Abstract
Background
Sepsis has a profound impact on the inflammatory and hemostatic systems. In addition to systemic inflammation, it can produce disseminated intravascular coagulation, microvascular thrombosis, consumptive coagulopathy, and multiple organ failure. We have shown that treatment with suberoylanilide hydroxamic acid (SAHA), a histone deacetylase inhibitor (HDACI), improves survival in a lethal model of cecal ligation and puncture (CLP) in mice. However, its effect on coagulation remains unknown. The goal of this study was to quantify the impact of SAHA treatment on coagulopathy in sepsis.
Methods
C57BL/6J mice were subjected to CLP, and 1 hour later given intraperitoneally either SAHA dissolved in dimethyl sulfoxide (DMSO) or DMSO only. Sham-operated animals were handled in similar manner without CLP. Blood samples were collected by cardiac puncture and evaluated using the TEG® 5000 Thrombelastograph® Hemostasis Analyzer System.
Results
Compared to the sham group, all animals in DMSO vehicle group died within 72 hrs, and developed coagulopathy that manifested as prolonged initial fibrin formation and fibrin cross-linkage time, and decreased clot formation speed, platelet function and clot rigidity. SAHA treatment significantly improved survival and was also associated with improvement in fibrin cross-linkage, clot formation, as well as platelet function and clot rigidity, without a significant impact on the clot initiation parameters.
Conclusions
SAHA treatment enhances survival and attenuates sepsis-associated coagulopathy by improving fibrin cross-linkage, rate of clot formation, platelet function and clot strength. HDACI may represent a novel therapeutic strategy for correcting sepsis-associated coagulopathy.
INTRODUCTION
Sepsis is a systemic inflammatory disorder, and its progression to septic shock is a serious clinical problem with high mortality. Despite of aggressive research efforts, effective treatments for septic shock remain elusive 1-4. Development of disseminated intravascular coagulation (DIC) in septic patients is associated with high mortality. Coagulation abnormalities, such as thrombocytopenia, prolonged clotting time and consumption coagulopathy, occur in nearly all patients with severe sepsis 5, 6, while DIC develops in 35% of severely septic patients7, 8. In Gram-negative sepsis, lipopolysaccharide (LPS) of Gram-negative bacilli induces tissue factor expression on endothelium and monocytes, resulting in coagulation activation and microvascular thrombosis, that subsequently leads to DIC and bleeding 9. Meanwhile, endotoxin-mediated release of cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) enhances coagulation and impairs fibrinolysis, further promoting widespread intravascular fibrin deposition, DIC and an enhanced risk of bleeding 10.
Thromboelastography (TEG) evaluates the dynamics of clot formation (and breakdown) and measures several parameters, including clot initiation, clot propagation, as well as platelet function and clot rigidity. TEG not only measures clot formation, but also its lysis. This allows for a more complete characterization of the coagulation system than classic clotting assays, such as the activated partial thromboplastin time (aPTT) and the activated clotting time (ACT) 11, 12. TEG has recently gained populatity and is increasingly being used in the setting of cardiac and liver surgery, trauma resuscitation, as well as monitoring of thrombin inhibitors and plasminogen activators 13-16. In patients with traumatic injuries, TEG is an effective tool to monitor early systemic changes in trauma-related coagulopathy 17. In addition, TEG has been used to evaluate characteristics of haemostatic disturbances in models of endotoxemia, as well as in critically ill septic patients 18-21.
We have previously demonstrated that treatment with suberoylanilide hydroxamic acid (SAHA), a histone deacetylase (HDAC) inhibitor, improves survival in rodent models of lipopolysaccharide (LPS)-induced endotoxemia 22, as well as in lethal sepsis due to cecum ligation and puncture (CLP) 23. We have also shown that administration of SAHA decreases LPS-mediated acute lung injury, and rapidly modulates several key genes involved in inflammation with an attenuation of inflammatory mediators derived from both cellular and humoral arms of the innate immune system 24. In addition, it suppresses nuclear factor (NF)-κB and hypoxia-inducible factor (HIF)-1α-mediated inflammatory pathways in LPS-stimulated macrophages, and attenuates production of numerous proinflammatory cytokines 25, 26. However, the effect of HDAC inhibitors on the coagulation system, especially in the setting of sepsis remains unknown. This study was performed to characterize the coagulation abnormalities in a lethal CLP model using TEG, and to assess the effects of SAHA treatment on these disturbances.
MATERIALS AND METHODS
Sepsis Model: Cecal Ligation and Puncture (CLP)
Mice were purchased from The Jackson Laboratory and were on a C57BL/6J background. Male mice weighing 18–26 g were housed for days before manipulations. The CLP murine model 27 modified by our laboratory was used to induce septic peritonitis. In brief, the peritoneal cavity was opened during isoflurane anesthesia, cecum was eviscerated and ligated below the ileocecal valve using a 5-0 suture, and punctured once with a 20 gauge needle. The punctured cecum was squeezed to expel a small amount of fecal material and relocated into the peritoneal cavity. The abdominal incision was closed in two layers with 4-0 silk suture. Animals were resuscitated by subcutaneous injection of 1 ml of saline. Sham-operated animals were handled in the same manner, except that the cecum was not ligated or punctured. All protocols were approved by the Animal Review Committee at Massachusetts General Hospital. The animals were euthanized with pentobarbital (200 mg/kg, i.p.) if they were deemed to be suffering as evidenced by inability to ambulate or feed, self-mutilation, bleeding, rapid weight loss (20% within 3 days), rough hair coat, hunched posture, distended abdomen, severe diarrhea, or lethargy, especially if debilitating or prolonged, or moribund.
Administration of SAHA and Experimental Design
Animals were randomly assigned to the following three groups (n=17-18/group): a) Sham-operated animals (SHAM); (b) vehicle [dimethyl sulfoxide (DMSO)] treated animals after CLP (CLP+DMSO), and (c) SAHA treated animals after CLP (CLP+SAHA). Mice received intra-peritoneal SAHA (50 mg/kg) dissolved in DMSO or vehicle (DMSO) alone 1 h after CLP. Sham-operated animals were subjected to laparotomy and intestinal manipulation, but the cecum was neither ligated nor punctured.
Thromboelastography (TEG)
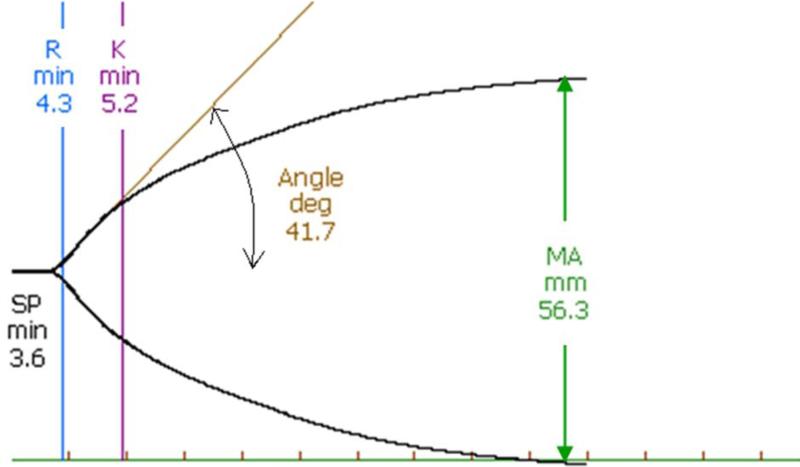
Whole blood samples were collected into heparin-rinsed tubes by cardiac puncture 48 h after CLP. Three hundred and forty μl of each sample was placed in a disposable heparinase-coated cuvette and analyzed by TEG® 5000 Thrombelastograph® Hemostasis Analyzer System (Haemonetics Corporation, Braintree, MA). The following parameters were evaluated: SP (Split Point, time from sample placement to first divergence of the trace; denotes the initial fibrin formation rate), R (Reaction time, time from sample placement until TEG® tracing amplitude reaches 2 mm; denotes the initial fibrin formation rate), K (time and Kinetics for fibrin cross-linkage, time from TEG® tracing amplitude reaches 2 mm until it reaches 20 mm; represents the time for development of fixed degree of viscoelasticity during clot formation), Angle (angle formed by the slope of the initial TEG® tracing; reflects speed at which solid clot forms), and MA (Maximum Amplitude, the greatest amplitude of the TEG® tracing; reflects platelet function and absolute strength of the fibrin clot) (Figure 1) 28.
Figure 1. Scheme of parameters obtained by thromboelastography (TEG).
Split Point (SP) is the time from sample placement to first divergence of the trace. Reaction time (R) is the time from sample placement until TEG tracing amplitude reaches 2 mm. Both of SP and R denote the initial fibrin formation rate. K value is the time from the end of R until the TEG tracing amplitude reaches 20 mm. It represents the time for development of fixed degree of viscoelasticity during clot formation. Angle is formed by the slope of the initial TEG tracing, and it reflects the speed at which solid clot forms. Maximum Amplitude (MA) is the greatest amplitude of the TEG tracing, and it reflects platelet function and the maximum strength of the fibrin clot.
Statistical Analysis
All data were summarized as mean ± standard error of mean (SEM). Differences between three groups were assessed using one-way analysis of variance (ANOVA) followed by Bonferroni post hoc test for multiple comparisons. Student's t test was used to compare the differences between two groups. All analyses were performed using GraphPad Prism. P values of 0.05 or less were considered as significance.
RESULTS
SAHA restores fibrin cross-linkage time in a lethal CLP-sepsis model
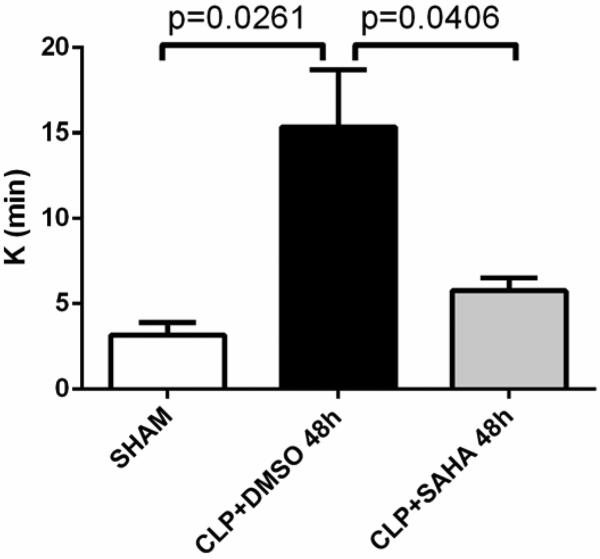
SAHA treatment significantly improved the survival after CLP (see Table 1). Compared to sham-operated group, animals subjected to CLP showed increased K (3.2 ± 0.7 versus 15.3 ± 3.4 min, p < 0.05) at 48 hrs, indicating that it took longer time for septic animals to develop fixed degree of viscoelasticity during clot formation and fibrin cross-linkage. This abnormality was not seen in SAHA treated animals, compared to those in the DMSO vehicle group (15.3 ± 3.4 versus 5.8 ± 0.7 min, p < 0.05; Figure 2 and 3).
Table 1.
Survival Outcomes of Different Animal Groups
| Groups | Survival rate at Day 10 after CLP (%) |
|---|---|
| Sham | 100 |
| CLP+DMSO | 0 |
| CLP+SAHA | 40 |
CLP: cecal ligation and puncture
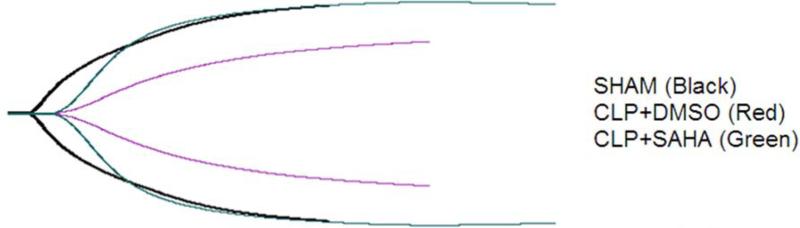
Figure 2. Representative TEG® tracings for animals from different groups.
Mice were intraperitoneally administered 50mg/kg SAHA or vehicle DMSO 1 h after CLP, while sham-operated animals served as controls. Whole blood samples were collected and coagulation status was monitored by TEG. Representative TEG® tracing for sham-operated group is in black, DMSO vehicle group in green, and SAHA treatment group in red. CLP: cecal ligation and puncture.
Figure 3. Clot propagation as represented by K.
K value is the time from the end of R until the clot reaches 20 mm, which indicates the time for development of fixed degree of viscoelasticity during clot formation and fibrin cross-linkage. The K values were recorded with the TEG® 5000 Thrombelastograph® Hemostasis Analyzer System and compared between three experimental groups (means ± SEM, n = 5-8 animals/group).
SAHA restores clot formation speed in lethal sepsis
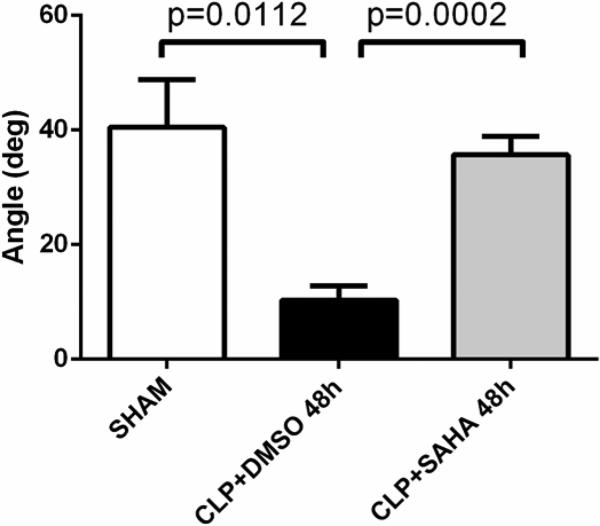
As reflected by Angle, clot formation speed in the DMSO vehicle group was slower than sham group (40.5 ± 8.3 versus 10.3 ± 2.5 deg, p < 0.05), which was consistent with tendency to bleed seen at decompensated DIC stage during septic shock. This was attenuated in the SAHA-treated animals, compared to those in the DMSO vehicle group (10.3 ± 2.5 versus 35.7 ± 3.2 deg, p < 0.001; Figure 2 and 4).
Figure 4. Clot propagation as represented by Angle.
Angle is formed by the slope of the initial TEG® tracing, and represents speed at which solid clot forms. The Angle data was recorded in TEG® 5000 Thrombelastograph® Hemostasis Analyzer System and compared between groups (means ± SEM; n = 5-8 animals/group). deg: degree
SAHA treatment improves platelet function and clot rigidity in lethal sepsis
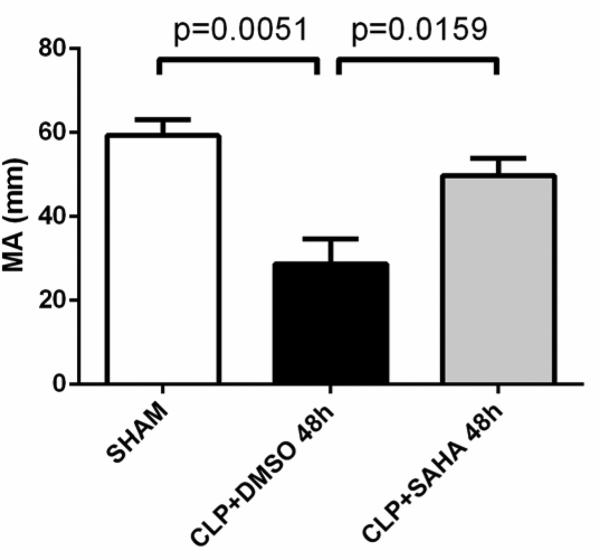
Platelet function and absolute strength of the fibrin clot, as indicated by Maximum Amplitude, were impaired in CLP animals compared to sham-operated animals (28.7 ± 6.0 versus 59.3 ± 3.8 mm, p < 0.01). These were preserved in the SAHA treatment group, compared to those in the DMSO vehicle group (28.7 ± 6.0 versus 49.8 ± 4.1 mm, p < 0.05; Figure 2 and 5).
Figure 5. Platelet function and clot rigidity as reflected by Maximum Amplitude (MA).
MA is the greatest amplitude of the TEG® tracing and reflects platelet function and absolute strength of the fibrin clot. The MA values were recorded with the TEG® 5000 Thrombelastograph® Hemostasis Analyzer System and compared between groups (means ± SEM, n = 5-8 animals/group).
SAHA does not significantly affect clot initiation
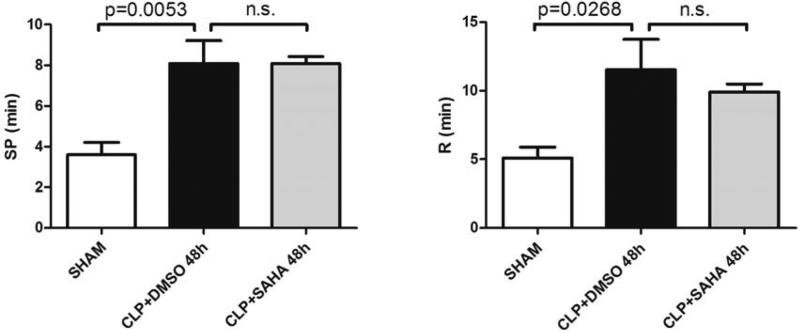
There were no significant differences between the SAHA treatment group and DMSO vehicle group for Split Point (8.1 ± 1.1 versus 8.1 ± 0.3 min), and Reaction Time (11.5 ± 2.2 versus 9.9 ± 0.6 min; Figure 2 and 6). These results indicate that SAHA treatment does not significantly affect clot initiation in this model.
Figure 6. Clot initiation as indicated by Split Point (SP) and Reaction time (R).
SP represents the time from sample placement to the first divergence of the trace, while R represents the time from sample placement until the TEG® tracing amplitude reaches 2 mm, both of which reflect the initial fibrin formation rate. The SP and R values were recorded with the TEG® 5000 Thrombelastograph® Hemostasis Analyzer System and compared between groups (means ± SEM, n = 5-8 animals/group). n.s.: not significant.
DISCUSSION
Sepsis is frequently associated with complications resulting from dysfunctional activation of the coagulation system. This may manifest as acute DIC characterized by widespread microvascular thrombosis, subsequent consumption of coagulation proteins and platelets, and an increased risk of bleeding. Our laboratory has recently discovered that treatment with SAHA improves long-term survival in a CLP-induced lethal sepsis model 23. In the current study, we evaluated the coagulation status in the same CLP model (with or without SAHA treatment) and discovered that: (1) CLP leads to a progressive hypocoagulable state which is characterized by longer initial fibrin cross-linkage time, decreased clot formation speed, poor platelet function and low clot rigidity; (2) Most of these abnormalities were not seen in the SAHA-treated animals.
Complex interactions between coagulation and inflammatory systems have been implicated in the pathogenesis of sepsis. The key event in the sepsis-associated DIC is the systemic inflammatory response (SIRS) to infectious agents 29. Overwhelming host responses to microorganisms and their derivatives, as well as to the overexpressed inflammatory mediators and complement activation products, are now believed to be responsible for massive thrombin formation and fibrin deposition, DIC, multiple organ dysfunction syndromes (MODS), coagulopathy, and bleeding 30-32. Enzymes generated during the clotting cascade, in turn, interact with specific cellular receptors, eliciting responses that amplify the inflammatory reactions 33. Meanwhile, recent evidence indicates that extracellular nuclear materials released from activated and especially apoptotic or necrotic cells, such as High Mobility Group Box-1 (HMGB-1) and histones, are endowed with cytotoxic, proinflammatory and thrombogenic properties. They propagate further inflammation, consumption of coagulation components, cell death and MODS 29, 34.
In sepsis, the causative agents and the associated inflammatory mediators drive fibrin formation and deposition by several simultaneously acting mechanisms: (1) aberrant expression of the tissue factor (TF) by different cells (especially monocytes-macrophages), (2) impairment of physiological anticoagulant pathways, orchestrated mainly by dysfunctional endothelial cells (3) the suppression of fibrinolysis due to overproduction of plasminogen activator inhibitor-1 (PAI-1) by endothelial cells, and an activation of thrombin activatable fibrinolysis inhibitor (TAFI). The ensuing microvascular thrombosis and ischemia are thought to contribute to tissue injury and MODS 30-32.
In the current study, we detected a progressive hypocoagulable state in the CLP animals, which was consistent with findings from reported in animal models of endotoxemia as well as critically ill septic patients. In a rat model of endotoxemia, fibrin and clot formation speed increased 1 h after intravenous LPS infusion. Thereafter, speed of clot formation, platelet function and clot rigidity decreased progressively, demonstrating a hypocoagulable state 18. TEG is also able to detect coagulation abnormalities and fibrinolysis in humans where low dose endotoxin infusion enhanced maximal lysis (ML) 3.9-fold within 2 hours19. Meanwhile, in a prospective cohort study of critically ill patients with sepsis, improved organ dysfunction upon discharge was shown to be associated with shortened coagulation time, accelerated clot formation, and increased firmness of the clot 20.
Anti-inflammatory properties of SAHA may contribute to prevention of the coagulation imbalance in this model, given the multiple interactions between the inflammatory and coagulation pathways. SAHA decreases numerous inflammatory mediators and pathways, which may suppress activation of coagulation cascades, attenuate consumption of coagulation proteins and platelets, and eventually decrease MODS and DIC during late stages of severe sepsis. We and others have demonstrated that SAHA attenuates inflammatory responses and inhibits the production of pro-inflammatory cytokines in vitro and in vivo 22, 25, 35. For example, SAHA improves survival in LPS-induced shock and CLP-induced lethal sepsis models 22, 23. It also significantly attenuates excessive TNF-α and IL-6 production in CLP animals, and decreases cytokine levels in the supernatant of macrophages that are stimulated with TLR4 agonist LPS or TLR1/2 agonist Pam3CSK4 43. SAHA treated animals also display a significant decrease in acute liver injury 23. In addition, SAHA reduces MyD88 expression at the gene and protein levels in the lungs after LPS insult, suggesting that inhibition of TLR4-MyD88-dependent pathway could be one explanation for the anti-inflammatory effects of SAHA 22, 24. Meanwhile, it has also been shown that anti-inflammatory properties of SAHA involve acetylation of heat shock protein 90 (HSP 90). In LPS-stimulated macrophages, SAHA acetylates HSP 90, resulting in dissociation and degradation of its client protein interleukin-1 receptor associated kinase 1 (IRAK1), subsequently decreasing nuclear translocation of NF-κB, and leading to attenuation of key pro-inflammatory cytokines 25. Moreover, SAHA attenuates hypoxia-inducible factor (HIF)-1[.alpha]-mediated inflammatory pathways in macrophages, and suppresses the release of proinflammatory nitric oxide and TNF-α 26. Other HDACI, such as valproic acid, have also been shown to attenuate inflammation and improve survival in a lethal two-hit model: hemorrhagic shock followed by an infection 36.
Cell death results in spillage of nuclear histones into the circulation, which results in severe inflammation. In vivo histone administration has been shown to result in neutrophil margination, vacuolated endothelium, intra-alveolar hemorrhage and vascular thrombosis 34. Extracellular histone release causes endothelial dysfunction, organ failure and death during sepsis, and its blockage reduces mortality in rodent models of sepsis 34. We have previously demonstrated that SAHA treatment decreases circulating histone H3 levels in a rodent model of endotoxemia, and attenuates its secretion from granulocytes in vitro37. This could be another possible explanation for the attenuation of coagulation imbalance with SAHA treatment in this CLP model. Interestingly, SAHA did not significantly affect the initiation of clot formation in this study. This finding also supports the possibility that SAHA modulates the coagulation system indirectly by suppressing the production of inflammatory mediators and inhibiting the release of toxic nuclear components.
Another possible explanation for the observed findings may be reduced endothelial activation and better preservation of endothelial functions in the SAHA treated animals. A number of studies in the literature support this explanation. An HDACI, trichostatin A (TSA), has been found to reduce monocyteendothelium interaction through a suppression of vascular cell adhesion molecule-1 (VCAM-1), suggesting attenuated endothelial activation.38. In addition, HDACIs, such as TSA, valproic acid and sodium butyrate, inhibit tissue factor (TF) production in cultured human umbilical vein endothelial cells (HUVECs) that have been stimulated with various agonists (TNF-α , IL1-β, LPS, et al). They also blunt TF induction in peripheral blood mononuclear cells (in vitro) and in thioglycolate-elicited murine peritoneal macrophages (in vivo)39. TSA does not alter the translational efficiency or degradation of TF, but inhibits the binding of NF-κB components (cRel/p65 and p65/p507) to the unique sites that are specific for VCAM and TF. Pulmonary vascular endothelial VCAM-1 and TF expression are also significantly inhibited by HDACIs40. Moreover, SAHA has been shown to down-regulate the expression of very late activation antigen-4 (VLA-4) on monocytes that is the counter-adhesion partner of VCAM-141. HDAC6 inhibitors, tubacin and MC1575, protect against the thrombin-induced endothelial dysfunction42.
This study has some limitations that should be acknowledged. This is essentially a proof-of-concept study that was not designed to generate detailed mechanistic information. Additional in vitro experiments need to be done to characterize the interactions between HDACIs and numerous coagulation components, such as platelets, tissue factor, thrombomodulin, and plasminogen activator inhibitor.
In conclusion, we have shown that lethal sepsis in a CLP model results in coagulopathy which can be prevented with SAHA treatment. Although the fundamental molecular and cellular signaling events still require further investigation, HDACI potentially represent a promising therapeutic strategy for improving survival and preventing coagulopathy in severe sepsis and septic shock.
Acknowledgements
This work was funded by a grant from NIH RO1 GM084127 to HBA. Data presented at the 9th Annual Academic Surgical Congress in San Diego, CA (February, 2014).
ABBREVIATIONS
- ACT
activated clotting time
- ALT
alanine aminotransferase
- ANOVA
one-way analysis of variance
- aPTT
activated partial thromboplastin time
- CLP
cecal ligation and puncture
- DIC
disseminated intravascular coagulation
- DMSO
dimethyl sulfoxide
- HDAC
histone deacetylase
- HDACI
HDAC inhibitor
- HIF
hypoxia-inducible factor
- HMGB-1
High Mobility Group Box-1
- HSP
heat shock protein
- ICU
intensive care unit
- IFN
interferon
- IL
interleukin
- IRAK1
interleukin-1 receptor associated kinase 1
- LPS
lipopolysaccharide
- MA
Maximum Amplitude
- ML
maximal lysis
- MODS
multiple organ dysfunction syndromes
- MyD88
myeloid differentiation factor 88
- NF-kB
nuclear factor-kappa B
- PAI-1
plasminogen activator inhibitor-1
- SAHA
suberoylanilide hydroxamic acid
- SIRS
systemic inflammatory responses
- SP
Split Point
- TAFI
thrombin activatable fibrinolysis inhibitor
- TEG
Thromboelastography
- TF
tissue factor
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor-α
- t-PA
tissue plasminogen activator
- TSA
trichostatin A
- VCAM-1
vascular cell adhesion molecule-1
- VLA-4
very late activation antigen-4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wenzel RP, Edmond MB. Septic shock--evaluating another failed treatment. N Engl J Med. 2012;366:2122–4. doi: 10.1056/NEJMe1203412. [DOI] [PubMed] [Google Scholar]
- 3.Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055–64. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 4.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 5.Kinasewitz GT, Yan SB, Basson B, Comp P, Russell JA, Cariou A, et al. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism [ISRCTN74215569]. Crit Care. 2004;8:R82–90. doi: 10.1186/cc2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levi M, de Jonge E, van der Poll T. Sepsis and disseminated intravascular coagulation. J Thromb Thrombolysis. 2003;16:43–7. doi: 10.1023/B:THRO.0000014592.27892.11. [DOI] [PubMed] [Google Scholar]
- 7.Bakhtiari K, Meijers JC, de Jonge E, Levi M. Prospective validation of the International Society of Thrombosis and Haemostasis scoring system for disseminated intravascular coagulation. Crit Care Med. 2004;32:2416–21. doi: 10.1097/01.ccm.0000147769.07699.e3. [DOI] [PubMed] [Google Scholar]
- 8.Dhainaut JF, Yan SB, Joyce DE, Pettila V, Basson B, Brandt JT, et al. Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J Thromb Haemost. 2004;2:1924–33. doi: 10.1111/j.1538-7836.2004.00955.x. [DOI] [PubMed] [Google Scholar]
- 9.Moldow CF, Bach RR, Staskus K, Rick PD. Induction of endothelial tissue factor by endotoxin and its precursors. Thromb Haemost. 1993;70:702–6. [PubMed] [Google Scholar]
- 10.Gando S. Microvascular thrombosis and multiple organ dysfunction syndrome. Crit Care Med. 2010;38:S35–42. doi: 10.1097/CCM.0b013e3181c9e31d. [DOI] [PubMed] [Google Scholar]
- 11.Salooja N, Perry DJ. Thrombelastography. Blood Coagul Fibrinolysis. 2001;12:327–37. doi: 10.1097/00001721-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Shore-Lesserson L. Evidence based coagulation monitors: heparin monitoring, thromboelastography, and platelet function. Semin Cardiothorac Vasc Anesth. 2005;9:41–52. doi: 10.1177/108925320500900105. [DOI] [PubMed] [Google Scholar]
- 13.Cvachovec K, Horacek M, Vislocky I. A retrospective survey of fibrinolysis as an indicator of poor outcome after cardiopulmonary bypass and a possible early sign of systemic inflammation syndrome. Eur J Anaesthesiol. 2000;17:173–6. doi: 10.1046/j.1365-2346.2000.00643.x. [DOI] [PubMed] [Google Scholar]
- 14.Viana JS, Pereira MG, Lozano L, Vieira H, Palmeiro A, Lourenco M, et al. Thrombelastographic evidence of hyperfibrinolysis during liver transplantation for familial amyloidotic polyneuropathy ATTR met 30. Transplant Proc. 2000;32:2645–6. doi: 10.1016/s0041-1345(00)01820-0. [DOI] [PubMed] [Google Scholar]
- 15.Liu G, Bowkett J, Przybylowski G, Bellomo R, McNicol PL. Postoperative fibrinolysis diagnosed by thrombelastography. Anaesth Intensive Care. 2000;28:77–81. doi: 10.1177/0310057X0002800115. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen VG, Crow JP, Zhou F, Parks DA. Peroxynitrite inactivates tissue plasminogen activator. Anesth Analg. 2004;98:1312–7. doi: 10.1213/01.ane.0000111105.38836.f6. table of contents. [DOI] [PubMed] [Google Scholar]
- 17.Rugeri L, Levrat A, David JS, Delecroix E, Floccard B, Gros A, et al. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost. 2007;5:289–95. doi: 10.1111/j.1538-7836.2007.02319.x. [DOI] [PubMed] [Google Scholar]
- 18.Tsai HJ, Tsao CM, Liao MH, Ka SM, Liaw WJ, Wu CC. Application of thrombelastography in liver injury induced by endotoxin in rat. Blood Coagul Fibrinolysis. 2012;23:118–26. doi: 10.1097/MBC.0b013e32834ee170. [DOI] [PubMed] [Google Scholar]
- 19.Spiel AO, Mayr FB, Firbas C, Quehenberger P, Jilma B. Validation of rotation thrombelastography in a model of systemic activation of fibrinolysis and coagulation in humans. J Thromb Haemost. 2006;4:411–6. doi: 10.1111/j.1538-7836.2006.01715.x. [DOI] [PubMed] [Google Scholar]
- 20.Daudel F, Kessler U, Folly H, Lienert JS, Takala J, Jakob SM. Thromboelastometry for the assessment of coagulation abnormalities in early and established adult sepsis: a prospective cohort study. Crit Care. 2009;13:R42. doi: 10.1186/cc7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins PW, Macchiavello LI, Lewis SJ, Macartney NJ, Saayman AG, Luddington R, et al. Global tests of haemostasis in critically ill patients with severe sepsis syndrome compared to controls. Br J Haematol. 2006;135:220–7. doi: 10.1111/j.1365-2141.2006.06281.x. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Liu B, Fukudome EY, Kochanek AR, Finkelstein RA, Chong W, et al. Surviving lethal septic shock without fluid resuscitation in a rodent model. Surgery. 2010;148:246–54. doi: 10.1016/j.surg.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao T, Li Y, Liu B, Liu Z, Chong W, Duan X, et al. Novel pharmacologic treatment attenuates septic shock and improves long-term survival. Surgery. 2013;154:206–13. doi: 10.1016/j.surg.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Liu B, Gu X, Kochanek AR, Fukudome EY, Liu Z, et al. Creating a “pro-survival” phenotype through epigenetic modulation. Surgery. 2012;152:455–64. doi: 10.1016/j.surg.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong W, Li Y, Liu B, Zhao T, Fukudome EY, Liu Z, et al. Histone deacetylase inhibitor suberoylanilide hydroxamic acid attenuates Toll-like receptor 4 signaling in lipopolysaccharide-stimulated mouse macrophages. J Surg Res. 2012;178:851–9. doi: 10.1016/j.jss.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chong W, Li Y, Liu B, Liu Z, Zhao T, Wonsey DR, et al. Anti-inflammatory properties of histone deacetylase inhibitors: a mechanistic study. J Trauma Acute Care Surg. 2012;72:347–53. doi: 10.1097/TA.0b013e318243d8b2. discussion 53-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–6. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waters JH, Anthony DG, Gottlieb A, Sprung J. Bleeding in a patient receiving platelet aggregation inhibitors. Anesth Analg. 2001;93:878–82. doi: 10.1097/00000539-200110000-00015. table of contents. [DOI] [PubMed] [Google Scholar]
- 29.Cinel I, Opal SM. Molecular biology of inflammation and sepsis: a primer. Crit Care Med. 2009;37:291–304. doi: 10.1097/CCM.0b013e31819267fb. [DOI] [PubMed] [Google Scholar]
- 30.Levi M, Schultz M, van der Poll T. Disseminated intravascular coagulation in infectious disease. Semin Thromb Hemost. 2010;36:367–77. doi: 10.1055/s-0030-1254046. [DOI] [PubMed] [Google Scholar]
- 31.Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis-associated disseminated intravascular coagulation and thromboembolic disease. Mediterr J Hematol Infect Dis. 2010;2:e2010024. doi: 10.4084/MJHID.2010.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levi M. The coagulant response in sepsis. Clin Chest Med. 2008;29:627–42, viii. doi: 10.1016/j.ccm.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Monroe DM, Key NS. The tissue factor-factor VIIa complex: procoagulant activity, regulation, and multitasking. J Thromb Haemost. 2007;5:1097–105. doi: 10.1111/j.1538-7836.2007.02435.x. [DOI] [PubMed] [Google Scholar]
- 34.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–21. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leoni F, Zaliani A, Bertolini G, Porro G, Pagani P, Pozzi P, et al. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc Natl Acad Sci U S A. 2002;99:2995–3000. doi: 10.1073/pnas.052702999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Z, Li Y, Liu B, Deperalta DK, Zhao T, Chong W, et al. Synergistic effects of hypertonic saline and valproic acid in a lethal rat two-hit model. J Trauma Acute Care Surg. 2013;74:991–7. doi: 10.1097/TA.0b013e31828583e3. discussion 7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Liu B, Fukudome EY, Lu J, Chong W, Jin G, et al. Identification of citrullinated histone H3 as a potential serum protein biomarker in a lethal model of lipopolysaccharide-induced shock. Surgery. 2011;150:442–51. doi: 10.1016/j.surg.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inoue K, Kobayashi M, Yano K, Miura M, Izumi A, Mataki C, et al. Histone deacetylase inhibitor reduces monocyte adhesion to endothelium through the suppression of vascular cell adhesion molecule-1 expression. Arterioscler Thromb Vasc Biol. 2006;26:2652–9. doi: 10.1161/01.ATV.0000247247.89787.e7. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Mahmud SA, Bitterman PB, Huo Y, Slungaard A. Histone deacetylase inhibitors suppress TF-kappaB-dependent agonist-driven tissue factor expression in endothelial cells and monocytes. J Biol Chem. 2007;282:28408–18. doi: 10.1074/jbc.M703586200. [DOI] [PubMed] [Google Scholar]
- 40.Hebbel RP, Vercellotti GM, Pace BS, Solovey AN, Kollander R, Abanonu CF, et al. The HDAC inhibitors trichostatin A and suberoylanilide hydroxamic acid exhibit multiple modalities of benefit for the vascular pathobiology of sickle transgenic mice. Blood. 2010;115:2483–90. doi: 10.1182/blood-2009-02-204990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahlknecht U, Schonbein C. Histone deacetylase inhibitor treatment downregulates VLA-4 adhesion in hematopoietic stem cells and acute myeloid leukemia blast cells. Haematologica. 2008;93:443–6. doi: 10.3324/haematol.11796. [DOI] [PubMed] [Google Scholar]
- 42.Saito S, Lasky JA, Guo W, Nguyen H, Mai A, Danchuk S, et al. Pharmacological inhibition of HDAC6 attenuates endothelial barrier dysfunction induced by thrombin. Biochem Biophys Res Commun. 2011;408:630–4. doi: 10.1016/j.bbrc.2011.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee WI, Yao TC, Yeh KW, Chen LC, Ou LS, Huang JL. PATCH Study Group. Stronger Toll-like receptor 1/2, 4, and 7/8 but less 9 responses in peripheral blood mononuclear cells in non-infectious exacerbated asthmatic children. Immunobiology. 2013;218(2):192–200. doi: 10.1016/j.imbio.2012.04.002. [DOI] [PubMed] [Google Scholar]