Abstract
Background
Phagocytes, especially monocytes, macrophages and dendritic cells, play a pivotal role in the innate as well as adaptive immune responses during sepsis. We have shown that inhibition of histone deacetylase (HDAC)-6 improves survival and increases bacterial clearance in a mouse model of cecal ligation and puncture (CLP). The aim of this study was to determine whether this effect was associated with changes in the number and composition of different blood cell types in the circulation.
Methods
C57BL/6J mice were subjected to CLP, and 1 hour later given an intraperitoneal injecton of either Tubastatin A dissolved in dimethyl sulfoxide (DMSO), or DMSO only. Sham-operated animals were treated in an identical fashion but not subjected to CLP. Forty-eight hours later peripheral blood was obtained via cardiac puncture and analyzed using a veterinary Hematrue hematology analyzer.
Results
Tubastatin A administration increased the number of circulating monocytes in the sham-operated as well as the CLP animals. In comparison to the sham, CLP animals displayed an increase in the granulocyte percentage in white blood cells, decrease in the lymphocyte number and percentage, with a resultant increase in the granulocyte to lymphocyte ratio. Treatment of CLP animals with Tubastatin A decreased the granulocyte percentage, and restored the lymphocyte number and percentage, which decreased the granulocyte to lymphocyte ratio. In the sham animals, Tubastatin A increased red blood cell (RBC) number, hematocrit and hemoglobin. This effect was not seen in CLP animals.
Conclusions
Tubastatin A treatment has significant impact on the composition of circulating blood cells. It increases the number of circulating monocytes and the RBC cell mass in sham-operated animals. In the CLP animals, it increases the monocyte count, decreases the percentage of granulocytes, restores the lymphocyte population, and decreases the granulocyte to lymphocyte ratio. These results may explain why Tubastatin A treatment improves survival in the septic models.
INTRODUCTION
Severe sepsis causes tremendous burden for health-care systems, with 750,000 new cases and more than 225,000 deaths annually in the United States 1–3. Severe sepsis and septic shock are among the most elusive syndromes in medicine for which all clinical trials have so far failed to show efficacy 4, 5.
Histone acetylation is an essential epigenetic mechanism that determines the amplitude of cellular and subcelluar signaling, by controlling the chromatin structure, accessibility of transcription factors to the DNA, and the subsequent gene transcription. This process regulated by the opposing actions of two families of enzymes: histone acetyltransferases (HATs) and histone deacetylases (HDACs). Histone acetylation relaxes the chromatin structure and promotes gene transcription, whereas histone deacetylation compacts the chromatin structure favoring gene silencing.
The 18 HDAC enzymes are grouped into four classes in humans and mice. Classical HDACs (class I, II and IV) are Zn2+ dependent, while the classes III sirtuins act through a NAD+-dependent mechanism 6. HDAC6 belongs to class IIb HDAC based on domain organization, and is unique among the classical HDAC family in that it is a cytoplasmic microtubule-associated enzyme. HDAC6 deacetylates tubulin, Hsp90 and cortactin, forms complexes with other partner proteins, and involves in a variety of biological processes 7.
Our lab was the first to demonstrate that administration of suberoylanilide hydroxamic acid (SAHA), a histone deacetylase inhibitor (HDACI), improved survival in lethal rodent models of lipopolysaccharide (LPS)-induced endotoxemia and cecal ligation and puncture (CLP)-induced severe sepsis 8–10. Selective inhibition of HDAC6 with Tubastatin A displays even better survival outcomes in the lethal CLP-sepsis model and increases bacterial clearance in circulation (unpublished data). However, the mechanisms underlying the increased survival outcomes and bacteria clearance after Tubastatin A treatment remain unclear. The current study was therefore designed to determine whether these effects are associated with changes in the number and composition of different blood cell types in the circulation.
MATERIALS AND METHODS
Sepsis Model: Cecal Ligation and Puncture (CLP)
Male C57BL/6J mice (18–26 gm) were purchased from The Jackson Laboratory and housed for 3 days before manipulations. The CLP murine model 11, modified by our laboratory, was used to induce fecal peritonitis. In brief, the peritoneal cavity was opened under inhaled isoflurane anesthesia. Cecum was eviscerated, ligated below the ileocecal valve using a 5-0 suture, and punctured through and through (2 holes) with a 20 gauge needle. The punctured cecum was squeezed to expel a small amount of fecal material and returned to the peritoneal cavity. The abdominal incision was closed in two layers with 4-0 silk suture. Animals were resuscitated by subcutaneous injection of 1 mL of saline. Sham-operated animals were handled in the same manner, except that the cecum was not ligated or punctured. This protocol was approved by the Animal Review Committee at the Massachusetts General Hospital. All surgery was performed under anesthesia, and all efforts were made to minimize suffering.
Administration of Tubastatin A and Experimental Design
Animals were randomly assigned to the following four groups (n = 5 for sham control, and 12/group for CLP): (a) Sham-operated animals (Sham Control); (b) Sham-operated animals injected with Tubastatin A (Sham + Tub.A); (c) Dimethyl sulfoxide (DMSO) vehicle treated animals after CLP (CLP Control), and (d) Tubastatin A treated animals after CLP (CLP + Tub.A). Mice received intra-peritoneal Tubastatin A dissolved in DMSO (70 mg/kg) or vehicle DMSO 1 h post-procedure. Sham-operated animals were subjected to laparotomy and intestinal manipulation, but the cecum was neither ligated nor punctured.
Peripheral Blood Analysis
Peripheral blood was obtained by via cardiac puncture 48 h post-procedure using a 1 mL heparinized syringe. Three hundred μl aliquots were then sampled and analyzed within 10 minutes of collection using a veterinary Hematrue hematology bench top analyzer (Heska Corporation, Loveland, CO) 12. The number and percentage of monocytes, granulocytes and lymphocytes in white blood cell, the number of red blood cells (RBCs) and platelets, hematocrit, and hemoglobin were measured in all animals.
Statistical Analysis
Results were represented as mean ± SEM. Differences between 4 groups were assessed using one way analysis of variance (ANOVA) followed by Bonferroni post hoc testing for multiple comparisons. Student’s t-test was used to compare the differences between two groups. All analyses were performed using GraphPad Prism. P values of 0.05 or less were considered significant.
RESULTS
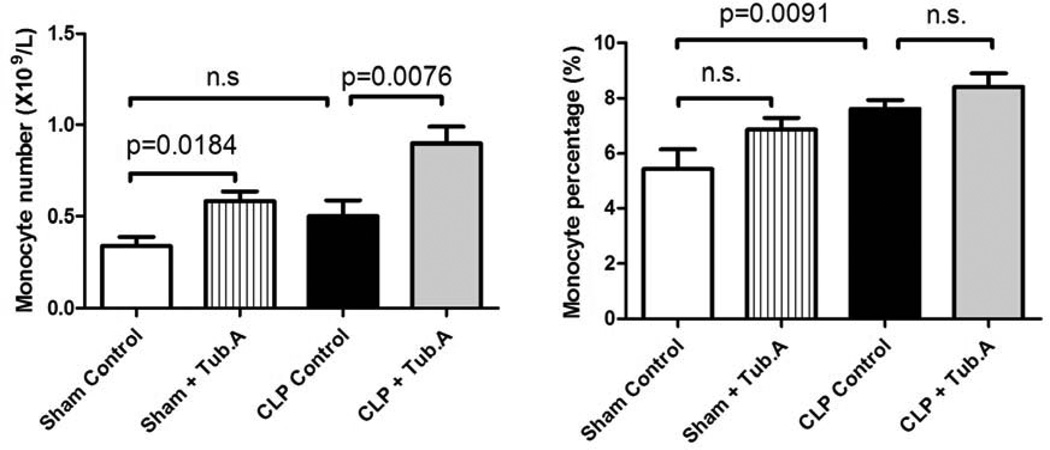
Tubastatin A increases circulating monocyte number in sham-operated and CLP animals 48 h post-procedure
HDAC6 inhibitor Tubastatin A increased circulating monocyte number in sham-operated (0.3±0.1 versus 0.6±0.1×109/L, P = 0.0184; Figure 1) and CLP animals (0.5±0.1 versus 0.9±0.1×109/L, P = 0.0076; Figure 1) 48 h post-procedure. The percent of monocytes in white blood cells was not significantly altered after the inhibition of HDAC6, either in sham-operated (5.4±0.7% versus 6.9±0.4%) or CLP animals (7.6±0.3% versus 8.4±0.5%).
Figure 1. Tubastatin A increases circulating monocyte number in sham-operated and CLP animals 48 h post-procedure.
Animals were randomly assigned to four groups as mentioned in Materials and Methods, and peripheral blood was obtained 48 h post-procedure. Three hundred μl aliquots were sampled and analyzed within 10 minutes of collection using a veterinary Hematrue hematology bench top analyzer. The number and percentage of monocytes in white blood cells were measured (means ± SEM, n = 5–12 mice/group). CLP: cecal ligation and puncture; Tub.A: tubastatin A.
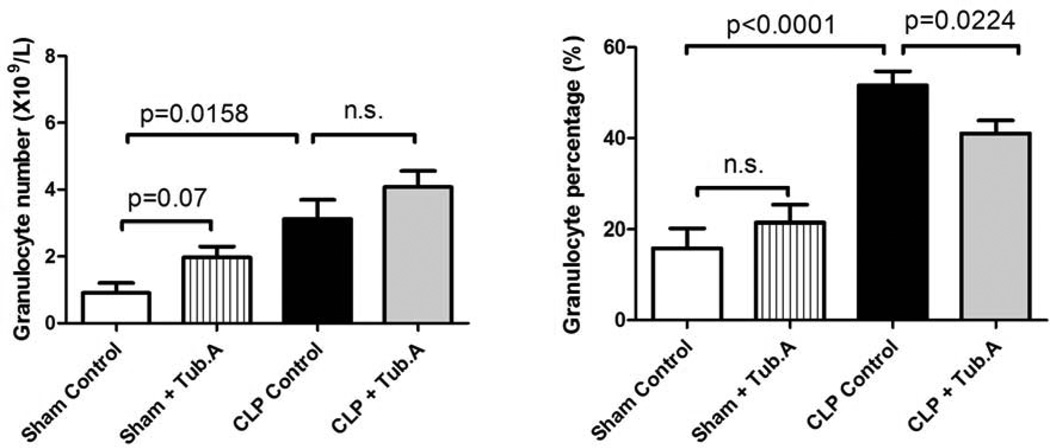
Tubastatin A decreases the granulocyte percentage in blood during severe sepsis
The granulocyte percentage in white blood cells increased 48 h after CLP (15.8±4.4% versus 51.7±2.9%, P < 0.0001; Figure 2), which was dramatically decreased after Tubastatin A treatment (51.7±2.9% versus 41.0±2.9%, P = 0.0224; Figure 2). In addition, Tubastatin A was not shown to affect the granulocyte percentage in sham-operated animals (15.8±4.4% versus 21.4±4.0%), and the number of granulocytes was not significantly affected by the inhibition of HDAC6 in sham-operated (0.9±0.3 versus 2.0±0.3) and CLP animals (3.1±0.6 versus 4.1±0.5).
Figure 2. Tubastatin A decreases the granulocyte percentage in blood during severe sepsis.
The number and percentage of granulocytes in white blood cells were determined 48 h post-procedure (means ± SEM, n = 5–12 mice/group).
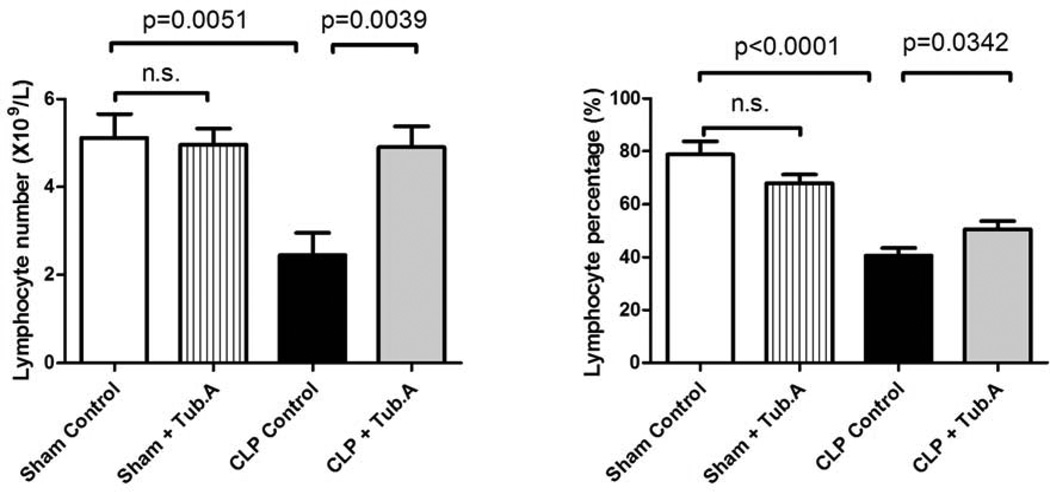
Tubastatin A restores the lymphocyte number and percentage in blood during severe sepsis
The number and percentage of lymphocytes decreased 48 h after CLP (lymphocyte number: 5.1±0.5 versus 2.5±0.5×109/L, P = 0.0051; lymphocyte percentage: 78.8±5.0% versus 40.7±2.9%, P < 0.0001; Figure 3), which were significantly restored by Tubastatin A treatment (lymphocyte number: 2.5±0.5 versus 4.9±0.5×109/L, P = 0.0039; lymphocyte percentage: 40.7±2.9% versus 50.6±3.1%, P = 0.0342; Figure 3). Tubastatin A did not alter lymphocyte number (5.1±0.5 versus 5.0±0.4) and percentage (78.8±5.0% versus 68.1±3.2%) in sham-operated animals.
Figure 3. Tubastatin A restores the lymphocyte number and percentage in blood during severe sepsis.
Lymphocyte number and percentage in white blood cells were measured 48 h post-procedure (means ± SEM, n = 5–12 mice/group).
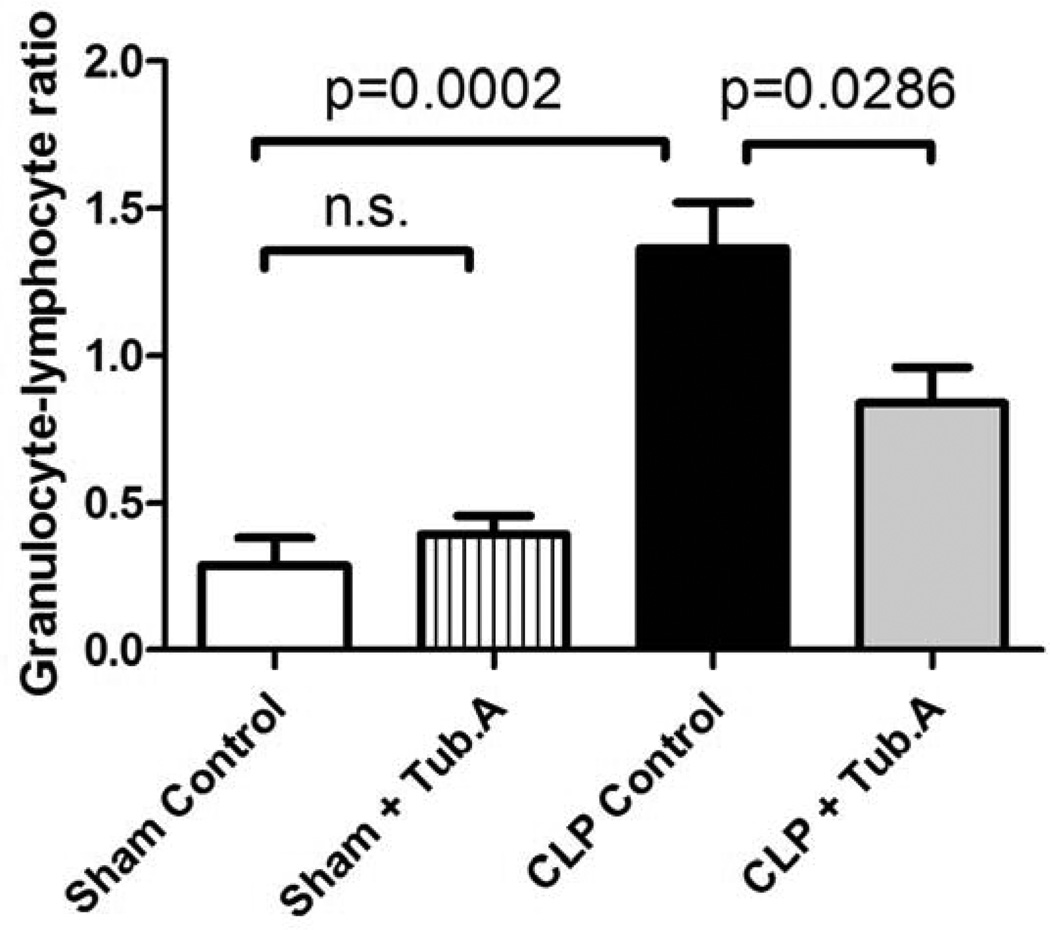
Tubastatin A decreases the granulocyte-lymphocyte ratio in a lethal septic model
The granulocyte-lymphocyte ratio increased 48 h after CLP (0.3±0.1 versus 1.4±0.2, P = 0.0002; Figure 4), and Tubastatin A attenuated the ratio markedly 1.4±0.2 versus 0.8±0.1, P = 0.0286; Figure 4). In sham-operated animals, the granulocyte-lymphocyte ratio was not affected by Tubastatin A treatment (0.3±0.1 versus 0.4±0.1).
Figure 4. Tubastatin A decreases the granulocyte-lymphocyte ratio in a lethal septic model.
The granulocyte-lymphocyte ratio was calculated by dividing the number of granulocytes by the number of lymphocyte 48 h post-procedure (means ± SEM, n = 5–12 mice/group).
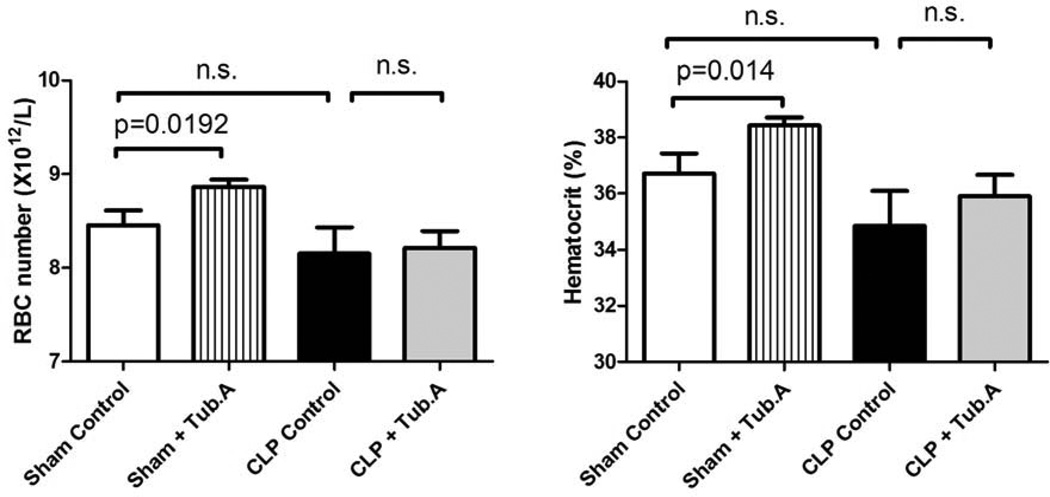
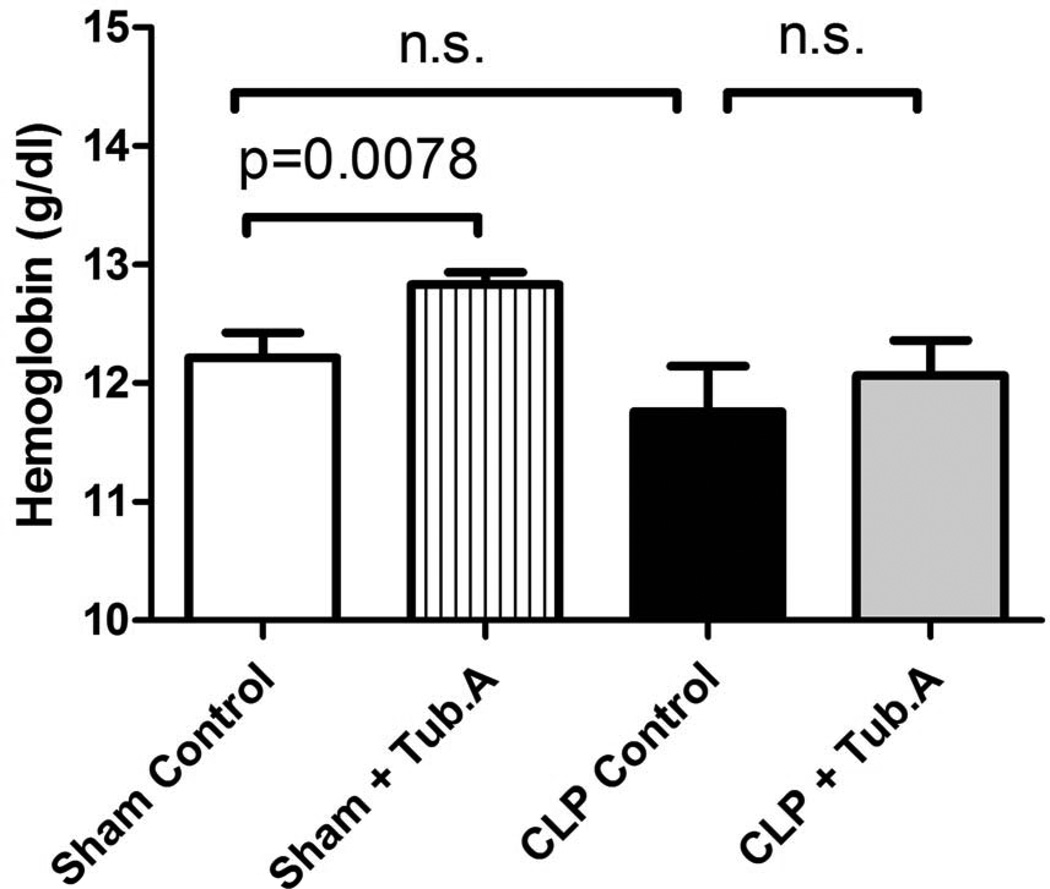
Tubastatin A increases RBC number, hematocrit and hemoglobin in sham-operated animals
Tubastatin A increased RBC number (8.5±0.2 versus 8.9±0.1×1012/L, P = 0.0192), hematocrit (36.7±0.7% versus 38.4±0.3% survival, P = 0.014) and hemoglobin (12.2±0.2 versus 12.8±0.1 g/dL, P = 0.0078) in sham-operated animals, but not in CLP animals (Figure 5 and 6).
Figure 5. Tubastatin A increases RBC number and hematocrit in sham-operated animals.
The RBC number and hematocrit were examined 48 h post-procedure (means ± SEM, n = 5–12 mice/group). RBC: red blood cell.
Figure 6. Tubastatin A increases hemoglobin in sham-operated animals.
Hemoglobin was measured 48 h post-procedure (means ± SEM, n = 5–12 mice/group).
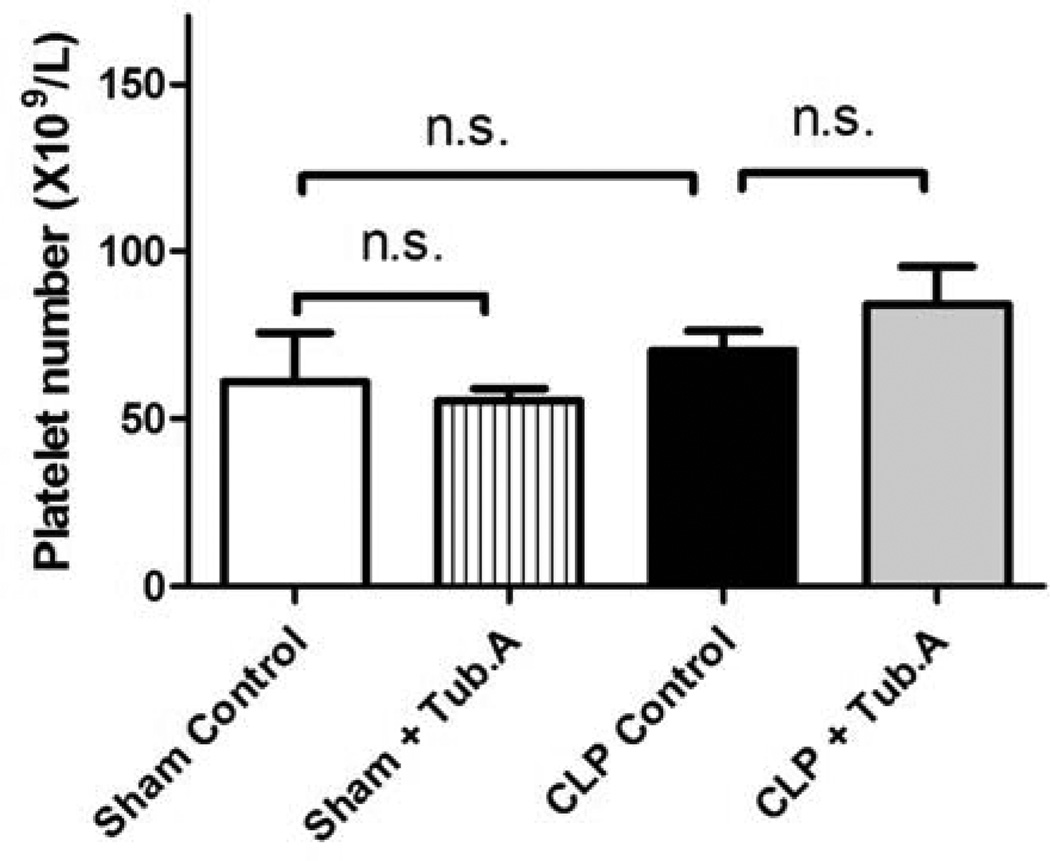
Platelet number was not significantly affected by Tubastatin A treatment
Tubastatin A treatment did not affect platelet number significantly in sham-operated (61.0±14.7 versus 55.5±3.4×109/L) and CLP animals (70.5±5.8 versus 84.2±10.9×109/L, Figure 7).
Figure 7. Tubastatin A does not affect platelet number significantly.
The platelet number was measured 48 h post-procedure (means ± SEM, n = 5–12 mice/group).
DISCUSSION
We have investigated the effects of HDAC6 inhibitor Tubastatin A on the number and composition of different blood cell types in circulation utilizing a lethal CLP septic model, and discovered some important findings: (1) HDAC6 inhibitor Tubastatin A increases circulating monocyte number in both sham-operated and CLP animals; (2) Tubastatin A decreased the granulocyte percentage, and restored the lymphocyte number and percentage, which decreased the granulocyte to lymphocyte ratio during severe sepsis; (3) Tubastatin A increases RBC number, hematocrit and hemoglobin in sham-operated animals.
Gene expression is turned on or off by transcriptional regulation and changes in chromatin structure, which are modulated by numerous posttranslational modifications at the amino-terminal tails of nucleosomal histones, such as acetylation/deacetylation, methylation, phosphorylation, and adenosine diphosphate (ADP) ribosylation. The acetylation status of chromatin is balanced by the activities of HATs and HDACs, which can activate or suppress the expression of specific genes 13–15.
HDAC6 is a unique HDAC localized in the cytoplasm, where it associates with non-histone substrates, including heat shock protein 90 (HSP90), α-tubulin and cortactin. It has an ubiquitin binding domain and two catalytic domains 16, 17. HDAC6 overexpression leads to tubulin deacetylation and increased cell motility 18, 19, whereas specific inhibition of HDAC6 activity or its downregulation by siRNA increases acetylation of α-tubulin and HSP90, reducing cellular motility and inducing degradation of HSP90 client proteins, such as Bcr-Abl, Raf-1, Akt, HER2/Neu, interleukin-1 receptor associated kinase 1 (IRAK1), and hypoxia-inducible factor (HIF)-1α 20–22.
HDAC6 has become a target for drug development to treat cancer, including acute myeloid leukemia, acute lymphocytic leukemia, multiple myeloma, and breast cancer 23–27. Cardiac HDAC6 catalytic activity is shown to increase in response to chronic hypertension, highlighting the need to determine whether HDAC6 inhibitors are protective in the setting of cardiovascular disease 28. Meanwhile, inhibition of HDAC6 promotes survival and regeneration of neurons, and has therapeutic potential to ameliorate injury of central nervous system 29. Notably, Tubastatin A, a selective HDAC6 inhibitor with a drug-like structure, simple synthesis and superior target selectivity, was generated in 2010 30. Our laboratory demonstrated that selective inhibition of HDAC6 with Tubastatin A improves long-term survival outcomes significantly in a lethal rodent model of CLP-sepsis, and increases bacteria clearance in circulation (unpublished data).
We discovered that pharmacological inhibition of HDAC6 by Tubastatin A increases circulating monocyte number remarkably in both sham-operated and CLP animals, which may explain the increased bacteria clearance and improved survival outcomes after Tubastatin A treatment in the lethal CLP-sepsis model. Monocytes and macrophages are critical effector cells contributing to the altered innate immune responses against infection, as the most efficient pathogen scavengers and the predominant source of inflammatory cytokines 31–33. Progressing monocyte and macrophage dysfunction impairs host defenses to invading pathogens, and results in immune dysfunction during severe sepsis and septic shock 34–36. Tubastatin A treatment replenishes circulating monocyte pool, which may potentially enhance host’s overall phagocytosis ability towards foreign pathogens and increases bacteria clearance, rendering significantly better survival outcomes in the lethal CLP-sepsis model.
The relationship between HDAC6 and monocyte development currently remains elusive. It is possible that inhibition of HDAC6 increases the number of monocytes by modulating epigenetic modifications and activating gene promoters of bone marrow mesenchymal stem cells, facilitating their differentiation into monocytes. Chromatin modifications were reported to be associated with transcriptional activity of monocytes. A genome-wide analysis of histone H3 trimethylation on lysine 4 (H3K4me3) and 27 (H3K27me3), and acetylation of H3 lysines (AcH3) in promoter regions indicated that H3K4me3 and AcH3 correlate with transcriptionally active genes significantly, whereas H3K27me3 is associated with inactive gene promoters 37. Meanwhile, monocyte acetylated histone H4 levels were significantly elevated in complication-free diabetic subjects, but not in diabetic patients with complications, suggesting that acetylation of monocytes may be a protective mechanism 38.
In addition, we discovered that Tubastatin A decreased the granulocyte percentage, and restored the lymphocyte number and percentage, which decreased the granulocyte to lymphocyte ratio in the lethal CLP model. An elevated preoperative neutrophil-lymphocyte ratio was revealed to be a convenient biomarker to identify patients with a poor prognosis after resection for primary gastric cancer 39. Preoperative neutrophil-lymphocyte ratio, in combination with CA125, may represent a simple and cost-effective method to identify ovarian cancers, and high neutrophil-lymphocyte ratio may predict adverse outcomes in ovarian cancer 40. Moreover, critically ill patients with severe sepsis or septic shock had significantly high neutrophil percentage and marked low lymphocyte counts. And the severity of clinical course, according to the Sequential Organ Failure Assessment (SOFA) as well as Acute Physiology And Chronic Health Evaluation II (APACHE II) score, correlated with the divergence of neutrophil and lymphocyte percentages in the white blood picture 41. Therefore, the decreased granulocyte to lymphocyte ratio after Tubastatin A treatment provides another explanation for the increased survival outcomes in the lethal septic model.
Anti-inflammatory host response during sepsis induces cell apoptosis of the innate and adaptive immune system, and these apoptotic cells result in immunosuppressive effect on surviving immune cells 42–44. Autopsies of patients with sepsis revealed extensive apoptosis of lymphocytes and gastrointestinal epithelial cells 45, which were similar to animal studies 46, 47. Our results demonstrated that Tubastatin A is protective against lymphocyte loss in the lethal septic model, resulting in better immune function in Tubastatin A-treated animals. Tubastatin A was also indicated to promote the activity Regulatory T cells (Tregs) in models of inflammation and autoimmunity, including experimental colitis and fully major histocompatibility complex (MHC)-incompatible cardiac allograft rejection, achieved by inhibition of the HDAC6-regulated protein HSP90 48.
Another important finding is that inhibition of HDAC6 increases RBC number, hematocrit and hemoglobin in sham-operated animals. Histone modifications have also been implicated in erythrocyte differentiation. Valproic acid (VPA), a non-selective HDACI, was reported to sustains the expression of stemness-related markers in hematopoietic stem/progenitor cells and enhance erythrocyte and megakaryocyte differentiation, by up-regulating genes of growth factor–independent protein 1B (GFI1B) and mixed-lineage leukemia translocated to chromosome 3 protein (MLLT3), mediated by the histone hyperacetylation at their promoter sites 49. HDACIs FK228 and VPA also enhances the potential of interleukin-3 to stimulate erythropoiesis and megakaryopoiesis 50, 51. Our results showed that Tubastatin A does not affect platelet number significantly, which indicates that selective inhibition of HDAC6 might have different effect on hematopoietic stem/progenitor cells, compared to non-selective HDACIs.
It is not clear if Tubastatin A has any impact on humoral immunity. We have explored the effects of Tubastatin A on immune organs during severe sepsis (unpublished data), and noted that selective inhibition of HDAC6 was associated with a significant attenuation of thymic and bone marrow atrophy, and splenic apoptosis. These results suggest that Tubastatin A may affect both humoral and cell-mediated immunity.
This study has certain limitation to be acknowledged. It would have been ideal to have cell differential data beyond the 48 hrs time-point. However, the high lethality of the model resulted in very few survivors beyond 48 hrs in the CLP control group. Without the matching data from the control group, any changes in the treated animals would have been difficult to interpret. Even when the animals survived, they were often too sick to permit repeated blood draws safely. Additional molecular mechanisms underlying epigenetic modifications of blood cells need to be investigated. And whether Tubastatin A affects the number and composition of other immune cells, such as bone marrow cells and splenocytes, need to be explored.
CONCLUSION
We have demonstrated that Tubastatin A, a selective inhibitor of HDAC6, has significant impact on the composition of circulating blood cells in a lethal polymicrobial sepsis model. Tubastatin A increases the number of circulating monocytes and the RBC cell mass in sham-operated animals. In addition, it increases the monocyte count, decreases the percentage of granulocytes, restores the lymphocyte population, and decreases the granulocyte to lymphocyte ratio in CLP animals. These results may explain why Tubastatin A treatment improves survival and increases bacteria clearance in the septic models.
Acknowledgements
This work was funded by a grant from NIH RO1 GM084127 to HBA.
ABBREVIATIONS
- AcH3
acetylation of H3 lysines
- ADP
adenosine diphosphate
- APACHE II
Acute Physiology And Chronic Health Evaluation II
- ANOVA
one way analysis of variance
- CLP
cecal ligation and puncture
- DMSO
dimethyl sulfoxide
- GFI1B
growth factor–independent protein 1B
- HATs
histone acetyltransferases
- HDAC
histone deacetylase
- HDACI
HDAC inhibitors
- HIF
hypoxia-inducible factor
- HSP
heat shock protein
- H3K4me3
histone H3 trimethylation on lysine 4
- IRAK1
interleukin-1 receptor associated kinase 1
- LPS
lipopolysaccharide
- MHC
major histocompatibility complex
- MLLT3
mixed-lineage leukemia translocated to chromosome 3 protein
- RBC
red blood cell
- SAHA
suberoylanilide hydroxamic acid
- SOFA
Sequential Organ Failure Assessment
- Tregs
Regulatory T cells
- VPA
Valproic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data presented at the 9th Annual Academic Surgical Congress in San Diego, CA (February, 2014).
Authors Contributions:
Conception and design (TZ, YL, HBA), analysis and interpretation (TZ, YL, HBA), data collection (TZ, BL), writing the article (TZ, YL), critical revision of the article (IH, YL, HBA) and obtaining funding (HBA).
REFERENCE
- 1.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2012;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 4.Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055–2064. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 5.Wenzel RP, Edmond MB. Septic shock--evaluating another failed treatment. N Engl J Med. 2012;366:2122–2124. doi: 10.1056/NEJMe1203412. [DOI] [PubMed] [Google Scholar]
- 6.Shakespear MR, Halili MA, Irvine KM, Fairlie DP, Sweet MJ. Histone deacetylases as regulators of inflammation and immunity. Trends in immunology. 2011;32:335–343. doi: 10.1016/j.it.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Valenzuela-Fernandez A, Cabrero JR, Serrador JM, Sanchez-Madrid F. HDAC6: a key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends in cell biology. 2008;18:291–297. doi: 10.1016/j.tcb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Liu B, Zhao H, Sailhamer EA, Fukudome EY, Zhang X, et al. Protective effect of suberoylanilide hydroxamic acid against LPS-induced septic shock in rodents. Shock. 2009;32:517–523. doi: 10.1097/SHK.0b013e3181a44c79. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Liu B, Fukudome EY, Kochanek AR, Finkelstein RA, Chong W, et al. Surviving lethal septic shock without fluid resuscitation in a rodent model. Surgery. 2010;148:246–254. doi: 10.1016/j.surg.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao T, Li Y, Liu B, Liu Z, Chong W, Duan X, et al. Novel pharmacologic treatment attenuates septic shock and improves long-term survival. Surgery. 2013;154:206–213. doi: 10.1016/j.surg.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohr AM, ElHassan IO, Hannoush EJ, Sifri ZC, Offin MD, Alzate WD, et al. Does beta blockade postinjury prevent bone marrow suppression? J Trauma. 2011;70:1043–1049. doi: 10.1097/TA.0b013e3182169326. discussion 9–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fullgrabe J, Hajji N, Joseph B. Cracking the death code: apoptosis-related histone modifications. Cell Death Differ. 2010;17:1238–1243. doi: 10.1038/cdd.2010.58. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Gilquin B, Khochbin S, Matthias P. Two catalytic domains are required for protein deacetylation. The Journal of biological chemistry. 2006;281:2401–2404. doi: 10.1074/jbc.C500241200. [DOI] [PubMed] [Google Scholar]
- 17.Zou H, Wu Y, Navre M, Sang BC. Characterization of the two catalytic domains in histone deacetylase 6. Biochemical and biophysical research communications. 2006;341:45–50. doi: 10.1016/j.bbrc.2005.12.144. [DOI] [PubMed] [Google Scholar]
- 18.Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4389–4394. doi: 10.1073/pnas.0430973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 20.Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, et al. Inhibition of histone deacetylase-6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. The Journal of biological chemistry. 2005;280:26729–26734. doi: 10.1074/jbc.C500186200. [DOI] [PubMed] [Google Scholar]
- 21.Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, et al. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Molecular cell. 2005;18:601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Triantafilou M, Triantafilou K. Heat-shock protein 70 and heat-shock protein 90 associate with Toll-like receptor 4 in response to bacterial lipopolysaccharide. Biochem Soc Trans. 2004;32:636–639. doi: 10.1042/BST0320636. [DOI] [PubMed] [Google Scholar]
- 23.Aldana-Masangkay GI, Sakamoto KM. The role of HDAC6 in cancer. J Biomed Biotechnol. 2011;2011:875824. doi: 10.1155/2011/875824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eklund EA, Platanias LC. Inhibition of histone deacetylase 6 as a therapeutic strategy for acute lymphocytic leukemia. Leuk Lymphoma. 2011;52:1421–1422. doi: 10.3109/10428194.2011.577259. [DOI] [PubMed] [Google Scholar]
- 25.Hackanson B, Rimmele L, Benkisser M, Abdelkarim M, Fliegauf M, Jung M, et al. HDAC6 as a target for antileukemic drugs in acute myeloid leukemia. Leuk Res. 2012;36:1055–1062. doi: 10.1016/j.leukres.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 26.Santo L, Hideshima T, Kung AL, Tseng JC, Tamang D, Yang M, et al. Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma. Blood. 2012;119:2579–2589. doi: 10.1182/blood-2011-10-387365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulz R, Marchenko ND, Holembowski L, Fingerle-Rowson G, Pesic M, Zender L, et al. Inhibiting the HSP90 chaperone destabilizes macrophage migration inhibitory factor and thereby inhibits breast tumor progression. J Exp Med. 2012;209:275–289. doi: 10.1084/jem.20111117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemon DD, Horn TR, Cavasin MA, Jeong MY, Haubold KW, Long CS, et al. Cardiac HDAC6 catalytic activity is induced in response to chronic hypertension. J Mol Cell Cardiol. 2011;51:41–50. doi: 10.1016/j.yjmcc.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivieccio MA, Brochier C, Willis DE, Walker BA, D'Annibale MA, McLaughlin K, et al. HDAC6 is a target for protection and regeneration following injury in the nervous system. Proc Natl Acad Sci U S A. 2009;106:19599–19604. doi: 10.1073/pnas.0907935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butler KV, Kalin J, Brochier C, Vistoli G, Langley B, Kozikowski AP. Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. J Am Chem Soc. 2010;132:10842–10846. doi: 10.1021/ja102758v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annual review of immunology. 2002;20:825–852. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 33.Mehta A, Brewington R, Chatterji M, Zoubine M, Kinasewitz GT, Peer GT, et al. Infection-induced modulation of m1 and m2 phenotypes in circulating monocytes: role in immune monitoring and early prognosis of sepsis. Shock. 2004;22:423–430. doi: 10.1097/01.shk.0000142184.49976.0c. [DOI] [PubMed] [Google Scholar]
- 34.Ayala A, Chaudry IH. Immune dysfunction in murine polymicrobial sepsis: mediators, macrophages, lymphocytes and apoptosis. Shock. 1996;6(Suppl 1):S27–S38. [PubMed] [Google Scholar]
- 35.Munoz C, Carlet J, Fitting C, Misset B, Bleriot JP, Cavaillon JM. Dysregulation of in vitro cytokine production by monocytes during sepsis. The Journal of clinical investigation. 1991;88:1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tserel L, Kolde R, Rebane A, Kisand K, Org T, Peterson H, et al. Genome-wide promoter analysis of histone modifications in human monocyte-derived antigen presenting cells. BMC Genomics. 2010;11:642. doi: 10.1186/1471-2164-11-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen SS, Jenkins AJ, Majewski H. Elevated plasma prostaglandins and acetylated histone in monocytes in Type 1 diabetes patients. Diabet Med. 2009;26:182–186. doi: 10.1111/j.1464-5491.2008.02658.x. [DOI] [PubMed] [Google Scholar]
- 39.Shimada H, Takiguchi N, Kainuma O, Soda H, Ikeda A, Cho A, et al. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer. 2010;13:170–176. doi: 10.1007/s10120-010-0554-3. [DOI] [PubMed] [Google Scholar]
- 40.Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009;58:15–23. doi: 10.1007/s00262-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5–14. [PubMed] [Google Scholar]
- 42.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 43.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 44.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Hotchkiss RS, Swanson PE, Cobb JP, Jacobson A, Buchman TG, Karl IE. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-cell-deficient mice. Crit Care Med. 1997;25:1298–1307. doi: 10.1097/00003246-199708000-00015. [DOI] [PubMed] [Google Scholar]
- 47.Oberholzer C, Oberholzer A, Clare-Salzler M, Moldawer LL. Apoptosis in sepsis: a new target for therapeutic exploration. FASEB J. 2001;15:879–892. doi: 10.1096/fj.00-058rev. [DOI] [PubMed] [Google Scholar]
- 48.de Zoeten EF, Wang L, Butler K, Beier UH, Akimova T, Sai H, et al. Histone deacetylase 6 and heat shock protein 90 control the functions of Foxp3(+) T-regulatory cells. Mol Cell Biol. 2011;31:2066–2078. doi: 10.1128/MCB.05155-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zini R, Norfo R, Ferrari F, Bianchi E, Salati S, Pennucci V, et al. Valproic acid triggers erythro/megakaryocyte lineage decision through induction of GFI1B and MLLT3 expression. Exp Hematol. 2012;40:1043–1054. e6. doi: 10.1016/j.exphem.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 50.Yamamura K, Ohishi K, Katayama N, Yu Z, Kato K, Masuya M, et al. Pleiotropic role of histone deacetylases in the regulation of human adult erythropoiesis. Br J Haematol. 2006;135:242–253. doi: 10.1111/j.1365-2141.2006.06275.x. [DOI] [PubMed] [Google Scholar]
- 51.Liu B, Ohishi K, Yamamura K, Suzuki K, Monma F, Ino K, et al. A potential activity of valproic acid in the stimulation of interleukin-3-mediated megakaryopoiesis and erythropoiesis. Exp Hematol. 2010;38:685–695. doi: 10.1016/j.exphem.2010.03.019. [DOI] [PubMed] [Google Scholar]