Abstract
Background:
Following various types of naturally occurring traumatic injury to an articular joint, the lubricating ability of synovial fluid is impaired, with a correlated alteration in the concentration and/or structure of lubricant molecules, hyaluronan, and proteoglycan-4 (PRG4). However, the effect of arthroscopic cartilage repair surgery on synovial fluid lubricant function and composition is unknown.
Hypothesis:
Arthroscopic treatment of full-thickness chondral defects in horses with (1) platelet-enriched fibrin or (2) platelet-enriched fibrin + mesenchymal stem cells leads to equine synovial fluid with impaired lubricant function and hyaluronan and PRG4 composition.
Study Design:
Controlled laboratory study.
Methods:
Equine synovial fluid was aspirated from normal joints at a preinjury state (0 days) and at 10 days and 3 months following fibrin or fibrin + mesenchymal stem cell repair of full-thickness chondral defects. Equine synovial fluid samples were analyzed for friction-lowering boundary lubrication of normal articular cartilage (static and kinetic friction coefficients) and concentrations of hyaluronan and PRG4, as well as molecular weight distribution of hyaluronan. Experimental groups deficient in lubrication function were also tested for the ability of exogenous high–molecular weight hyaluronan to restore lubrication function.
Results:
Lubrication and biochemical data varied with time after surgery but generally not between repair groups. Relative to preinjury, kinetic friction was higher (+94%) at 10 days but returned to baseline levels at 3 months, while static friction was not altered. Correspondingly, hyaluronan concentration was transiently lower (−64%) and shifted toward lower molecular weight forms, while PRG4 concentration was increased (+210%) in 10-day samples relative to preinjury levels. Regression analysis revealed that kinetic friction decreased with increasing total and high–molecular weight hyaluronan. Addition of high–molecular weight hyaluronan to bring 10-day hyaluronan levels to 2.0 mg/mL restored kinetic friction to preinjury levels.
Conclusion:
Following arthroscopic surgery for cartilage defect repair, synovial fluid lubrication function is transiently impaired, in association with decreased hyaluronan concentration. This functional deficiency in synovial fluid lubrication can be counteracted in vitro by addition of high–molecular weight hyaluronan.
Clinical Relevance:
Synovial fluid lubrication is deficient shortly after arthroscopic cartilage repair surgery, and supplementation with high–molecular weight hyaluronan may be beneficial.
Keywords: cartilage repair, synovial fluid, lubrication, hyaluronan, proteoglycan-4
In healthy synovial joints, the normal, low friction between articulating cartilage surfaces is achieved through various lubrication mechanisms, including those acting in the boundary mode to reduce surface-to-surface interaction.4,33 Boundary mode lubrication is mediated by interactions of synovial fluid (SF) biomolecules with the articular cartilage surface and can be detected with high sensitivity when load support by interstitial fluid pressurization subsides.4 When such lubrication is diminished, cartilage may undergo increased wear12 and shear strain.41 Such diminished lubrication has been observed for SF following acute injuries in horses2 and humans6 and in experiments with rabbits12 and guinea pigs38 where articular cartilage degeneration ensued. The elucidation of situations in which SF lubrication properties and lubricant biomolecules are diminished could lead to the development of treatments to correct such lubrication deficiency.
Previous investigations of cartilage lubrication have identified proteoglycan-4 (PRG4) and hyaluronan (HA) as boundary lubricant molecules that are variably altered after acute injury (AI) in SF. Increasing concentrations of HA and PRG4, either individually or in combination, leads to decreased cartilage-on-cartilage friction in vitro relative to saline alone.32 Relative to concentrations in normal (NL) joints, that of HA (CHA) in AI-SF has both been reported as lower than2,3,6 or not different27,30,40 from CHA of NL-SF. The molecular weight (MW) distribution of HA for AI equine SF (eSF) has been reported as either shifted toward lower MW forms2 or not different relative to NL-eSF.40 The concentration of PRG4 (CPRG4) in AI-SF has been reported as both lower11,12,38 and higher2,6 than that in NL-SF. This variation may be due to a variety of factors, including study design, injury type, SF sampling times, and analytical methods.
Few studies have simultaneously examined the mechanical and biochemical properties of AI-SF and NL-SF, allowing for investigation of relationships between SF function and composition. In AI-eSF at within 3 weeks of injury, diminished (worse) lubrication was correlated with decreased CHA, even as CPRG4 increased2; there, addition of high-MW HA to poorly lubricating AI-eSF to bring the HA concentration to 1.0 mg/mL (physiological values for horses2) restored normal friction-reducing lubrication function. In contrast, after anterior cruciate ligament transection (ACLT) in rabbits12 and guinea pigs,38 diminished lubricating ability was correlated with decreased CPRG4, but CHA was not reported. In previous studies of cartilage repair using chondrocyte-fibrin matrices in horses, eSF HA concentration was decreased transiently.14 Delineation of the concomitant changes in lubricating and lubricant properties of SF after joint injury or repair would further define states in which SF lubricating function is diminished, and cartilage damage may occur.
Such alterations in SF lubricating function may occur after surgical repairs of cartilage defects, since such procedures markedly alter the joint environment. Microfracture is commonly used for cartilage repair in the knee and involves forming small holes in the subchondral bone to stimulate bone marrow cells to enter the defect and form repair tissue.35 However, the regenerated tissue is fibrocartilaginous, with load-bearing properties inferior to those of native hyaline articular cartilage.21 The application to the defect site of autogenously derived mesenchymal stem cells (MSCs) within a platelet-enriched fibrin scaffold (F + MSC) may augment cartilage repair. Bone marrow aspirate concentrate provides a source of MSCs capable of regenerating cartilage,15 and previous equine studies indicate that injection of bone marrow aspirate16 or MSCs26 results in cartilaginous repair that is superior to microfracture. However, in the postsurgery joint, SF lubrication properties may be abnormal.
The hypothesis of this study was that acute cartilage injury and subsequent arthroscopic cartilage repair procedures (fibrin and F + MSC) lead to alterations in the lubrication quality and composition of SF in a manner dependent on time after treatment. The objectives were to compare eSF at a preinjury state (t = 0 d), at 10 days (t = 10 d), and at 3 months (t = 3 mo) following fibrin or F + MSC surgery of induced full-thickness chondral defects for (1) the coefficients of friction at a normal cartilage-on-cartilage interface in the boundary lubrication mode, (2) the concentration and quality of HA and PRG4 and molecular weight, (3) the relationship between lubrication function and composition, and (4) the ability of high-MW HA to restore lubricating ability in samples deficient in lubrication function.
Materials and Methods
Materials
Reagents for lubrication testing materials were as described previously,2,6,33 including HA in a 4000-kDa form (Healon; Advanced Medical Optics). The antibody to PRG4 was anti-Lubricin from AbCam,2 nonspecific rabbit IgG was from Pierce, and mouse anti-rabbit IgG secondary antibody was from Jackson ImmunoResearch. Streptomyces hyaluronidase was from Seikagaku, SeaKem gold agarose was from Lonza; 50X TAE (2M Tris, 0.5 M ethylenediamine tetraacetic acid [EDTA]) electrophoresis buffer was from Life Technologies, Hybond-P polyvinylidene difluoride (PVDF) membrane for Western blotting was from GE Healthcare, and Stains-All was from Sigma-Aldrich.
Operative Technique and Synovial Fluid Collection
With Institutional Animal Care and Use Committee approval, bilateral experimental cartilage defects (15 mm in diameter) were created (L.R.G. and C.W.M.) by removing cartilage including the calcified layer and extending down to, but not through, the subchondral bone in the midlateral trochlear ridge of adult horses (age range, 2-6 years; n = 12). Autologous nucleated cells from iliac crest bone marrow aspirate were pelleted by centrifugation, cultured for 1 day, and separated from nonadherent cells.22 Stromal colonies were allowed to form, trypsinized, and reseeded into monolayer cultures in α-minimal essential medium with 10% fetal bovine serum and 2 ng/mL fibroblast growth factor–2 for 2 days.22 MSCs were collected, expanded an additional 4 to 5 days, and then cryopreserved until the day before surgery when they were thawed and plated at 10,000 cells/cm2 in culture medium.22 Cells were then trypsinized, washed in phosphate-buffered saline (PBS), and combined with fibrinogen.22 Cells isolated in this manner have been previously shown to have chondrogenic and osteogenic differentiation potential.22 Fibrinogen and platelets were harvested from horse plasma28 and formed into a fibrin-based scaffold on injection with bovine thrombin to achieve a final platelet concentration of 1010/mL. For each horse (Figure 1), 1 patellofemoral joint (side randomized) was treated with fibrin (n = 12), while the contralateral joint was treated with F + MSC (n = 12). The defect was created as a part of the repair procedure.27 From each of these 2 joints, eSF was aspirated at 3 times, just (˜15 minutes) before making a portal for the arthroscopic defect creation (0 d), at 10 days (10 d), and at 3 months (3 mo) following surgery. The total eSF volume aspirated was noted (±0.1 mL), and the eSF was clarified by centrifugation (3000g, 30 minutes, 4°C) and stored at −80°C for subsequent analysis.
Figure 1.
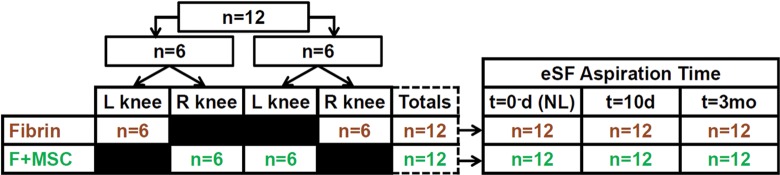
Study design. Adult horses (n = 12) were treated with fibrin (n = 12) in the left (L) or right (R) knee and F + MSC (n = 12) in the contralateral knee for full-thickness, 15 mm–diameter chondral defects in the midlateral trochlear ridge. Each joint was aspirated initially, t = 0 days (NL), and at 10 days and 3 months following surgery. eSF, equine synovial fluid; F, fibrin; MSC, mesenchymal stem cell, NL, normal.
Experimental Design
The effects of treatment (fibrin or F + MSC) or duration (t = 0 d, 10 d, 3 mo) postsurgery on eSF lubricant function and composition were assessed. Portions of eSF samples were assessed for friction-lowering properties by lubrication tests in the boundary mode. Other portions were analyzed for the concentrations of total protein, PRG4, HA, and HA in MW ranges of 2.5 to 7, 1 to 2.5, 0.5 to 1, and 0.12 to 0.5 MDa, respectively.
Based on deficient lubricating properties and lower concentration of HA in t = 10 d samples, to determine whether HA supplementation alone could restore eSF lubricating function, pools (n = 8) of t = 10 d eSF were analyzed further. Fibrin (n = 4) and F + MSC (n = 4) pools were made by combining equal SF volumes from 2 to 3 individual fibrin or F + MSC samples, respectively. Samples were then tested for friction-lowering properties alone and after in vitro supplementation with high-MW HA (Healon) to 0.8 mg/mL (physiological) and 2.0 mg/mL (more typical of CHA normally in humans and following therapeutic HA injection8) to develop a dosage response.
Lubrication Tests
Portions of eSF were analyzed for start-up (static, μstatic) and steady-state (kinetic, μkinetic) coefficients of friction as measures of boundary lubrication function in a cartilage-on-cartilage articulation test described previously.33 Intact articular surface pairs used were osteochondral cores and annuli (n = 65 pairs) paired from the same adult bovine knees (n = 22 harvested), stored in PBS supplemented with protease inhibitors (PIs) (2 mM Na-EDTA, 1 mM PMSF [phenylmethanesulfonyl fluoride], 5 mM Benz-HCl [benzamidine hydrochloride], and 10 mM NEM [N-ethylmaleimide]) at −80°C prior to testing. Osteochondral substrates were bathed in test lubricant for approximately 24 hours at 4°C prior to lubrication testing with 18% cartilage compression, an effective sliding velocity of 0.3 mm/s, and prespin pause time (Tps) of 1.2, 12, and 120 seconds. Friction coefficients were calculated using the equilibrium axial load following 30-minute stress relaxation and, for µstatic, using the peak torque (|τ|) measured either within the first 10° of the start of rotation and after the 120-second prespin pause, or, for µkinetic, from an averaged |τ| during steady-state sliding. Consistent with previous work, µkinetic did not vary significantly with Tps, so that µkinetic data are presented as the average for all Tps.
Biochemical Analyses of Lubricant Molecules
Portions of eSF were assayed for the concentrations of protein, HA, and PRG4.2,6 Protein concentration (CProtein) was determined by the bicinchoninic acid assay per the manufacturer’s protocol. PRG4 concentration (CPRG4) was quantified by Western blot using anti-Lubricin antibody.2 Briefly, Streptomyces hyaluronidase–digested portions of eSF were run (0.5-2.0 µL/lane) on a 2% agarose gel in TAE (40 mM Tris-acetate and 1 mM EDTA at pH 8.3) with 0.1% SDS then transferred to a PVDF membrane. The membrane was probed with anti-Lubricin antibody or nonspecific rabbit IgG, followed by a mouse anti-rabbit light chain–specific secondary antibody conjugated to horseradish peroxidase, and then quantified by ECL-Plus detection and digital scanning using a STORM 840 Imaging System (Molecular Dynamics). From the digitized data, PRG4 concentrations were determined with ImageQuant software (Molecular Dynamics) and comparison to an equine PRG4 standard.7 The CHA of proteinase K–digested eSF was quantified by an ELISA-like assay using recombinant human aggrecan.19 HA MW distribution was determined by horizontal agarose gel electrophoresis of proteinase K–digested eSF (300 ng/lane) through a 1% agarose gel in TAE. Gels were stained with Stains-all, and image processing was performed to determine the distribution of HA in 7.0 to 2.5, 2.5 to 1.0, 1.0 to 0.5, and 0.50 to 0.12 MDa ranges.7
Statistical Analysis
Data are expressed as mean ± SEM. Differences between treatment groups were assessed by a repeated-measures analysis of variance (ANOVA), implemented as a linear mixed model with a fixed factor of treatment, covariate of time, and random effect of animal to account for the inclusion of left and right joints. This implementation allows incorporation of data even from individual joints where 1 or 2 eSF samples of the 3 time points were missing, which was the case in 6 and 1 of the 24 joints, respectively. For the preinjury state (t = 0 d), similarity of biochemical compositions of left and right knee SFs was analyzed by correlation analysis for the Pearson correlation coefficient, paired t tests, and Bland-Altman analysis.1 Since these analyses confirmed the similarity of eSF biochemical compositions from left and right knees of an individual animal at t = 0 d, as expected, post hoc planned comparisons by Student t tests for lubrication and biochemical data were made to assess the effect of time postsurgery (0 d, 10 d, 3 mo) within each treatment group (fibrin or F + MSC) and to assess the effect of treatment within each time postsurgery (10 d, 3 mo). The dependencies of µkinetic and µstatic on CHA (total and within defined MW ranges) were analyzed by univariate regression. The effect of HA supplementation to deficiently lubricating eSF was assessed by repeated-measures ANOVA, with pairwise comparisons made by paired t tests. Bonferroni corrections were applied when multiple comparisons were performed with data from an individual experimental group.
Results
Left Versus Right Knee Comparisons
The biochemical composition of eSF samples from left and right knees of individual animals were similar in the initial preinjury state (t = 0 d). This similarity between eSF from left and right knees at t = 0 d was indicated by both high Pearson correlation coefficients (r) and statistically nonsignificant paired t tests for volume, protein, PRG4, and HA (total and in MW ranges of 2.5-7 and 1-2.5 MDa) (Table 1). Furthermore, Bland-Altman plots (representative example, CProtein) (Figure 2) indicated that each biochemical component was similar between left and right joints, with individual left-right differences for each horse being within ±2 SD of the mean difference. Values of t = 0 d CHA(0.5-1.0MDa) and CHA(0.12-0.50MDa) were somewhat different between left and right knees (Table 1). CHA(0.5-1.0MDa) and CHA(0.12-0.50MDa) at t = 0 d were on average 0.008 ± 0.001 and 0.014 ± 0.001 mg/mL, respectively (and <4.3% of the total HA for all samples), with average differences between left and right knees being small (0.009 and 0.013 mg/mL, respectively). Due to this similarity in most biochemical components (volume, protein, PRG4, total HA, and HA in 2.5-7.0 and 1.0-2.5 MDa regions), data from contralateral joints were not treated as independent measures in statistical analyses.
TABLE 1.
Analysis of Dependence Between Left (L) and Right (R) t = 0 Days Data
| Assay | L vs R: Correlation | L vs R: t Test |
|---|---|---|
| Pearson r | P Value | |
| Volume | 0.57 | .60 |
| CProtein | 0.78 | .22 |
| CPRG4 | 0.45 | .58 |
| CHA | 0.76 | .92 |
| CHA(2.5-7.0MDa) | 0.79 | .98 |
| CHA(1.0-2.5MDa) | 0.47 | .69 |
| CHA(0.5-1.0MDa) | −0.36 | .86 |
| CHA(0.25-0.50MDa) | −0.56 | .34 |
Figure 2.
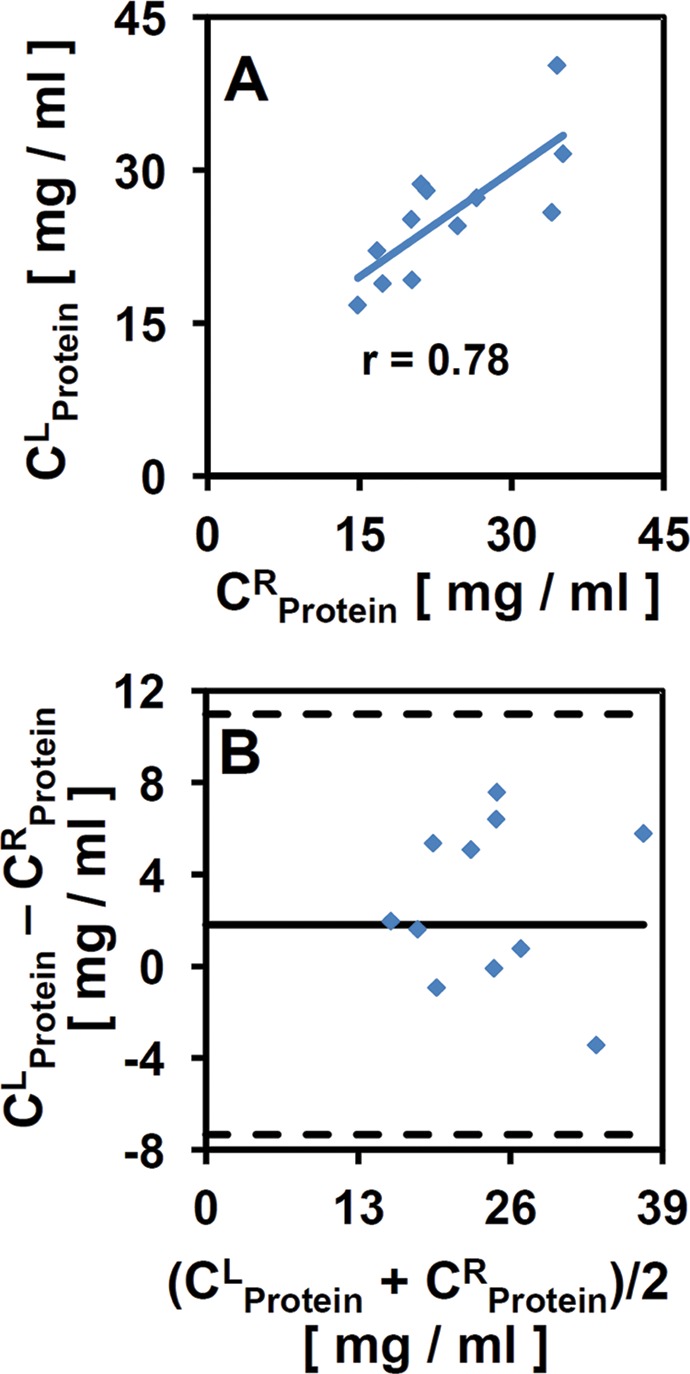
Graphical representation of left versus right joint similarity of equine synovial fluid (eSF) at day 0. (A) Linear regression of left (L) versus right (R) knee CProtein. (B) Bland-Altman plots of individual differences between left and right knee CProtein plotted against their mean. Solid line represents the mean difference and dashed lines represent ±2 SDs (σ) of the individual differences. All individual differences were within 2σ of the mean difference.
Lubrication Function of eSF
μstatic (Figure 3A) and µkinetic (Figure 3B) varied with time after surgery (each, P < .01) without an interaction effect with treatment (each, P > .65), with µkinetic varying between treatments (P = .009) while μstatic,Tps=120s did not (P = .95). μstatic,Tps=120s (Figure 3A) at t = 0 d averaged 0.094 ± 0.008, and relative to that, at t = 10 d for F + MSC tended to increase (+37%), but did not reach statistical significance (P = .033, α = .05/3). µkinetic (Figure 3B) at t = 0 d averaged 0.024, and relative to that value, increased (+94%) at t = 10 d for fibrin and F + MSC treatments (each, P < .01). In contrast, at t = 3 mo, μstatic,Tps=120s (for both treatments) and µkinetic (for F + MSC) were similar to t = 0 d levels (P = .057-.41) (Figure 3, A and B), while µkinetic (Figure 3B) for fibrin tended to be elevated (+28%), but did not reach statistical significance (P = .029, α = .05/3). Post hoc analysis of µkinetic did not reveal specific differences between the 2 treatment groups (fibrin, F + MSC) at each time (t = 10 d and 3 mo) postsurgery (each, P = .14-.22).
Figure 3.
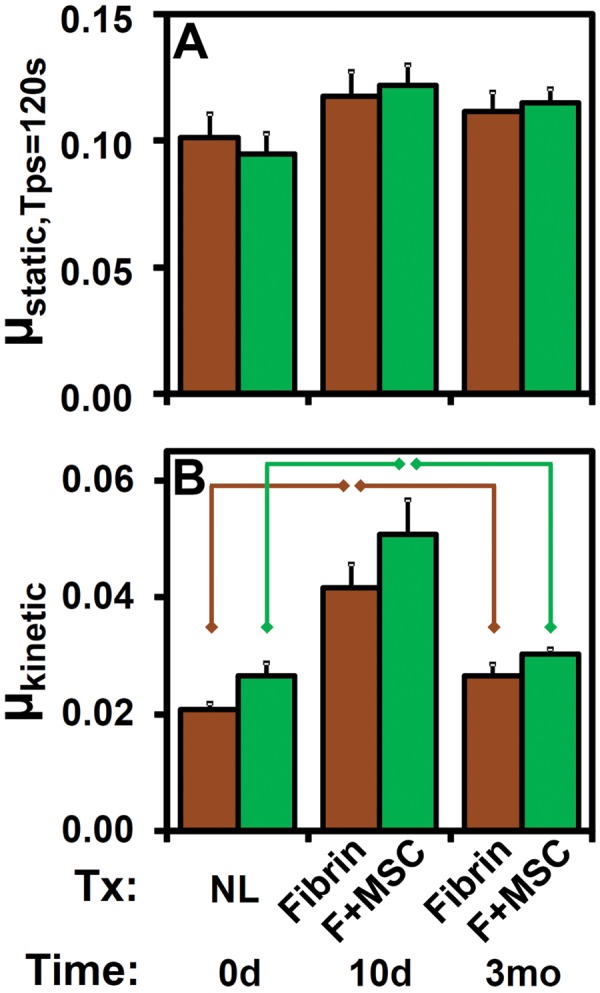
Effects of time postsurgery (0 days, 10 days, 3 months) and treatment (NL, fibrin, F + MSC) on friction-lowering lubricant properties of synovial fluid. (A) Start-up (μstatic) and (B) steady-state (μkinetic) friction coefficients for treatment groups ( ) fibrin and (
) fibrin and ( ) F + MSC. n = 9-12. Data are represented as mean ± SEM. Lines indicate significant difference between groups (P < .05/3). F, fibrin; MSC, mesenchymal stem cell; NL, normal.
) F + MSC. n = 9-12. Data are represented as mean ± SEM. Lines indicate significant difference between groups (P < .05/3). F, fibrin; MSC, mesenchymal stem cell; NL, normal.
Biochemical Analysis of eSF
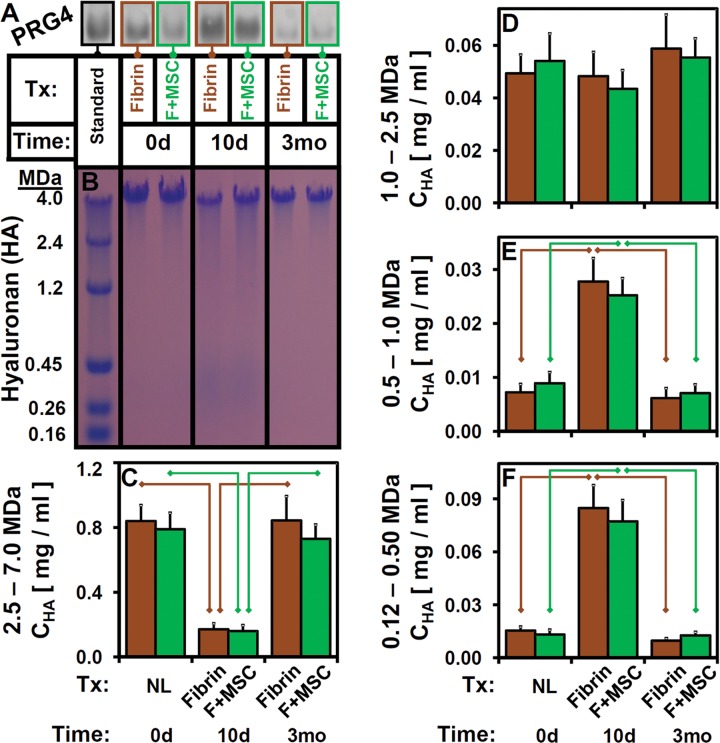
Consistent with lubrication function results, biochemical properties of eSF were transiently altered at 10 days postsurgery. The normal eSF at t = 0 d averaged 2.73 mL in volume, with Cprotein of 24.8 mg/mL and CPRG4 of 32.6 µg/mL. Aspirated eSF volume (Figure 4A), as well as concentrations of total protein (Figure 4B), PRG4 (Figure 4C), and HA (Figure 4D) varied with time after surgery (all, P < .001) in a treatment-independent manner (P = .40-.96). While there was no interaction effect between treatment and time postsurgery for volume, CPRG4, and CHA (each, P = .72-.98), a significant interaction effect was observed for CProtein (P = .016). Relative to presurgery values, volume, CProtein (for F + MSC), and CPRG4 at t = 10 d were higher (+150%, +55%, +210%; all, P < .005), although CProtein (fibrin) did not reach statistical significance between t = 0 d and 10 d (P = .054). While CPRG4 returned to normal levels at t = 3 mo (P > .069), CProtein was diminished (–39%) in t = 3 mo samples relative to normal (P < .005), and volume tended to be slightly elevated (+91%) at t = 3 mo relative to normal but did not reach statistical significance (P = .026-.046, α = .05/3). Western blot of individual samples for PRG4 identified a specific immunoreactive band that varied substantially in intensity but not in migration distance (Figure 5A).
Figure 4.
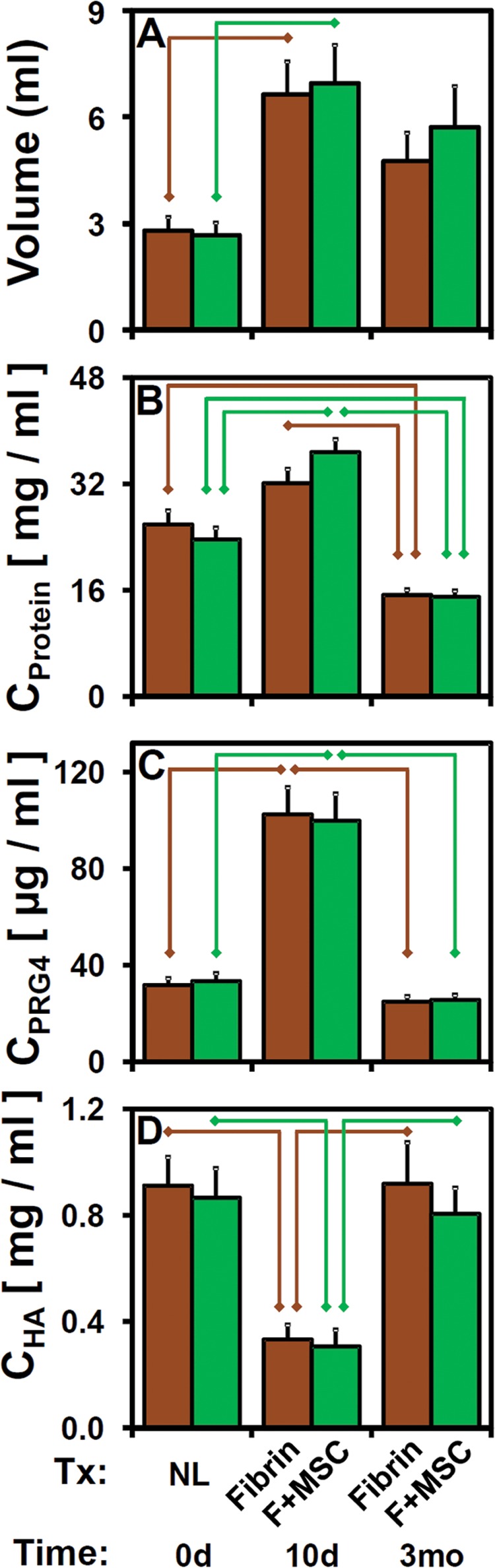
Effects of time postsurgery and treatment on eSF properties as described in Figure 3. (A) eSF volume. Concentrations of (B) protein, (C) PRG4, and (D) HA. n = 10-12. eSF, equine synovial fluid; HA, hyaluronan; PRG4, proteoglycan-4.
Figure 5.
Effects of time postsurgery and treatment on eSF properties as described in Figure 3. Representative (A) PRG4 Western Blot and (B) agarose gels of eSF with HA MW markers at left. (C-F) CHA for indicated MW ranges for fibrin and F + MSC. n = 10-12. eSF, equine synovial fluid; F, fibrin; HA, hyaluronan; MSC, mesenchymal stem cell; MW, molecular weight; PRG4, proteoglycan-4.
Correspondingly, the concentration of HA (Figure 4D) in eSF was transiently decreased by surgery at t = 10 d compared with baseline. Relative to CHA = 0.889 mg/mL at t = 0 d, CHA at t = 10 d was lower (−64%, P < .05/4) and returned to normal levels at t = 3 mo (each, P > .69). Quantification of gel electrophoresis images of HA (Figure 5B) demonstrated that HA MW distribution shifted correspondingly from baseline high levels of high-MW HA toward increasing levels of low-MW HA at t = 10 d and returned to the baseline distribution at t = 3 mo (Figure 5, C-F). CHA within 2.5 to 7.0, 0.5 to 1.0, and 0.12 to 0.50 MDa bins varied with time postsurgery (all, P < .001), but not with treatment (P = .42-.84) or with an interaction (P = .43-.8) (Figure 5, C, E, and F). CHA(1.0-2.5MDa) did not vary with time, treatment, or their interaction (all, P > .45) (Figure 5D). Relative to t = 0 d levels, CHA(2.5−7.0MDa) (Figure 5C) were lower (−79%) at t = 10 d (all, P < .001) and similar at t = 3 mo (all, P > .67). CHA(0.5-1.0MDa) and CHA(0.12-0.50MDa) t = 10 d values were higher (+229% and +470%, respectively; all, P < .005; Figure 5, E and F) relative to normal values and returned to baseline values at t = 3 mo (P = .097-.90).
Univariate and Multivariate Linear Regression Analysis
Regression analysis indicated certain correlations between lubrication properties and eSF biochemical composition. Univariate regression showed that µkinetic decreased as CHA increased (P < .001) (Figure 6). This was associated with the size distribution of HA, such that µkinetic decreased as CHA(2.5-7.0MDa) increased (P < .001). µkinetic did not correlate with CHA(1.0-2.5MDa) (P = .27) and increased with CHA(0.5-1.0MDa) and CHA(0.12-0.50MDa) (all, P < .001).
Figure 6.
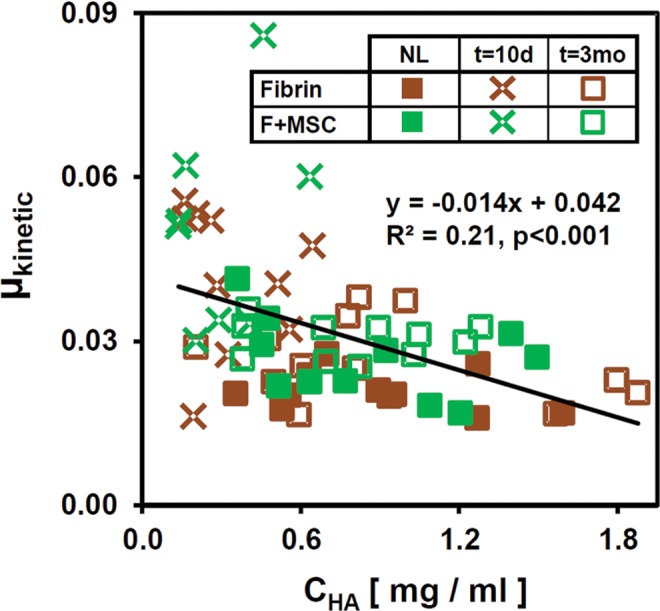
Univariate regression of μkinetic versus CHA.
Restoration of Lubrication Function by Supplementation With High-MW HA
Lubricating function of t = 10 d eSF samples (mean CHA, 0.32 mg/mL) with initially deficient lubrication properties (µkinetic) was restored by in vitro HA supplementation (to 0.8 or 2.0 mg/mL by addition of 10 mg/mL Healon) for both fibrin and F + MSC groups, resulting in a composition of 95% eSF for 0.8 mg/mL groups and 81% eSF for 2.0 mg/mL groups. μkinetic was altered by added CHA (P < .001) (Figure 7B) independent of treatment (P = .42) and without an interaction effect (P = .90). With supplementation to CHA = 2.0 mg/mL, the elevated μkinetic of fibrin and F + MSC eSF (+120% of NL-eSF, P < .01) (Figure 7B) was lowered (P < .01) to levels indistinguishable from that of NL-eSF (P = .67). Supplementation to CHA = 0.8 mg/mL tended to reduce µkinetic but did not reach statistical significance (P = .019, α = .05/4). µstatic,Tps=120s was not modulated by CHA or treatment, and no interaction effect was evident (all, P > .10) (Figure 7A).
Figure 7.
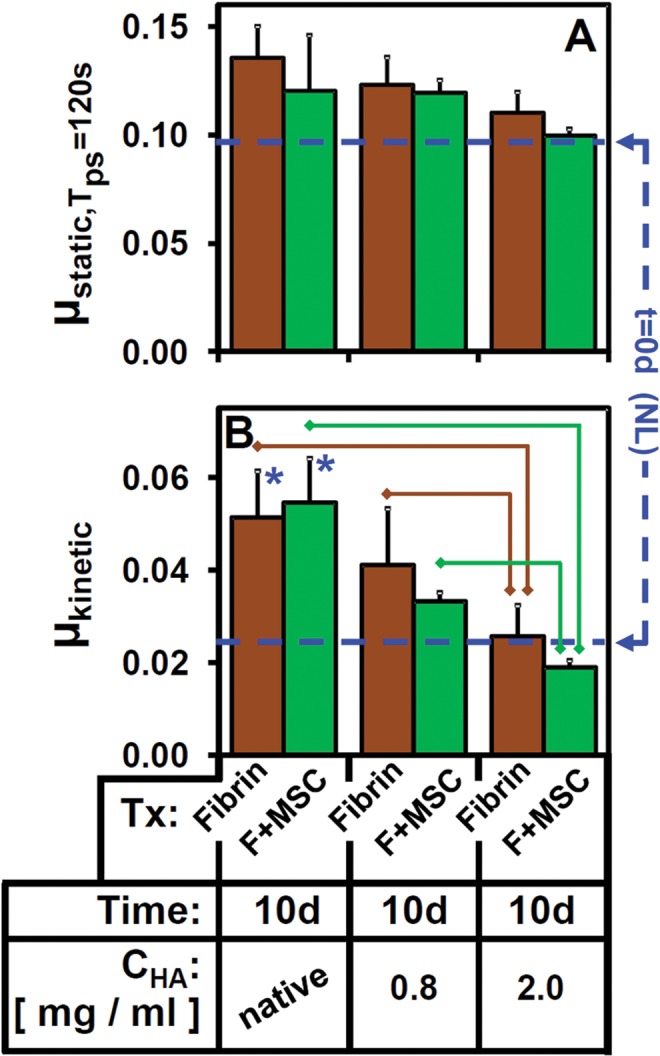
Effect of supplementing t = 10 days equine synovial fluid (eSF) pools on (A) μstatic and (B) μkinetic with 4 MDa Healon. CHA was at native levels or increased to a final concentration of 0.8 or 2.0 mg/mL. Lines indicate groups that are significantly different (P < .05/4).  : versus t = 0 days (P < .05/4). n = 4 for Native, 0.6 and 2.0 mg/mL groups. Average for t = 0 days groups (n = 11) is also shown as dashed lines for reference. F, fibrin; MSC, mesenchymal stem cell; NL, normal.
: versus t = 0 days (P < .05/4). n = 4 for Native, 0.6 and 2.0 mg/mL groups. Average for t = 0 days groups (n = 11) is also shown as dashed lines for reference. F, fibrin; MSC, mesenchymal stem cell; NL, normal.
Discussion
These results indicate that SF lubrication function and biochemical composition are modulated over time following arthroscopic repair of a cartilage defect for 2 types of repair. Boundary lubrication function was diminished shortly at t = 10 d after surgery and returned to normal at t = 3 mo, as indicated by increased steady-state friction coefficients for t = 10 d samples relative to t = 0 d and 3 mo samples (see Figure 3). This lubrication deficiency of eSF for t = 10 d samples was associated with decreased concentration of HA (Figures 4D and 6) and a shift toward lower MW forms of HA (Figure 5), as well as increases in volume (Figure 4A), concentration of protein (Figure 4B), and concentration of PRG4 (Figure 4C). High-MW HA supplementation of lubricant-deficient t = 10 d eSF samples resulted in a dose-dependent restoration of lubrication function (Figure 7).
The equine experimental model of the present study has a number of advantages but also some limitations. It allowed for a repeated-measures study design to investigate changes in the lubricating ability and composition of SF, as it transitioned from normal to injury and repair states. The patellofemoral joint of horses can be operated on arthroscopically, analogous to surgical repair of cartilage damage in the human knee joint. The comparison between normal (t = 0 d), injury (t = 10 d), and repair (t = 3 mo) states within each animal obviates the interanimal variability that is substantial across populations of different animals. The bilateral design ensured that similarities between left and right joints in the initial preinjury state could be evaluated. One limitation of this design is that without a sham surgery, untreated chondral defect, or microfracture treatment (commonly used cartilage repair procedure), it is unclear whether the effects at times after surgery were specific to the type of cartilage repair procedure or may have been due to the surgery, creation of the defect, or treatment of the defect. However, the focus of this study was to investigate the combined effect of defect and surgery, as relevant in the postinjury and/or postsurgery state, at which time lubrication function is diminished and further cartilage damage may occur. Further study of the effect of an untreated defect or arthroscopy alone could help to define ways to augment this surgical modality to minimize alterations to SF properties. Also, the time points (t = 0 d, 10 d, 3 mo) were limited but similar to those (4 d, 7 d, 21 d) of a previous study on equine cartilage repair.14
The finding of time-dependent alterations in lubrication after surgery is consistent with and extends previous studies of properties of SF after joint injury. The elevated t = 10 d coefficient of friction was consistent with observations of diminished lubricating ability relative to the normal state of SF obtained from acutely injured horses2 and patients shortly following tibial plateau fracture,6 tested under the same cartilage-on-cartilage lubrication test employed here, as well as rabbits following ACLT tested under a cartilage-on-glass system,12 and guinea pigs following ACLT tested under a whole-joint pendulum system.38 The restoration of normal lubricating ability by the long (t = 3 mo) duration postsurgery may be analogous to the normal lubricating properties of eSF after chronic injury2 and human SF (hSF) from patients with various grades of osteoarthritis.9
Certain baseline (t = 0 d) and injury properties of eSF were consistent with previous studies. The µkinetic of t = 0 d eSF samples was comparable to that of normal SF in previous studies in horses (0.026) and humans (0.022), using the same cartilage-on-cartilage lubrication test employed here,2,6 and also to results from latex-on-glass lubrication tests for normal bovine SF (0.021), as well as to hSF from patients with degenerative joint disease (0.024), who were proposed to have normal lubricating ability.9 The increased volume and protein for t = 10 d eSF samples was in agreement with previous studies.18,25
Variations in concentrations of the lubricant molecules HA and PRG4 were also somewhat consistent with those of previous studies. Alterations of SF composition following an arthroscopic repair procedure have been reported previously in humans37 and horses,14,20 and the present study builds on those by defining the time-dependent effect on eSF lubricant molecules and concomitant effects on eSF lubricating function. The HA concentration in t = 0 d samples (CHA = 0.89 mg/mL) was within the published range of 0.26 to 1.3 mg/mL for normal eSF.2,29 The decrease in CHA for t = 10 d samples, relative to t = 0 d values, that returned by t = 3 mo was consistent with the time course and magnitude of decreased HA concentrations of eSF from joints following cartilage repair14 and was consistent with diminished HA concentrations for both eSF from acutely injured carpal or metacarpophalangeal joints2 and hSF from patients with traumatic arthritis and hydrarthrosis3 or shortly following tibial plateau fracture.6 On the other hand, other eSF analyses have not detected effects on CHA, relative to normal, for horses at 1 to 2 weeks following experimental treatment with fibrin matrices with or without IGF-1 for an analogous cartilage defect27 and horses with acute traumatic synovitis.30,40 The elevated PRG4 concentration in t = 10 d samples, relative to t = 0 d samples, is consistent with previous studies on hSF from patients shortly following tibial plateau fracture,6 eSF from acutely injured horses.2 However, the opposite change, of decreased PRG4 concentration, was observed in studies on SF obtained from rabbits12 and guinea pigs38 following ACLT or from patients with ACL injury.11 The differences may be due to differences in experimental design, acute injury type, joint type, species, and method of determining HA and PRG4 content and quality. For example, in addition to characterizing PRG4 directly from SF, analyses of protein and carbohydrate components may reveal differences between joint states.13,36 Also, joint destabilization injuries, such as ACLT, may alter the synovial mechanical environment34 compared with the cartilage defect injuries here, and such differences may have differential modulatory effects on the rates of secretion and joint efflux of HA and PRG4. The present study expands on these previous works by detailing the alterations in HA and PRG4 following cartilage defect repair surgery.
The finding of the decrease in HA concentration and shift in HA toward low-MW forms at 10 days postsurgery provides new information with potential therapeutic implications. One previous study on eSF HA MW had an upper detection limit of 3 MDa with high-performance liquid chromatography40 and resolved normal HA MW as being predominantly in the 2 to 3 MDa range. This technical limitation may have led to the apparent similarity in HA MW distribution between normal and acute traumatic arthritis eSF40 since, as found in the present study, HA MW extends up to 3 to 7 MDa.5 The current findings on altered structure of HA after joint injury and repair are consistent with our recent study on AI-eSF.2
The finding that the in vitro addition of high-MW HA to equine SF that is deficient in lubricating ability restores lubricant function suggests that intra-articular lubricant supplementation may help maintain and/or restore the boundary lubrication function of SF following arthroscopic joint repair surgery. While there is a vast amount of information regarding the possible mechanisms of action of HA17 and the use of HA as a clinical treatment for osteoarthritis,39 there is limited information on clinical studies of HA as a treatment for acute joint injury10,42 or cartilage repair.31 To evaluate a possible role of HA injections to augment lubrication after surgery for acute knee injury, the time-dependent concentrations of HA and its contribution to cartilage lubrication needs to be clarified at various times postsurgery. The dose dependence of the lubricating ability of eSF following supplementation of high-MW HA to 2.0 mg/mL extends our previous study of chemical augmentation of AI-eSF.2 The finding that in vitro HA supplementation restores SF lubrication function for t = 10 d samples supports the correlative data, implicating low concentration of high-MW HA as the cause of lubrication deficiency. However, this does not eliminate the possibility that other unstudied molecules in the t = 10 d eSF may interfere with the individual or combined lubricating mechanisms of PRG4 and HA. In studies on osteoarthritis hSF with diminished lubricating ability and normal CHA but diminished CPRG4, normal lubricating ability was restored following supplementation with PRG4 to normal levels.23 On the other hand, in the current study, CPRG4 was elevated in deficiently lubricating postsurgery eSF. Thus, there may be applications for either HA or PRG4 as a viscosupplement, depending on the independent and interactive effects of molecules in SF. However, injection therapies may require repeated applications or extended-release formulations, since the residence time of HA in synovial joints is approximately 24 hours and this decreases following joint injury.24
Footnotes
One of more of the authors declared the following potential conflict of interest or source of funding: This work was supported by grants from the National Institutes of Health: RC2 AR058929 (to C.R.C.) and R01 AR055637 (to R.L.S.). Additional individual support was received from a UCSD Chancellor’s Research Scholarship (to M.J.G.) and the Medical Student Training in Aging Research Program (to M.J.G.).
References
- 1. Altman DG, Bland JM. Measurement in medicine—the analysis of method comparison studies. Statistician. 1983;32:307–317. [Google Scholar]
- 2. Antonacci JM, Schmidt TA, Serventi LA, et al. Effects of equine joint injury on boundary lubrication of articular cartilage by synovial fluid: role of hyaluronan. Arthritis Rheum. 2012;64:2917–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asari A, Miyauchi S, Sekiguchi T, et al. Hyaluronan, cartilage destruction and hydrarthrosis in traumatic arthritis. Osteoarthritis Cartilage. 1994;2:79–89. [DOI] [PubMed] [Google Scholar]
- 4. Ateshian GA. The role of interstitial fluid pressurization in articular cartilage lubrication. J Biomech. 2009;42:1163–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balazs EA, Watson D, Duff IF, Roseman S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritis human fluids. Arthritis Rheum. 1967;10:357–376. [DOI] [PubMed] [Google Scholar]
- 6. Ballard BL, Antonacci JM, Temple-Wong MM, et al. Effect of tibial plateau fracture on lubrication function and composition of synovial fluid. J Bone Joint Surg Am. 2012;94:e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blewis ME, Lao BJ, Schumacher BL, Bugbee WD, Sah RL, Firestein GS. Interactive cytokine regulation of synoviocyte lubricant secretion. Tissue Eng Part A. 2010;16:1329–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blewis ME, Nugent-Derfus GE, Schmidt TA, Schumacher BL, Sah RL. A model of synovial fluid lubricant composition in normal and injured joints. Eur Cell Mater. 2007;13:26–39. [DOI] [PubMed] [Google Scholar]
- 9. Davis WHJ, Lee SL, Sokoloff L. Boundary lubricating ability of synovial fluid in degenerative joint disease. Arthritis Rheum. 1978;21:754–760. [DOI] [PubMed] [Google Scholar]
- 10. Di Marco C, Letizia GA. Hyaluronic acid in the treatment of pain due to knee joint immobilisation. Clin Drug Invest. 1995;10:191–197. [Google Scholar]
- 11. Elsaid KA, Fleming BC, Oksendahl HL, et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58:1707–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elsaid KA, Jay GD, Warman ML, Rhee DK, Chichester CO. Association of articular cartilage degradation and loss of boundary-lubricating ability of synovial fluid following injury and inflammatory arthritis. Arthritis Rheum. 2005;52:1746–1755. [DOI] [PubMed] [Google Scholar]
- 13. Estrella RP, Whitelock JM, Packer NH, Karlsson NG. The glycosylation of human synovial lubricin: implications for its role in inflammation. Biochem J. 2010;429:359–367. [DOI] [PubMed] [Google Scholar]
- 14. Fortier LA, Mohammed HO, Lust G, Nixon AJ. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br. 2002;84:276–288. [DOI] [PubMed] [Google Scholar]
- 15. Fortier LA, Nixon AJ, Williams J, Cable CS. Isolation and chondrocytic differentiation of equine bone marrow-derived mesenchymal stem cells. Am J Vet Res. 1998;59:1182–1187. [PubMed] [Google Scholar]
- 16. Fortier LA, Potter HG, Rickey EJ, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92:1927–1937. [DOI] [PubMed] [Google Scholar]
- 17. Ghosh P, Guidolin D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent? Semin Arthritis Rheum. 2002;32:10–37. [DOI] [PubMed] [Google Scholar]
- 18. Gibson KT, Hodge H, Whittem T. Inflammatory mediators in equine synovial fluid. Aust Vet J. 1996;73:148–151. [DOI] [PubMed] [Google Scholar]
- 19. Haserodt S, Aytekin M, Dweik RA. A comparison of the sensitivity, specificity, and molecular weight accuracy of three different commercially available hyaluronan ELISA-like assays. Glycobiology. 2011;21:175–183. [DOI] [PubMed] [Google Scholar]
- 20. Hendrickson DA, Nixon AJ, Grande DA, et al. Chondrocyte-fibrin matrix transplants for resurfacing extensive articular cartilage defects. J Orthop Res. 1994;12:485–497. [DOI] [PubMed] [Google Scholar]
- 21. Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–463. [DOI] [PubMed] [Google Scholar]
- 22. Kisiday JD, Hale BW, Almodovar JL, et al. Expansion of mesenchymal stem cells on fibrinogen-rich protein surfaces derived from blood plasma. J Tissue Eng Regen Med. 2011;5:600–611. [DOI] [PubMed] [Google Scholar]
- 23. Ludwig TE, McAllister JR, Lun V, Wiley JP, Schmidt TA. Diminished cartilage lubricating ability of human osteoarthritic synovial fluid deficient in proteoglycan 4: restoration through proteoglycan 4 supplementation. Arthritis Rheum. 2012;64:3963–3971. [DOI] [PubMed] [Google Scholar]
- 24. McCarty WJ, Cheng JC, Hansen BC, Yamaguchi T, Masuda K, Sah RL. The biophysical mechanisms of altered hyaluronan concentration in synovial fluid after anterior cruciate ligament transection. Arthritis Rheum. 2012;64:3993–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McIlwraith CW, Billinghurst RC, Frisbie DD. Current and future diagnostic means to better characterize osteoarthritis in the horse - routine synovial fluid analysis and synovial fluid and serum markers. AAEP Proc. 2001;47:171–191. [Google Scholar]
- 26. McIlwraith CW, Frisbie DD, Rodkey WG, et al. Evaluation of intra-articular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthroscopy. 2011;27:1552–1561. [DOI] [PubMed] [Google Scholar]
- 27. Nixon AJ, Fortier LA, Williams J, Mohammed H. Enhanced repair of extensive articular defects by insulin-like growth factor-I-laden fibrin composites. J Orthop Res. 1999;17:475–487. [DOI] [PubMed] [Google Scholar]
- 28. Qiu LL, Levinson SS, Keeling KL, Elin RJ. Convenient and effective method for removing fibrinogen from serum specimens before protein electrophoresis. Clin Chem. 2003;49 (6 pt 1):868–872. [DOI] [PubMed] [Google Scholar]
- 29. Rowley G, Antonas KN, Hilbert BJ. Quantitation of hyaluronic acid in equine synovia. Am J Vet Res. 1982;43:1096–1099. [PubMed] [Google Scholar]
- 30. Saari H, Konttinen YT, Tulamo RM, Antti-Poika I, Honkanen V. Concentration and degree of polymerization of hyaluronate in equine synovial fluid. Am J Vet Res. 1989;50:2060–2063. [PubMed] [Google Scholar]
- 31. Saw KY, Anz A, Siew-Yoke Jee C, et al. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy. 2013;29:684–694. [DOI] [PubMed] [Google Scholar]
- 32. Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 2007;56:882–891. [DOI] [PubMed] [Google Scholar]
- 33. Schmidt TA, Sah RL. Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthritis Cartilage. 2007;15:35–47. [DOI] [PubMed] [Google Scholar]
- 34. Setton LA, Elliott DM, Mow VC. Altered mechanics of cartilage with osteoarthritis: human osteoarthritis and an experimental model of joint degeneration. Osteoarthritis Cartilage. 1999;7:2–14. [DOI] [PubMed] [Google Scholar]
- 35. Steadman JR, Rodkey WG, Briggs KK, Rodrigo JJ. The microfracture technic in the management of complete cartilage defects in the knee joint [in German]. Orthopade. 1999;28:26–32. [DOI] [PubMed] [Google Scholar]
- 36. Steele BL, Alvarez-Veronesi MC, Schmidt TA. Molecular weight characterization of PRG4 proteins using multi-angle laser light scattering (MALLS). Osteoarthritis Cartilage. 2013;21:498–504. [DOI] [PubMed] [Google Scholar]
- 37. Taskiran E, Taskiran D, Duran T, Lok V. Articular cartilage homeostasis after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 1998;6:93–98. [DOI] [PubMed] [Google Scholar]
- 38. Teeple E, Elsaid KA, Fleming BC, et al. Coefficients of friction, lubricin, and cartilage damage in the anterior cruciate ligament-deficient guinea pig knee. J Orthop Res. 2008;26:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trigkilidas D, Anand A. The effectiveness of hyaluronic acid intra-articular injections in managing osteoarthritic knee pain. Ann R Coll Surg Engl. 2013;95:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tulamo RM, Heiskanen T, Salonen M. Concentration and molecular weight distribution of hyaluronate in synovial fluid from clinically normal horses and horses with diseased joints. Am J Vet Res. 1994;55:710–715. [PubMed] [Google Scholar]
- 41. Wong BL, Kim SH, Antonacci JM, McIlwraith CW, Sah RL. Cartilage shear dynamics during tibio-femoral articulation: effect of acute joint injury and tribosupplementation on synovial fluid lubrication. Osteoarthritis Cartilage. 2010;18:464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zattoni G, Cabrioli A, Brunelli G, Pergellini A. Efficacy and tolerability of hyaluronic acid in acute knee injury: a controlled clinical study. Eur J Rheumatol Inflam. 1995;15:63–69. [Google Scholar]