.1 (erg) potassium current and slows proliferation in the infant acute monocytic leukemia cell line THP-1. Cell Physiol Biochem. 2011;28:1169–1180. doi: 10.1159/000335850. [DOI] [PubMed] [Google Scholar]
- 187.Lin H, Xiao J, Luo X, Wang H, Gao H, et al. Overexpression HERG K(+) channel gene mediates cell-growth signals on activation of oncoproteins SP1 and NF-kappaB and inactivation of tumor suppressor Nkx3.1. J Cell Physiol. 2007;1:137–47. doi: 10.1002/jcp.21015. [DOI] [PubMed] [Google Scholar]
- 188.Wang H, Zhang Y, Cao L, Han H, Wang J, et al. HERG K+ channel, a regulator of tumor cell apoptosis and proliferation. Cancer Res. 2002;62:4843–4848. [PubMed] [Google Scholar]
- 189.Lastraioli E, Guasti L, Crociani O, Polvani S, Hofmann G, et al. herg1 gene and HERG1 protein are overexpressed in colorectal cancers and regulate cell invasion of tumor cells. Cancer Res. 2004;64:606–611. doi: 10.1158/0008-5472.can-03-2360. [DOI] [PubMed] [Google Scholar]
- 190.Wang Z. Roles of K+ channels in regulating tumour cell proliferation and apoptosis. Pflugers Arch. 2004;448:274–286. doi: 10.1007/s00424-004-1258-5. [DOI] [PubMed] [Google Scholar]
- 191.Arcangeli A. Expression and role of hERG channels in cancer cells. Novartis Found Symp. 2005;266:225–232. [PubMed] [Google Scholar]
- 192.Klezovitch O, Risk M, Coleman I, Lucas JM, Null M, et al. A causal role for ERG in neoplastic transformation of prostate epithelium. Proc Natl Acad Sci USA. 2008;105:2105–2110. doi: 10.1073/pnas.0711711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mu D, Chen L, Zhang X, See LH, Koch CM, et al. Genomic amplification and oncogenic properties of the KCNK9 potassium channel gene. Cancer Cell. 2003;3:297–302. doi: 10.1016/s1535-6108(03)00054-0. [DOI] [PubMed] [Google Scholar]
- 194.Pei L, Wiser O, Slavin A, Mu D, Powers S, et al. Oncogenic potential of TASK3 (Kcnk9) depends on K+ channel function. Proc Natl Acad Sci USA. 2003;100:7803–7807. doi: 10.1073/pnas.1232448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bennett ES, Smith BA, Harper JM. Voltage-gated Na+ channels confer invasive properties on human prostate cancer cells. Pflugers Arch. 2004;447:908–914. doi: 10.1007/s00424-003-1205-x. [DOI] [PubMed] [Google Scholar]
- 196.Pardo LA, del Camino D, Sánchez A, Alves F, Brüggemann A, et al. Oncogenic potential of EAG K(+) channels. EMBO J. 1999;18:5540–5547. doi: 10.1093/emboj/18.20.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lin H, Li Z, Chen C, Luo X, Xiao J, et al. Transcriptional and post-transcriptional mechanisms for oncogenic overexpression of ether à go-go K+ channel. PLoS One. 2011;6:e20362. doi: 10.1371/journal.pone.0020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Klimatcheva E, Wonderlin WF. An ATP-sensitive K(+) current that regulates progression through early G1 phase of the cell cycle in MCF-7 human breast cancer cells. J Membr Biol. 1999;171:35–46. doi: 10.1007/s002329900556. [DOI] [PubMed] [Google Scholar]
- 199.Strobl JS, Wonderlin WF, Flynn DC. Mitogenic signal transduction in human breast cancer cells. Gen Pharmacol. 1995;26:1643–1649. doi: 10.1016/0306-3623(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 200.Brackenbury WJ, Djamgoz MB. Activity-dependent regulation of voltage-gated Na+ channel expression in Mat-LyLu rat prostate cancer cell line. J Physiol. 2006;573:343–356. doi: 10.1113/jphysiol.2006.106906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Onganer PU, Seckl MJ, Djamgoz MB. Neuronal characteristics of small-cell lung cancer. Br J Cancer. 2005;93:1197–1201. doi: 10.1038/sj.bjc.6602857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Onganer PU, Djamgoz MB. Small-cell lung cancer (human): potentiation of endocytic membrane activity by voltage-gated Na(+) channel expression in vitro. J Membr Biol. 2005;204:67–75. doi: 10.1007/s00232-005-0747-6. [DOI] [PubMed] [Google Scholar]
- 203.Fraser SP, Diss JK, Chioni AM, Mycielska ME, Pan H, et al. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res. 2005;11:5381–5389. doi: 10.1158/1078-0432.CCR-05-0327. [DOI] [PubMed] [Google Scholar]
- 204.Liu LY, Hu CL, Ma LJ, Zhang ZH, Mei YA. ET-1 inhibits B-16 murine melanoma cell migration by decreasing K(+) currents. Cell Motil Cytoskeleton. 2004;58:127–136. doi: 10.1002/cm.20002. [DOI] [PubMed] [Google Scholar]
- 205.Fraser SP, Ozerlat-Gunduz I, Onkal R, Diss JK, Latchman DS, et al. Estrogen and non-genomic upregulation of voltage-gated Na(+) channel activity in MDA-MB-231 human breast cancer cells: role in adhesion. J Cell Physiol. 2010;224:527–539. doi: 10.1002/jcp.22154. [DOI] [PubMed] [Google Scholar]
- 206.Onkal R, Mattis JH, Fraser SP, Diss JK, Shao D, et al. Alternative splicing of Nav1.5: an electrophysiological comparison of ‘neonatal’ and ‘adult’ isoforms and critical involvement of a lysine residue. J Cell Physiol. 2008;216:716–726. doi: 10.1002/jcp.21451. [DOI] [PubMed] [Google Scholar]
- 207.Diss JK, Fraser SP, Walker MM, Patel A, Latchman DS, et al. Beta-subunits of voltage-gated sodium channels in human prostate cancer: quantitative in vitro and in vivo analyses of mRNA expression. Prostate Cancer Prostatic Dis. 2008;11:325–333. doi: 10.1038/sj.pcan.4501012. [DOI] [PubMed] [Google Scholar]
- 208.Ding Y, Brackenbury WJ, Onganer PU, Montano X, Porter LM, et al. Epidermal growth factor upregulates motility of Mat-LyLu rat prostate cancer cells partially via voltage-gated Na+ channel activity. J Cell Physiol. 2008;215:77–81. doi: 10.1002/jcp.21289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Brackenbury WJ, Djamgoz MB, Isom LL. An emerging role for voltage-gated Na+ channels in cellular migration: regulation of central nervous system development and potentiation of invasive cancers. Neuroscientist. 2008;14:571–583. doi: 10.1177/1073858408320293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Uysal-Onganer P, Djamgoz MB. Epidermal growth factor potentiates in vitro metastatic behaviour of human prostate cancer PC-3M cells: involvement of voltage-gated sodium channel. Mol Cancer. 2007;6:76. doi: 10.1186/1476-4598-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Brackenbury WJ, Djamgoz MB. Nerve growth factor enhances voltage-gated Na+ channel activity and Transwell migration in Mat-LyLu rat prostate cancer cell line. J Cell Physiol. 2007;210:602–608. doi: 10.1002/jcp.20846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Takamori M. An autoimmune channelopathy associated with cancer: Lambert-Eaton myasthenic syndrome. Intern Med. 1999;38:86–96. doi: 10.2169/internalmedicine.38.86. [DOI] [PubMed] [Google Scholar]
- 213.Weksberg R, Shuman C, Caluseriu O, Smith AC, Fei YL, et al. Discordant KCNQ1OT1 imprinting in sets of monozygotic twins discordant for Beckwith-Wiedemann syndrome. Hum Mol Genet. 2002;11:1317–1325. doi: 10.1093/hmg/11.11.1317. [DOI] [PubMed] [Google Scholar]
- 214.Weksberg R, Nishikawa J, Caluseriu O, Fei YL, Shuman C, et al. Tumor development in the Beckwith-Wiedemann syndrome is associated with a variety of constitutional molecular 11p15 alterations including imprinting defects of KCNQ1OT1. Hum Mol Genet. 2001;10:2989–3000. doi: 10.1093/hmg/10.26.2989. [DOI] [PubMed] [Google Scholar]
- 215.Lee MP, Hu RJ, Johnson LA, Feinberg AP. Human KVLQT1 gene shows tissue-specific imprinting and encompasses Beckwith-Wiedemann syndrome chromosomal rearrangements. Nat Genet. 1997;15:181–185. doi: 10.1038/ng0297-181. [DOI] [PubMed] [Google Scholar]
- 216.Feldman JD, Vician L, Crispino M, Tocco G, Marcheselli VL, et al. KID-1, a protein kinase induced by depolarization in brain. J Biol Chem. 1998;273:16535–16543. doi: 10.1074/jbc.273.26.16535. [DOI] [PubMed] [Google Scholar]
- 217.Liu W, Feldman JD, Machado HB, Vician LJ, Herschman HR. Expression of depolarization-induced immediate early gene proteins in PC12 cells. J Neurosci Res. 2003;72:670–678. doi: 10.1002/jnr.10626. [DOI] [PubMed] [Google Scholar]
- 218.Saris CJ, Domen J, Berns A. The pim-1 oncogene encodes two related protein-serine/threonine kinases by alternative initiation at AUG and CUG. EMBO J. 1991;10:655–664. doi: 10.1002/j.1460-2075.1991.tb07994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Arcangeli A, Becchetti A, Mannini A, Mugnai G, De Filippi P, et al. Integrin-mediated neurite outgrowth in neuroblastoma cells depends on the activation of potassium channels. J Cell Biol. 1993;122:1131–1143. doi: 10.1083/jcb.122.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hofmann G, Bernabei PA, Crociani O, Cherubini A, Guasti L, et al. HERG K+ channels activation during beta(1) integrin-mediated adhesion to fibronectin induces an up-regulation of alpha(v)beta(3) integrin in the preosteoclastic leukemia cell line FLG 29.1. J Biol Chem. 2001;276:4923–4931. doi: 10.1074/jbc.M005682200. [DOI] [PubMed] [Google Scholar]
- 221.McSheehy PM, Troy H, Kelland LR, Judson IR, Leach MO, et al. Increased tumour extracellular pH induced by Bafilomycin A1 inhibits tumour growth and mitosis in vivo and alters 5-fluorouracil pharmacokinetics. Eur J Cancer. 2003;39:532–540. doi: 10.1016/s0959-8049(02)00671-8. [DOI] [PubMed] [Google Scholar]
- 222.Ohta T, Arakawa H, Futagami F, Fushida S, Kitagawa H, et al. Bafilomycin A1 induces apoptosis in the human pancreatic cancer cell line Capan-1. J Pathol. 1998;185:324–330. doi: 10.1002/(SICI)1096-9896(199807)185:3<324::AID-PATH72>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 223.Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, et al. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 1998;23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- 224.Rhodes T, Barrand MA, Twentyman PR. Modification by brefeldin A, bafilomycin A1 and 7-chloro-4-nitrobenz-2-oxa-1,3-diazole (NBD) of cellular accumulation and intracellular distribution of anthracyclines in the non-P-glycoprotein-mediated multidrug-resistant cell line COR-L23/R. Br J Cancer. 1994;70:60–66. doi: 10.1038/bjc.1994.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Prasad PD, Mahesh VB, Leibach FH, Ganapathy V. Functional coupling between a bafilomycin A1-sensitive proton pump and a probenecid-sensitive folate transporter in human placental choriocarcinoma cells. Biochim Biophys Acta. 1994;1222:309–314. doi: 10.1016/0167-4889(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 226.Kinoshita K, Hidaka H, Ohkuma S. Induction of phagocytic activity of M1 cells by an inhibitor of vacuolar H+-ATPase, bafilomycin A1. FEBS Lett. 1994;337:221–225. doi: 10.1016/0014-5793(94)80195-9. [DOI] [PubMed] [Google Scholar]
- 227.Henomatsu N, Yoshimori T, Yamamoto A, Moriyama Y, Tashiro Y. Inhibition of intracellular transport of newly synthesized prolactin by bafilomycin A1 in a pituitary tumor cell line, GH3 cells. Eur J Cell Biol. 1993;62:127–139. [PubMed] [Google Scholar]
- 228.Heinle S, Stünkel K, Zähner H, Drautz H, Bessler WG. Immunosuppressive effects of the macrolide antibiotic bafilomycin towards lymphocytes and lymphoid cell lines. Arzneimittelforschung. 1988;38:1130–1133. [PubMed] [Google Scholar]
- 229.Jäger H, Dreker T, Buck A, Giehl K, Gress T, et al. Blockage of intermediate-conductance Ca2+ -activated K+ channels inhibit human pancreatic cancer cell growth in vitro. Mol Pharmacol. 2004;65:630–638. doi: 10.1124/mol.65.3.630. [DOI] [PubMed] [Google Scholar]
- 230.Schuller HM, Al-Wadei HA. Neurotransmitter receptors as central regulators of pancreatic cancer. Future Oncol. 2010;6:221–228. doi: 10.2217/fon.09.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Entschladen F, Lang K, Drell TL, Joseph J, Zaenker KS. Neurotransmitters are regulators for the migration of tumor cells and leukocytes. Cancer Immunol Immunother. 2002;51:467–482. doi: 10.1007/s00262-002-0300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ortega A. A new role for GABA: inhibition of tumor cell migration. Trends Pharmacol Sci. 2003;24:151–154. doi: 10.1016/S0165-6147(03)00052-X. [DOI] [PubMed] [Google Scholar]
- 233.Schuller HM, Al-Wadei HA, Majidi M. Gamma-aminobutyric acid, a potential tumor suppressor for small airway-derived lung adenocarcinoma. Carcinogenesis. 2008;29:1979–1985. doi: 10.1093/carcin/bgn041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Schuller HM, Al-Wadei HA, Majidi M. GABA B receptor is a novel drug target for pancreatic cancer. Cancer. 2008;112:767–778. doi: 10.1002/cncr.23231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Frede J, Fraser SP, Oskay-Özcelik G, Hong Y, Ioana Braicu E, et al. Ovarian cancer: Ion channel and aquaporin expression as novel targets of clinical potential. Eur J Cancer. 2013;49:2331–2344. doi: 10.1016/j.ejca.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 236.Speyer CL, Smith JS, Banda M, DeVries JA, Mekani T, et al. Metabotropic glutamate receptor-1: a potential therapeutic target for the treatment of breast cancer. Breast Cancer Res Treat. 2012;132:565–573. doi: 10.1007/s10549-011-1624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Yildirim S, Altun S, Gumushan H, Patel A, Djamgoz MB. Voltage-gated sodium channel activity promotes prostate cancer metastasis in vivo. Cancer Lett. 2012;323:58–61. doi: 10.1016/j.canlet.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 238.Spugnini EP, Citro G, Fais S. Proton pump inhibitors as anti vacuolar-ATPases drugs: a novel anticancer strategy. J Exp Clin Cancer Res. 2010;29:44. doi: 10.1186/1756-9966-29-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Schickling BM, Aykin-Burns N, Leslie KK, Spitz DR, Korovkina VP. An inhibitor of K+ channels modulates human endometrial tumor-initiating cells. Cancer Cell Int. 2011;11:25. doi: 10.1186/1475-2867-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kometiani P, Liu L, Askari A. Digitalis-induced signaling by Na+/K+-ATPase in human breast cancer cells. Mol Pharmacol. 2005;67:929–936. doi: 10.1124/mol.104.007302. [DOI] [PubMed] [Google Scholar]
- 242.Blackiston DJ, McLaughlin KA, Levin M. Bioelectric controls of cell proliferation: ion channels, membrane voltage and the cell cycle. Cell Cycle. 2009;8:3519–3528. doi: 10.4161/cc.8.21.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Rubin H. Mechanisms for enduring biological change. Am J Physiol. 1992;262:L111–113. doi: 10.1152/ajplung.1992.262.1.L111. [DOI] [PubMed] [Google Scholar]
- 244.Rubin H. On the nature of enduring modifications induced in cells and organisms. Am J Physiol. 1990;258:L19–24. doi: 10.1152/ajplung.1990.258.2.L19. [DOI] [PubMed] [Google Scholar]
- 245.Hahn WC, Weinberg RA. Rules for making human tumor cells. N Engl J Med. 2002;347:1593–1603. doi: 10.1056/NEJMra021902. [DOI] [PubMed] [Google Scholar]
- 246.Huang S, Ingber DE. A non-genetic basis for cancer progression and metastasis: self-organizing attractors in cell regulatory networks. Breast Dis. 2006;26:27–54. doi: 10.3233/bd-2007-26104. [DOI] [PubMed] [Google Scholar]
- 247.Cone CD., Jr Unified theory on the basic mechanism of normal mitotic control and oncogenesis. J Theor Biol. 1971;30:151–181. doi: 10.1016/0022-5193(71)90042-7. [DOI] [PubMed] [Google Scholar]
- 248.Cone CD., Jr The role of the surface electrical transmembrane potential in normal and malignant mitogenesis. Ann N Y Acad Sci. 1974;238:420–435. doi: 10.1111/j.1749-6632.1974.tb26808.x. [DOI] [PubMed] [Google Scholar]
- 249.Binggeli R, Weinstein RC. Membrane potentials and sodium channels: hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions. J Theor Biol. 1986;123:377–401. doi: 10.1016/s0022-5193(86)80209-0. [DOI] [PubMed] [Google Scholar]
- 250.Binggeli R, Cameron IL. Cellular potentials of normal and cancerous fibroblasts and hepatocytes. Cancer Res. 1980;40:1830–1835. [PubMed] [Google Scholar]
- 251.Burr HS. Biologic Organization and the Cancer Problem. Yale J Biol Med. 1940;12:277–282. [PMC free article] [PubMed] [Google Scholar]
- 252.Burr HS, Strong LC, Smith GM. Bio-Electric Correlates of Methylcolanthrene-Induced Tumors in Mice. Yale J Biol Med. 1938;10:539–544. [PMC free article] [PubMed] [Google Scholar]
- 253.House CD, Vaske CJ, Schwartz AM, Obias V, Frank B, et al. Voltage-gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Res. 2010;70:6957–6967. doi: 10.1158/0008-5472.CAN-10-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Stojadinovic A, Nissan A, Gallimidi Z, Lenington S, Logan W, et al. Electrical impedance scanning for the early detection of breast cancer in young women: preliminary results of a multicenter prospective clinical trial. J Clin Oncol. 2005;23:2703–2715. doi: 10.1200/JCO.2005.06.155. [DOI] [PubMed] [Google Scholar]
- 255.Bauer J, Capra S, Davies PS. Estimation of total body water from foot-to-foot bioelectrical impedance analysis in patients with cancer cachexia - agreement between three prediction methods and deuterium oxide dilution. J Hum Nutr Diet. 2005;18:295–300. doi: 10.1111/j.1365-277X.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 256.Aberg P, Geladi P, Nicander I, Hansson J, Holmgren U, et al. Non-invasive and microinvasive electrical impedance spectra of skin cancer - a comparison between two techniques. Skin Res Technol. 2005;11:281–286. doi: 10.1111/j.0909-725X.2005.00125.x. [DOI] [PubMed] [Google Scholar]
- 257.Gupta D, Lis CG, Dahlk SL, Vashi PG, Grutsch JF, et al. Bioelectrical impedance phase angle as a prognostic indicator in advanced pancreatic cancer. Br J Nutr. 2004;92:957–962. doi: 10.1079/bjn20041292. [DOI] [PubMed] [Google Scholar]
- 258.Gupta D, Lammersfeld CA, Burrows JL, Dahlk SL, Vashi PG, et al. Bioelectrical impedance phase angle in clinical practice: implications for prognosis in advanced colorectal cancer. Am J Clin Nutr. 2004;80:1634–1638. doi: 10.1093/ajcn/80.6.1634. [DOI] [PubMed] [Google Scholar]
- 259.Gatta L Study group on the application of extracellular bioimpedance tomography (Gastro-Mida(x)) in the diagnosis of colorectal diseases. The clinical role of extracellular bioimpedance tomography (Gastro-Mida(x)) in the diagnosis of colorectal diseases. Minerva Med. 2004;95:541–556. [PubMed] [Google Scholar]
- 260.Aberg P, Nicander I, Hansson J, Geladi P, Holmgren U, et al. Skin cancer identification using multifrequency electrical impedance--a potential screening tool. IEEE Trans Biomed Eng. 2004;51:2097–2102. doi: 10.1109/TBME.2004.836523. [DOI] [PubMed] [Google Scholar]
- 261.Toso S, Piccoli A, Gusella M, Menon D, Bononi A, et al. Altered tissue electric properties in lung cancer patients as detected by bioelectric impedance vector analysis. Nutrition. 2000;16:120–124. doi: 10.1016/s0899-9007(99)00230-0. [DOI] [PubMed] [Google Scholar]
- 262.Konishi Y, Morimoto T, Kinouchi Y, Iritani T, Monden Y. Electrical properties of extracted rat liver tissue. Res Exp Med (Berl) 1995;195:183–192. doi: 10.1007/BF02576787. [DOI] [PubMed] [Google Scholar]
- 263.Kimura S, Morimoto T, Uyama T, Monden Y, Kinouchi Y, et al. Application of electrical impedance analysis for diagnosis of a pulmonary mass. Chest. 1994;105:1679–1682. doi: 10.1378/chest.105.6.1679. [DOI] [PubMed] [Google Scholar]
- 264.Morimoto T, Kimura S, Konishi Y, Komaki K, Uyama T, et al. A study of the electrical bio-impedance of tumors. J Invest Surg. 1993;6:25–32. doi: 10.3109/08941939309141189. [DOI] [PubMed] [Google Scholar]
- 265.Morimoto T, Kinouchi Y, Iritani T, Kimura S, Konishi Y, et al. Measurement of the electrical bio-impedance of breast tumors. Eur Surg Res. 1990;22:86–92. doi: 10.1159/000129087. [DOI] [PubMed] [Google Scholar]
- 266.Davies RJ, Joseph R, Asbun H, Sedwitz M. Detection of the cancer-prone colon, using transepithelial impedance analysis. Arch Surg. 1989;124:480–484. doi: 10.1001/archsurg.1989.01410040090021. [DOI] [PubMed] [Google Scholar]
- 267.Cuzick J, Holland R, Barth V, Davies R, Faupel M, et al. Electropotential measurements as a new diagnostic modality for breast cancer. Lancet. 1998;352:359–363. doi: 10.1016/s0140-6736(97)10002-2. [DOI] [PubMed] [Google Scholar]
- 268.Faupel M, Vanel D, Barth V, Davies R, Fentiman IS, et al. Electropotential evaluation as a new technique for diagnosing breast lesions. Eur J Radiol. 1997;24:33–38. doi: 10.1016/s0720-048x(96)01113-8. [DOI] [PubMed] [Google Scholar]
- 269.Moyer MP, Moyer RC, Waite MR. A survey of intracellular Na+ and K+ of various normal, transformed, and tumor cells. J Cell Physiol. 1982;113:129–133. doi: 10.1002/jcp.1041130121. [DOI] [PubMed] [Google Scholar]
- 270.Ouwerkerk R, Jacobs MA, Macura KJ, Wolff AC, Stearns V, et al. Elevated tissue sodium concentration in malignant breast lesions detected with non-invasive 23Na MRI. Breast Cancer Res Treat. 2007;106:151–160. doi: 10.1007/s10549-006-9485-4. [DOI] [PubMed] [Google Scholar]
- 271.Beech JA. Bioelectric potential gradients may initiate cell cycling: ELF and zeta potential gradients may mimic this effect. Bioelectromagnetics. 1997;18:341–348. doi: 10.1002/(sici)1521-186x(1997)18:5<341::aid-bem1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 272.Dahmane N, Lee J, Robins P, Heller P, Ruiz i Altaba A. Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature. 1997;389:876–881. doi: 10.1038/39918. [DOI] [PubMed] [Google Scholar]
- 273.Wallingford JB, Seufert DW, Virta VC, Vize PD. p53 activity is essential for normal development in Xenopus. Curr Biol. 1997;7:747–757. doi: 10.1016/s0960-9822(06)00333-2. [DOI] [PubMed] [Google Scholar]
- 274.Yang S, Lockwood A, Hollett P, Ford R, Kao K. Overexpression of a novel Xenopus rel mRNA gene induces tumors in early embryos. J Biol Chem. 1998;273:13746–13752. doi: 10.1074/jbc.273.22.13746. [DOI] [PubMed] [Google Scholar]
- 275.Le X, Langenau DM, Keefe MD, Kutok JL, Neuberg DS, et al. Heat shock-inducible Cre/Lox approaches to induce diverse types of tumors and hyperplasia in transgenic zebrafish. Proc Natl Acad Sci USA. 2007;104:9410–9415. doi: 10.1073/pnas.0611302104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Stratton MR, Fisher C, Gusterson BA, Cooper CS. Detection of point mutations in N-ras and K-ras genes of human embryonal rhabdomyosarcomas using oligonucleotide probes and the polymerase chain reaction. Cancer Res. 1989;49:6324–6327. [PubMed] [Google Scholar]
- 277.Gilmore TD, Kalaitzidis D, Liang MC, Starczynowski DT. The c-Rel transcription factor and B-cell proliferation: a deal with the devil. Oncogene. 2004;23:2275–2286. doi: 10.1038/sj.onc.1207410. [DOI] [PubMed] [Google Scholar]
- 278.McNulty SE, del Rosario R, Cen D, Meyskens FL, Jr, Yang S. Comparative expression of NFkappaB proteins in melanocytes of normal skin vs. benign intradermal naevus and human metastatic melanoma biopsies. Pigment Cell Res. 2004;17:173–180. doi: 10.1111/j.1600-0749.2004.00128.x. [DOI] [PubMed] [Google Scholar]
- 279.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17:165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wolff C, Fuks B, Chatelain P. Comparative study of membrane potential-sensitive fluorescent probes and their use in ion channel screening assays. J Biomol Screen. 2003;8:533–543. doi: 10.1177/1087057103257806. [DOI] [PubMed] [Google Scholar]
- 281.Lobikin M, Chernet B, Lobo D, Levin M. Resting potential, oncogene-induced tumorigenesis, and metastasis: the bioelectric basis of cancer in vivo. Phys Biol. 2012;9:065002. doi: 10.1088/1478-3975/9/6/065002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 283.Smith DS, Humphrey PA, Catalona WJ. The early detection of prostate carcinoma with prostate specific antigen: the Washington University experience. Cancer. 1997;80:1852–1856. [PubMed] [Google Scholar]
- 284.Shin EH, Li Y, Kumar U, Sureka HV, Zhang X, et al. Membrane potential mediates the cellular binding of nanoparticles. Nanoscale. 2013;5:5879–5886. doi: 10.1039/c3nr01667f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Boddapati SV, Tongcharoensirikul P, Hanson RN, D’Souza GG, Torchilin VP, et al. Mitochondriotropic liposomes. J Liposome Res. 2005;15:49–58. doi: 10.1081/lpr-64958. [DOI] [PubMed] [Google Scholar]
- 286.BURR HS. Electrometrics of atypical growth. Yale J Biol Med. 1952;25:67–75. [PMC free article] [PubMed] [Google Scholar]
- 287.Burr HS. Changes in the Field Properties of Mice with Transplanted Tumors. Yale J Biol Med. 1941;13:783–788. [PMC free article] [PubMed] [Google Scholar]
- 288.Nordenström BE. The paradigm of biologically closed electric circuits (BCEC) and the formation of an International Association (IABC) for BCEC systems. Eur J Surg Suppl. 1994:7–23. [PubMed] [Google Scholar]
- 289.Nordenstrom B, Naslund I, Nordenstrom J, Wersall P. Activation of Bcec-Channels for Electrochemical Therapy (Ect) of Cancer - Foreword. Proceedings of the Iabc International-Association-for- Biologically-Closed-Electric-Circuits (Bcec) in Medicine and Biology; Stockholm. September 12–15, 1993; 1994. [PubMed] [Google Scholar]
- 290.Nordenström BE. Impact of biologically closed electric circuits (BCEC) on structure and function. Integr Physiol Behav Sci. 1992;27:285–303. doi: 10.1007/BF02691165. [DOI] [PubMed] [Google Scholar]
- 291.Pandiella A, Magni M, Lovisolo D, Meldolesi J. The effect of epidermal growth factor on membrane potential. Rapid hyperpolarization followed by persistent fluctuations. J Biol Chem. 1989;264:12914–12921. [PubMed] [Google Scholar]
- 292.Maffini MV, Soto AM, Calabro JM, Ucci AA, Sonnenschein C. The stroma as a crucial target in rat mammary gland carcinogenesis. J Cell Sci. 2004;117:1495–1502. doi: 10.1242/jcs.01000. [DOI] [PubMed] [Google Scholar]
- 293.Sun YJ, Long DX, Li W, Hou WY, Wu YJ, et al. Effects of avermectins on neurite outgrowth in differentiating mouse neuroblastoma N2a cells. Toxicol Lett. 2010;192:206–211. doi: 10.1016/j.toxlet.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 294.Sharmeen S, Skrtic M, Sukhai MA, Hurren R, Gronda M, et al. The antiparasitic agent ivermectin induces chloride-dependent membrane hyperpolarization and cell death in leukemia cells. Blood. 2010;116:3593–3603. doi: 10.1182/blood-2010-01-262675. [DOI] [PubMed] [Google Scholar]
- 295.Ottesen EA, Campbell WC. Ivermectin in human medicine. J Antimicrob Chemother. 1994;34:195–203. doi: 10.1093/jac/34.2.195. [DOI] [PubMed] [Google Scholar]
- 296.Duke BO, Marty AM, Peett DL, Gardo J, Pion SD, et al. Neoplastic change in Onchocerca volvulus and its relation to ivermectin treatment. Parasitology. 2002;125:431–444. doi: 10.1017/s0031182002002305. [DOI] [PubMed] [Google Scholar]
- 297.Vafa B, Lewis TM, Cunningham AM, Jacques P, Lynch JW, et al. Identification of a new ligand binding domain in the alpha1 subunit of the inhibitory glycine receptor. J Neurochem. 1999;73:2158–2166. [PubMed] [Google Scholar]
- 298.Aw S, Adams DS, Qiu D, Levin M. H,K-ATPase protein localization and Kir4.1 function reveal concordance of three axes during early determination of left-right asymmetry. Mech Dev. 2008;125:353–372. doi: 10.1016/j.mod.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.O’Connor PJ, MacNaught F, Butler WH, Cooper DP, Margison GP, et al. Increased pathology incidence in the forestomach of rats maintained on a diet containing ivermectin and given a single dose of N-methyl-N1-nitro-N-nitrosoguanidine. J Environ Pathol Toxicol Oncol. 2001;20:223–227. [PubMed] [Google Scholar]
- 300.Miyauchi S, Gopal E, Fei YJ, Ganapathy V. Functional identification of SLC5A8, a tumor suppressor down-regulated in colon cancer, as a Na(+)-coupled transporter for short-chain fatty acids. J Biol Chem. 2004;279:13293–13296. doi: 10.1074/jbc.C400059200. [DOI] [PubMed] [Google Scholar]
- 301.Ueno M, Toyota M, Akino K, Suzuki H, Kusano M, et al. Aberrant methylation and histone deacetylation associated with silencing of SLC5A8 in gastric cancer. Tumour Biol. 2004;25:134–140. doi: 10.1159/000079145. [DOI] [PubMed] [Google Scholar]
- 302.Hu S, Liu D, Tufano RP, Carson KA, Rosenbaum E, et al. Association of aberrant methylation of tumor suppressor genes with tumor aggressiveness and BRAF mutation in papillary thyroid cancer. Int J Cancer. 2006;119:2322–2329. doi: 10.1002/ijc.22110. [DOI] [PubMed] [Google Scholar]
- 303.Paroder V, Spencer SR, Paroder M, Arango D, Schwartz S, Jr, et al. Na(+)/monocarboxylate transport (SMCT) protein expression correlates with survival in colon cancer: molecular characterization of SMCT. Proc Natl Acad Sci USA. 2006;103:7270–7275. doi: 10.1073/pnas.0602365103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Ganapathy V, Thangaraju M, Gopal E, Martin PM, Itagaki S, et al. Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J. 2008;10:193–199. doi: 10.1208/s12248-008-9022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Park JY, Helm JF, Zheng W, Ly QP, Hodul PJ, et al. Silencing of the candidate tumor suppressor gene solute carrier family 5 member 8 (SLC5A8) in human pancreatic cancer. Pancreas. 2008;36:e32–39. doi: 10.1097/MPA.0b013e3181630ffe. [DOI] [PubMed] [Google Scholar]
- 306.Li H, Myeroff L, Smiraglia D, Romero MF, Pretlow TP, et al. SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc Natl Acad Sci USA. 2003;100:8412–8417. doi: 10.1073/pnas.1430846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Siavoshian S, Segain JP, Kornprobst M, Bonnet C, Cherbut C, et al. Butyrate and trichostatin A effects on the proliferation/differentiation of human intestinal epithelial cells: induction of cyclin D3 and p21 expression. Gut. 2000;46:507–514. doi: 10.1136/gut.46.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Archer SY, Meng S, Shei A, Hodin RA. p21(WAF1) is required for butyrate-mediated growth inhibition of human colon cancer cells. Proc Natl Acad Sci USA. 1998;95:6791–6796. doi: 10.1073/pnas.95.12.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Morozova N, Shubin M. Pattern Formation in Morphogenesis. Springer; Berlin Heidelberg: 2013. [Google Scholar]
- 310.Beloussov LV, Opitz JM, Gilbert SF. Life of Alexander G. Gurwitsch and his relevant contribution to the theory of morphogenetic fields. Int J Dev Biol. 1997;41:771–777. [PubMed] [Google Scholar]
- 311.Balls M, Ruben Ln. Variation In The Response Of Xenopus Laevis To Normal Tissue Homografts. Dev Biol. 1964;10:92–104. doi: 10.1016/0012-1606(64)90006-5. [DOI] [PubMed] [Google Scholar]
- 312.Stevens LC. The development of transplantable teratocarcinomas from intratesticular grafts of pre- and postimplantation mouse embryos. Dev Biol. 1970;21:364–382. doi: 10.1016/0012-1606(70)90130-2. [DOI] [PubMed] [Google Scholar]
- 313.Parodi S, Brambilla G. Relationships between mutation and transformation frequencies in mammalian cells treated “in vitro” with chemical carcinogens. Mutat Res. 1977;47:53–74. doi: 10.1016/0165-1110(77)90017-3. [DOI] [PubMed] [Google Scholar]
- 314.Clark WH., Jr The nature of cancer: morphogenesis and progressive (self)-disorganization in neoplastic development and progression. Acta Oncol. 1995;34:3–21. doi: 10.3109/02841869509093632. [DOI] [PubMed] [Google Scholar]
- 315.Kraemer PM, Travis GL, Ray FA, Cram LS. Spontaneous neoplastic evolution of Chinese hamster cells in culture: multistep progression of phenotype. Cancer Res. 1983;43:4822–4827. [PubMed] [Google Scholar]
- 316.Cram LS, Bartholdi MF, Ray FA, Travis GL, Kraemer PM. Spontaneous neoplastic evolution of Chinese hamster cells in culture: multistep progression of karyotype. Cancer Res. 1983;43:4828–4837. [PubMed] [Google Scholar]
- 317.Dolberg DS, Bissell MJ. Inability of Rous sarcoma virus to cause sarcomas in the avian embryo. Nature. 1984;309:552–556. doi: 10.1038/309552a0. [DOI] [PubMed] [Google Scholar]
- 318.Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70:537–546. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Barcellos-Hoff MH. It takes a tissue to make a tumor: epigenetics, cancer and the microenvironment. J Mammary Gland Biol Neoplasia. 2001;6:213–221. doi: 10.1023/a:1011317009329. [DOI] [PubMed] [Google Scholar]
- 320.Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, et al. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci USA. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Oppenheimer BS, Oppenheimer ET, Stout AP. Sarcomas induced in rodents by imbedding various plastic films. Proc Soc Exp Biol Med. 1952;79:366–369. doi: 10.3181/00379727-79-19380. [DOI] [PubMed] [Google Scholar]
- 322.Bischoff F, Bryson G. Carcinogenesis through Solid State Surfaces. Prog Exp Tumor Res. 1964;5:85–133. doi: 10.1159/000385997. [DOI] [PubMed] [Google Scholar]
- 323.Oppenheimer BS, Oppenheimer ET, Stout AP. Carcinogenic Effect Of Imbedding Various Plastic Films In Rats And Mice. Surg Forum. 1953;4:672–676. [PubMed] [Google Scholar]
- 324.OPPENHEIMER BS, OPPENHEIMER ET, STOUT AP, DANISHEFSKY I. Malignant tumors resulting from embedding plastics in rodents. Science. 1953;118:305–306. doi: 10.1126/science.118.3063.305. [DOI] [PubMed] [Google Scholar]
- 325.Wade MH, Trosko JE, Schindler M. A fluorescence photobleaching assay of gap junction-mediated communication between human cells. Science. 1986;232:525–528. doi: 10.1126/science.3961495. [DOI] [PubMed] [Google Scholar]
- 326.Ruch RJ, Trosko JE. Gap-junction communication in chemical carcinogenesis. Drug Metab Rev. 2001;33:117–124. doi: 10.1081/dmr-100000137. [DOI] [PubMed] [Google Scholar]
- 327.Duflot-Dancer A, Mesnil M, Yamasaki H. Dominant-negative abrogation of connexin-mediated cell growth control by mutant connexin genes. Oncogene. 1997;15:2151–2158. doi: 10.1038/sj.onc.1201393. [DOI] [PubMed] [Google Scholar]
- 328.Yamasaki H, Krutovskikh V, Mesnil M, Tanaka T, Zaidan-Dagli ML, et al. Role of connexin (gap junction) genes in cell growth control and carcinogenesis. C R Acad Sci III. 1999;322:151–159. doi: 10.1016/s0764-4469(99)80038-9. [DOI] [PubMed] [Google Scholar]
- 329.Omori Y, Zaidan Dagli ML, Yamakage K, Yamasaki H. Involvement of gap junctions in tumor suppression: analysis of genetically-manipulated mice. Mutat Res. 2001;477:191–196. doi: 10.1016/s0027-5107(01)00120-8. [DOI] [PubMed] [Google Scholar]
- 330.Yamasaki H, Mesnil M, Omori Y, Mironov N, Krutovskikh V. Intercellular communication and carcinogenesis. Mutat Res. 1995;333:181–188. doi: 10.1016/0027-5107(95)00144-1. [DOI] [PubMed] [Google Scholar]
- 331.Temme A, Buchmann A, Gabriel HD, Nelles E, Schwarz M, et al. High incidence of spontaneous and chemically induced liver tumors in mice deficient for connexin32. Curr Biol. 1997;7:713–716. doi: 10.1016/s0960-9822(06)00302-2. [DOI] [PubMed] [Google Scholar]
- 332.Loewenstein WR. Junctional cell-to-cell communication and growth control. Ann N Y Acad Sci. 1980;339:39–45. doi: 10.1111/j.1749-6632.1980.tb15966.x. [DOI] [PubMed] [Google Scholar]
- 333.Loewenstein WR. Junctional intercellular communication and the control of growth. Biochim Biophys Acta. 1979;560:1–65. doi: 10.1016/0304-419x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- 334.Loewenstein WR. Transfer of information through cell junctions and growth control. Proc Can Cancer Conf. 1969;8:162–170. [PubMed] [Google Scholar]
- 335.Loewenstein WR, Kanno Y. Intercellular communication and the control of tissue growth: lack of communication between cancer cells. Nature. 1966;209:1248–1249. doi: 10.1038/2091248a0. [DOI] [PubMed] [Google Scholar]
- 336.Rose B, Mehta PP, Loewenstein WR. Gap-junction protein gene suppresses tumorigenicity. Carcinogenesis. 1993;14:1073–1075. doi: 10.1093/carcin/14.5.1073. [DOI] [PubMed] [Google Scholar]
- 337.Mesnil M, Crespin S, Avanzo JL, Zaidan-Dagli ML. Defective gap junctional intercellular communication in the carcinogenic process. Biochim Biophys Acta. 2005;1719:125–145. doi: 10.1016/j.bbamem.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 338.Stoletov K, Strnadel J, Zardouzian E, Momiyama M, Park FD, et al. Role of connexins in metastatic breast cancer and melanoma brain colonization. J Cell Sci. 2013;126:904–913. doi: 10.1242/jcs.112748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Moreno E. Is cell competition relevant to cancer? Nat Rev Cancer. 2008;8:141–147. doi: 10.1038/nrc2252. [DOI] [PubMed] [Google Scholar]
- 340.Dobrowolski R, Hertig G, Lechner H, Wörsdörfer P, Wulf V, et al. Loss of connexin43-mediated gap junctional coupling in the mesenchyme of limb buds leads to altered expression of morphogens in mice. Hum Mol Genet. 2009;18:2899–2911. doi: 10.1093/hmg/ddp227. [DOI] [PubMed] [Google Scholar]
- 341.Jin EJ, Lee SY, Jung JC, Bang OS, Kang SS. TGF-beta3 inhibits chondrogenesis of cultured chick leg bud mesenchymal cells via downregulation of connexin 43 and integrin beta4. J Cell Physiol. 2008;214:345–353. doi: 10.1002/jcp.21202. [DOI] [PubMed] [Google Scholar]
- 342.Pizard A, Burgon PG, Paul DL, Bruneau BG, Seidman CE, et al. Connexin 40, a target of transcription factor Tbx5, patterns wrist, digits, and sternum. Mol Cell Biol. 2005;25:5073–5083. doi: 10.1128/MCB.25.12.5073-5083.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Levin M, Mercola M. Expression of connexin 30 in Xenopus embryos and its involvement in hatching gland function. Dev Dyn. 2000;219:96–101. doi: 10.1002/1097-0177(200009)219:1<96::AID-DVDY1034>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 344.Lecanda F, Warlow PM, Sheikh S, Furlan F, Steinberg TH, et al. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol. 2000;151:931–944. doi: 10.1083/jcb.151.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Warner A. Interactions between growth factors and gap junctional communication in developing systems. Novartis Found Symp. 1999;219:60–72. doi: 10.1002/9780470515587.ch5. [DOI] [PubMed] [Google Scholar]
- 346.Levin M. Gap junctional communication in morphogenesis. Prog Biophys Mol Biol. 2007;94:186–206. doi: 10.1016/j.pbiomolbio.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Schiffmann Y. The Turing-Child energy field as a driver of early mammalian development. Prog Biophys Mol Biol. 2008;98:107–117. doi: 10.1016/j.pbiomolbio.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 348.Gray RS, Roszko I, Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev Cell. 2011;21:120–133. doi: 10.1016/j.devcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Lee M, Vasioukhin V. Cell polarity and cancer--cell and tissue polarity as a non-canonical tumor suppressor. J Cell Sci. 2008;121:1141–1150. doi: 10.1242/jcs.016634. [DOI] [PubMed] [Google Scholar]
- 350.Klezovitch O, Fernandez TE, Tapscott SJ, Vasioukhin V. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 2004;18:559–571. doi: 10.1101/gad.1178004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Wodarz A, Näthke I. Cell polarity in development and cancer. Nat Cell Biol. 2007;9:1016–1024. doi: 10.1038/ncb433. [DOI] [PubMed] [Google Scholar]
- 352.Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat Cell Biol. 2008;10:1257–1268. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.SCHARRER B. Insect tumors induced by nerve severance: incidence and mortality. Cancer Res. 1953;13:73–76. [PubMed] [Google Scholar]
- 354.SCHARRER B, LOCHHEAD MS. Tumors in the invertebrates: a review. Cancer Res. 1950;10:403–419. [PubMed] [Google Scholar]
- 355.Pawlowski A, Weddell G. Induction of tumors in denervated skin. Nature. 1967;213:1234–1236. [Google Scholar]
- 356.Outzen HC, Custer RP, Prehn RT. Influence of regenerative capacity and innervation on oncogenesis in the adult frog (Rana pipiens) J Natl Cancer Inst. 1976;57:79–84. doi: 10.1093/jnci/57.1.79. [DOI] [PubMed] [Google Scholar]
- 357.Burr HS. The Meaning of Bio-Electric Potentials. Yale J Biol Med. 1944;16:353–360. [PMC free article] [PubMed] [Google Scholar]
- 358.Becker RO. The basic biological data transmission and control system influenced by electrical forces. Ann N Y Acad Sci. 1974;238:236–241. doi: 10.1111/j.1749-6632.1974.tb26793.x. [DOI] [PubMed] [Google Scholar]
- 359.Burr HS. Field Properties of the Developing Frog’s Egg. Proc Natl Acad Sci USA. 1941;27:276–281. doi: 10.1073/pnas.27.6.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Burr HS, Northrop FS. Evidence for the Existence of an Electro-Dynamic Field in Living Organisms. Proc Natl Acad Sci USA. 1939;25:284–288. doi: 10.1073/pnas.25.6.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Camphausen K, Purow B, Sproull M, Scott T, Ozawa T, et al. Influence of in vivo growth on human glioma cell line gene expression: convergent profiles under orthotopic conditions. Proc Natl Acad Sci USA. 2005;102:8287–8292. doi: 10.1073/pnas.0502887102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Seilern-Aspang F, Kratochwill L. Regeneration in animals and related problems. North-Holland Publishing Company; 1965. [Google Scholar]
- 363.Auerbach R, Morrissey LW, Kubai L, Sidky YA. Regional differences in tumor growth: studies of the vascular system. Int J Cancer. 1978;22:40–46. doi: 10.1002/ijc.2910220110. [DOI] [PubMed] [Google Scholar]
- 364.Auerbach R, Morrissey LW, Sidky YA. Gradients in tumour growth. Nature. 1978;274:697–699. doi: 10.1038/274697a0. [DOI] [PubMed] [Google Scholar]
- 365.Auerbach R, Morrissey LW, Sidky YA. Regional differences in the incidence and growth of mouse tumors following intradermal or subcutaneous inoculation. Cancer Res. 1978;38:1739–1744. [PubMed] [Google Scholar]
- 366.Auerbach R, Auerbach W. Regional differences in the growth of normal and neoplastic cells. Science. 1982;215:127–134. doi: 10.1126/science.7053564. [DOI] [PubMed] [Google Scholar]
- 367.Kubai L, Auerbach R. Regional differences in the growth of skin transplants. Transplantation. 1980;30:128–131. doi: 10.1097/00007890-198008000-00010. [DOI] [PubMed] [Google Scholar]
- 368.Morrissey LW, Sidky YA, Auerbach R. Regional differences in the growth of tumor cells injected intraperitoneally into syngeneic adult mice. Cancer Res. 1980;40:2197–2201. [PubMed] [Google Scholar]
- 369.Biskind MS, Biskind GR. Tumor of rat testis produced by heterotransplantation of infantile testis to spleen of adult castrate. Proc Soc Exp Biol Med. 1945;59:4–8. [Google Scholar]
- 370.Biskind MS, Biskind GS. Development of tumors in the rat ovary after transplantation into the spleen. Proc Soc Exp Biol Med. 1944;55:176–179. [Google Scholar]
- 371.Mintz B, Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci USA. 1975;72:3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Illmensee K, Mintz B. Totipotency and normal differentiation of single teratocarcinoma cells cloned by injection into blastocysts. Proc Natl Acad Sci USA. 1976;73:549–553. doi: 10.1073/pnas.73.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Astigiano S, Damonte P, Fossati S, Boni L, Barbieri O. Fate of embryonal carcinoma cells injected into postimplantation mouse embryos. Differentiation. 2005;73:484–490. doi: 10.1111/j.1432-0436.2005.00043.x. [DOI] [PubMed] [Google Scholar]
- 374.Webb CG, Gootwine E, Sachs L. Developmental potential of myeloid leukemia cells injected into midgestation embryos. Dev Biol. 1984;101:221–224. doi: 10.1016/0012-1606(84)90132-5. [DOI] [PubMed] [Google Scholar]
- 375.Li L, Connelly MC, Wetmore C, Curran T, Morgan JI. Mouse embryos cloned from brain tumors. Cancer Res. 2003;63:2733–2736. [PubMed] [Google Scholar]
- 376.Pierce GB, Wells RS. The blastocyst in control of embryonal carcinoma. Prog Clin Biol Res. 1982;85(Pt B):593–600. [PubMed] [Google Scholar]
- 377.Pierce GB, Arechaga J, Wells RS. Embryonic control of cancer. Prog Clin Biol Res. 1986;226:67–77. [PubMed] [Google Scholar]
- 378.Podesta AH, Mullins J, Pierce GB, Wells RS. The neurula stage mouse embryo in control of neuroblastoma. Proc Natl Acad Sci USA. 1984;81:7608–7611. doi: 10.1073/pnas.81.23.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Pierce GB, Aguilar D, Hood G, Wells RS. Trophectoderm in control of murine embryonal carcinoma. Cancer Res. 1984;44:3987–3996. [PubMed] [Google Scholar]
- 380.Pierce GB. The cancer cell and its control by the embryo. Rous-Whipple Award lecture. Am J Pathol. 1983;113:117–124. [PMC free article] [PubMed] [Google Scholar]
- 381.Pierce GB, Pantazis CG, Caldwell JE, Wells RS. Specificity of the control of tumor formation by the blastocyst. Cancer Res. 1982;42:1082–1087. [PubMed] [Google Scholar]
- 382.Haldi M, Ton C, Seng WL, McGrath P. Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis. 2006;9:139–151. doi: 10.1007/s10456-006-9040-2. [DOI] [PubMed] [Google Scholar]
- 383.Lee LM, Seftor EA, Bonde G, Cornell RA, Hendrix MJ. The fate of human malignant melanoma cells transplanted into zebrafish embryos: assessment of migration and cell division in the absence of tumor formation. Dev Dyn. 2005;233:1560–1570. doi: 10.1002/dvdy.20471. [DOI] [PubMed] [Google Scholar]
- 384.Kasemeier-Kulesa JC, Teddy JM, Postovit LM, Seftor EA, Seftor RE, et al. Reprogramming multipotent tumor cells with the embryonic neural crest microenvironment. Dev Dyn. 2008;237:2657–2666. doi: 10.1002/dvdy.21613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Hendrix MJ, Seftor EA, Seftor RE, Kasemeier-Kulesa J, Kulesa PM, et al. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat Rev Cancer. 2007;7:246–255. doi: 10.1038/nrc2108. [DOI] [PubMed] [Google Scholar]
- 386.Kulesa PM, Kasemeier-Kulesa JC, Teddy JM, Margaryan NV, Seftor EA, et al. Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment. Proc Natl Acad Sci USA. 2006;103:3752–3757. doi: 10.1073/pnas.0506977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Hochedlinger K, Blelloch R, Brennan C, Yamada Y, Kim M, et al. Reprogramming of a melanoma genome by nuclear transplantation. Genes Dev. 2004;18:1875–1885. doi: 10.1101/gad.1213504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Kenny PA, Bissell MJ. Tumor reversion: correction of malignant behavior by microenvironmental cues. Int J Cancer. 2003;107:688–695. doi: 10.1002/ijc.11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Zhang W, Remenyik E, Zelterman D, Brash DE, Wikonkal NM. Escaping the stem cell compartment: sustained UVB exposure allows p53-mutant keratinocytes to colonize adjacent epidermal proliferating units without incurring additional mutations. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:13948–13953. doi: 10.1073/pnas.241353198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Chao DL, Eck JT, Brash DE, Maley CC, Luebeck EG. Preneoplastic lesion growth driven by the death of adjacent normal stem cells. Proc Natl Acad Sci USA. 2008;105:15034–15039. doi: 10.1073/pnas.0802211105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Pierce GB, Speers WC. Tumors as caricatures of the process of tissue renewal: prospects for therapy by directing differentiation. Cancer Res. 1988;48:1996–2004. [PubMed] [Google Scholar]
- 392.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 393.Tsonis PA. Effects of carcinogens on regenerating and non-regenerating limbs in amphibia (review) Anticancer Res. 1983;3:195–202. [PubMed] [Google Scholar]
- 394.Wolsky A. Regeneration and cancer. Growth. 1978;42:425–426. [PubMed] [Google Scholar]
- 395.Donaldson DJ, Mason JM. Cancer-related aspects of regeneration research: a review. Growth. 1975;39:475–496. [PubMed] [Google Scholar]
- 396.Ruben LN, Balls M, Stevens J. Cancer and super-regeneration in Triturus viridescens limbs. Experientia. 1966;22:260–261. doi: 10.1007/BF01900949. [DOI] [PubMed] [Google Scholar]
- 397.ROSE SM, WALLINGFORD HM. Transformation of renal tumors of frogs to normal tissues in regenerating limbs of salamanders. Science. 1948;107:457. [PubMed] [Google Scholar]
- 398.Brockes JP. Regeneration and cancer. Biochim Biophys Acta. 1998;1377:M1–11. doi: 10.1016/s0304-419x(97)00029-2. [DOI] [PubMed] [Google Scholar]
- 399.Tsonis PA, Eguchi G. Carcinogens on regeneration. Effects of N-methyl-N′-nitro-N-nitrosoguanidine and 4-nitroquinoline-1-oxide on limb regeneration in adult newts. Differentiation. 1981;20:52–60. doi: 10.1111/j.1432-0436.1981.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 400.Pizzarello DJ, Wolsky A. Carcinogenesis and regeneration in newts. Experientia. 1966;22:387–388. doi: 10.1007/BF01901153. [DOI] [PubMed] [Google Scholar]
- 401.Okamoto M. Simultaneous demonstration of lens regeneration from dorsal iris and tumour production from ventral iris in the same newt eye after carcinogen administration. Differentiation. 1997;61:285–292. doi: 10.1046/j.1432-0436.1997.6150285.x. [DOI] [PubMed] [Google Scholar]
- 402.Zilakos NP, Zafiratos CS, Parchment RE. Stage-dependent genetically-based deformities of the regenerating newt limb from 4-nitroquinoline-N-oxide mutagenesis: potential embryonic regulation of cancer. Differentiation. 1996;60:67–74. doi: 10.1046/j.1432-0436.1996.6020067.x. [DOI] [PubMed] [Google Scholar]
- 403.SHEREMETIEVA EA. SPONTANEOUS MELANOMA IN REGENERATING TAILS OF AXOLOTLS. J Exp Zool. 1965;158:101–121. doi: 10.1002/jez.1401580110. [DOI] [PubMed] [Google Scholar]
- 404.Prehn RT. Regeneration versus neoplastic growth. Carcinogenesis. 1997;18:1439–1444. doi: 10.1093/carcin/18.8.1439. [DOI] [PubMed] [Google Scholar]
- 405.BREEDIS C. Induction of accessory limbs and of sarcoma in the Newt (Triturus viridescens) with carcinogenic substances. Cancer Res. 1952;12:861–866. [PubMed] [Google Scholar]
- 406.Farber E. Pre-cancerous steps in carcinogenesis. Their physiological adaptive nature. Biochim Biophys Acta. 1984;738:171–180. doi: 10.1016/0304-419x(83)90002-1. [DOI] [PubMed] [Google Scholar]
- 407.Farber E. The multistep nature of cancer development. Cancer Res. 1984;44:4217–4223. [PubMed] [Google Scholar]
- 408.Coleman WB, Wennerberg AE, Smith GJ, Grisham JW. Regulation of the differentiation of diploid and some aneuploid rat liver epithelial (stemlike) cells by the hepatic microenvironment. Am J Pathol. 1993;142:1373–1382. [PMC free article] [PubMed] [Google Scholar]
- 409.McCullough KD, Coleman WB, Ricketts SL, Wilson JW, Smith GJ, et al. Plasticity of the neoplastic phenotype in vivo is regulated by epigenetic factors. Proc Natl Acad Sci USA. 1998;95:15333–15338. doi: 10.1073/pnas.95.26.15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Tatematsu M, Nagamine Y, Farber E. Redifferentiation as a basis for remodeling of carcinogen-induced hepatocyte nodules to normal appearing liver. Cancer Res. 1983;43:5049–5058. [PubMed] [Google Scholar]
- 411.Ogawa K, Solt DB, Farber E. Phenotypic diversity as an early property of putative preneoplastic hepatocyte populations in liver carcinogenesis. Cancer Res. 1980;40:725–733. [PubMed] [Google Scholar]
- 412.Enomoto K, Farber E. Kinetics of phenotypic maturation of remodeling of hyperplastic nodules during liver carcinogenesis. Cancer Res. 1982;42:2330– 2335. [PubMed] [Google Scholar]
- 413.Zupanc GK. Adult neurogenesis and neuronal regeneration in the brain of teleost fish. J Physiol Paris. 2008;102:357–373. doi: 10.1016/j.jphysparis.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 414.Richardson J, Zeng Z, Ceol C, Mione M, Jackson IJ, et al. A zebrafish model for nevus regeneration. Pigment Cell Melanoma Res. 2011;24:378–381. doi: 10.1111/j.1755-148X.2011.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Pflugfelder VERHANDL DEUTSCH ZOOL GES-ZOOL ANZ. 1938;11(Suppl) [Google Scholar]
- 416.Pflugfelder Wechselwirkungen von Drusen innerer Sekretion bei Dixippus morosus. Br Z Wiss Zool. 1939;152:384–408. [Google Scholar]
- 417.Pflugfelder Z Krebsforsch 1950 [Google Scholar]
- 418.PFLUGFELDER O. Tumor formations in invertebrates and lower vertebrates. Strahlentherapie. 1954;93:181–195. [PubMed] [Google Scholar]
- 419.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 420.Nakagawara A. Molecular basis of spontaneous regression of neuroblastoma: role of neurotrophic signals and genetic abnormalities. Hum Cell. 1998;11:115–124. [PubMed] [Google Scholar]
- 421.Marshall WF. Origins of cellular geometry. BMC Biol. 2011;9:57. doi: 10.1186/1741-7007-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Noble D. A theory of biological relativity: no privileged level of causation. Interface Focus. 2012;2:55–64. doi: 10.1098/rsfs.2011.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Ellis GFR, Noble D, O’Connor T. Top-down causation: an integrating theme within and across the sciences? INTRODUCTION. Interface Focus. 2012;2:1–3. [Google Scholar]
- 424.Noble D. Genes and causation. Philos Trans A Math Phys Eng Sci. 2008;366:3001–3015. doi: 10.1098/rsta.2008.0086. [DOI] [PubMed] [Google Scholar]
- 425.Garrett PE, Kurtz SR. Clinical utility of oncofetal proteins and hormones as tumor markers. Med Clin North Am. 1986;70:1295–1306. doi: 10.1016/s0025-7125(16)30899-9. [DOI] [PubMed] [Google Scholar]
- 426.Adinolfi M, Lessof MH. Cancer, oncogenes and oncofetal antigens. Q J Med. 1985;54:193–204. [PubMed] [Google Scholar]
- 427.Calvo R, Drabkin HA. Embryonic genes in cancer. Ann Oncol. 2000;11(Suppl 3):207–218. doi: 10.1093/annonc/11.suppl_3.207. [DOI] [PubMed] [Google Scholar]
- 428.Mutyal NN, Radosevich A, Tiwari AK, Stypula Y, Wali R, et al. Biological mechanisms underlying structural changes induced by colorectal field carcinogenesis measured with low-coherence enhanced backscattering (LEBS) spectroscopy. PloS one. 2013;8:e57206. doi: 10.1371/journal.pone.0057206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Damania D, Roy HK, Kunte D, Hurteau JA, Subramanian H, et al. Insights into the field carcinogenesis of ovarian cancer based on the nanocytology of endocervical and endometrial epithelial cells. Int J Cancer. 2013;133:1143–1152. doi: 10.1002/ijc.28122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Backman V, Roy HK. Advances in biophotonics detection of field carcinogenesis for colon cancer risk stratification. J Cancer. 2013;4:251–261. doi: 10.7150/jca.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Subramanian H, Roy HK, Pradhan P, Goldberg MJ, Muldoon J, et al. Nanoscale cellular changes in field carcinogenesis detected by partial wave spectroscopy. Cancer Res. 2009;69:5357–5363. doi: 10.1158/0008-5472.CAN-08-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Kopelovich L, Henson DE, Gazdar AF, Dunn B, Srivastava S, et al. Surrogate anatomic/functional sites for evaluating cancer risk: an extension of the field effect. Clin Cancer Res. 1999;5:3899–3905. [PubMed] [Google Scholar]
- 433.Farinella-Ferruzza N. The transformation of a tail into a limb after xenoplastic transformation. Experientia. 1956;12:304–305. [Google Scholar]
- 434.Holtfreter J. Transformation of a Tail into a Limb or Gill-Like Structures. J EXP ZOOL. 1955;129:623–648. [Google Scholar]
- 435.FARINELLA-FERRUZZA N. Reciprocal transplantations of tail bud between Discoglossus and Triton. Riv Biol. 1953;45:523–557. [PubMed] [Google Scholar]
- 436.Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Vincent M. Cancer: a de-repression of a default survival program common to all cells?: a life-history perspective on the nature of cancer. Bioessays. 2012;34:72–82. doi: 10.1002/bies.201100049. [DOI] [PubMed] [Google Scholar]
- 438.Dean M. Cancer as a complex developmental disorder--nineteenth Cornelius P. Rhoads Memorial Award Lecture. Cancer Res. 1998;58:5633–5636. [PubMed] [Google Scholar]
- 439.Chabner BA, Roberts TG., Jr Timeline: Chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5:65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- 440.Nooter K, Herweijer H. Multidrug resistance (mdr) genes in human cancer. Br J Cancer. 1991;63:663–669. doi: 10.1038/bjc.1991.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci. 2000;11:265–283. doi: 10.1016/s0928-0987(00)00114-7. [DOI] [PubMed] [Google Scholar]
- 442.Shah NP, Skaggs BJ, Branford S, Hughes TP, Nicoll JM, et al. Sequential ABL kinase inhibitor therapy selects for compound drug-resistant BCR-ABL mutations with altered oncogenic potency. J Clin Invest. 2007;117:2562–2569. doi: 10.1172/JCI30890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Deisboeck TS, Berens ME, Kansal AR, Torquato S, Stemmer-Rachamimov AO, et al. Pattern of self-organization in tumour systems: complex growth dynamics in a novel brain tumour spheroid model. Cell Prolif. 2001;34:115–134. doi: 10.1046/j.1365-2184.2001.00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Rosen R. Anticipatory systems: philosophical, mathematical, and methodological foundations. Pergamon Press; 1985. [Google Scholar]
- 445.Bertschinger N, Olbrich E, Ay N, Jost J. Autonomy: an information theoretic perspective. Biosystems. 2008;91:331–345. doi: 10.1016/j.biosystems.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 446.Olsen ML, Sontheimer H. Mislocalization of Kir channels in malignant glia. Glia. 2004;46:63–73. doi: 10.1002/glia.10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Axelrod R, Axelrod DE, Pienta KJ. Evolution of cooperation among tumor cells. Proc Natl Acad Sci USA. 2006;103:13474–13479. doi: 10.1073/pnas.0606053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Bidard FC, Pierga JY, Vincent-Salomon A, Poupon MF. A “class action” against the microenvironment: do cancer cells cooperate in metastasis? Cancer Metastasis Rev. 2008;27:5–10. doi: 10.1007/s10555-007-9103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Guthrie PB, Lee RE, Rehder V, Schmidt MF, Kater SB. Self-recognition: a constraint on the formation of electrical coupling in neurons. J Neurosci. 1994;14:1477–1485. doi: 10.1523/JNEUROSCI.14-03-01477.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Firme CP, 3rd, Natan RG, Yazdani N, Macagno ER, Baker MW. Ectopic expression of select innexins in individual central neurons couples them to pre-existing neuronal or glial networks that express the same innexin. J Neurosci. 2012;32:14265–14270. doi: 10.1523/JNEUROSCI.2693-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.McCusker C, Gardiner DM. The axolotl model for regeneration and aging research: a mini-review. Gerontology. 2011;57:565–571. doi: 10.1159/000323761. [DOI] [PubMed] [Google Scholar]
- 452.Maden M. Axolotl/newt. Methods Mol Biol. 2008;461:467–480. doi: 10.1007/978-1-60327-483-8_32. [DOI] [PubMed] [Google Scholar]
- 453.Lobo D, Malone TJ, Levin M. Towards a bioinformatics of patterning: a computational approach to understanding regulative morphogenesis. Biol Open. 2013;2:156–169. doi: 10.1242/bio.20123400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Barkai N, Ben-Zvi D. ‘Big frog, small frog’--maintaining proportions in embryonic development: delivered on 2 July 2008 at the 33rd FEBS Congress in Athens, Greece. FEBS J. 2009;276:1196–1207. doi: 10.1111/j.1742-4658.2008.06854.x. [DOI] [PubMed] [Google Scholar]
- 455.Slack JM. A serial threshold theory of regeneration. J Theor Biol. 1980;82:105–140. doi: 10.1016/0022-5193(80)90092-2. [DOI] [PubMed] [Google Scholar]
- 456.Koni PA, Khanna R, Chang MC, Tang MD, Kaczmarek LK, et al. Compensatory anion currents in Kv1.3 channel-deficient thymocytes. J Biol Chem. 2003;278:39443–39451. doi: 10.1074/jbc.M304879200. [DOI] [PubMed] [Google Scholar]
- 457.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Adams DS, Tseng AS, Levin M. Light-activation of the Archaerhodopsin H(+)-pump reverses age-dependent loss of vertebrate regeneration: sparking system-level controls in vivo. Biol Open. 2013;2:306–313. doi: 10.1242/bio.20133665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Levin M. The wisdom of the body: future techniques and approaches to morphogenetic fields in regenerative medicine, developmental biology and cancer. Regen Med. 2011;6:667–673. doi: 10.2217/rme.11.69. [DOI] [PubMed] [Google Scholar]
- 460.Auletta G, Ellis GF, Jaeger L. Top-down causation by information control: from a philosophical problem to a scientific research programme. J R Soc Interface. 2008;5:1159–1172. doi: 10.1098/rsif.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Juarrero A. Top-Down Causation and Autonomy in Complex Systems. Underst Complex Syst. 2009:83–102. [Google Scholar]
- 462.Soto AM, Sonnenschein C, Miquel PA. On physicalism and downward causation in developmental and cancer biology. Acta Biotheor. 2008;56:257–274. doi: 10.1007/s10441-008-9052-y. [DOI] [PubMed] [Google Scholar]
- 463.Davies PC, Demetrius L, Tuszynski JA. Cancer as a dynamical phase transition. Theor Biol Med Model. 2011;8:30. doi: 10.1186/1742-4682-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Davies PC. The epigenome and top-down causation. Interface Focus. 2012;2:42–48. doi: 10.1098/rsfs.2011.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Levin M. Molecular bioelectricity in developmental biology: new tools and recent discoveries: control of cell behavior and pattern formation by transmembrane potential gradients. Bioessays. 2012;34:205–217. doi: 10.1002/bies.201100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Gurwitsch AG. A Biological Field Theory 1944 [Google Scholar]
- 467.Sachdeva G, Kalyanasundaram K, Krishnan J, Chakravarthy VS. Bistable dynamics of cardiac cell models coupled by dynamic gap junctions linked to cardiac memory. Biol Cybern. 2010;102:109–121. doi: 10.1007/s00422-009-0352-3. [DOI] [PubMed] [Google Scholar]
- 468.Gallaher J, Bier M, van Heukelom JS. First order phase transition and hysteresis in a cell’s maintenance of the membrane potential--An essential role for the inward potassium rectifiers. Biosystems. 2010;101:149–155. doi: 10.1016/j.biosystems.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 469.Gorostiza P, Volgraf M, Numano R, Szobota S, Trauner D, et al. Mechanisms of photoswitch conjugation and light activation of an ionotropic glutamate receptor. Proc Natl Acad Sci USA. 2007;104:10865–10870. doi: 10.1073/pnas.0701274104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain’s spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- 471.Hafting T, Fyhn M, Bonnevie T, Moser MB, Moser EI. Hippocampus-independent phase precession in entorhinal grid cells. Nature. 2008;453:1248–1252. doi: 10.1038/nature06957. [DOI] [PubMed] [Google Scholar]
- 472.Grossberg S. Progress in Theoretical Biology. Academic Press; New York: 1978. [Google Scholar]
- 473.Pulver SR, Griffith LC. Spike integration and cellular memory in a rhythmic network from Na+/K+ pump current dynamics. Nat Neurosci. 2010;13:53–59. doi: 10.1038/nn.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Daoudal G, Debanne D. Long-term plasticity of intrinsic excitability: learning rules and mechanisms. Learn Mem. 2003;10:456–465. doi: 10.1101/lm.64103. [DOI] [PubMed] [Google Scholar]
- 475.Debanne D, Daoudal G, Sourdet V, Russier M. Brain plasticity and ion channels. J Physiol Paris. 2003;97:403–414. doi: 10.1016/j.jphysparis.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 476.Krishnan J, Sachdeva G, Chakravarthy VS, Radhakrishnan S. Interpreting voltage-sensitivity of gap junctions as a mechanism of cardiac memory. Math Biosci. 2008;212:132–148. doi: 10.1016/j.mbs.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 477.Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu Rev Neurosci. 2004;27:393–418. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- 478.Bennett MV. Gap junctions as electrical synapses. J Neurocytol. 1997;26:349–366. doi: 10.1023/a:1018560803261. [DOI] [PubMed] [Google Scholar]
- 479.Buznikov GA, Shmukler YB. Possible role of “prenervous” neurotransmitters in cellular interactions of early embryogenesis: a hypothesis. Neurochem Res. 1981;6:55–68. doi: 10.1007/BF00963906. [DOI] [PubMed] [Google Scholar]
- 480.Teh JL, Chen S. Glutamatergic signaling in cellular transformation. Pigment Cell Melanoma Res. 2012;25:331–342. doi: 10.1111/j.1755-148X.2012.00983.x. [DOI] [PubMed] [Google Scholar]
- 481.Martino JJ, Wall BA, Mastrantoni E, Wilimczyk BJ, La Cava SN, et al. Metabotropic glutamate receptor 1 (Grm1) is an oncogene in epithelial cells. Oncogene. 2013;32:4366–4376. doi: 10.1038/onc.2012.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Schiffner S, Chen S, Becker JC, Bosserhoff AK. Highly pigmented Tg(Grm1) mouse melanoma develops non-pigmented melanoma cells in distant metastases. Exp Dermatol. 2012;21:786–788. doi: 10.1111/j.1600-0625.2012.01560.x. [DOI] [PubMed] [Google Scholar]
- 483.Bagot RC, Zhang TY, Wen X, Nguyen TT, Nguyen HB, et al. Variations in postnatal maternal care and the epigenetic regulation of metabotropic glutamate receptor 1 expression and hippocampal function in the rat. Proc Natl Acad Sci USA. 2012;109(Suppl 2):17200–17207. doi: 10.1073/pnas.1204599109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, et al. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372:237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- 485.Vicaut E, Laemmel E, Stücker O. Impact of serotonin on tumour growth. Ann Med. 2000;32:187–194. doi: 10.3109/07853890008998826. [DOI] [PubMed] [Google Scholar]
- 486.Dizeyi N, Bjartell A, Nilsson E, Hansson J, Gadaleanu V, et al. Expression of serotonin receptors and role of serotonin in human prostate cancer tissue and cell lines. Prostate. 2004;59:328–336. doi: 10.1002/pros.10374. [DOI] [PubMed] [Google Scholar]
- 487.Pai VP, Marshall AM, Hernandez LL, Buckley AR, Horseman ND. Altered serotonin physiology in human breast cancers favors paradoxical growth and cell survival. Breast Cancer Res. 2009;11:R81. doi: 10.1186/bcr2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Yang M, Brackenbury WJ. Membrane potential and cancer progression. Front Physiol. 2013;4:185. doi: 10.3389/fphys.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Reid B, Song B, McCaig CD, Zhao M. Wound healing in rat cornea: the role of electric currents. FASEB J. 2005;19:379–386. doi: 10.1096/fj.04-2325com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Lange CS, Steele VE. The mechanism of anterior-posterior polarity control in planarians. Differentiation. 1978;11:1–12. doi: 10.1111/j.1432-0436.1978.tb00965.x. [DOI] [PubMed] [Google Scholar]
- 491.Burr HS, Hovland CI. Bio-Electric Correlates of Development in Amblystoma. Yale J Biol Med. 1937;9:540–549. [PMC free article] [PubMed] [Google Scholar]
- 492.Yamashita M. Fluctuations in nuclear envelope’s potential mediate synchronization of early neural activity. Biochem Biophys Res Commun. 2011;406:107–111. doi: 10.1016/j.bbrc.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 493.Mazzanti M, Bustamante JO, Oberleithner H. Electrical dimension of the nuclear envelope. Physiol Rev. 2001;81:1–19. doi: 10.1152/physrev.2001.81.1.1. [DOI] [PubMed] [Google Scholar]
- 494.Onkal R, Djamgoz MB. Molecular pharmacology of voltage-gated sodium channel expression in metastatic disease: clinical potential of neonatal Nav1.5 in breast cancer. Eur J Pharmacol. 2009;625:206–219. doi: 10.1016/j.ejphar.2009.08.040. [DOI] [PubMed] [Google Scholar]
- 495.Saito T, Schlegel R, Andresson T, Yuge L, Yamamoto M, et al. Induction of cell transformation by mutated 16K vacuolar H+-atpase (ductin) is accompanied by down-regulation of gap junctional intercellular communication and translocation of connexin 43 in NIH3T3 cells. Oncogene. 1998;17:1673–1680. doi: 10.1038/sj.onc.1202092. [DOI] [PubMed] [Google Scholar]
- 496.Gupta N, Martin PM, Prasad PD, Ganapathy V. SLC5A8 (SMCT1)-mediated transport of butyrate forms the basis for the tumor suppressive function of the transporter. Life Sci. 2006;78:2419–2425. doi: 10.1016/j.lfs.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 497.Roepke TK, Purtell K, King EC, La Perle KM, Lerner DJ, et al. Targeted deletion of Kcne2 causes gastritis cystica profunda and gastric neoplasia. PLoS One. 2010;5:e11451. doi: 10.1371/journal.pone.0011451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.House CD, Vaske CJ, Schwartz AM, Obias V, Frank B, et al. Voltage-gated Na+ channel SCN5A is ‘a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Res. 2010;70:6957–6967. doi: 10.1158/0008-5472.CAN-10-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Song Z, He CD, Liu J, Sun C, Lu P, et al. Blocking glutamate-mediated signalling inhibits human melanoma growth and migration. Exp Dermatol. 2012;21:926–931. doi: 10.1111/exd.12048. [DOI] [PubMed] [Google Scholar]