Abstract
Background: Thyroid cancer is an increasingly common malignancy. Although likelihood of survival from well-differentiated thyroid cancer can vary by disease severity, it is not known how patients' life expectancies change the farther they are from time of diagnosis.
Methods: Using data from the Surveillance, Epidemiology, End Results (SEER) registry, we selected patients diagnosed with well-differentiated thyroid cancer (N=43,392) between 1998 and 2005. Patients were followed for up to 12 years. Conditional survival estimates by SEER stage and age were obtained based on Cox proportional hazards regression model of disease-specific survival.
Results: Patients with localized thyroid cancer have excellent conditional 5-year survival, irrespective of where they are in their survivorship phase. Patients with regional thyroid cancer have relatively stable conditional 5-year survival, whereas for patients with distant thyroid cancer there is gradual improvement the farther from time of diagnosis. Age and gender influence conditional survival. Similarly, age has a strong effect on disease-specific survival for patients with thyroid cancer with localized (hazard ratio [HR] 88.7 [95% confidence interval {CI} 26.3–552), comparing age ≥80 with <30 years), regional (HR 105 [95% CI 52.6–250]), and distant disease [HR 86.8 (95% CI 32.5–354)]. Male gender is also associated with a significantly worse disease-specific survival among patients with regional disease (HR 1.56 [95% CI 1.31–1.85]) but not among patients with localized or distant disease.
Conclusion: Cancer stage, gender, age at diagnosis, and length of time already survived can influence conditional survival for patients with thyroid cancer. Understanding the conditional 5-year disease-specific survival of well-differentiated thyroid cancer is key to creating treatment plans and tailoring surveillance.
Introduction
In the past 35 years, the incidence rate of thyroid cancer has risen by nearly 200% (1–6). This increasingly common cancer has variable prognoses, with most patients having an excellent likelihood of long-term survival and others with an increased risk of death secondary to their cancer (7–9). Although the 5- and 10-year prognosis at time of diagnoses is known (3), the likelihood of survival based on how long a patient has already survived (i.e., conditional survival) remains understudied.
Understanding conditional survival is important for patients and providers. It is the key to creating treatment plans, to determining long-term follow-up/surveillance strategies, and to giving patients realistic expectations for their future. For some cancers, patients who survive the first few years after diagnosis have a much improved life expectancy (10). For other cancers, likelihood of death persists for many years (10). In the context of steadily increasing numbers of low-risk thyroid cancer (3,5), it is important to understand conditional survival rates based on cancer stage, patient age, and patient gender. This will allow more tailored treatment and surveillance.
In this study, we used data from Surveillance, Epidemiology, and End Results (SEER) registry to determine conditional 5-year disease-specific survival based on patient age, gender, and stage. Because patient age has an important role in determining thyroid cancer outcome (11,12), we hypothesized that age at diagnosis would also be important to thyroid cancer conditional survival.
Materials and Methods
Data source and study population
Using SEER data we selected patients diagnosed with well-differentiated thyroid cancer (N=43,392) between 1998 and 2005. Well-differentiated thyroid cancer included patients with papillary thyroid cancer, follicular thyroid cancer, and Hürthle cell cancer. Patients were followed for up to 12 years with a median follow up of 7.5 years. Tumor stage was categorized according to SEER summary stage as localized, regional, or distant. Localized includes single or multifocal invasive tumor(s) confined to the thyroid or into the capsule but not beyond. Regional includes direct extension into blood vessels, cricoid cartilage, esophagus, larynx, nerves, muscles, and parathyroid. It also includes tumor described as “fixed to adjacent tissues” and regional lymph node involvement. Distant includes distant lymph nodes, metastasis, and extension into bone, mediastinal tissues, etc. (3). Age was categorized by decades, as <30, 30–39, 40–49, 50–59, 60–69, 70–79, or ≥80 years.
Because this study uses publically available data that cannot be tracked to human subjects, per the University of Michigan Institutional Review Board, IRB approval was not needed.
Statistical analysis
The primary end point was disease-specific survival, defined as the time from diagnosis to death from thyroid cancer, as determined by SEER. We used Cox proportional hazards regression to model disease-specific survival as a function of age and gender.
Given that a patient has survived t years past diagnosis, the conditional 5-year survival function CS5(t) is the probability that he or she will survive an additional 5 years. Thus, the conditional 5-year survival function can formally be defined as CS5(t)=Pr(T>5+t|T>t), where T represents a patient's true disease-specific survival (time in years) since diagnosis. Applying the definition of conditional probability, one can write CS5(t)=Pr(T>5+t)/Pr(T>t)=S(5+t)/S(t) where S is the disease-specific survival function. We estimated the conditional 5-year survival based on estimates of S(5+t) and S(t) from the Cox regression model, using the Breslow estimator of the baseline cumulative hazard function.
Results
Table 1 shows the characteristics of the study population. For thyroid cancer, a large proportion of the patients have localized disease (62.8%). Only 3.7% of all patients with thyroid cancer have distant metastases. Thyroid cancer is far more common in females (76.7%) and among white patients (83%). At the time of last follow-up, only 2.6% of all thyroid cancer patients died from their cancers.
Table 1.
Characteristics of Study Population
| Thyroid | ||||
|---|---|---|---|---|
| Cancer | Localized n (%) | Regional n (%) | Distant n (%) | Total n (%) |
| Stage | 27,267 (62.8) | 14,526 (33.5) | 1599 (3.7) | 43,392 (100.0) |
| Patient characteristics | ||||
| Age | ||||
| <30 | 2728 (10.0) | 2270 (15.6) | 196 (12.3) | 5194 (12.0) |
| 30–39 | 5487 (20.1) | 2989 (20.6) | 184 (11.5) | 8,660 (20.0) |
| 40–49 | 7049 (25.9) | 3407 (23.5) | 201 (12.6) | 10,657 (24.6) |
| 50–59 | 5971 (21.9) | 2588 (17.8) | 272 (17.0) | 8831 (20.4) |
| 60–69 | 3419 (12.5) | 1625 (11.2) | 277 (17.3) | 5321 (12.3) |
| 70–79 | 2037 (7.5) | 1171 (8.1) | 298 (18.6) | 3506 (8.1) |
| >80 | 576 (2.1) | 476 (3.3) | 171 (10.7) | 1223 (2.8) |
| Gender | ||||
| Female | 21,796 (79.9) | 10,535 (72.5) | 930 (58.2) | 33,261 (76.7) |
| Male | 5471 (20.1) | 3991 (27.5) | 669 (41.8) | 10,131 (23.3) |
| Race | ||||
| White | 22,752 (83.4) | 12,009 (82.7) | 1243 (77.7) | 36,004 (83.0) |
| Black | 1899 (7.0) | 700 (4.8) | 121 (7.6) | 2720 (6.3) |
| Other | 2400 (8.8) | 1719 (11.8) | 225 (14.1) | 4344 (10.0) |
| Unknown | 216 (0.8) | 98 (0.7) | 10 (0.6) | 324 (0.7) |
| Year of diagnosis | ||||
| 1998 | 1331 (4.9) | 869 (6.0) | 85 (5.3) | 2285 (5.3) |
| 1999 | 1483 (5.4) | 905 (6.2) | 105 (6.6) | 2493 (5.7) |
| 2000 | 3,177 (11.7) | 1826 (12.6) | 211 (13.2) | 5214 (12.0) |
| 2001 | 3524 (12.9) | 1930 (13.3) | 198 (12.4) | 5652 (13.0) |
| 2002 | 3923 (14.4) | 2037 (14.0) | 253 (15.8) | 6213 (14.3) |
| 2003 | 4270 (15.7) | 2121 (14.6) | 234 (14.6) | 6625 (15.3) |
| 2004 | 4606 (16.9) | 2363 (16.3) | 235 (14.7) | 7204 (16.6) |
| 2005 | 4953 (18.2) | 2475 (17.0) | 278 (17.4) | 7706 (17.8) |
| Disease-specific survival | ||||
| Dead | 130 (0.5) | 525 (3.6) | 471 (29.5) | 1126 (2.6) |
| Censored | 27,137 (99.5) | 14,001 (96.4) | 1128 (70.5) | 42,266 (97.4) |
| Overall survival | ||||
| Dead | 2,129 (7.8) | 1664 (11.5) | 752 (47.0) | 4,545 (10.5) |
| Censored | 25,138 (92.2) | 12,862 (88.5) | 847 (53.0) | 38,847 (89.5) |
The last date of follow-up was November 2010. Patients who were alive at their last time of follow-up or November 2010 (whichever came earlier) were considered to be censored at that time point.
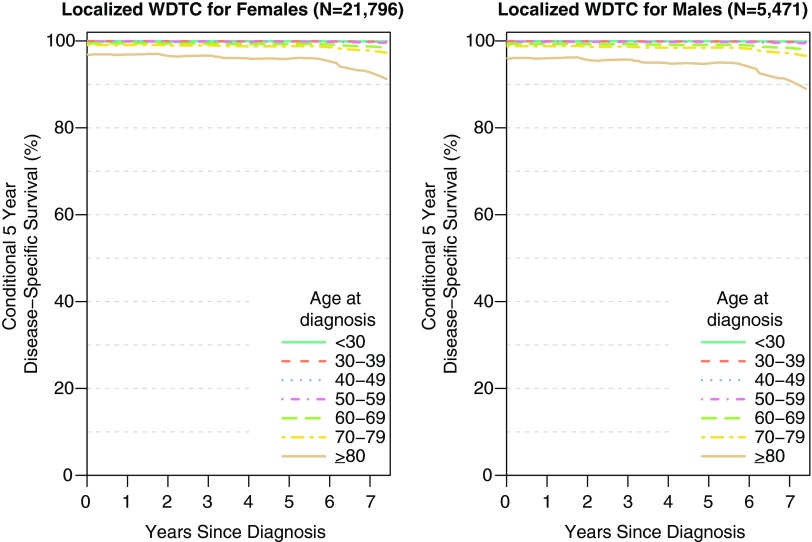
Prognosis is excellent if the disease is localized. Figure 1 illustrates the conditional 5-year disease-specific survival of male versus female patients with localized thyroid cancer. A small age gradient is seen for both genders, demonstrating better survival for younger patients. For example, for females with localized disease, the probability of surviving an additional 5 years, after surviving 2 years past diagnosis [denoted CS5(2)], is 99.9%, 99.4%, and 96.6% for ages 30–39, 60–69, and ≥80, respectively. For females, the probability of surviving an additional 5 years after surviving 5 years past diagnosis [denoted CS5(5)], is 99.9%, 99.3%, and 95.9% for ages 30–39, 60–69, and ≥80, respectively.
FIG. 1.
Conditional 5-year disease-specific survival of female and male patients with localized thyroid cancer. The conditional 5-year disease-specific survival is near 100% for most patients and is relatively stable the further a patient is from time of diagnosis. Color images available online at www.liebertpub.com/thy
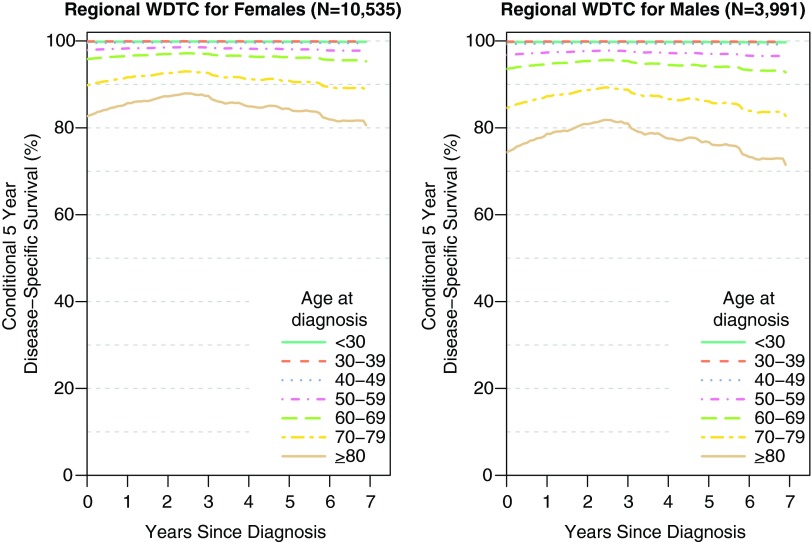
Figure 2 shows the conditional 5-year disease-specific survival of patients with regional thyroid cancer. For thyroid cancer, the conditional 5-year survival remains relatively stable over time. For both genders, there is a clear age gradient, with older patients faring worse than younger patients. For example, for females with regional disease, CS5(2) is 99.9%, 97.0%, and 87.3% for ages 30–39, 60–69, and ≥80, respectively. Five years past diagnosis CS5(5) is 99.9%, 96.3%, and 84.4% for females ages 30–39, 60–69, and ≥80, respectively. However, males generally have a slightly lower conditional 5-year disease-specific survival.
FIG. 2.
Conditional 5-year disease-specific survival of female and male patients with regional thyroid cancer. The conditional 5-year disease-specific survival differs by age at diagnosis and is relatively stable the further a patient is from time of diagnosis. Color images available online at www.liebertpub.com/thy
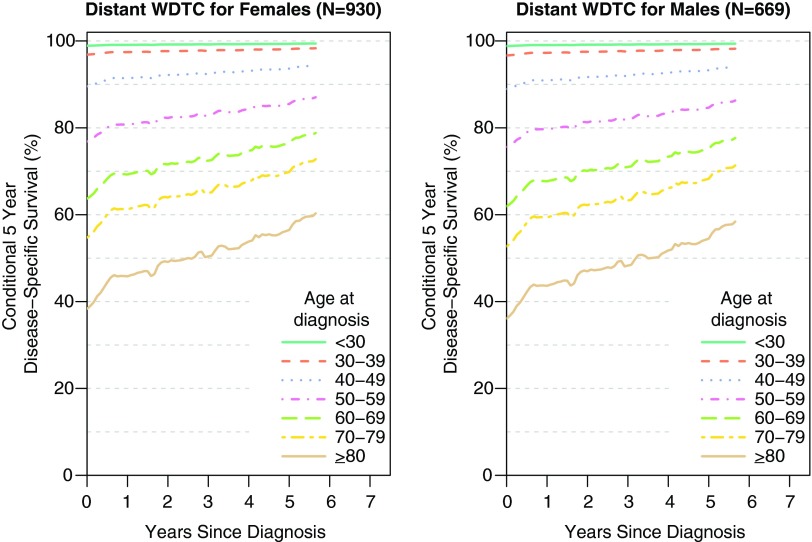
The conditional 5-year disease-specific survival of patients with distant metastases from thyroid cancer shows that for both genders, older patients with thyroid cancer with distant metastases have a worse conditional 5-year survival than younger patients (Fig. 3). For example, for females with distant disease, CS5(2) is 97.7%, 71.6%, and 49.2% for ages 30–39, 60–69, and ≥80, respectively. Five years past diagnosis CS5(5) is 98.1%, 76.4%, and 56.5% for females ages 30–39, 60–69, and ≥80, respectively. For both genders, the conditional 5-year disease-specific survival improves gradually over time.
FIG. 3.
Conditional 5-year disease-specific survival of female and male patients with distant thyroid cancer. The conditional 5-year disease-specific survival differs by age at diagnosis and improves the further a patient is from time of diagnosis. Color images available online at www.liebertpub.com/thy
Table 2 demonstrates disease-specific mortality by age and gender. For all stages of thyroid cancer, there is a very strong, linear association between age and disease-specific survival (for example, age 70–79 versus <30 years, localized (hazard ratio [HR] 26.58 [95% confidence interval {CI} 8.08–163.08)], regional [HR 59.39 (95% CI 30.12–139.87)], and distant [HR 54.54 (95% CI 20.67–221.42)]. For thyroid cancer, male gender is associated with a significantly worse disease-specific survival among patients with regional disease [HR 1.56 (95% CI 1.31–1.85)] but not among patients with localized or distant disease.
Table 2.
Disease-Specific Mortality by Age and Gender
| Thyroid (N=43,392) | |||
|---|---|---|---|
| CancerStage | Localized HR (95% CI) | Regional HR (95% CI) | Distant HR (95% CI) |
| Patient characteristics | |||
| Age | |||
| <30 | 1.00 | 1.00 | 1.00 |
| 30–39 | 1.99 (0.50, 13.21) | 0.53 (0.16, 1.68) | 2.90 (0.84, 13.23) |
| 40–49 | 1.77 (0.46, 11.62) | 2.63 (1.21, 6.54) | 9.98 (3.54, 41.77) |
| 50–59 | 4.03 (1.16, 25.44) | 11.69 (5.83, 27.78) | 23.81 (8.92, 97.21) |
| 60–69 | 15.05 (4.58, 92.78) | 23.53 (11.81, 55.76) | 40.82 (15.42, 165.97) |
| 70–79 | 26.58 (8.08, 163.90) | 59.39 (30.12, 139.87) | 54.54 (20.67, 221.42) |
| >80 | 88.67 (26.33, 551.83) | 105.20 (52.57, 249.95) | 86.80 (32.48, 354.46) |
| Gender | |||
| Female | 1.00 | 1.00 | 1.00 |
| Male | 1.28 (0.86, 1.85) | 1.56 (1.31, 1.85) | 1.06 (0.89, 1.27) |
CI, confidence interval; HR, hazard ratio.
Discussion
The results of this study provide information to patients and providers regarding the conditional 5-year disease-specific survival of patients with thyroid cancer. We found that age at diagnosis was important for the conditional 5-year disease-specific survival in all stages of thyroid cancer. We found that the conditional 5-year disease-specific survival for localized thyroid cancer was excellent. For patients with regional thyroid cancer the conditional 5-year disease-specific survival was relatively static over time whereas for distant thyroid cancer, there was a gradual improvement the longer a patient lived past time of diagnosis. Male gender was associated with significantly worse disease-specific survival for regional disease (but not for localized or distant disease), which translates to worse conditional survival for males (an effect seen primarily in the older age groups).
Although conditional survival has been studied in other common malignancies, to date, studies have not focused on the implications of conditional survival on patients with thyroid cancer with varying age, gender, and stage at time of diagnosis (10,13–15). Thyroid cancer is the ninth most common cancer in the United States (16). Despite overall declines in cancer rates, the incidence of thyroid cancer is rising (1,2,4). Prognosis for thyroid cancers is generally excellent, but not all patients have uniformly good long-term survival. Since incidence is rising and outcome is variable, it is becoming increasingly important to understand conditional survival in this cancer.
The importance of age in thyroid cancer outcome has been studied (17–19). However, this study illustrates the fact that the importance of age to thyroid cancer outcome is linear and persists years after diagnosis. Even in patients who are 5 years since the time of diagnosis, age at diagnosis has implications for their conditional 5-year disease-specific survival, with older patients having worse outcomes than younger patients.
A possible limitation of this study is that conditional 5-year disease-specific survival may change over time, especially with the advent of new therapeutics. However, during the time span of this study thyroid cancer did not benefit from a novel therapeutic that would have influenced life expectancy in a significant and universal manner. Another potential limitation of this study is the fact that although disease-specific survival rates are standard outcome measures, they can underestimate actual cancer-related deaths (20). This would have the effect of minimizing our findings in the context of how conditional survival influences long-term outcomes. Theoretically, reported disease-specific survival rates may even overestimate cancer-related deaths. However, disease-specific survival remains the gold standard outcome measure with large cancer registries.
Despite limitations, this study has relevant findings for both physicians and patients. Understanding the conditional 5-year disease-specific survival allows physicians to create management plans. For example, the mortality rate for localized thyroid cancer is extremely low and the conditional 5-year disease-specific survival excellent. Many of these patients may be able to be discharged from thyroid cancer clinics after a few years of surveillance. In contrast, patients with distant thyroid cancer have a conditional 5-year disease-specific survival curve that suggests that some patients still die because of thyroid cancer many years after initial diagnosis. These patients likely need long-term surveillance in a dedicated thyroid cancer clinic. Knowledge of conditional survival may have implications for changing paradigms in survivorship care for an increasing number of patients. Understanding the conditional 5-year disease-specific survival also allows patients to have a more accurate assessment of their life expectancy.
The findings from this study are important because the further a patient is from time of diagnosis, the less relevant the initial survival estimation and the more pertinent the conditional survival estimation. The results inform both patients and their providers and may lead to more tailored cancer surveillance. Since the number of patients diagnosed with thyroid cancer continues to increase (4), conditional survival is becoming increasingly relevant to a greater number of patients and physicians.
Acknowledgments
Dr. Haymart is supported by NIH 1K07CA154595-02. Dr. Wong is supported by AHRQ 1K08 HS20937-01. Brittany Gay assisted with creating the figures and tables. The thyroid cancer research was supported by the University of Michigan MCubed Seed Funding Program and by The Punya Foundation for Thyroid Cancer Research.
Author Disclosure Statement
The authors have no conflict of interest to disclose.
References
- 1.Davies L, Welch HG.2006Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167 [DOI] [PubMed] [Google Scholar]
- 2.Chen AY, Jemal A, Ward EM.2009Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer 115:3801–3807 [DOI] [PubMed] [Google Scholar]
- 3.www.seer.cancer.gov2013. (accessed November1, 2013)
- 4.Simard EP, Ward EM, Siegel R, Jemal A.2012Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin 2012January4 [Epub ahead of print]; DOI: 10.3322/caac.20141 [DOI] [PubMed] [Google Scholar]
- 5.Esserman LJ, Thompson IM, Jr, Reid B.2013Overdiagnosis and overtreatment in cancer: an opportunity for improvement. JAMA 310:797–798 [DOI] [PubMed] [Google Scholar]
- 6.Davies L, Welch HG.2014Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 140:317–322 [DOI] [PubMed] [Google Scholar]
- 7.Hundahl SA, Fleming ID, Fremgen AM, Menck HR.1998A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995 [see comments]. Cancer 83:2638–2648 [DOI] [PubMed] [Google Scholar]
- 8.Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, Cooper DS, Haugen BR, Ladenson PW, Magner J, Robbins J, Ross DS, Skarulis M, Maxon HR, Sherman SI.2006Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 16:1229–1242 [DOI] [PubMed] [Google Scholar]
- 9.Gilliland FD, Hunt WC, Morris DM, Key CR.1997Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer 79:564–573 [DOI] [PubMed] [Google Scholar]
- 10.Janssen-Heijnen ML, Gondos A, Bray F, Hakulinen T, Brewster DH, Brenner H, Coebergh JW.2010Clinical relevance of conditional survival of cancer patients in Europe: age-specific analyses of 13 cancers. J Clin Oncol 28:2520–2528 [DOI] [PubMed] [Google Scholar]
- 11.Verburg FA, Mader U, Tanase K, Thies ED, Diessl S, Buck AK, Luster M, Reiners C.2013Life expectancy is reduced in differentiated thyroid cancer patients ≥45 years old with extensive local tumor invasion, lateral lymph node, or distant metastases at diagnosis and normal in all other DTC patients. J Clin Endocrinol Metab 98:172–180 [DOI] [PubMed] [Google Scholar]
- 12.Yang L, Shen W, Sakamoto N.2013Population-based study evaluating and predicting the probability of death resulting from thyroid cancer and other causes among patients with thyroid cancer. J Clin Oncol 31:468–474 [DOI] [PubMed] [Google Scholar]
- 13.Merrill RM, Hunter BD.2010Conditional survival among cancer patients in the United States. Oncologist 15:873–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merrill RM, Henson DE, Barnes M.1999Conditional survival among patients with carcinoma of the lung. Chest 116:697–703 [DOI] [PubMed] [Google Scholar]
- 15.Bouvier AM, Remontet L, Hédelin G, Launoy G, Jooste V, Grosclaude P, Belot A, Lacour B, Estève J, Bossard N, Faivre J; Association of the French Cancer Registries (FRANCIM) 2009Conditional relative survival of cancer patients and conditional probability of death: a French National Database analysis. Cancer 115:4616–4624 [DOI] [PubMed] [Google Scholar]
- 16.American Cancer Society 2013www.cancer.org (accessed July2, 2013)
- 17.Johnston LE, Tran Cao HS, Chang DC, Bouvet M.2012Sociodemographic predictors of survival in differentiated thyroid cancer: results from the SEER Database. ISRN Endocrinol 2012:384707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonklaas J, Nogueras-Gonzalez G, Munsell M, Litofsky D, Ain KB, Bigos ST, Brierley JD, Cooper DS, Haugen BR, Ladenson PW, Magner J, Robbins J, Ross DS, Skarulis MC, Steward DL, Maxon HR, Sherman SI.2012The impact of age and gender on papillary thyroid cancer survival. J Clin Endocrinol Metab 97:E878–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vini L, Hyer SL, Marshall J, A'Hern R, Harmer C.2003Long-term results in elderly patients with differentiated thyroid carcinoma. Cancer 97:2736–2742 [DOI] [PubMed] [Google Scholar]
- 20.Hoel DG, Ron E, Carter R, Mabuchi K.1993Influence of death certificate errors on cancer mortality trends. J Natl Cancer Inst 85:1063–1068 [DOI] [PubMed] [Google Scholar]