Abstract
Previous studies have reported that chronic increases in dietary salt intake enhance sympathetic nerve activity (SNA) and arterial blood pressure (ABP) responses evoked from brainstem nuclei of normotensive, salt-resistant rats. The purpose of the present study was to determine whether this sensitization results in exaggerated SNA and ABP responses during activation of various cardiovascular reflexes and also increases ABP variability (BPV). Male Sprague-Dawley rats were fed 0.1% NaCl chow (low), 0.5% NaCl chow (medium), 4.0% NaCl chow (high) for 14–17 days. Then, animals were prepared for recordings of lumbar, renal, and splanchnic SNA and ABP. The level of dietary salt intake directly correlated with the magnitude of SNA and ABP responses to electrical stimulation of sciatic afferents or intracerebroventricular infusion of 0.6M or 1.0M NaCl. Similarly, there was a direct correlation between the level of dietary salt intake and the sympathoinhibitory responses produced by acute volume expansion, stimulation of the aortic depressor nerve or cervical vagal afferents. In contrast, dietary salt intake did not affect the sympathetic and ABP responses to chemoreflex activation produced by hypoxia or hypercapnia. Chronic lesion of the anteroventral 3rd ventricle region eliminated the ability of dietary salt intake to modulate these cardiovascular reflexes. Finally, rats chronically instrumented with telemetry units indicate that increased dietary salt intake elevated BPV but not mean ABP. These findings indicate that dietary salt intake works through the forebrain hypothalamus to modulate various centrally-mediated cardiovascular reflexes and increase BPV.
Keywords: NaCl, salt-resistant, lamina terminalis, blood pressure, sympathetic nerve activity
INTRODUCTION
Several lines of evidence suggest excess dietary salt (NaCl) intake adversely affects cardiovascular function independent of changes in arterial blood pressure (ABP) [1–8]. First, population studies indicate that normotensive humans with a greater level of salt intake have an increased risk for an adverse cardiovascular event [1, 2, 4]. Second, evidence from humans and animal models indicates that excess dietary salt intake promotes endothelial and microvascular dysfunction [9, 10], left ventricular hypertrophy [11], fibrosis of the heart, kidney, and arteries [11], and enhances cardiovascular responses evoked by chemical excitation or inhibition of several brainstem structures [12–17]. This latter effect has been largely attributed to a central sensitization of brainstem autonomic pathways as stimulation of the dorsolateral funiculus to activate downstream pathways produced similar changes in sympathetic nerve activity (SNA) and/or ABP of rats fed low versus high NaCl diets [13, 17]. Collectively, these observations suggest that dietary salt intake modulates the responsiveness or gain of central autonomic pathways to alter the functional regulation of SNA and ABP in normotensive, salt-resistant rats.
A limitation of the above-mentioned studies is that the experimental manipulations were largely limited to exogenous application of various neurotransmitters into brainstem nuclei. A key question is whether the ability of dietary salt to modulate the responsiveness of central autonomic circuits results in functional changes in the regulation of SNA and ABP during activation of physiological reflexes. Evidence of this idea arises from a limited number of studies that have reported greater pressor responses to stimulation of sciatic afferents [6, 16], intracerebroventricular injection of angiotensin II [18] or hypertonic NaCl [19], and the exercise pressor reflex [20]. In addition, the depressor response to electrical activation of the aortic depressor nerve was also enhanced by increased dietary salt intake [17]. Since the majority of these previous studies lacked direct recordings of SNA, the differences in ABP cannot be attributed to differences in SNA or the ability of dietary salt intake to alter the responsiveness of central autonomic circuits versus a change in vascular reactivity.
Therefore, the purpose of the present study was several-fold. First, we simultaneously recorded SNA to a number of end-organs during activation of various sympathetic reflexes in animals fed low, moderate, and high NaCl diets. Second, since a prior study reported that the ability of dietary salt intake to modulate centrally-evoked responses depends on the integrity of the anteroventral 3rd ventricular region (AV3V) [12], the above experiments were repeated in animals with chronic AV3V lesions. Finally, if increased dietary salt intake exaggerates a number of cardiovascular reflexes in normotensive, salt-resistant rats, we hypothesized that dietary salt intake would alter blood pressure variability (BPV). Increased BPV has been identified as a predictor of subsequent cardiovascular disease [21].
METHODS
All of the experimental procedures conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the Pennsylvania State College of Medicine.
Animals
Male Sprague-Dawley rats (225–275g, Charles River Laboratories) were singly-housed in a temperature-controlled room (22–23°C) with a 12:12h light:dark cycle. Sham or AV3V lesions were performed as described previously in our laboratory [12] (Figure S1, please see http://hyper.ahajournals.org). After water and food intake returned to pre-surgical levels (~3–5 days), these animals and additional control rats were fed 0.1% NaCl chow (low, D17020, Research Diets) or 4.0% NaCl chow (high, D17013, Research Diets) with ad libitum access to de-ionized water for 14–17 days. Then, cardiovascular reflexes were evaluated as described below. Food and fluid intake were measured daily. In addition, we assessed a subset of cardiovascular reflexes in two other dietary NaCl groups. First, control animals were fed 0.5% NaCl chow (D14020401, Research Diets) to test the effect of a moderate NaCl diet. Second, control animals were fed 0.1% NaCl chow but water was replaced by 0.9% NaCl solution as prior studies have supplemented NaCl to the drinking water rather than chow [12–14, 17]. Again, these latter two groups were on the respective diet for 14–17 days.
Sympathetic Reflexes
After 14–17 days of the respective diet, sympathetic and cardiovascular responses to various reflexes were examined in Inactin-anesthetized rats at least 60 min after all surgical procedures were completed (please see http://hyper.ahajournals.org). To maintain stable levels of SNA and ABP, only 3–4 reflexes were tested per animal. Responses to chemoreceptor stimulation or volume expansion were not tested in sham or AV3V-lesioned animals. 1) Somatosympathetic Reflex. The sciatic afferent nerve was placed onto a bipolar, stainless steel electrode and stimulated electrically (1ms pulse, 500µA, 5ms duration) over a range of frequencies (1, 2, 5, 10, 20 Hz) in a random order while animals were paralyzed with pancuronium (0.5 mg/kg, IV) [17]. 2) Chemoreceptor. Rats were ventilated with a hypoxic (10% O2, 90% N2, 30 s) or hypercapnic (33% O2, 7% CO2, 60 N2, 30 s) gas mixture. Responses were tested twice separated by a minimum of 5 min. 3) Volume Expansion. A double-lumen jugular catheter was placed into the veno-atrial junction as verified by the presence of atrial pressure waveforms. Volume expansion was produced by infusion of isotonic saline (20% estimated blood volume of 60mL/kg body weight) over 5min through the veno-atrial catheter [22]. Atrial pressure was measured through the second veno-atrial catheter. 4) Aortic Depressor Nerve. The aortic depressor nerve was placed onto a bipolar, stainless steel electrode and stimulated electrically (2 ms pulse, 500 µA, 15s duration) at various frequencies (1, 2, 5, 10, and 20 Hz) [17]. 5) Vagus Afferent Nerve. The left cervical vagus nerve was placed onto a bipolar, stainless steel electrode, crushed distally, and stimulated electrically (1 ms pulse, 500µA, 5s duration) at various frequencies (1, 2, 5, 10, 20 Hz). 6) Increased Cerebrospinal Fluid Sodium Concentration. Animals were pretreated with the vasopressin antagonist Manning compound (10 µg/kg, IV). Approximately 10 min later, 0.6M or 1.0M NaCl (5uL/10 min) was infused through a 23 gauge cannula implanted into the lateral ventricle. Infusions were separated by 60 min. The ICV infusions were performed last. The cannula placement was confirmed at the end of experiments by the presence of dye in the third and fourth ventricle after injection of Evan’s Blue Dye (2µL, 0.5%).
Analysis of Blood Pressure Variability (BPV)
BPV was analyzed in a separate group of rats chronically instrumented with PA-C40 telemetry units (Data Sciences International) and fed 0.1% and 4.0% NaCl chow in 2 week intervals (please see http://hyper.ahajournals.org). Systolic, diastolic, and mean ABP and heart rate were sampled at 2KHz and analyzed on a beat-to-beat basis using Spike2 software for both day (1–3 PM) and night (1–3 AM) periods (lights on 7AM–7PM) at Day 14 of the respective diet.
Statistical Analysis
All data are expressed as mean±SEM. Changes in SNA were calculated after background noise (assessed after nerve crush) was subtracted. Peak changes (1s) were calculated for all responses, compared to a 30s (sciatic, aortic depressor, cervical vagus, and chemoreceptor experiments) or 5 min (volume expansion, central NaCl infusions) baseline segment. Data was analyzed by a 1- or 2-way ANOVA with repeated measures when appropriate. When significant F values were obtained, post-hoc comparisons were performed using independent (between groups) or paired (within group) t-tests with a layered Bonferroni correction. Linear regression analysis was performed using SigmaPlot. A P value <0.05 was significant.
RESULTS
For purposes of presentation, results of control and AV3V-lesioned rats fed 0.1% or 4.0% NaCl are illustrated here whereas data for those animals fed 0.5% NaCl chow or drinking 0.9% NaCl and tested for a subset of reflexes are located within the online supplement (please see http://hyper.ahajournals.org). Since there were no differences in any variable between control versus sham-lesioned groups, the data sets were collapsed into a single control group for the respective diet. As expected, rats ingested significantly more NaCl and water when fed 4.0% versus 0.1% NaCl chow (Table 1). Importantly, daily NaCl intake did not differ between control versus AV3V-lesioned rats fed the same diet. Plasma [Na+] and [Cl−] were significantly higher in control and AV3V-lesioned rats fed 4.0% versus 0.1% NaCl.
Table 1.
Characteristics of control and AV3V-lesioned rats.
| Control Group | AV3V Lesion | |||
|---|---|---|---|---|
| Characteristic | 0.1% | 4.0% | 0.1% | 4.0% |
| Total # of animals | 31 (5) | 27 (7) | 8 | 9 |
| (# of sham) | ||||
| NaCl Intake, mg/day | 27±1 | 954±28 * | 25±1 | 922±60 * |
| Fluid Intake, mL/day | 26±2 | 65±2 * | 25±2 | 67±5 * |
| Mean ABP, mmHg | 107±3 | 107±3 | 101±7 | 107±5 |
| Heart Rate, bpm | 377±12 | 398±8 | 440±12 | 413±22 |
| Lumbar SNA, µV | 1.98±0.29 | 1.66±0.20 | 2.53±0.44 | 1.75±0.38 |
| Renal SNA, µV | 3.52±0.52 | 3.09±0.46 | 2.44±0.83 | 3.31±1.21 |
| Splanchnic SNA, µV | 2.22±0.28 | 1.62±0.19 | 1.79±0.31 | 1.34±0.41 |
| Plasma [Na+], mM | 141.5±1.0 | 143.3±1.0 * | 142.1±1.2 | 146.0±1.3 *† |
| Plasma [K+], mM | 3.4±0.1 | 3.3±0.2 | 3.1±0.1 | 3.1±0.2 |
| Plasma [Cl−], mM | 109.7±1.3 | 113.8±1.0 * | 110.7±1.3 | 115.4±1.5 * |
Values are mean±SEM.
P<0.05 vs control group fed 0.1% NaCl,
P<0.05 vs control group fed same diet.
Somatosympathetic Reflex
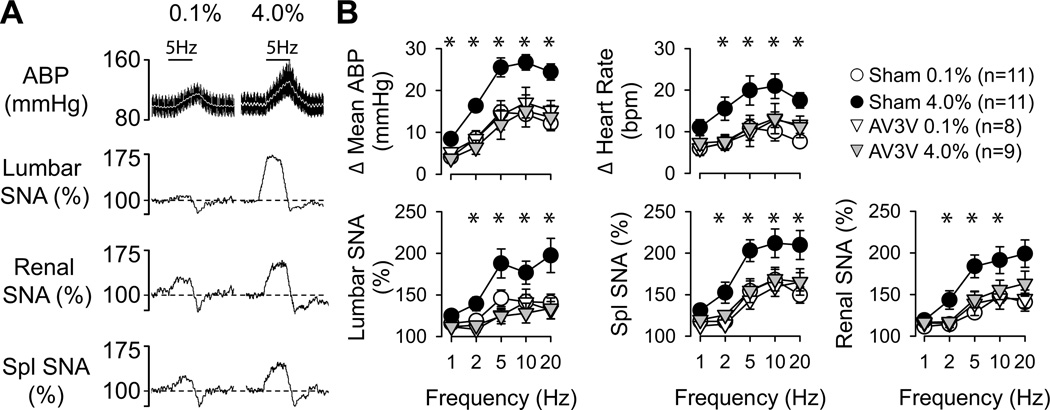
Electrical activation of the sciatic nerve produced frequency-dependent increases in mean ABP, heart rate, and lumbar, splanchnic, and renal SNA (Figure 1). These responses were significantly greater in control rats fed 4.0% versus 0.1% NaCl (Figure 1). Similar findings were observed in control rats fed 0.5% NaCl or drinking 0.9% NaCl (Figure S2, http://hyper.ahajournals.org). Moreover, a linear regression analysis revealed there was a NaCl-dependent relationship as the magnitude of these responses progressively increased with the average daily intake of NaCl across rats fed 0.1%, 0.5%, and 4.0% NaCl (5Hz: r≥0.412 for all variables, P<0.05). However, these differences were completely absent after AV3V lesions as AV3V-lesioned rats fed 4.0% NaCl displayed changes in SNA, HR, and mean ABP that were not statistically different from control or AV3V-lesioned rats fed 0.1% NaCl (Figure 1).
Figure 1.
(A) Example of mean (grey line) and pulsatile ABP, heart rate and rectified/integrated (1s time constant) lumbar, renal, and splanchnic SNA during electrical stimulation of sciatic afferent nerves (5Hz, 5s duration) in control rats fed 0.1% or 4.0% NaCl diets. (B) Mean±SEM of peak (1s) changes reflect greater increase in ABP, heart rate, and SNA of control rats fed 4.0% versus 0.1% NaCl diets (*P<0.05). The greater responses were absent in AV3V-lesioned rats.
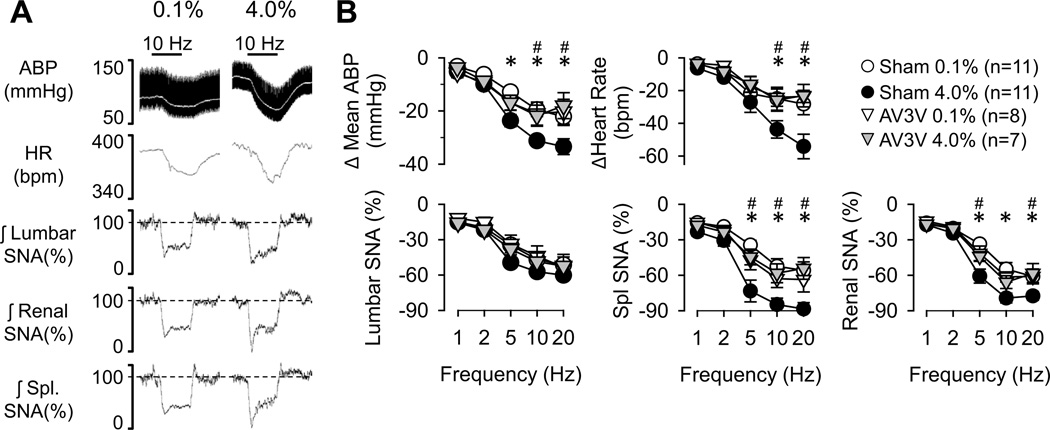
Aortic Depressor Nerve
Sympathoinhibitory responses produced by stimulation of the aortic depressor nerve at higher frequencies (5–20 Hz) were significantly greater in rats fed 4.0% versus 0.1% NaCl (Figure 2). The only variable unaffected by dietary salt intake was lumbar SNA; the decrease in lumbar SNA was not statistically different between rats fed 0.1% versus 4.0% NaCl. Similar findings were observed in control rats fed 0.5% NaCl or drinking 0.9% NaCl (Figure S3, http://hyper.ahajournals.org). Again, there was a NaCl-dependent relationship as the magnitude of these responses progressively increased with the average daily intake of NaCl across rats fed 0.1, 0.5, and 4.0% NaCl chow (5Hz: r≥0.397 for all variables except lumbar SNA, P<0.05). These exaggerated sympathoinhibitory responses in rats fed 4.0% NaCl were absent in AV3V-lesioned rats (Figure 2).
Figure 2.
(A) Example of mean (grey line) and pulsatile ABP, heart rate and rectified/integrated (1s time constant) lumbar, renal, and splanchnic SNA during electrical stimulation of aortic depressor nerve (10Hz, 15 s duration) in rats fed 0.1% or 4.0% NaCl diets. (B) Mean±SEM of peak (1s) changes reflect greater decreases in ABP, heart rate, and splanchnic and renal SNA of rats fed 4.0% versus 0.1% NaCl diets. The greater responses were absent in AV3V-lesioned rats. *P<0.05, 0.1% versus 4.0% NaCl in control/sham rats; #P<0.05, control versus AV3V-lesioned rats fed 4.0% NaCl
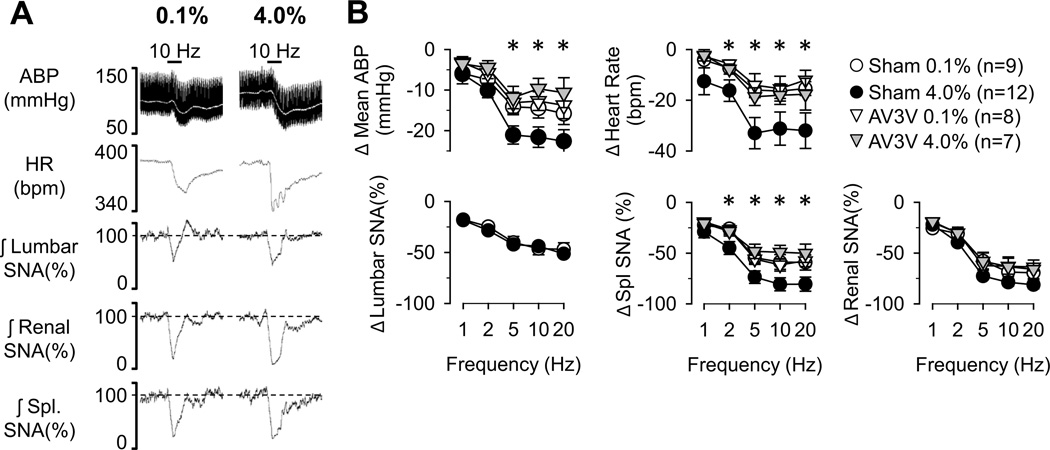
Vagal Afferent Nerve
The decreases in mean ABP, heart rate, and splanchnic SNA during stimulation of vagal afferent nerves were significantly greater in rats fed 4.0% versus 0.1% NaCl (Figure 3). Although lumbar and renal SNA decreased in a frequency-dependent manner, the magnitude of these responses were not statistically different between rats fed 0.1% versus 4.0% NaCl. The responses were not tested in rats fed 0.5% NaCl or drinking 0.9% NaCl. Again, these differences were absent in AV3V-lesioned animals (Figure 3).
Figure 3.
(A) Example of mean (grey line) and pulsatile ABP, heart rate and rectified/integrated (1s time constant) lumbar, renal, and splanchnic SNA during electrical stimulation of vagal afferents (10Hz, 5 s duration) in rats fed 0.1% or 4.0% NaCl diets. (B) Mean±SEM of peak (1s) changes reflect greater decreases in ABP, heart rate, and splanchnic SNA of rats fed 4.0% versus 0.1% NaCl diets. The greater responses were absent in AV3V-lesioned rats. *P<0.05, 0.1% versus 4.0% NaCl in control rats
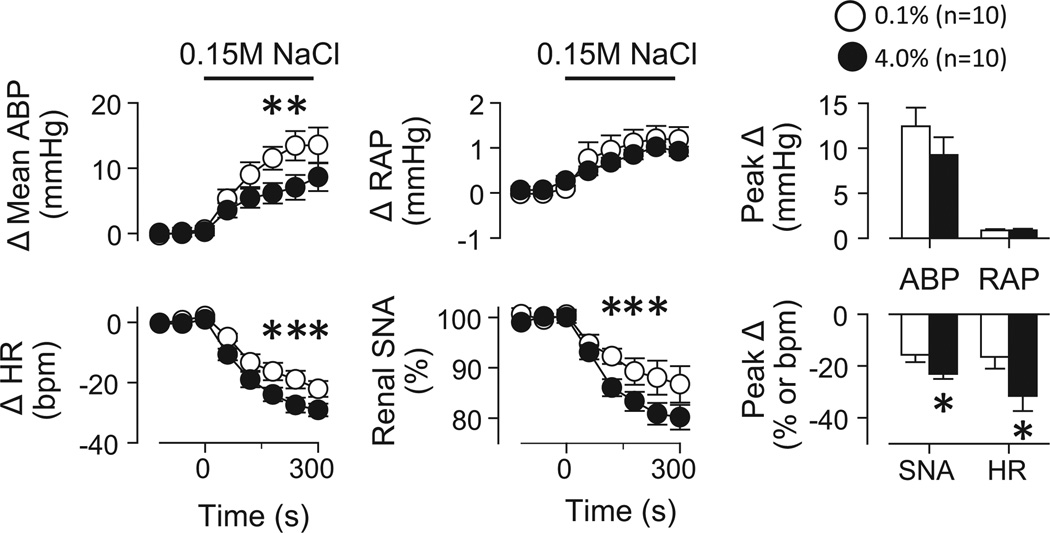
Volume Expansion
Acute volume expansion produced significant increases in mean ABP and right atrial pressure but decreased heart rate and renal SNA (Figure 4). The decreases in heart rate and renal SNA were significantly greater in rats fed 4.0% versus 0.1% NaCl. Splanchnic and lumbar sympathoinhibitory responses tended to be significantly greater in rats fed 4.0% versus 0.1% NaCl (P=0.09, data not shown). Baseline right atrial pressure was not different between groups (0.1%: 3.61±0.22 vs 4.0%: 4.00±0.12 mmHg, P>0.2). The responses were not tested in control rats fed 0.5% NaCl or drinking 0.9% NaCl or AV3V-lesioned rats.
Figure 4.
(A) Mean±SEM changes in mean ABP, heart rate (HR), right atrial pressure (RAP), and renal SNA during volume expansion of rats fed 0.1% versus 4.0% NaCl. (B) Peak 1s responses.*P<0.05 versus 0.1% NaCl
Intracerebroventricular (ICV) Infusion of Hypertonic NaCl
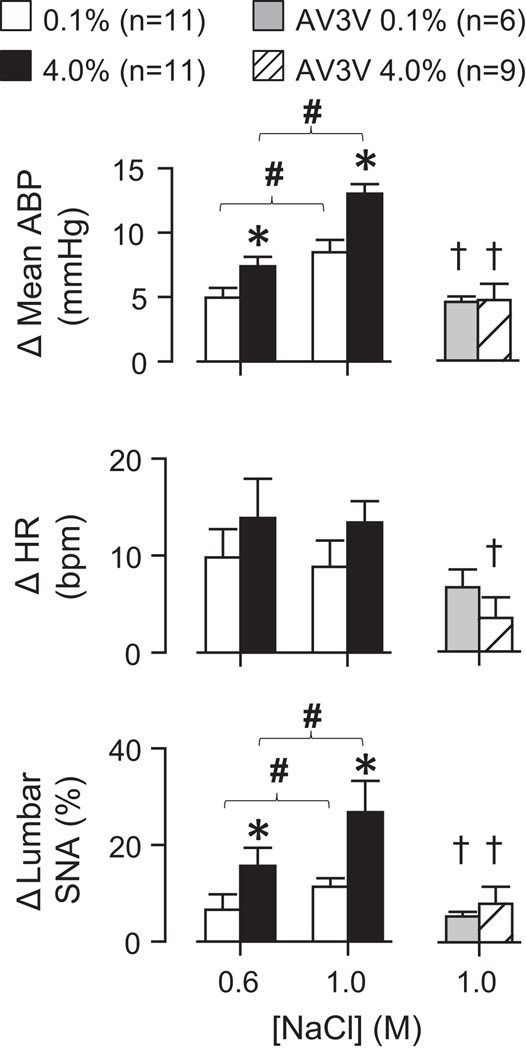
ICV infusion of 0.6M or 1.0M NaCl produced dose-dependent increases in mean ABP and lumbar SNA (Figure 5). Again, the magnitude of these responses was significantly greater in rats fed 4.0% versus 0.1% NaCl. Similar findings were observed in control rats fed 0.5% NaCl or drinking 0.9% NaCl (Figure S4, http://hyper.ahajournals.org). Again, there was a significant linear correlation between the changes in lumbar SNA and mean ABP and the average daily intake of NaCl across rats fed 0.1%, 0.5% and 4.0% NaCl (1.0M: r≥0.433 for mean ABP and lumbar SNA, P<0.05). A3V3 lesion significantly attenuated this sympathoexcitatory response in rats fed 0.1% and 4.0% NaCl (Figure 5). In addition, there were no longer differences in the magnitude of these responses between AV3V-lesioned rats fed 0.1% versus 4.0% NaCl.
Figure 5.
ICV infusion of 0.6M or 1.0M NaCl produced significantly greater increases in mean ABP and lumbar SNA of rats fed 4.0% versus 0.1% NaCl. Lesion of the AV3V significantly attenuated these responses and abolished the differences between rats fed 0.1% versus 4.0% NaCl chow. *P<0.05 versus 0.1% NaCl; #P<0.05, 0.6M versus 1.0M NaCl within same diet; †P<0.05 versus control rats.
Chemoreflex
Chemoreceptor activation by ventilation with a hypoxic or hypercapnic gas mixture decreased mean ABP and increased SNA. However, the magnitude of these changes were not statistically different between control rats fed 0.1% versus 4.0% NaCl (Figure S5, http://hyper.ahajournals.org).
Analysis of BPV
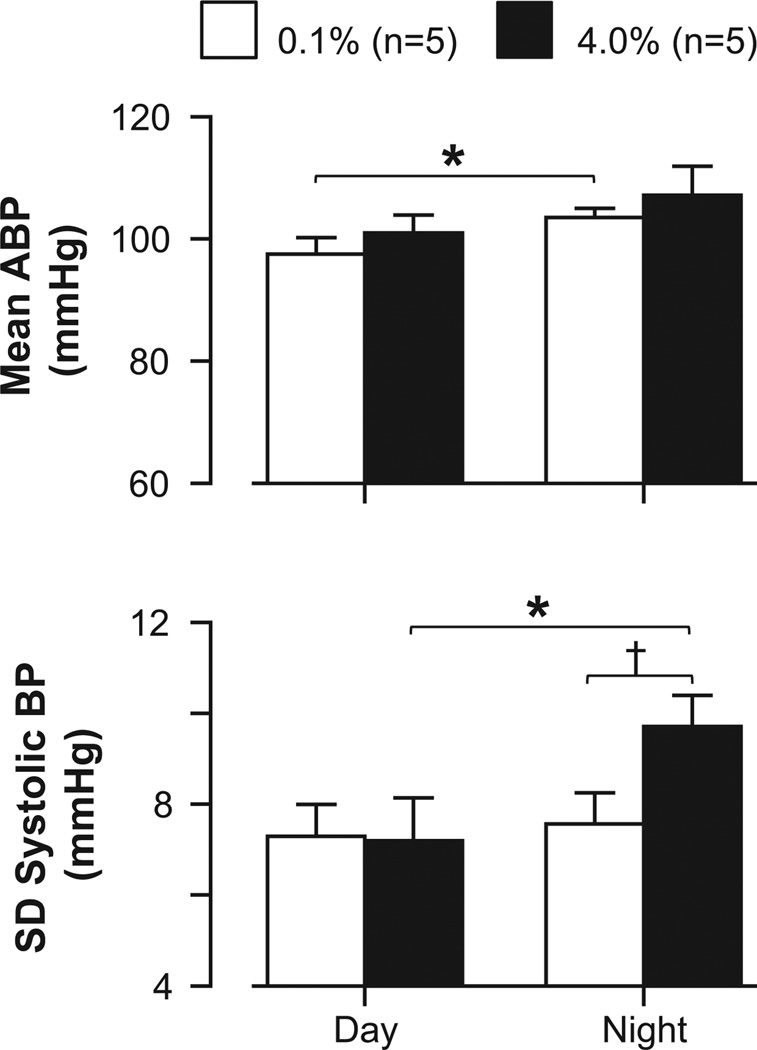
In a final set of experiments, we analyzed ABP and heart rate at day and night periods of control rats instrumented with telemetry units and fed 0.1% or 4.0% NaCl for 2 week intervals. Mean ABP did not differ at day or night periods between rats fed 0.1% versus 4.0% NaCl (Figure 6); however, the standard deviation of beat-to-beat systolic ABP was significantly higher at night when rats were fed 4.0% versus 0.1% NaCl. Similar findings were observed with mean, diastolic, and systolic ABP (Table S2, http://hyper.ahajournals.org). Heart rate did not differ between dietary salt conditions (Table S2, http://hyper.ahajournals.org).
Figure 6.
Mean±SEM of mean ABP and standard deviation of systolic ABP of rats instrumented with telemetry and fed 0.1% or 4.0% NaCl for 2 weeks. ABP was analyzed on a beat-to-beat basis for day (1–3 PM) and night (1–3 AM) periods (lights on/off at 7AM/7PM).*P<0.05, †P<0.01
DISCUSSION
The present study indicates that dietary salt intake modulates the responsiveness of central autonomic circuits to result in functional changes in the regulation of SNA and ABP. This conclusion is based on several novel observations to indicate that the level of dietary NaCl intake was directly correlated to the magnitude of the SNA and ABP responses during several experimental manipulations including: 1) activation of sciatic afferents, 2) stimulation of aortic depressor nerve, 3) activation of vagal nerve afferents, 4) volume expansion, and 5) elevation in cerebrospinal fluid [NaCl]. The enhanced SNA and ABP responses in rats fed a high NaCl diet were prevented by prior lesion of the AV3V. Consistent with a sensitization of central autonomic pathways, a high NaCl diet was also found to increase BPV independent of mean ABP. Altogether, these findings suggest that dietary NaCl intake, via the AV3V region, modulates the central gain of sympathetic circuits to alter the functional regulation of SNA and cardiovascular function in normotensive, salt-resistant rats.
A number of experiments in the present study utilized electrical stimulation of a peripheral nerve to control afferent neural input and directly assess central gain or responsiveness by changes in SNA and ABP. Rats fed 4.0% versus 0.1% NaCl displayed greater changes in SNA and ABP during electrical stimulation of sciatic afferents, aortic depressor nerve, or vagal afferents. Similar exaggerated responses were observed in response to ICV infusion of hypertonic NaCl or volume expansion. These findings are consistent with previous observations in which a high NaCl diet enhanced baroreflex gain in rats [23] and humans [24], the exercise pressor response [20], and sympathoinhibitory response to volume expansion [25]. The novelty of the current findings is that dietary NaCl intake exaggerated the magnitude of the SNA response thereby suggesting the dietary NaCl affects central autonomic networks versus a change in vascular reactivity. While it is difficult to define a “normal” NaCl intake in rodents, the SNA and ABP responses examined in a subset of reflexes displayed a NaCl-dependent relationship over a range of NaCl diets (0.1%, 0.5%, or 4.0% NaCl). In a few instances, dietary NaCl did not modulate the SNA response in all sympathetic nerves. The reason for this result is unclear but suggests a complex interaction. Altogether, these findings suggest that the level of dietary NaCl intake modulates the gain of central sympathetic networks to functionally alter the regulation of SNA and ABP in normotensive, salt-resistant animals.
It is noteworthy that the present findings contradict a few previous studies in which dietary NaCl intake was not found to alter sympathetic and cardiovascular reflexes. In rabbits, 6-day access to 0.9% NaCl drinking solution did not alter renal SNA and cardiovascular responses to stressful stimuli (handling, gentle restraint, or nasopharyngeal activation with cigarette smoke) [26]. In rats, electrical stimulation of the aortic depressor nerve or cervical vagal afferents did not produce greater decreases in renal SNA and ABP of rats given access to 0.9% NaCl versus water for 1 week [27]. Interestingly, previous studies from our laboratory have documented that enhanced SNA and ABP responses to RVLM activation are observed after two, but not one, weeks of access to 0.9% NaCl diet [13]. Therefore, the lack of a potentiation by increased NaCl intake in the above-mentioned studies may simply reflect too short duration of increased dietary NaCl.
The majority, if not all, of the reflexes tested in the current study depend on neurotransmission in the rostral ventrolateral medulla (RVLM) [28–32]. Although prior studies have reported that increased dietary NaCl intake enhances sympathoexcitatory and sympathoinhibitory responses evoked from the RVLM [12–14, 16, 17], there is currently no direct evidence to indicate that the enhanced responses can be directly attributed to selective changes in the excitability of RVLM neurons versus other neuronal populations. Indeed, the dependence of this effect on the AV3V region raises the possibility that dietary salt may affect multiple neuronal populations extending from the hypothalamus to the hindbrain. However, the absence of exaggerated responses to chemoreflex activation suggests that dietary salt intake does not modulate every central autonomic pathway or reflex.
The AV3V region and lamina terminalis play a pivotal role in body fluid homeostasis and cardiovascular regulation [33]. AV3V lesions disrupt thirst, antidiuretic hormone secretion, and changes in SNA to body fluid homeostatic challenges [33, 34]. These lesions also prevent or attenuate several salt-senstive models of hypertension [35–39] and the enhanced SNA and/or ABP responses to stimulation of the RVLM of rats fed high salt diets [12]. In the present study, rats with chronic AV3V lesions fed 4.0% NaCl chow did not show exaggerated sympathetic and cardiovascular responses to any stimulus. In fact, the responses of AV3V lesioned rats were not different between those fed 0.1% versus 4.0% NaCl or control rats fed 0.1% NaCl. Consistent with a previous report [40], AV3V lesion attenuated the sympathetic and/or pressor response to ICV infusion of hypertonic NaCl but there were no differences between dietary groups. These observations cannot be explained by differences in NaCl intake between control and AV3V lesioned animals. Altogether, these findings suggest that dietary salt intake affects neurons in the AV3V region to subsequently alter the excitability of central sympathetic circuits. The factor that links dietary salt intake to the AV3V region and gain of sympathetic networks is unknown; however, several studies have reported that dietary salt intake produces small changes in plasma electrolytes and osmolality [12, 41]. The present findings confirm this notion as plasma sodium and chloride concentrations were elevated in control and AV3V-lesioned rats fed 4.0% NaCl versus control rats fed 0.1% NaCl. In addition, chronic ICV NaCl infusions have also been reported to exaggerate sympathetic and cardiovascular responses to air-jet stress in Dahl-resistant rats [19]. Since the AV3V region contains osmosensitive neurons [42], it is interesting to speculate that dietary salt intake may activate these neurons through changes in electrolyte concentrations or osmolality. However, changes in dietary NaCl intake will also affect other circulating factors such as the renin-angiotensin system. Therefore, we cannot exclude the possibility that both increases and/or decreases in such factors also contribute.
Accumulating evidence suggests that short- and long-term BPV are associated with the development of end-organ damage [21]. Moreover, long-term BPV may predict adverse cardiovascular events. Numerous mechanisms contribute to BPV including modulation of central autonomic function, fluctuations in humoral systems, emotion, and behavior [21]. Since dietary NaCl intake was found to exaggerate numerous sympathoinhibitory and sympathoexcitatory reflexes, we hypothesized that dietary NaCl intake, independent of mean ABP, may increase BPV. Indeed, BPV was significantly higher in rats fed 4.0% NaCl versus 0.1% NaCl at night but not during the day. The reason for the circadian effect is not clear but could be related to when the animals are active, and plasma electrolytes concentrations are highest [12, 41]. Interestingly, these findings are consistent with population studies that indicate normotensive humans with a greater level of salt intake have an increased risk for an adverse cardiovascular event [1, 2, 4].
Perspectives
Although dietary salt intake is recognized as a contributing factor to the pathogenesis of hypertension [1–8, 43–45], recent evidence suggests dietary salt intake may increase the risk for adverse cardiovascular events independent of changes in ABP [1–8]. The present study suggests that one such mechanism for this adverse effect may be a central sensitization of sympathetic circuits to result in exaggerated cardiovascular reflexes and increase BPV in normotensive, salt-resistant animals. Moreover, this central sensitization may also explain why dietary salt intake works with other factors (ie, angiotensin II) to synergistically increase ABP in chronic experimental models of hypertension. How dietary salt intake produces these adverse effects either in normotensive, salt-resistant animals or synergistically in salt-sensitive models is unknown.
Novelty and Significance.
1) What is New?
Dietary salt intake modulated the sympathetic and cardiovascular responses to a number of stimuli in normotensive, salt-resistant rats.
Lesion of the AV3V region prevented these exaggerated responses.
Elevated dietary salt intake significantly increased BPV independent of changes in mean ABP.
What is Relevant?
The current findings demonstrate that dietary salt intake can promote adverse effects by modulation of central autonomic circuits in normotensive, salt-resistant rodents.
Summary
The findings of this study suggest that dietary salt intake can sensitize central brain pathways to exaggerate sympathetic and cardiovascular responses and increase BPV in normotensive, salt-resistant subjects. This sensitization may also underlie the synergistic effects of dietary salt intake in a variety of salt-sensitive and hypertensive models.
ACKNOWLEDGEMENTS
None.
Sources of Funding
This research was supported by NIH NHLBI R01 HL-113270 (S.D.S.), an American Heart Association Established Investigator Grant (S.D.S.), and the Pennsylvania Department of Health using Tobacco CURE Funds (S.D.S.). The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP) BMJ. 2007;334:885–888. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook NR, Appel LJ, Whelton PK. Lower Levels of Sodium Intake and Reduced Cardiovascular Risk. Circulation. 2014;129:981–989. doi: 10.1161/CIRCULATIONAHA.113.006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotchen TA, Cowley AW, Jr, Frohlich ED. Salt in health and disease--a delicate balance. N Engl J Med. 2013;368:1229–1237. doi: 10.1056/NEJMra1212606. [DOI] [PubMed] [Google Scholar]
- 4.Strazzullo P, D'Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567. doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appel LJ, Frohlich ED, Hall JE, Pearson TA, Sacco RL, Seals DR, Sacks FM, Smith SC, Jr, Vafiadis DK, Van Horn LV. The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: a call to action from the American Heart Association. Circulation. 2011;123:1138–1143. doi: 10.1161/CIR.0b013e31820d0793. [DOI] [PubMed] [Google Scholar]
- 6.Stocker SD, Madden CJ, Sved AF. Excess dietary salt intake alters the excitability of central sympathetic networks. Physiol Behav. 2010;100:519–524. doi: 10.1016/j.physbeh.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Donnell MJ, Mente A, Smyth A, Yusuf S. Salt intake and cardiovascular disease: why are the data inconsistent? Eur Heart J. 2013;34:1034–1040. doi: 10.1093/eurheartj/ehs409. [DOI] [PubMed] [Google Scholar]
- 8.O'Donnell MJ, Yusuf S, Mente A, Gao P, Mann JF, Teo K, McQueen M, Sleight P, Sharma AM, Dans A, Probstfield J, Schmieder RE. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306:2229–2238. doi: 10.1001/jama.2011.1729. [DOI] [PubMed] [Google Scholar]
- 9.DuPont JJ, Greaney JL, Wenner MM, Lennon-Edwards SL, Sanders PW, Farquhar WB, Edwards DG. High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. J Hypertens. 2013;31:530–536. doi: 10.1097/HJH.0b013e32835c6ca8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greaney JL, DuPont JJ, Lennon-Edwards SL, Sanders PW, Edwards DG, Farquhar WB. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. J Physiol. 2012;590:5519–5528. doi: 10.1113/jphysiol.2012.236992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frohlich ED. The salt conundrum: a hypothesis. Hypertension. 2007;50:161–166. doi: 10.1161/HYPERTENSIONAHA.107.088328. [DOI] [PubMed] [Google Scholar]
- 12.Adams JM, Bardgett ME, Stocker SD. Ventral lamina terminalis mediates enhanced cardiovascular responses of rostral ventrolateral medulla neurons during increased dietary salt. Hypertension. 2009;54:308–314. doi: 10.1161/HYPERTENSIONAHA.108.127803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams JM, Madden CJ, Sved AF, Stocker SD. Increased dietary salt enhances sympathoexcitatory and sympathoinhibitory responses from the rostral ventrolateral medulla. Hypertension. 2007;50:354–359. doi: 10.1161/HYPERTENSIONAHA.107.091843. [DOI] [PubMed] [Google Scholar]
- 14.Adams JM, McCarthy JJ, Stocker SD. Excess dietary salt alters angiotensinergic regulation of neurons in the rostral ventrolateral medulla. Hypertension. 2008;52:932–937. doi: 10.1161/HYPERTENSIONAHA.108.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isogai O, Tsukamoto K, Masubuchi Y, Tomioka S, Suzuki T, Kawato H, Yajima Y, Kasamaki Y, Ito S, Kanmatsuse K. High salt diet enhances cardiovascular responses from the nucleus tractus solitarius and ventrolateral medulla of Sprague-Dawley rats. Clin Exp Hypertens. 2005;27:33–44. doi: 10.1081/ceh-200044252. [DOI] [PubMed] [Google Scholar]
- 16.Ito S, Gordon FJ, Sved AF. Dietary salt intake alters cardiovascular responses evoked from the rostral ventrolateral medulla. Am J Physiol. 1999;276:R1600–R1607. doi: 10.1152/ajpregu.1999.276.6.R1600. [DOI] [PubMed] [Google Scholar]
- 17.Pawloski-Dahm CM, Gordon FJ. Increased dietary salt sensitizes vasomotor neurons of the rostral ventrolateral medulla. Hypertension. 1993;22:929–933. doi: 10.1161/01.hyp.22.6.929. [DOI] [PubMed] [Google Scholar]
- 18.Mann JF, Schiffrin EL, Schiller PW, Rascher W, Boucher R, Genest J. Central actions and brain receptor binding of angiotensin II: Influence of sodium intake. Hypertension. 1980;2:437–443. doi: 10.1161/01.hyp.2.4.437. [DOI] [PubMed] [Google Scholar]
- 19.Huang BS, Wang H, Leenen FH. Enhanced sympathoexcitatory and pressor responses to central Na+ in Dahl salt-sensitive vs. -resistant rats. Am J Physiol Heart Circ Physiol. 2001;281:H1881–H1889. doi: 10.1152/ajpheart.2001.281.5.H1881. [DOI] [PubMed] [Google Scholar]
- 20.Yamauchi K, Tsuchimochi H, Stone AJ, Stocker SD, Kaufman MP. Increased dietary salt intake enhances the exercise pressor reflex. Am J Physiol Heart Circ Physiol. 2014;306:H450–H454. doi: 10.1152/ajpheart.00813.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol. 2013;10:143–155. doi: 10.1038/nrcardio.2013.1. [DOI] [PubMed] [Google Scholar]
- 22.LaGrange LP, Toney GM, Bishop VS. Chronic angiotensin II infusion attenuates the renal sympathoinhibitory response to acute volume expansion. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1098–R1107. doi: 10.1152/ajpregu.00165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang BS, Leenen FH. Dietary Na and baroreflex modulation of blood pressure and RSNA in normotensive vs. spontaneously hypertensive rats. Am J Physiol. 1994;266:H496–H502. doi: 10.1152/ajpheart.1994.266.2.H496. [DOI] [PubMed] [Google Scholar]
- 24.Grassi G, Dell'Oro R, Seravalle G, Foglia G, Trevano FQ, Mancia G. Short- and long-term neuroadrenergic effects of moderate dietary sodium restriction in essential hypertension. Circulation. 2002;106:1957–1961. doi: 10.1161/01.cir.0000033519.45615.c7. [DOI] [PubMed] [Google Scholar]
- 25.Victor RG, Morgan DA, Thoren P, Mark AL. High salt diet sensitizes cardiopulmonary baroreflexes in Dahl salt-resistant rats. Hypertension. 1986;8:II21–II127. doi: 10.1161/01.hyp.8.6_pt_2.ii21. [DOI] [PubMed] [Google Scholar]
- 26.McBryde FD, Malpas SC, Guild SJ, Barrett CJ. A high-salt diet does not influence renal sympathetic nerve activity: a direct telemetric investigation. Am J Physiol Regul Integr Comp Physiol. 2009;297:R396–R402. doi: 10.1152/ajpregu.90741.2008. [DOI] [PubMed] [Google Scholar]
- 27.DiBona GF, Jones SY. Endogenous angiotensin affects responses to stimulation of baroreceptor afferent nerves. J Hypertens. 2003;21:1539–1546. doi: 10.1097/00004872-200308000-00019. [DOI] [PubMed] [Google Scholar]
- 28.Kiely JM, Gordon FJ. Non-NMDA receptors in the rostral ventrolateral medulla mediate somatosympathetic pressor responses. J Auton Nerv Syst. 1993;43:231–239. doi: 10.1016/0165-1838(93)90329-s. [DOI] [PubMed] [Google Scholar]
- 29.Sun MK, Guyenet PG. GABA-mediated baroreceptor inhibition of reticulospinal neurons. Am J Physiol. 1985;249:R672–R680. doi: 10.1152/ajpregu.1985.249.6.R672. [DOI] [PubMed] [Google Scholar]
- 30.Sun MK, Guyenet PG. Arterial baroreceptor and vagal inputs to sympathoexcitatory neurons in rat medulla. Am J Physiol. 1987;252:R699–R709. doi: 10.1152/ajpregu.1987.252.4.R699. [DOI] [PubMed] [Google Scholar]
- 31.Lang SM, Stocker SD. Glutamate receptor activation in the rostral ventrolateral medulla (RVLM) mediates the sympathetic response to increased cerebrospinal fluid (CSF) sodium concentration. FASEB J. 2013;27:695.4. [Google Scholar]
- 32.Sun MK, Reis DJ. NMDA receptor-mediated sympathetic chemoreflex excitation of RVL-spinal vasomotor neurones in rats. J Physiol. 1995;482(Pt 1):53–68. doi: 10.1113/jphysiol.1995.sp020499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson AK, Loewy AD. Circumventricular Organs and Their Role in Visceral Functions. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Functions. New York: Oxford University Press; 1990. pp. 247–267. [Google Scholar]
- 34.Toney GM, Stocker SD. Hyperosmotic activation of CNS sympathetic drive: implications for cardiovascular disease. J Physiol. 2010;588:3375–3384. doi: 10.1113/jphysiol.2010.191940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goto A, Ganguli M, Tobian L, Johnson MA, Iwai J. Effect of an anteroventral third ventricle lesion on NaCl hypertension in Dahl salt-sensitive rats. Am J Physiol. 1982;243:H614–H618. doi: 10.1152/ajpheart.1982.243.4.H614. [DOI] [PubMed] [Google Scholar]
- 36.Berecek KH, Barron KW, Webb RL, Brody MJ. Vasopressin-central nervous system interactions in the development of DOCA hypertension. Hypertension. 1982;4:131–137. [PubMed] [Google Scholar]
- 37.Buggy J, Fink GD, Haywood JR, Johnson AK, Brody MJ. Interruption of the maintenance phase of established hypertension by ablation of the anteroventral third ventricle (AV3V) in rats. Clin Exp Hypertens. 1978;1:337–353. doi: 10.3109/10641967809068612. [DOI] [PubMed] [Google Scholar]
- 38.Buggy J, Fink GD, Johnson AK, Brody MJ. Prevention of the development of renal hypertension by anteroventral third ventricular tissue lesions. Circ Res. 1977;40:I110–I117. [PubMed] [Google Scholar]
- 39.Haywood JR, Fink GD, Buggy J, Boutelle S, Johnson AK, Brody MJ. Prevention of two-kidney, one-clip renal hypertension in rat by ablation of AV3V tissue. Am J Physiol. 1983;245:H683–H689. doi: 10.1152/ajpheart.1983.245.4.H683. [DOI] [PubMed] [Google Scholar]
- 40.Veerasingham SJ, Leenen FH. Excitotoxic lesions of the ventral anteroventral third ventricle and pressor responses to central sodium, ouabain and angiotensin II. Brain Res. 1997;749:157–160. doi: 10.1016/s0006-8993(96)01381-9. [DOI] [PubMed] [Google Scholar]
- 41.Habecker BA, Grygielko ET, Huhtala TA, Foote B, Brooks VL. Ganglionic tyrosine hydroxylase and norepinephrine transporter are decreased by increased sodium chloride in vivo and in vitro. Auton Neurosci. 2003;107:85–98. doi: 10.1016/S1566-0702(03)00133-4. [DOI] [PubMed] [Google Scholar]
- 42.Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008;9:519–531. doi: 10.1038/nrn2400. [DOI] [PubMed] [Google Scholar]
- 43.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 44.Weinberger MH. Salt sensitivity is associated with an increased mortality in both normal and hypertensive humans. J Clin Hypertens (Greenwich) 2002;4:274–276. doi: 10.1111/j.1524-6175.2002.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]