Abstract
Extracellular stresses influence transcription factor (TF) expression and therefore lineage identity in the peri-implantation mouse embryo and its stem cells. This potentially affects pregnancy outcome. To understand the effects of stress signaling during this critical period of pregnancy, we exposed cultured murine embryonic stem cells (mESCs) to hyperosmotic stress. We then measured stress-enzyme-dependent regulation of key pluripotency and lineage TFs. Hyperosmotic stress slowed mESC accumulation due to slowing of the cell cycle over 72 h, after a small apoptotic response within 12 h. Phosphoinositide 3-kinase (PI3K) enzymatic signaling was responsible for stem cell survival under stressed conditions. Stress initially triggered mESC differentiation after 4 h through MEK1, c-Jun N-terminal kinase (JNK), and PI3K enzymatic signaling, which led to proteasomal degradation of Oct4, Nanog, Sox2, and Rex1 TF proteins. Concurrent with this post-transcriptional effect was the decreased accumulation of potency TF mRNA transcripts. After 12–24 h of stress, cells adapted, cell cycle resumed, and Oct4 and Nanog mRNA and protein expression returned to approximately normal levels. The TF protein recovery was mediated by p38MAPK and PI3K signaling, as well as by MEK2 and/or MEK1. However, due to JNK signaling, Rex1 expression did not recover. Probing for downstream lineages revealed that although mESCs did not differentiate morphologically during 24 h of stress, they were primed to differentiate by upregulating markers of the first lineage differentiating from mESCs, extraembryonic endoderm. Thus, although two to three TFs that mark pluripotency recover expression by 24 h of stress, there is nonetheless sustained Rex1 suppression and a priming of mESCs for differentiation to the earliest lineage.
Introduction
Transcription factor (TF) expression and therefore lineage identity in the peri-implantation embryo and its stem cells may be influenced by extracellular stresses [1,2]. Perturbations of the embryo during the critical period of implantation frequently lead to loss of the pregnancy [3,4]. Understanding the integration of stress enzyme signaling of the developing embryo may help to improve early pregnancy success rates, and avoid or mitigate long-term negative effects on the health of offspring.
In vivo, the earliest placental lineage to differentiate after embryo implantation is trophoblast giant cells (TGCs). TGCs maintain early pregnancy by producing the hormones that stimulate uterine changes necessary to support an embryo. When placental trophoblast stem cells (TSCs), precursors to TGCs, were confronted with hyperosmotic stress in vitro, the stress enzymes that were activated modulated lineage TF expression [2,5–7]. Nearly all surviving TSCs terminally differentiated to first-lineage TGCs [5,7,8] and later lineages were suppressed [5,8]. This would hypothetically provide for the nutritional needs of the implanting embryo but leave insufficient stem cells to populate the other necessary placental lineages, jeopardizing long-term survival of the embryo.
Murine embryonic stem cells (mESCs) derived from the inner cell mass (ICM) of an E3.5 blastocyst are also highly sensitive to extrinsic signaling [9]. Extracellular signal regulated kinase (ERK) signaling can induce differentiation of mESCs; its suppression allows pluripotent stem cells to be derived from refractory mouse strains, and also allows the self-renewal of mESCs in culture [10]. Phosphoinositide 3-kinase (PI3K) regulates both the proliferation and pluripotency of mESCs, in part by its ability to maintain Nanog expression [11]. p38MAPK signaling is necessary for mesoderm development [12,13], and mESCs lacking c-Jun N-terminal kinase (JNK)1 fail to undergo neuronal differentiation [14]. All of these enzymes may be activated by external stressors, such as hyperosmotic stress [15]. Therefore extrinsic stress signaling through stress enzymes may influence the kinetics and/or lineage allocation of differentiating mESCs.
Pluripotency in both mESCs and hESCs is maintained by a network of TFs—Oct4, Sox2, and Nanog—which suppress the differentiated state [16,17]. The TF Rex1 is another common marker of the pluripotent state [18]. Toxicological stressors can decrease potency in hESCs via a decrease in Oct4, Sox2, and Rex1 that potentially leads to abnormal differentiation [19].
Oct4 maintains pluripotency in part by suppressing trophectoderm in both the ICM of the embryo and in the derivative mESCs in culture [20,21]. A loss of 50% of Oct4 levels results in differentiation to trophectoderm, while a 50% increase above normal expression triggers differentiation to the early appearing primitive endoderm (PrEndo) [21]. This is a reflection of the transient higher levels of Oct4 in the delaminating primitive endoderm derived from ICM of the E3.5 blastocyst [22]. Recent evidence suggests that Oct4 is required for differentiation of extraembryonic endoderm (ExEndo) by non-cell autonomous fibroblast growth factor (FGF)4 function and by cell autonomous upregulation of ExEndo TFs [23,24]. Thus, small, transient changes in Oct4 levels change the potency of mESCs and stress may contribute to transient Oct4 regulation.
Nanog suppresses PrEndo and its derivative ExEndo expression in the blastocyst. High Nanog expression is found only in pluripotent cells; low expression sensitizes mESCs to differentiation signals, committing them to PrEndo and later ExEndo lineages [25,26]. Rex1 expression correlates strongly with pluripotency in mESCs [18]; its expression is lost as mESCs differentiate to either PrEndo/ExEndo or the later-appearing embryonic ectoderm (EmEcto) [27–29]. In contrast to Oct4, Rex1-homozygous-null-mutant mESCs can be isolated, but have a higher spontaneous differentiation rate to all lineages [30]. In vivo, Rex1-null embryos express some visceral endoderm markers at lower levels [31], suggesting a role for Rex1 in ExEndo differentiation.
Therefore, cells of the ICM and its immediate successor lineages, PrEndo and EmEcto, may be identified by measuring the relative quantitative expression of potency TFs. The current study used hyperosmotic stress to concurrently activate multiple stress enzyme signaling pathways in mESCs. We subsequently measured the stress-enzyme-dependent changes in TFs Oct4, Nanog, Sox2, and Rex1 to reveal the impact of stress on pluripotency. After screening 21 signaling kinases in 11 subfamilies using 14 inhibitors, 5 protein kinases (PKs) were revealed to affect the kinetics and/or the allocation of lineages from differentiating mESCs. While hyperosmotic stress did not trigger morphological differentiation of mESCs cultured in monolayer, it primed mESCs to initiate first-lineage PrEndo/ExEndo differentiation, altering the response of mESCs to differentiation cues.
Materials and Methods
Reagents
MG132, lactacystin, and sorbitol were from Sigma (St. Louis, MO). Enzyme inhibitors LY294002, U0126, PD98059, SB202190, AKTi, and L-JNKi-1 were from Calbiochem (La Jolla, CA). Amido black was from MP Biomedicals (Solon, OH). Rabbit polyclonal to mouse Oct4 (sc-5279), goat anti-human Sox2 (sc-17320), anti-rabbit MEK1 (sc-219), and anti-mouse MEK2 (sc-13159) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-rabbit Nanog was from Chemicon/Millipore (AB5731; Billerica, MA) and Rex1 antibodies were from Abcam (AB28141; Cambridge, MA). Anti-rabbit p38 (CS9212), phospho-p38 (Thr180/Tyr182 CS9211), JNK (CS9252), phospho-SAPK (Thr183/Tyr185 CS9251), phospho-MEK1/2 (Ser217/221 CS9121), ERK1/2 (CS9102), phospho-ERK1/2 (Thr202/Tyr204, CS9106), phospho-AKT (Ser473 CS2965), β-actin (CS4967), and cleaved-caspase 3 (CS9664) antibodies were from Cell Signaling (Beverly, MA). For real-time quantitative PCR (RT-qPCR) we used the RNeasy Mini Kit for RNA isolation and QuantiTect Reverse Transcription Kit, both from Qiagen (Germantown, MD), and Fast SYBR Green Master Mix from Applied Biosystems (Foster City, CA). RNA primers (Oct4, Nanog, Rex1, Dab2, Lrp2, and Fgf5) were from Integrated DNA Technologies (Coralville, IA).
Cell culture and stimulation
mESC-D3 cells (ATCC, Manassas, VA) were cultured in the absence of feeder cells in Dulbecco's modified Eagle's medium (Gibco, Grand Island, NY) supplemented with 15% mESC-screened fetal bovine serum (HyClone, Logan, UT), 2 mM l-glutamine, 1 mM sodium pyruvate, 1 mM nonessential amino acids, 0.1 mM 2-mercaptoethanol (Sigma), and 1,000 U/mL murine leukemia inhibitory factor (LIF; Millipore, Temecula, CA) on 0.1% gelatin-coated dishes at 37°C in humidified air with 5% CO2 [31]. mESCs were cultured overnight after passaging before stimulation with sorbitol. Osmolality was determined by freezing point depression using Advanced Instruments Wide Range Osmometer 3W2 (Advanced Instruments, Norwood, MA). One liter of a very dilute aqueous solution at room temperature very nearly has a mass of 1 kg, so at low solute concentrations (approximately <0.50 M), osmolarity and osmolality are considered equivalent.
Enzyme inhibition
Enzyme inhibitors were chosen according to the specificity reported in the kinase inhibitor literature [32,33]. Inhibitor concentrations were determined following dose response experiments for each inhibitor based on the range of concentrations determined from the mESC and kinase inhibitor literature [32–37]. The single doses selected for use in ongoing experiments and shown herein were the lowest doses impacting Oct4 protein expression following 4 h of sorbitol exposure (as determined by western blot) with minimal toxicity (as determined by phase microscopy).
Inhibitors were suspended in dimethyl sulfoxide (DMSO), diluted to the proper concentrations, and preloaded for 1 h prior to sorbitol stimulation. Vehicle-only control experiments were performed and expression of Oct4, Nanog, and Rex1 was determined by western blot to be not significantly different from nonvehicle control (data not shown). Inhibitor-only experiments were performed for 24 h to determine the impact of enzyme inhibition on Oct4, Nanog, and Rex1 expression (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd). In 14/18 conditions, inhibitors had no effect on potency TF levels; in 2 conditions, the inhibitors decreased expression by 20% and, in the remaining 2 conditions, TF expression was increased above baseline. Inhibitor-only experiments were performed for 4 and 24 h to determine the impact of enzyme inhibition on Oct4, Nanog, and Rex1 expression. There were no significant differences in expression after 4 h of inhibitor-only treatment (Oct4 P=0.78, Nanog P=0.81, and Rex1 P=0.55; data not shown). There were no significant differences in expression of Oct4 (P=0.23), Rex1 (P=0.053), or 5/6 of the Nanog inhibitors (P=0.19) after 24 h of inhibitor-only inhibition (Supplementary Table S1). There was a significant effect on Nanog expression during 24 h of PD98059-inhibitor-only treatment (P=0.0007). However in Table 2, PD98059 targets had no significant effect on the recovery of Nanog during 24 h of stress, the biological significance of PD98059 inhibitor only is nil.
Table 2.
Transcription Factor Expression Levels During 24h of Hyperosmotic Stress ± Enzyme Inhibition in mESC Monolayer Culture
| 24-h Inhibition | Oct4 | Nanog | Rex1 |
|---|---|---|---|
| Unstressed | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 |
| Stress only (S) | 1.03±0.16 | 1.15±0.20 | 0.35±0.08a |
| PD98059 (MEK1)+S | 0.78±0.06 | 0.80±0.09 | 0.51±0.01a |
| U0126 (MEK1/2)+S | 0.27±0.03a,b | 0.67±0.32 | 0.31±0.12a |
| SB202190 (p38)+S | 0.51±0.07a,b | 0.10±0.01a,b | 0.19±0.07a |
| L-JNKi-1 (JNK)+S | 0.91±0.15 | 0.71±0.15 | 0.89±0.08b |
| LY294002 (PI3K)+S | 0.65±0.08 | 0.44±0.07a,b | 0.00a,b |
| AKT1+S | 0.84±0.19 | 0.97±0.03 | 0.44±0.04a |
Values represent percent of unstressed expression.
Means statistically different from unstressed.
Means statistically different from “stress only,” n≥3, ANOVA and Student-Newman-Keuls post hoc tests, P<0.05.
DMSO-only controls were also done. DMSO at 0.6% did not impact Oct4, Nanog, and Rex1 expression at 4 or 25 h. This (0.6%) was higher than the highest diluent concentration in any of our experiments (0.4% in the U0126 experiments).
Cell accumulation and apoptosis
Cell accumulation was assayed by counting cells using a hemocytometer following trypan blue exclusion. mESCs were trypsinized, plated, and cultured overnight to allow for adaptation after passage. Time-zero counts were taken at least 1 day after passage and all subsequent counts were normalized to this. Apoptosis was measured by immunoblot for cleaved caspase 3 [38].
Microscopy
Indirect immunocytochemistry was performed as described previously [39,40]. Photomicrography was performed using a Leica DM IRE2 automated epifluorescence microscope (Wetzlar, Germany) controlled electronically by Simple PCI AI software (Hamamatsu Corporation, Sewickley, PA). All micrographs were taken at a magnification of 100×, except where indicated.
Western blot analysis
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and western immunoblot analysis of mESC lysates were performed as previously described [41]. Cells were harvested with cold lysis buffer (Cell Signaling) and protein was quantified by BCA assay (Pierce, Rockford, IL). Ten to twenty micrograms of aliquots was fractionated on 10% polyacrylamide precast gels (Bio-Rad, Hercules, CA), transferred to nitrocellulose membranes (Amersham Biosciences, Aylesbury, United Kingdom), probed overnight with primary antibodies, and developed as previously described [40]. Protein bands were visualized using the ECL Advance Western Blotting Detection Kit (GE Healthcare, Waukesha, WI), blot was scanned to obtain an electronic image, and intensity of protein bands was quantified using Image J analysis software (rsbweb.nih.gov) and normalized to amido black staining [42]. Data are expressed as the change in expression relative to no treatment at time zero.
RT-qPCR analysis
mESCs were trypsinized and harvested for RT-qPCR analysis using a 7500 Fast Real Time PCR System (Applied Biosystems). Total RNA was isolated from cell lysates using RNeasy Mini Kit (Qiagen). RNA content was measured using ND-1000 Spectrophotometer (NanoDrop and ThermoScientific, Wilmington, DE). Complementary DNA (cDNA) was synthesized from 50 to 100 ng of total RNA using QuantiTect Reverse Transcription Kit (Qiagen) according to manufacturer's instructions, and diluted 1:5. One microliter of cDNA template was added to 1 μL of both the forward and reverse primers for each specific transcript and 10 μL of Fast SYBR Green Master Mix (Applied Biosystems) for the RT-qPCR. Primer sequences are shown in Supplementary Table S2. Primer pairs were checked for specificity using BLAST analysis and were checked by both agarose gel electrophoresis and thermal dissociation curves to ensure amplification of a single product, and to rule out formation of primer dimers during the RT-qPCR. The RT-qPCR cycling parameters were as follows: enzyme activation, 95°C for 20 s; denature, 95°C for 3 s; and anneal/extend, 60°C for 30 s for a total of 40 cycles.
The expression of the target genes was quantified against that of two internal reference genes, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and 18s ribosomal RNA subunit (18s rRNA). GAPDH was determined to be the most stable of 10 reference genes tested during mESC differentiation [43,44], and 18s rRNA was selected due to its stability during hyperosmolar conditions in TSCs [8]. Fold change was determined using the ddCt method [45]. Data are expressed as the fold change in expression relative to no treatment at time zero.
Statistical analysis
Results of these investigations were described as the mean±standard error of at least three independent experiments. Data were analyzed using SPSS v. 19.0. In some cases, hypotheses were restated and additional replicates with increased numbers of controls were done to obtain higher statistical confidence. Thus, the sample size for each data point in some figures may vary. Statistical analysis consisted of ANOVA with Student-Newman-Keuls post hoc tests, or Kruskal–Wallis nonparametric ANOVA tests (due to non-normal distribution of data), followed by Mann–Whitney tests on each pair of groups with Bonferroni correction of the P-value. Groups were considered to be significantly different if P<0.05.
Results
mESC growth and colony morphology during hyperosmotic stress
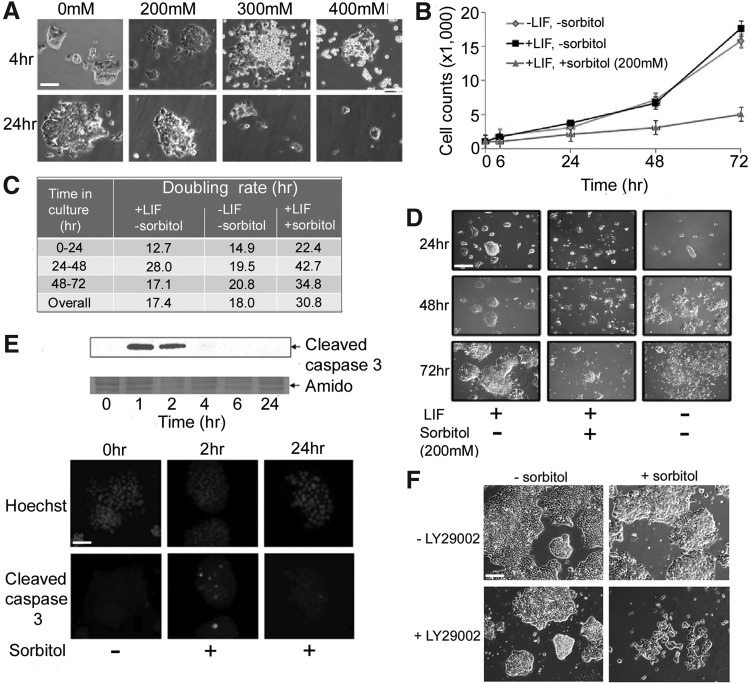
Previous work in our lab established 400 mM sorbitol as the dose that induced the highest levels of stress enzyme activity and function in mouse TSCs and embryos [41,46,47]. Our mESC studies therefore began with this dose, but it proved to be lethal in mESCs (Fig. 1A). We then established 200 mM sorbitol as the nonlethal experimental dose, and tested effects of this dose on mESC proliferation and apoptosis. Osmolality was measured at 330±4 mOsm/kg H2O before sorbitol addition (described in “Materials and Methods” section) [46], and 531±7 mOsm/kg H2O after addition of 200 mM sorbitol. At low solute concentrations, osmolality and osmolarity can be considered equivalent, because the mass of 1 L of a dilute aqueous solution is very close to 1 kg. The osmolarity of mouse uterine fluid has been reported as 330 mOsm [48]. Thus our baseline media were isotonic with uterine fluid.
FIG. 1.
Hyperosmotic stress effects on cell proliferation in murine embryonic stem cells (mESCs). (A) mESCs were cultured in the presence of 0–400 mM sorbitol for either 4 or 24 h and transmitted light micrographs were taken. (B) mESCs were cultured ±leukemia inhibitory factor (LIF) and ±200 mM sorbitol for 0–72 h. mESCs were trypsinized and counted with a hemocytometer following trypan blue exclusion. Error flags represent standard error of the mean, n≥3. (C) Doubling rates were calculated and tabulated from cell counts in (B). (D) mESCs were incubated in the presence of LIF ±200 mM sorbitol for 24, 48, or 72 h and transmitted light micrographs were taken. (E) mESCs were cultured in the presence of 200 mM sorbitol for 0–24 h. In some wells, cells were lysed, and proteins were fractionated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), blotted, and probed for the presence of the caspase 3 cleavage product (n=3). Time-matched cells were fixed, and stained for the cleavage product of caspase 3 with Hoechst staining of the nuclei. (F) mESCs were cultured for 24 h ±sorbitol (200 mM),±phosphoinositide 3-kinase (PI3K) inhibitor, and LY294002 (25 μM); transmitted light micrographs were taken. All micrographs were taken at 100×. Scale bar=50 μm.
To establish the optimal, nonmorbid hyperosmotic dose, mESCs were cultured for 72 h in three experimental conditions: isosmotic media+LIF, isosmotic media−LIF, and hyperosmotic media+LIF. Cell counts were done (Fig. 1B) and micrographs were taken at 24, 48, and 72 h of culture (Fig. 1D). Stress slowed growth rates from an overall doubling rate of 17.4 h in untreated cells+LIF to 30.8 h in treated mESCs (Fig. 1C).
To test whether the reduced accumulation of cells during stress was the result of slowing of the cell cycle or apoptosis, we assayed for apoptosis by probing for the small cleavage product generated when caspase 3 is activated. It has been reported that mESCs do not express a functional death ligand (Fas/FasL) system [49]. Nevertheless, caspase 3 is activated in both the extrinsic (ie, extracellular induction) and intrinsic (mitochondrial) apoptotic pathways. This cleavage product was detected following 1–2 h of stress (14% of cells; Fig. 1E), and occasionally at 4 h, but by 6–24 h the remaining cells had adapted to the stress with cleaved-caspase 3 detected in fewer than 5% of cells by immunofluorescence, and no protein detected by immunoblot. mESC survival in hyperosmotic conditions was dependent on PI3K signaling (Fig. 1F); inhibition of PI3K with LY294002 led to massive cell death of up to 90% of mESCs during 24 h of hyperosmotic stress. Thus this level of hyperosmotic stress produced transient apoptosis followed by diminished but positive cell growth and survival that was PI3K dependent.
Hyperosmotic activation of signaling enzymes
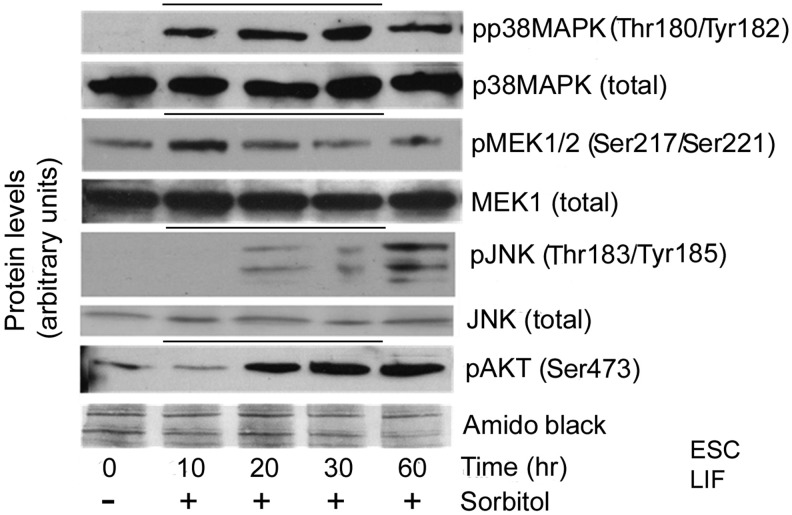
A small subset of the 500 PKs in the kinome typically respond to stress stimuli, suggesting these as candidates for mediating cellular responses to hyperosmotic stress [2,50]. After screening 21 signaling kinases in 11 subfamilies using 14 inhibitors, 5 kinases in the mitogen activated protein kinase (MAPK) and PI3K families were identified as producing differentiation-priming effects in hyperosmotic conditions. To determine the kinetics of activation of these five kinases in our system, mESCs were treated with 200 mM sorbitol for 1 h. Expression levels of the activated (ie, phosphorylated) enzymes were assayed by western blot analysis (Fig. 2), including phospho-p38MAPK, phospho-JNK, phospho-MEK1/2, and phospho-AKT (the downstream effector of PI3K signaling). Activated p38MAPK was first detected at 10 min of stimulation; activation persisted throughout 1 h of stimulation. Similarly, activated JNK was first detected at 20 min of stimulation, and persisted throughout 1 h of stimulation. Activated MEK1/2 and activated AKT were present endogenously at low levels at time zero; sorbitol stimulated higher activation levels of each. Increased MEK1/2 activation was stimulated within the first 10 min of stress exposure, but returned to baseline levels by 20 min. In contrast, increased AKT activation occurred by 20–30 min of stimulation, and persisted through 1 h of sustained stress exposure.
FIG. 2.
Activation of p38, MEK1/2, c-Jun N-terminal kinase (JNK), and AKT by hyperosmolarity. mESCs were incubated in 200 mM sorbitol for 0–60 min to detect kinetics of enzyme activation. Cells were lysed and proteins were fractionated using SDS-PAGE, blotted, and probed for phospho-p38 (Thr180/Tyr182), phospho-JNK (Thr183/Tyr185), phospho-MEK1/2 (Ser217/221), or phospho-AKT (Ser473). Blots are representative of triplicate experiments. Phospho-proteins and their loading controls are grouped between black lines.
Efficacy of enzyme inhibitors
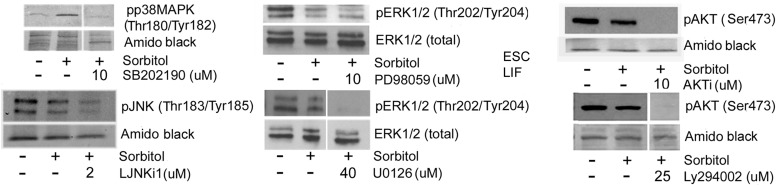
Efficacy of the pharmacological inhibitors for these enzymes was tested under stress conditions (Fig. 3). LY294002 and AKTi inhibited AKT activation by an average of 88% and 95%, respectively. PD98059 directly inhibits new MEK1 activation; we determined its efficacy by measuring the activation of MEK1's downstream targets, ERK1/2. PD98059 inhibited ERK1/2 activation by an average of 30%. This is consistent with the literature that describe the mechanism of action of PD98059; it binds to inactive MEK1 thus preventing new activation, but does not shut off endogenously active MEK1 [51]. U0126 blocks MEK1/2 activity, preventing phosphorylation of downstream targets, ERK1/2 [52]. U0126 inhibited ERK1/2 activation by an average of 87%. SB202190 inhibited p38MAPK activation by an average of 60%. L-JNKi-1 inhibited JNK activation by an average of 50%. Thus highest inhibition and specificity was identified and attained in line with the encyclopedic testing by Bain et al. [53,54].
FIG. 3.
Efficacy of enzyme inhibitors during hyperosmotic stress. mESCs were incubated for 4 h in the presence of 200 mM sorbitol with or without one of the enzyme inhibitors SB202190 (p38), PD98059 (MEK1), U0126 (MEK1/2), L-JNKi-1 (JNK), AKTi (AKT), or LY294002 (PI3K). Cells were lysed and proteins were fractionated using SDS-PAGE, blotted, and probed for phospho-p38 (Thr180/Tyr182), phospho-JNK (Thr183/Tyr185), phospho-ERK1/2 (Thr202/Tyr204), or phospho-AKT (Ser473). Blots are representative of triplicate experiments.
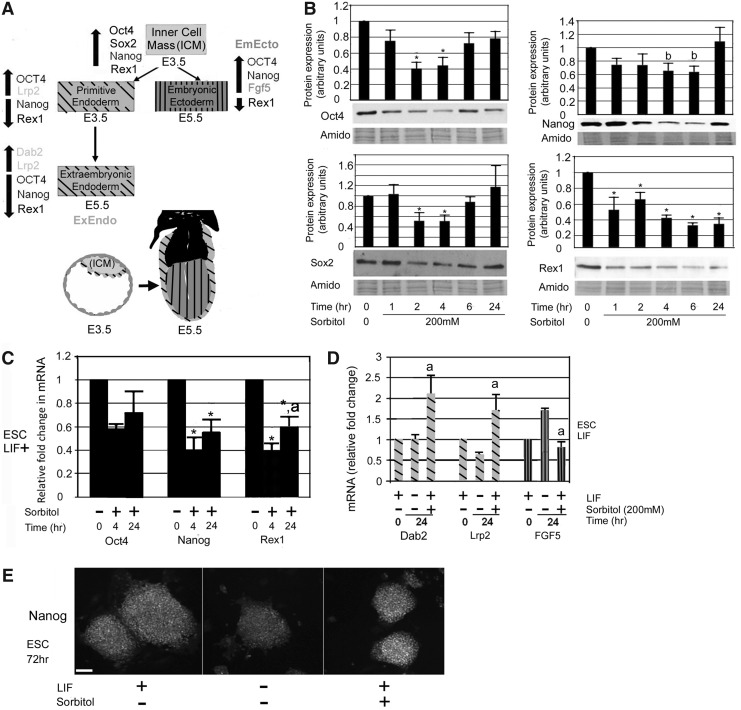
Hyperosmotic stress modulated expression of TF markers of pluripotency
mESCs were treated with 200 mM sorbitol for 24 h to test for stress-induced differentiation through loss of potency TFs and/or gain of TFs that mark differentiated lineages PrEndo/ExEndo or EmEcto. Figure 4A illustrates the relative expression of these TFs in the undifferentiated ICM, modeled by mESCs, and each of the potential downstream lineages of mESCs, PrEndo that gives rise to other ExEndo lineages, and EmEcto. Expression levels of Oct4, Sox2, Nanog, and Rex1 were determined at multiple time points up to 24 h by western blot analysis and RT-qPCR. Hyperosmotic stress rapidly activated the differentiation program, with expression of Oct4, Nanog, Rex1, and Sox2 TF proteins decreasing within the first hour and reaching a nadir between 2 and 4 h of continued stress (Fig. 4B). The nadir of Nanog expression was more variable than the other three TFs, occurring as early as 2 h in some experiments or as late as 6 h of stress. By 6 h, the overt differentiation program was aborted, and the decline in Oct4, Sox2, and Nanog expression halted. Over the next 2–20 h, expression of these three TF proteins rebounded toward the unstressed baseline with both Sox2 and Nanog achieving complete, robust recovery to their prestressed protein levels (Fig. 4B). Because Nanog, Oct4, and Rex1 expression was adequate to identify mESC or subsequent lineages, we did not continue to measure Sox2 expression. Nanog and Oct4 recovery was maintained through 72 h of ongoing stress (Fig. 4E, Oct4, data not shown). Recovery at the mRNA level also occurred, with both Oct4 and Nanog moving toward their unstressed baseline, although this recovery had not reached significance at 24 h (Fig. 4C).
FIG. 4.
Hyperosmotic stress in mESCs impacts transcription factor expression at protein and mRNA levels. (A) The inner cell mass (ICM) of the blastocyst coexpresses Oct4/Rex1/Nanog in pluripotent cells at E3.5. After E3.5 a Rex1 subpopulation delaminates from the ICM and expresses primitive endoderm (PrEndo) marker, Lrp2, at E3.5 and extraembryonic endoderm (ExEndo) marker, Dab2, at E4.5. At E4.75, Rex1 decreases in the remaining cells of the ICM and, by E5.5, fibroblast growth factor (FGF)5 is expressed in embryonic ectoderm (EmEcto). Upward arrow indicates that expression is maintained or increasing; downward arrow means that expression is decreasing or absent. (B) mESCs were incubated in 200 mM sorbitol for 0–24 h and lysed. Proteins were fractionated using SDS-PAGE, blotted, and probed for Oct4, Sox2, Nanog, or Rex1. Histograms show relative expression of each protein when normalized to amido black. (C) Total RNA was isolated and subjected to reverse transcription to form complementary DNA (cDNA). Real-time quantitative PCR (RT-qPCR) was performed with Oct4, Nanog, and Rex1 primers. Histograms represent relative fold change in mRNA expression using the ddCt method. (D) mESCs in monolayer were incubated ±sorbitol and ±LIF for 24 h. Dab2, Lrp2, and Fgf5 mRNA transcript levels were examined by RT-qPCR. Histogram shows the relative fold changes when compared with time zero, no stress. Error flags are the standard error of the mean (n=3). “a” Indicates significant difference for sorbitol +LIF compared with LIF−at 24 h. (P<0.05, ANOVA and Student-Newman-Keuls post hoc tests). (E) mESCs were treated with sorbitol for 72 h, fixed, and probed for Nanog. Micrographs taken at 40×. Scale bar=50 μm. Error bars represent standard error of the mean (n=3); “*” Denotes a significant difference from the 0 h untreated control; “a” denotes significant difference when compared with 4 h+sorbitol time point. “b” Indicates that the expression nadir (variable in Nanog) was significantly different from the unstressed mESCs at time zero (4 and 6 h were chosen to represent the nadir in this histogram). ANOVA+Student-Newman-Keuls post hoc tests, P<0.05. In (A) and (D) diagonal lines indicate primitive endoderm starting at E3.5 through extraembryonic endoderm at E5.5 and vertical lines indicate primitive ectoderm starting at E5.5.
In contrast, Rex1 protein levels did not rebound, remaining at <40% of their unstressed levels throughout the 24-h time course (Fig. 4B). Rex1 mRNA levels, however, recovered to about 70% of their unstressed levels by 24 h (Fig. 4C). Overall, stress induced a rapid, transient loss of protein and mRNA in potency TFs, but this loss was reversed in some TFs as mESCs adapted to the stress. Rex1 protein was exceptional in its persistent stress-induced suppression through 24 h.
Hyperosmotic effects on lineage markers during monolayer cell culture
The persistent suppression of Rex1 during hyperosmotic stress suggested that some mESCs were differentiating to PrEndo/ExEndo or EmEcto. We therefore used RT-qPCR to look at markers of these lineages after 24 h of hyperosmotic stress in a monolayer culture system (Fig. 4D). We selected Lrp2, which arises in the E3.5 PrEndo [24,55], and Dab2, which arises in the E4.5 ExEndo [24,56], as PrEndo/ExEndo markers, and Fgf5, which arises in the E5.5 EmEcto [57,58], as the EmEcto marker (Fig. 4A). We compared mRNA expression in stressed mESCs with LIF with that of both unstressed mESCs cultured in the presence of LIF (a pluripotent control) and unstressed mESCs cultured following LIF removal (a differentiation control).
Sorbitol induced significantly higher levels of Lrp2 and Dab2 expression compared with either of the time-matched controls (Fig. 4D). In contrast, although 24 h of LIF removal was sufficient for unstressed cells to upregulate the EmEcto marker Fgf5, the presence of sorbitol suppressed this upregulation in stressed cells. Thus, the stress-induced loss of Rex1 correlated with upregulation of markers of the early appearing PrEndo/ExEndo lineages, but suppression of the later EmEcto lineage marker.
Hyperosmotic-stress-induced loss of potency TFs due to proteasomal degradation
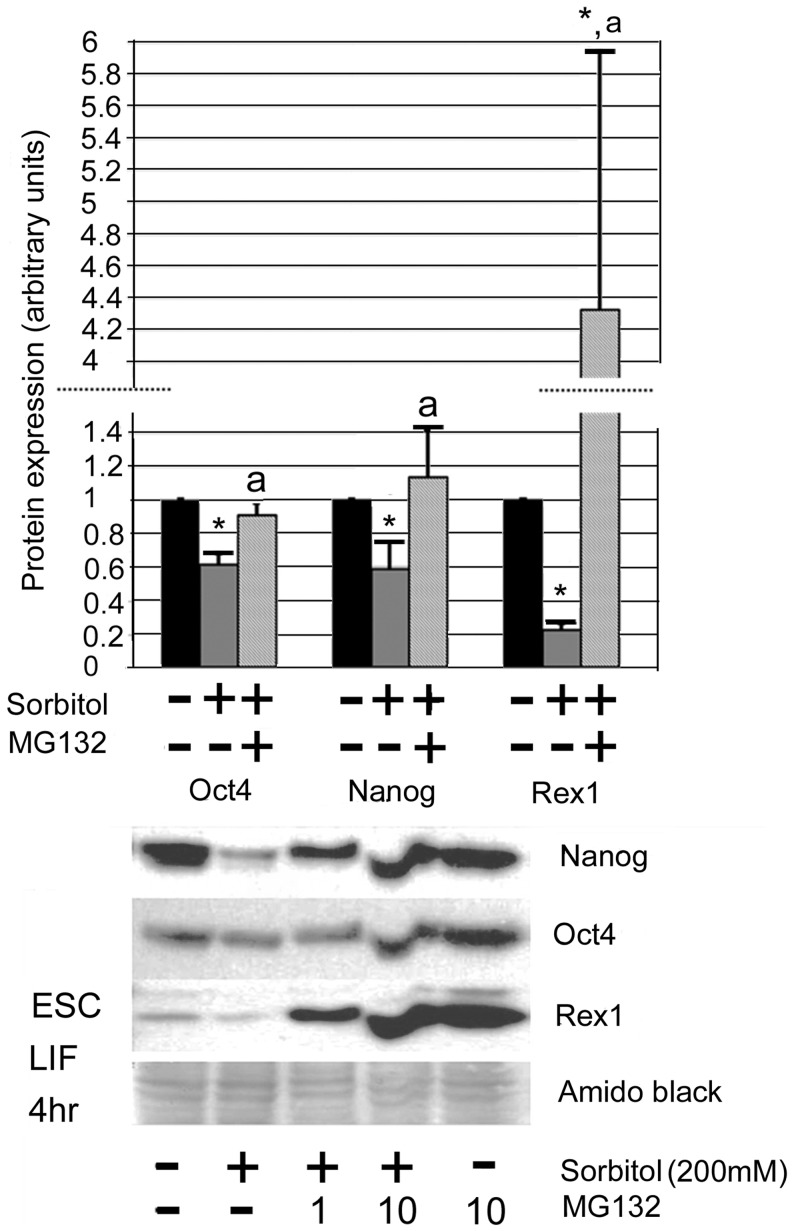
To determine the mechanism of TF protein loss during stress, we treated mESCs with one of two proteasome inhibitors, MG132 (Fig. 5) and lactacystin (Supplementary Fig. S1). Both inhibitors prevented the stress-induced loss of Oct4, Nanog, and Rex1. Oct4 loss was reversed by 75%, Nanog by 93%, and Rex1 by 310% in 4 h of proteasome inhibition by MG132.
FIG. 5.
MG132 proteasome inhibitor effects on Oct4, Nanog, and Rex1 during sorbitol stimulation of mESCs. mESCs were treated for 4 h with 0 or 200 mM sorbitol±10 μM MG132 and then lysed. Total cellular protein was fractionated using SDS-PAGE, blotted, and probed for Oct4, Nanog, or Rex1. Error bars represent standard error of the mean (n=3); “*” Denotes a significant difference from the untreated control; “a” denotes significant difference when compared with 4 h+sorbitol time point. ANOVA and Student-Newman-Keuls post hoc tests: Oct4 P=0.002; Nanog P=0.005; and Rex1 P=0.001.
MEK1 and other enzymes trigger mESC initial stress response: initiation of differentiation program
mESCs were cultured in the presence of pharmacological inhibitors and hyperosmotic stress for 4 h to identify the enzymes that mediated the stress-induced loss of potency TF proteins, reported in Figure 4B. The complete results of p38, JNK, MEK1/2, and PI3K inhibition during 4 h of hyperosmotic stress in mESCs are summarized in Table 1.
Table 1.
Transcription Factor Expression Levels During 4h of Hyperosmotic Stress ± Enzyme Inhibition in mESC Monolayer Culture
| 4-h Inhibition | Oct4 | Nanog | Rex1 |
|---|---|---|---|
| Unstressed | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 |
| Stress only (S) | 0.52±0.03a | 0.65±0.07a | 0.63±0.06a |
| PD98059 (MEK1)+S | 0.93±0.09b | 2.57±0.50a,b | 1.20±0.05b |
| U0126 (MEK1/2)+S | 0.44±0.13a | 0.24±0.02a,b | 0.98±0.04b |
| SB202190 (p38)+S | 0.81±0.21 | 0.52±0.12a | 0.59±0.23a |
| L-JNKi-1 (JNK)+S | 0.21±0.04a,b | 1.08±0.11b | 0.47±0.04a |
| LY294002 (PI3K)+S | 0.92±0.12b | 0.55±0.10a | 0.36±0.14a |
| AKTi+S | 0.57±0.05a | 0.47±0.06a | 0.43±0.14a |
Values represent percent of unstressed expression.
Means statistically different from unstressed.
Means statistically different from “stress only,” n≥3, ANOVA and Student-Newman-Keuls post hoc tests, P<0.05.
JNK, c-Jun N-terminal kinase; PI3K, phosphoinositide 3-kinase.
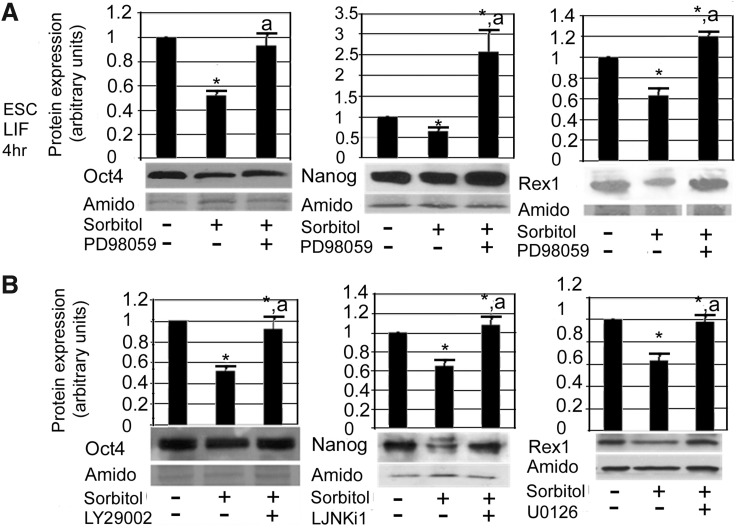
MEK1 activation triggered a loss of expression of all three TFs; when its stress-induced activation was prevented by PD98059, expression of both Oct4 and Rex1 remained at the unstressed baseline, while Nanog expression increased to 2.5 times its unstressed level (Fig. 6A). This effect was repeated for Rex1 during inhibition with the MEK1/2 inhibitor U0126 (Fig. 6B); due to inhibition of both MEK1 and MEK2 during stress exposure, Rex1 expression remained at unstressed levels. This suggests that MEK1 was the regulator of Rex1 protein destruction during stress.
FIG. 6.
Enzymes activated during 4 h of hyperosmotic stress initiate the differentiation program in mESCs. mESCs were incubated in 200 mM sorbitol for 0–4 h with or without the presence of (A) PD98059 (10 μM), or (B) LY294002 (25 μM), L-JNKi-1 (2 μM), or U0126 (40 μM). mESCs were lysed, and proteins were fractionated using SDS-PAGE, blotted, and probed for Oct4, Nanog, or Rex1. Histograms show relative expression of each protein when normalized to amido black expression. Error flags represent standard error of the mean (n≥3). “*” Denotes significant difference from unstressed mESCs. “a” Indicates significant difference from stress-only time point. ANOVA and Student-Newman-Keuls post hoc tests (P<0.05).
In contrast, MEK1/2 inhibition with U0126 did not prevent Oct4 or Nanog loss during stress for 4 h (Table 1). Instead, inhibition of MEK1/2 kinase activity allowed even greater loss of these two TFs. Therefore a U0126 target that is not targeted by PD98059, putatively MEK2, protected Oct4 and Nanog from being completely lost during stress. This indicates that the targets of U0126 and PD98059 produce opposite effects on pluripotency, and perhaps interact negatively with each other.
Several other enzymes were also implicated in the stress-induced loss of potency TFs. PI3K signaling led to loss of Oct4 expression during the first 4 h of stress; its inhibition with LY294002 prevented the stress-induced loss of Oct4 (Fig. 6B). This effect was not mediated through the AKT pathway, as specific inhibition of AKT did not prevent Oct4 loss (Table 1). When both PI3K and MEK1 signaling was inhibited simultaneously, Oct4 levels did not rise significantly above baseline (data not shown). This suggests that both PI3K and MEK1 use a common pathway to destroy Oct4 protein under stress conditions. A third stress enzyme, JNK, was involved in the loss of Nanog expression during stress; its inhibition allowed Nanog expression to be preserved during stress (Fig. 6B).
p38MAPK and other signaling kinases produced mESC adaptive response to hyperosmotic stress: differentiation program aborted, and pluripotency restored at 24 h
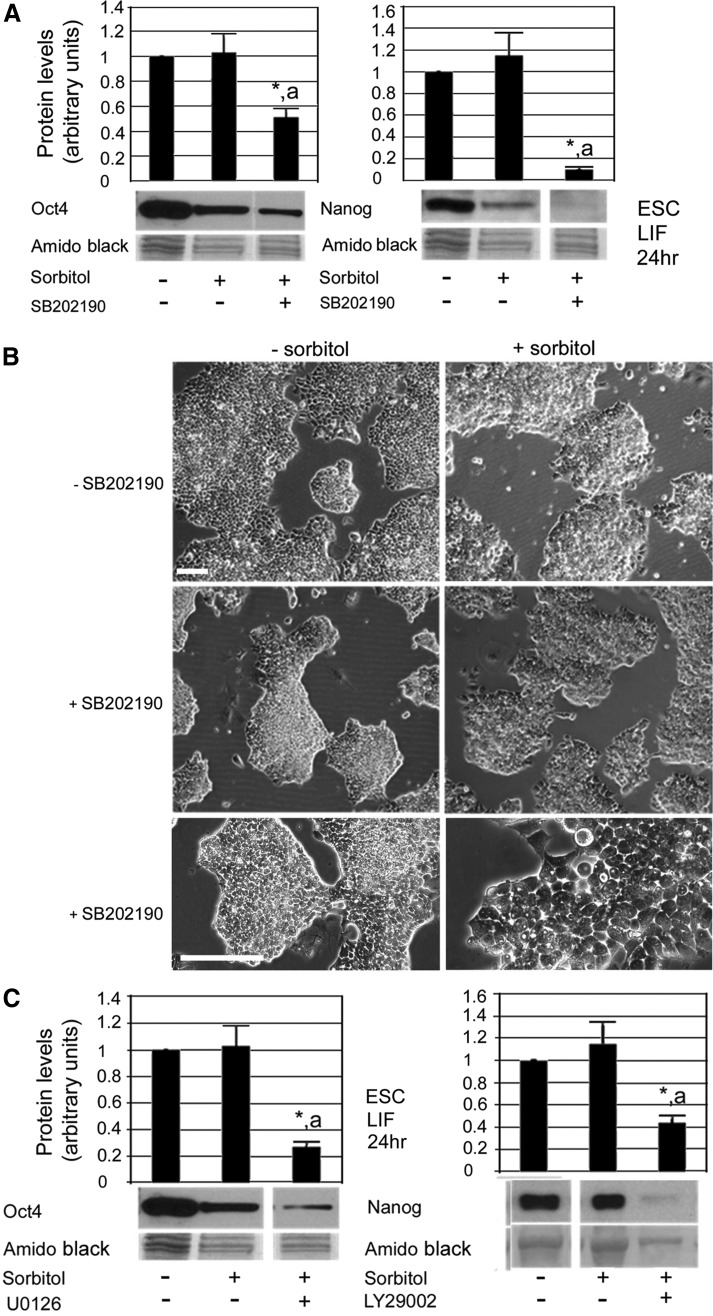
We next tested the enzymatic mechanisms that mediated the recovery of potency TF proteins during stress that persisted for 24 h. mESCs were cultured in the presence of hyperosmotic stress for 24 h with or without pharmacological inhibitors of PKs; the complete results of p38MAPK, JNK, MEK1/2, and PI3K inhibition during 24 h of hyperosmotic stress are summarized in Table 2. p38MAPK signaling mediated the recovery of Oct4 and Nanog proteins to their unstressed baselines during 24 h of stress (Fig. 7A). When stress-induced activation of p38MAPK was prevented by SB202190, Oct4 expression did not recover from its 4-h nadir of 50% of unstressed levels. Similarly, Nanog expression during ongoing stress decreased to 10% during p38MAPK inhibition. Micrographs of p38MAPK-inhibited mESCs are shown in Figure 7B; although 24 h is not adequate to see outright differentiation, the colonies do appear to show the initial signs of differentiation, with some migration of cells away from the colonies, larger cobblestone cells characteristic of ExEndo [59], and more projections of the cells along the colony borders. Thus, p38MAPK inhibition affected the stress-induced changes in morphology and appeared to enable stress-induced differentiation.
FIG. 7.
p38MAPK rescues pluripotency of mESCs during 24 h of hyperosmotic stress. (A) mESCs were incubated ±200 mM sorbitol for 0–24 h and ±p38 inhibitor SB202190 (10 μM). mESCs were lysed, and proteins were fractionated using SDS-PAGE, blotted, and probed for Oct4 or Nanog. (B) Phase micrographs of mESCs following 24 h of culture in the presence/absence of stress and p38MAPK inhibitor, SB202190. Scale bars=50 μm. (C) mESCs were incubated ±200 mM sorbitol for 0–24 h±either U0126 or LY294002 (25 μM). mESCs were lysed and proteins were fractionated using SDS-PAGE, blotted, and probed for Oct4 or Nanog. Histograms show relative expression of each protein when normalized to amido black expression. Error flags represent standard error of the mean (n≥3). “*” Denotes significant difference from unstressed mESCs. “a” Indicates significant difference from stress-only time point. ANOVA and Student-Newman-Keuls post hoc tests (P<0.05).
Inhibition of both MEK1 and MEK2 with U0126 also prevented Oct4 recovery (Fig. 7C), whereas inhibition of only MEK1 with PD98059 did not prevent Oct4 recovery (Table 2). When taken together with the U0126 results at 4 h of hyperosmotic stress, this suggests that the unique U0126 target(s), putatively MEK2, plays a role in preserving pluripotency during adaptation to stress conditions after 4 h. Similarly, PI3K signaling played a role in Nanog recovery during ongoing stress; its inhibition prevented Nanog recovery.
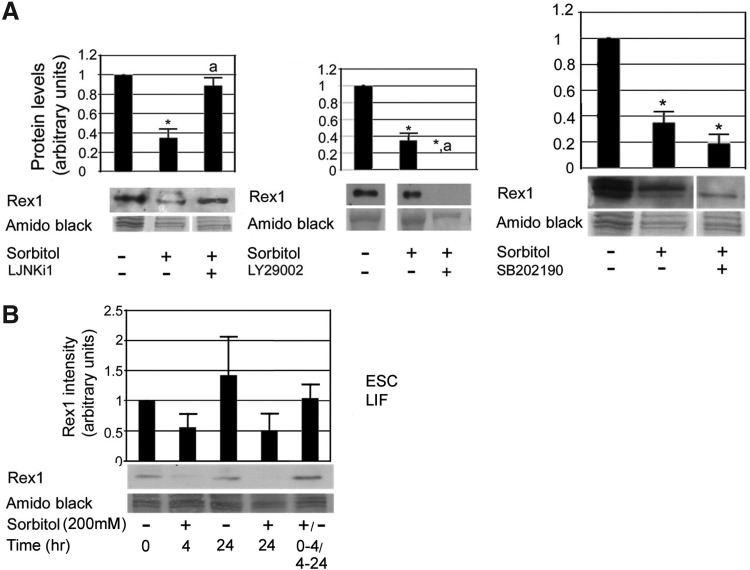
JNK-dependent signaling produced persistent suppression of Rex1 during 24 h of hyperosmotic stress
In contrast to the p38MAPK-dependent effects on Oct4 and Nanog during 24 h of hyperosmotic stress, p38MAPK signaling was not able to reverse Rex1 suppression, neither was PI3K signaling (Fig. 8A). Instead, JNK signaling suppressed Rex1 expression during 24 h of hyperosmotic stress (Fig. 8A). To test whether the stress-induced suppression of Rex1 was reversible, mESCs were subjected to only 4 h of hyperosmotic stress and then returned to iso-osmotic media. Rex1 expression at 24 h recovered to baseline; this indicates that continued stress was required for persistent Rex1 suppression (Fig. 8B). The persistent suppression of Rex1 throughout the duration of stress stimulation suggests that at least a subpopulation of mESCs may be primed by stress for differentiation by maintaining chronic Rex1 loss as well as transient loss of Oct4, Nanog, and Sox2.
FIG. 8.
JNK-dependent signaling suppressed Rex1 expression during 24 h of hyperosmotic stress. (A) mESCs were incubated±200 mM sorbitol for 0–24 h±(A) p38MAPK inhibitor SB202190 (10 μM) or PI3K inhibitor LY294002 (25 μM); or JNK inhibitor L-JNKi-1 (2 μM). mESCs were lysed, and proteins were fractionated using SDS-PAGE, blotted, and probed for Rex1. Histograms show relative expression of each protein when normalized to amido black expression. Error flags represent standard error of the mean (n≥3). “*” Denotes significant difference from unstressed mESCs. “a” Indicates significant difference from stress-only time point. ANOVA and Student-Newman-Keuls post hoc tests (P<0.05). (B) To test the reversibility of the Rex1 loss during sorbitol stress, mESCs were incubated±200 mM sorbitol for 0–4 h and then sorbitol was removed from some dishes for the remaining 20 h (lane 5). Histogram is representative of two independent experiments.
Discussion
In this study, the level of hyperosmotic stress analyzed slowed mESC accumulation due to slowing of the cell cycle, not ongoing apoptosis. PI3K signaling was responsible for cell survival under these stress conditions. Stress initially triggered mESC differentiation through MEK1, JNK, and PI3K signaling, leading to proteasomal degradation of Oct4, Nanog, Sox2, and Rex1 proteins. Concurrent with this post-transcriptional effect were the stress-induced decreased levels of their mRNA transcripts. In addition to the increase in degradation of potency TF proteins and decrease in mRNA transcript levels reported here, suppression of new translation is also caused by the levels of hyperosmotic stress used here [60]. Simultaneous increases in protein degradation rates and decreases in synthesis rates enable rapid changes in the cellular “stemness” program. However, as stress at levels studied here continued beyond 4 h, cells adapted, cell cycle resumed, and Oct4 and Nanog mRNA and protein expression returned to near normal levels by 24 h. The recovery of potency TF proteins was mediated by p38MAPK and PI3K signaling, as well as by that of an unknown MEK1/2 inhibitor target. Rex1 protein levels, however, did not recover; its persistent suppression was due to stress-induced JNK signaling. Table 3 summarizes the enzyme effects reported in this study.
Table 3.
Summary of Enzyme Effects on Oct4, Nanog, and Rex1 Following Hyperosmotic Stress in mESC Monolayer Culture
| Transcription factor | Enzymes regulating initial stress responses; loss of TF expression at 4 h | Enzymes regulating long-term adaptive stress responses at 24 h |
|---|---|---|
| Oct4 | Mek1>Mek2 PI3K | p38MAPK (rescue of expression) Mek2 (putatively) |
| Nanog | Mek>Mek2 JNK | p38MAPK (rescue of expression) PI3K |
| Rex1 | Mek1 | JNK (ongoing suppression) |
TF, transcription factor.
Stress-induced loss and regain of four potency TFs in mESCs by enzymes was characterized for the first time in these studies. MEK1 mediated potency loss at the 4-h nadir of Oct4 and played a role in regain of Oct4, Nanog, and Rex1 from 4 to 24 h. The mediator of regain of Oct4 by MEK1/2 is not clear. However, MEK1 is a weaker enzyme that blocks the stronger enzyme MEK2 in a heterodimer [61], and both MEK1 and MEK2 are expressed in TSCs/ESCs/blastocysts [39] and should heterodimerize. One possible interpretation is that PD98059 suppresses MEK1 and reactivates MEK2 that reverses stress-induced Oct4 loss from 0 to 4 h. U0126 inhibits both but importantly MEK2, blocking Oct4 recovery from 4 to 24 h. We hypothesize that MEK2 protects Oct4 from 0 to 4 h unless MEK1 activity is dominant and reverses Oct4 loss from 4 to 24 h if MEK2 activity becomes dominant (Table 3). Activity and chemical inhibitor data for MEK1 and MEK2 support this but further attempts to test this hypothesis using siRNA to MEK1 and MEK2 failed due to insufficient specificity of the knockdowns (data not shown).
JNK mediates both Nanog loss from 0 to 4 h and continuing Rex1 loss between 4 and 24 h. Both JNK-dependent effects would mediate predisposition toward endoderm, which is suppressed by Nanog [24,62,63], although Rex1 may mediate later ectoderm and specific subtypes of extraembryonic endoderm [30,31]. Interestingly, JNK prioritizes differentiation of TSCs stressed by hyperosmotic sorbitol, benzopyrene, or hypoxic O2 below 2%, by inducing first lineage [5,7,64] and suppressing later lineages [5]. JNK appears to mediate stress-induced endoderm, the first lineage arising from ESCs, but it remains to be determined whether JNK suppresses later lineages.
In stressed ESCs, first-lineage markers show that nutrient acquisition is a function associated with the increased expression of Lrp2 and Dab2 in the primitive endoderm. Lrp2 marks the earliest allocation of primitive endoderm in the E3.5 blastocyst and is accompanied by a suite of other proteins, such as cubilin and megalin, that are also expressed in the earliest ExEndo in the blastocyst and are part of lipid uptake mechanisms shared by many absorptive epithelia [23,65–68]. Dab2 arises during primitive endoderm lineage fixation at E4.5 and anchors the absorptive complex in the apical surface where it may function to feed the endoderm and adjacent ICM in the blastocyst or inner stem cells subjacent to the outer visceral endoderm of embryoid bodies [23,69]. Biochemical evidence and evidence from the first peri-implantation-null lethals that were endoderm specific resulted in death of the overlying primitive ectoderm by E6.0 suggest that the early endoderm feeds the embryonic ectoderm (reviewed in Rappolee [70]). Thus, induction of both Lrp2 and Dab2 by stress suggests that a primary function of stress-induced differentiation is to provide function that becomes essential rapidly after blastocyst implantation. Hyperosmotic stress begins to induce this absorptive capability in mESCs in monolayers, similar to the induction by several stressors of the placental hormone PL1 from TSCs [5,7,8,71] that increases nutritional supply to the fetal-maternal interface in vivo.
PI3K and p38MAPK protect potency after adaptation to stress, leading to recovery of Nanog/Rex1 and Nanog/Oct4 between 4 and 24 h, respectively (Table 3). Oct4 recovery may occur for several reasons. One is that a sufficient, successful stress response occurs during the first 4 h and allows substantial ESC proliferation and accumulation, and thus induction of full differentiation is not required to compensate for fewer cells. Alternately, the function of early stem cells is to retain potency and divide exponentially between days E4.0 and E11.0 [72,73] so stress-induced differentiation of all cells would not be tolerated. A third hypothesis is that Oct4 function is required to mediate the ongoing stress response as established also for Oct1 [74–76]. If the function of transient Oct4 loss is to enable sufficient ExEndo differentiation, then this may have been accomplished and longer culture in monolayer of embryoid bodies would show that a larger subpopulation of these cells arises. The ESC survival effects corroborate previous studies for PI3K function in ESCs, TSCs, and embryos [34,77], but the role of mediating Oct4 loss from 0 to 4 h is novel and unexpected.
As in our study, Mao et al. found that mESCs maintained a pluripotent phenotype during long-term exposure to hyperosmolarity, although proteins involved in both protein synthesis and degradation via the ubiquitin-proteasome system were decreased [78]. In many cell types, such as cardiomyocytes, hyperosmotic stress at these levels causes an 80% decrease in new translation within the first 30 min [60]. When stress decreases new translation and increases potency factor degradation, rapid, adaptive programmatic changes are possible.
The ubiquitin-proteasome system has been reported to have a role in the self-renewal and differentiation of both human and mouse ESCs (reviewed in Naujokat and Saric [79]). Inhibition of proteasomes decreased levels of four Oct4, Sox2, Nanog, and Rex1 mRNAs [80] and Oct4 and Nanog mRNAs in unstressed human ESCs and thus proteasome function is proposed to be part of potency maintenance. It is not clear from these studies or our studies whether stress would increase proteasome-dependent protein destruction in human ESCs or proteasome-dependent increase in potency mRNA occurs in unstressed mouse ESCs, respectively. The activity of the 20S subunit of the proteasome is normally upregulated during mESC differentiation [81]; its suppression during hyperosmotic stress supports the finding that mESCs are not enabled to differentiate fully during extended hyperosmotic stress.
The reported half-life of Oct4 protein ranges from 1.5 to 8 h. A half-life of ∼90 min has been reported for Oct4 protein in undifferentiated P19 [82], 6–8 h in NIH3T3-overexpressing Oct4 protein [83], 6.9 h for Oct4 mRNA in mouse ESCs [18], and a few hours for Oct4 mRNA in differentiated cells undergoing reprogramming [84]. Oct4 is required to exclude Cdx2 in nonpolar inner cells of the blastocyst [85] and is required for FGF4 secretion that sustains adjacent trophectoderm [86,87] and primitive endoderm cells in the three-dimensional (3D) blastocyst [23,24,88,89]. However, Oct4 also plays a transient, cell-autonomous role in establishing primitive endoderm [23]. Importantly, Oct4 is a stress response factor that controls stem cell metabolism in mESCs in culture before and during stress [74,75] and metabolism of cultured mouse embryos [23]. Thus, the cohort of potency TFs with Oct4 at its apex is dynamically poised under normal circumstances to regulate potency, allocation, stem cell metabolism, and stress responses.
Nanog has a half-life of ∼2 h in human ESCs, and is controlled by proteasomal regulation via the PEST motif [90]. In mESCs, a half-life of 5.2 h has been reported for Nanog mRNA [18]. Rex1 half-life has been reported to be from 30 min [91] to 2.2 h [18] in mESCs [91]. In our mESC system, inhibition of the proteasome for 4 h led to a 3-fold increase in Rex1 expression, suggesting a short half-life of this protein. The rapid turnover of Rex1 as compared with Oct4 and Nanog allows it to respond more quickly to changing conditions. That Rex1 was the only pluripotency marker that did not recover to normal expression levels during stress is unique, as it normally recovers quickly during fluctuations in expression [29]. This may be due to increased protein destruction, JNK-dependent silencing of the Rex1 promoter despite Oct4 [16], or JNK-dependent phosphorylation of Oct4 that decreases binding to the Rex1 promoter. These data suggest that the stressed mESC monolayer may act more like the epithelial embryonic ectoderm of E5.5 that has lost Rex1 since differentiating from the ICM, but still expresses Oct4, Nanog, and Sox2. During normal, unstressed mESC culture, subpopulations of both low-Rex1- and high-Rex1-expressing cells exist [29]. Low-Rex1-expressing cells have poor ability to differentiate into primitive endoderm, and predominantly differentiate to primitive ectoderm lineages. High-Rex1-expressing mESCs were pluripotent and, upon reinjection to embryos, they contributed to multilineage chimeras [29]. These populations were interconvertible during culture without added stress; low Rex1 expressors could revert back to high Rex1 expression regaining developmental potential, and vice versa.
In the current study, hyperosmotic stress maintains low Rex1 expression in mESCs and suppresses interconversion back to high Rex1 expression. This result led us to expect that the stressed mESCs would induce epiblast markers, such as FGF5, due to their known inverse regulation [92]. This did not prove to be the case; however, as epiblast was suppressed while primitive endoderm markers were induced. Rex1 expression is required for complete development of extraembryonic lineages. Rex1-negative mESCs were defective in some visceral endoderm markers although the major marker, α-feto protein (AFP), was expressed [31] and Rex1−/− F9 teratocarcinoma stem cells were only able to differentiate to parietal endoderm [93]. The data suggest that Rex1 plays a role in directing lineages of endoderm differentiation in F9 cells but data should be interpreted cautiously as, unlike mESCs, AFP requires Rex1 expression and F9 cells may not emulate complex regulatory mechanisms of mESCs. In this study, persistence of some Rex1 expression in stressed cells presumably allows mESC priming toward primitive endoderm. However, the persistent suppression of Rex1 expression raises the question of whether visceral endoderm downstream of primitive endoderm will be decreased in favor of parietal endoderm [59].
In the current study, JNK activation by continuous hyperosmotic stress suppressed Rex1 expression, but was not adequate to trigger outright differentiation of mESCs. JNK may not be activated long enough to irreversibly commit ESCs to differentiation, or there may be insufficient activation of other enzymes needed to complement JNK-induced differentiation, or insufficient 3D interactions may occur. Unlike TSCs, ESCs may require the 3D interactions available in embryoid bodies. However, it was not defined whether JNK directly decreased Rex1 protein or indirectly suppressed it during induction of differentiation. In each case of stress-induced differentiation of mESCs and TSCs, JNK was active within a pathway required to produce a new lineage, but in none of the cases was it capable of initiating differentiation on its own. JNK may work with other differentiation cues and enzymatic mechanisms. But during the stress response, the activation of multiple pathways with competing effects prevented outright or complete differentiation of large cell subpopulations. For example, JNK suppressed Rex1 expression during 24 h of stress, while PI3K signaling was simultaneously maintaining Rex1, preventing total Rex1 protein loss. This additional signaling masked but did not negate JNK's action on Rex1. Of course if a single ESC lost the three potency TFs undergoing transient stress-induced loss, and the continuing loss of Rex1 for a long enough period, then it might differentiate irreversibly. Clearly the analysis of the fate of subpopulations of stressed mESCs and hESCs in monolayer, embryoid bodies, and in embryos is needed.
Finally, the stress-induced differentiation of TSCs occurred even in the presence of FGF4 signaling that sustains their multipotent state [5–8,94]. Stress signaling was dominant over FGF4 signaling at doses where stem cell populations expanded, but at diminished rates. In the current study, mESCs integrated stress-response signals with potentially competing signals from exogenous LIF and bone morphogenetic protein (BMP). The cytokine LIF promotes mESC self-renewal by activating the TF STAT3. BMPs are a serum component that induces expression of inhibitor of differentiation genes that block expression-lineage-specific TF function and facilitate the self-renewal response to LIF/STAT3 [95]. In our system, the integration of all these signals during hyperosmotic stress led to preservation of a pluripotent population; stress was not dominant in overt changes in mESC monolayers. However, in vivo the pluripotent ICM is a transient stage that stem cells move through to populate the lineages that will eventually make up the embryo and its support cells. LIF in the four-cell-stage embryo and interleukin-6 at the blastocyst stage are necessary to maintain phosphorylated STAT3 and Oct4 in order to maintain pluripotency in vivo [96]. If stress occurred in an environment that was more characteristic of the nonepithelial ICM, one where differentiation was not repressed, then the stress activation of the differentiation program may proceed unimpeded. Thus, stress may drive preimplantation ICM to ExEndo but suppress other lineages that arise at gastrulation.
It is probable, but remains to be determined, whether these mechanisms revealed by the average activity per cell assayed by the biochemical assays here take place in individual cells and occur in vivo. The data suggest that the period when stem cells first allocate at E3.5, differentiate soon after, and mediate gastrulation at E6.5 is critical and can be affected critically by stress. The understanding of how stress enzymes mount successful adaptation to stress through regulation of potency and differentiation-mediating TFs during this critical period is of profound importance. The goal is to understand the mechanisms and thresholds between healthy, adaptive and unhealthy, maladaptive responses that are important in the general population but also during medical procedures such as in vitro fertilization that alleviate infertility.
Supplementary Material
Acknowledgments
The authors thank Mike Kruger and Jing Dai for advice on statistical analysis. We are also indebted to Drs. Jose CIbelli, Jerry Sanders, Joseph Dunbar, Don DeGracia, Assia Shisheva, and Todd Leff for helpful discussion and criticisms of the article. This research was supported by grants to D.A.R. from NIH (R01 HD40972A and 1R03HD061431) and from the Office of the Vice President for Research at Wayne State University.
Author Disclosure Statement
All authors acknowledge that there are no conflicts of interest or competing financial interests involved with submission of or interpretation of data in this article.
References
- 1.Fauque P, Mondon F, Letourneur F, Ripoche MA, Journot L, Barbaux S, Dandolo L, Patrat C, Wolf JP, et al. (2010). In vitro fertilization and embryo culture strongly impact the placental transcriptome in the mouse model. PLoS One 5:e9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhong W, Xie Y, Abdallah M, Awonuga AO, Slater JA, Sipahi L, Puscheck EE. and Rappolee DA. (2010). Cellular stress causes reversible, PRKAA1/2-, and proteasome-dependent ID2 protein loss in trophoblast stem cells. Reproduction 140:921–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smart YC, Fraser IS, Roberts TK, Clancy RL. and Cripps AW. (1982). Fertilization and early pregnancy loss in healthy women attempting conception. Clin Reprod Fertil 1:177–184 [PubMed] [Google Scholar]
- 4.Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG. and Nisula BC. (1988). Incidence of early loss of pregnancy. N Engl J Med 319:189–194 [DOI] [PubMed] [Google Scholar]
- 5.Xie Y, Zhou S, Jiang Z, Dai J, Puscheck E, Lee I, Parker GC, Huttemann M. and Rappolee D. (2014). Hypoxic stress induces SAPK-dependent, imbalanced trophoblast stem cell differentiation but does not sustain it due to mitochondrial insufficiency. Stem Cell Res In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie Y, Awonuga A, Liu J, Rings E, Puscheck EE. and Rappolee DA. (2013). Stress induces AMP-dependent loss of potency factors Id2 and Cdx2 in early embryos and stem cells. Stem Cells Dev 22:1564–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Awonuga AO, Zhong W, Abdallah ME, Slater JA, Zhou SC, Xie YF, Puscheck EE. and Rappolee DA. (2011). Eomesodermin, HAND1, and CSH1 proteins are induced by cellular stress in a stress-activated protein kinase-dependent manner. Mol Reprod Dev 78:519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Xu W, Sun T, Wang F, Puscheck E, Brigstock D, Wang QT, Davis R. and Rappolee DA. (2009). Hyperosmolar stress induces global mRNA responses in placental trophoblast stem cells that emulate early post-implantation differentiation. Placenta 30:66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lander AD. (2009). The ‘stem cell’ concept: is it holding us back?. J Biol 8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burdon T, Chambers I, Stracey C, Niwa H. and Smith A. (1999). Signaling mechanisms regulating self-renewal and differentiation of pluripotent embryonic stem cells. Cells Tissues Organs 165:131–143 [DOI] [PubMed] [Google Scholar]
- 11.Storm MP, Bone HK, Beck CG, Bourillot PY, Schreiber V, Damiano T, Nelson A, Savatier P. and Welham MJ. (2007). Regulation of Nanog expression by phosphoinositide 3-kinase-dependent signaling in murine embryonic stem cells. J Biol Chem 282:6265–6273 [DOI] [PubMed] [Google Scholar]
- 12.Duval D, Malaise M, Reinhardt B, Kedinger C. and Boeuf H. (2004). A p38 inhibitor allows to dissociate differentiation and apoptotic processes triggered upon LIF withdrawal in mouse embryonic stem cells. Cell Death Differ 11:331–341 [DOI] [PubMed] [Google Scholar]
- 13.Barruet E, Hadadeh O, Peiretti F, Renault VM, Hadjal Y, Bernot D, Tournaire R, Negre D, Juhan-Vague I, Alessi MC. and Binetruy B. (2011). p38 mitogen activated protein kinase controls two successive-steps during the early mesodermal commitment of embryonic stem cells. Stem Cells Dev 20:1233–1246 [DOI] [PubMed] [Google Scholar]
- 14.Amura CR, Marek L, Winn RA. and Heasley LE. (2005). Inhibited neurogenesis in JNK1-deficient embryonic stem cells. Mol Cell Biol 25:10791–10802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheikh-Hamad D. and Gustin MC. (2004). MAP kinases and the adaptive response to hypertonicity: functional preservation from yeast to mammals. Am J Physiol Renal Physiol 287:F1102–F1110 [DOI] [PubMed] [Google Scholar]
- 16.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, et al. (2005). Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122:947–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, Chevalier B, Johnstone SE, Cole MF, et al. (2006). Control of developmental regulators by Polycomb in human embryonic stem cells. Cell 125:301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharova LV, Sharov AA, Piao Y, Shaik N, Sullivan T, Stewart CL, Hogan BL. and Ko MS. (2007). Global gene expression profiling reveals similarities and differences among mouse pluripotent stem cells of different origins and strains. Dev Biol 307:446–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pal R, Mamidi MK, Das AK. and Bhonde R. (2011). Human embryonic stem cell proliferation and differentiation as parameters to evaluate developmental toxicity. J Cell Physiol 226:1583–1595 [DOI] [PubMed] [Google Scholar]
- 20.Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H. and Smith A. (1998). Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95:379–391 [DOI] [PubMed] [Google Scholar]
- 21.Niwa H, Miyazaki J. and Smith AG. (2000). Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 24:372–376 [DOI] [PubMed] [Google Scholar]
- 22.Palmieri SL, Peter W, Hess H. and Scholer HR. (1994). Oct-4 transcription factor is differentially expressed in the mouse embryo during establishment of the first two extraembryonic cell lineages involved in implantation. Dev Biol 166:259–267 [DOI] [PubMed] [Google Scholar]
- 23.Frum T, Halbisen MA, Wang C, Amiri H, Robson P. and Ralston A. (2013). Oct4 cell-autonomously promotes primitive endoderm development in the mouse blastocyst. Dev Cell 25:610–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frankenberg S, Gerbe F, Bessonnard S, Belville C, Pouchin P, Bardot O. and Chazaud C. (2011). Primitive endoderm differentiates via a three-step mechanism involving Nanog and RTK signaling. Dev Cell 21:1005–1013 [DOI] [PubMed] [Google Scholar]
- 25.Chambers I, Silva J, Colby D, Nichols J, Nijmeijer B, Robertson M, Vrana J, Jones K, Grotewold L. and Smith A. (2007). Nanog safeguards pluripotency and mediates germline development. Nature 450:1230–1234 [DOI] [PubMed] [Google Scholar]
- 26.Singh AM, Hamazaki T, Hankowski KE. and Terada N. (2007). A heterogeneous expression pattern for Nanog in embryonic stem cells. Stem Cells 25:2534–2542 [DOI] [PubMed] [Google Scholar]
- 27.Rathjen J, Lake JA, Bettess MD, Washington JM, Chapman G. and Rathjen PD. (1999). Formation of a primitive ectoderm like cell population, EPL cells, from ES cells in response to biologically derived factors. J Cell Sci 112(Pt 5):601–612 [DOI] [PubMed] [Google Scholar]
- 28.Lake J, Rathjen J, Remiszewski J. and Rathjen PD. (2000). Reversible programming of pluripotent cell differentiation. J Cell Sci 113(Pt 3):555–566 [DOI] [PubMed] [Google Scholar]
- 29.Toyooka Y, Shimosato D, Murakami K, Takahashi K. and Niwa H. (2008). Identification and characterization of subpopulations in undifferentiated ES cell culture. Development 135:909–918 [DOI] [PubMed] [Google Scholar]
- 30.Scotland KB, Chen S, Sylvester R. and Gudas LJ. (2009). Analysis of Rex1 (zfp42) function in embryonic stem cell differentiation. Dev Dyn 238:1863–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masui S, Ohtsuka S, Yagi R, Takahashi K, Ko MS. and Niwa H. (2008). Rex1/Zfp42 is dispensable for pluripotency in mouse ES cells. BMC Dev Biol 8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alessi DR, Cuenda A, Cohen P, Dudley DT. and Saltiel AR. (1995). PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem 270:27489–27494 [DOI] [PubMed] [Google Scholar]
- 33.Davies SP, Reddy H, Caivano M. and Cohen P. (2000). Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351:95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gross VS, Hess M. and Cooper GM. (2005). Mouse embryonic stem cells and preimplantation embryos require signaling through the phosphatidylinositol 3-kinase pathway to suppress apoptosis. Mol Reprod Dev 70:324–332 [DOI] [PubMed] [Google Scholar]
- 35.Hamazaki T, Kehoe SM, Nakano T. and Terada N. (2006). The Grb2/Mek pathway represses Nanog in murine embryonic stem cells. Mol Cell Biol 26:7539–7549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR. and Cohen P. (2007). The selectivity of protein kinase inhibitors: a further update. Biochem J 408:297–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SH, Lee MY. and Han HJ. (2008). Short-period hypoxia increases mouse embryonic stem cell proliferation through cooperation of arachidonic acid and PI3K/Akt signalling pathways. Cell Prolif 41:230–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mullen SF. and Critser JK. (2004). Using TUNEL in combination with an active caspase-3 immunoassay to identify cells undergoing apoptosis in preimplantation mammalian embryos. Methods Mol Biol 254:393–406 [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Wang F, Sun T, Trostinskaia A, Wygle D, Puscheck E. and Rappolee DA. (2004). Entire mitogen activated protein kinase (MAPK) pathway is present in preimplantation mouse embryos. Dev Dyn 231:72–87 [DOI] [PubMed] [Google Scholar]
- 40.Xie Y, Wang Y, Sun T, Wang F, Trostinskaia A, Puscheck E. and Rappolee DA. (2005). Six post-implantation lethal knockouts of genes for lipophilic MAPK pathway proteins are expressed in preimplantation mouse embryos and trophoblast stem cells. Mol Reprod Dev 71:1–11 [DOI] [PubMed] [Google Scholar]
- 41.Xie Y, Zhong W, Wang Y, Trostinskaia A, Wang F, Puscheck EE. and Rappolee DA. (2007). Using hyperosmolar stress to measure biologic and stress-activated protein kinase responses in preimplantation embryos. Mol Hum Reprod 13:473–481 [DOI] [PubMed] [Google Scholar]
- 42.Aldridge GM, Podrebarac DM, Greenough WT. and Weiler IJ. (2008). The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J Neurosci Methods 172:250–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy CL. and Polak JM. (2002). Differentiating embryonic stem cells: GAPDH, but neither HPRT nor beta-tubulin is suitable as an internal standard for measuring RNA levels. Tissue Eng 8:551–559 [DOI] [PubMed] [Google Scholar]
- 44.Willems E, Mateizel I, Kemp C, Cauffman G, Sermon K. and Leyns L. (2006). Selection of reference genes in mouse embryos and in differentiating human and mouse ES cells. Int J Dev Biol 50:627–635 [DOI] [PubMed] [Google Scholar]
- 45.Livak KJ. and Schmittgen TD. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 46.Zhong W, Xie Y, Wang Y, Lewis J, Trostinskaia A, Wang F, Puscheck EE. and Rappolee DA. (2007). Use of hyperosmolar stress to measure stress-activated protein kinase activation and function in human HTR cells and mouse trophoblast stem cells. Reprod Sci 14:534–547 [DOI] [PubMed] [Google Scholar]
- 47.Xie Y, Liu J, Proteasa S, Proteasa G, Zhong W, Wang Y, Wang F, Puscheck EE. and Rappolee DA. (2008). Transient stress and stress enzyme responses have practical impacts on parameters of embryo development, from IVF to directed differentiation of stem cells. Mol Reprod Dev 75:689–697 [DOI] [PubMed] [Google Scholar]
- 48.Harris SE, Gopichandran N, Picton HM, Leese HJ. and Orsi NM. (2005). Nutrient concentrations in murine follicular fluid and the female reproductive tract. Theriogenology 64:992–1006 [DOI] [PubMed] [Google Scholar]
- 49.Brunlid G, Pruszak J, Holmes B, Isacson O. and Sonntag KC. (2007). Immature and neurally differentiated mouse embryonic stem cells do not express a functional Fas/Fas ligand system. Stem Cells 25:2551–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie Y, Awonuga AO, Zhou S, Puscheck EE. and Rappolee DA. (2011). Interpreting the stress response of early mammalian embryos and their stem cells. Int Rev Cell Mol Biol 287:43–95 [DOI] [PubMed] [Google Scholar]
- 51.Dudley DT, Pang L, Decker SJ, Bridges AJ. and Saltiel AR. (1995). A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci U S A 92:7686–7689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goueli SA, Hsiao K, Lu T. and Simpson D. (1998). U0126: a novel, selective and potent inhibitor of MAP kinase kinase (MEK). Promega Notes 69:6–9 [Google Scholar]
- 53.Bain J, McLauchlan H, Elliott M. and Cohen P. (2003). The specificities of protein kinase inhibitors: an update. Biochem J 371:199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arbiser JL, Kau T, Konar M, Narra K, Ramchandran R, Summers SA, Vlahos CJ, Ye K, Perry BN, et al. (2007). Solenopsin, the alkaloidal component of the fire ant (Solenopsis invicta), is a naturally occurring inhibitor of phosphatidylinositol-3-kinase signaling and angiogenesis. Blood 109:560–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerbe F, Cox B, Rossant J. and Chazaud C. (2008). Dynamic expression of Lrp2 pathway members reveals progressive epithelial differentiation of primitive endoderm in mouse blastocyst. Dev Biol 313:594–602 [DOI] [PubMed] [Google Scholar]
- 56.Yang DH, Smith ER, Roland IH, Sheng Z, He J, Martin WD, Hamilton TC, Lambeth JD. and Xu XX. (2002). Disabled-2 is essential for endodermal cell positioning and structure formation during mouse embryogenesis. Dev Biol 251:27–44 [DOI] [PubMed] [Google Scholar]
- 57.Haub O. and Goldfarb M. (1991). Expression of the fibroblast growth factor-5 gene in the mouse embryo. Development 112:397–406 [DOI] [PubMed] [Google Scholar]
- 58.Hebert JM, Boyle M. and Martin GR. (1991). mRNA localization studies suggest that murine FGF-5 plays a role in gastrulation. Development 112:407–415 [DOI] [PubMed] [Google Scholar]
- 59.Brown K, Legros S, Artus J, Doss MX, Khanin R, Hadjantonakis AK. and Foley A. (2010). A comparative analysis of extra-embryonic endoderm cell lines. PLoS One 5:e12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patel J, McLeod LE, Vries RG, Flynn A, Wang X. and Proud CG. (2002). Cellular stresses profoundly inhibit protein synthesis and modulate the states of phosphorylation of multiple translation factors. Eur J Biochem 269:3076–3085 [DOI] [PubMed] [Google Scholar]
- 61.Catalanotti F, Reyes G, Jesenberger V, Galabova-Kovacs G, de Matos Simoes R, Carugo O. and Baccarini M. (2009). A Mek1-Mek2 heterodimer determines the strength and duration of the Erk signal. Nat Struct Mol Biol 16:294–303 [DOI] [PubMed] [Google Scholar]
- 62.Hamazaki T, Oka M, Yamanaka S. and Terada N. (2004). Aggregation of embryonic stem cells induces Nanog repression and primitive endoderm differentiation. J Cell Sci 117:5681–5686 [DOI] [PubMed] [Google Scholar]
- 63.Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M. and Yamanaka S. (2003). The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113:631–642 [DOI] [PubMed] [Google Scholar]
- 64.Abdallah M, Xie Y, Puscheck EE, Rappolee DA. and Awonuga AO. (2009). Benzopyrene activates SAPK and induces HAND1 that favors differentiation of trophoblast stem cells. Fertil Steril 92:S136–S137 [Google Scholar]
- 65.Maurer ME. and Cooper JA. (2005). Endocytosis of megalin by visceral endoderm cells requires the Dab2 adaptor protein. J Cell Sci 118:5345–5355 [DOI] [PubMed] [Google Scholar]
- 66.Assemat E, Vinot S, Gofflot F, Linsel-Nitschke P, Illien F, Chatelet F, Verroust P, Louvet-Vallee S, Rinninger F. and Kozyraki R. (2005). Expression and role of cubilin in the internalization of nutrients during the peri-implantation development of the rodent embryo. Biol Reprod 72:1079–1086 [DOI] [PubMed] [Google Scholar]
- 67.Smith BT, Mussell JC, Fleming PA, Barth JL, Spyropoulos DD, Cooley MA, Drake CJ. and Argraves WS. (2006). Targeted disruption of cubilin reveals essential developmental roles in the structure and function of endoderm and in somite formation. BMC Dev Biol 6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Drake CJ, Fleming PA, Larue AC, Barth JL, Chintalapudi MR. and Argraves WS. (2004). Differential distribution of cubilin and megalin expression in the mouse embryo. Anat Rec A Discov Mol Cell Evol Biol 277:163–170 [DOI] [PubMed] [Google Scholar]
- 69.Yang DH, Cai KQ, Roland IH, Smith ER. and Xu XX. (2007). Disabled-2 is an epithelial surface positioning gene. J Biol Chem 282:13114–13122 [DOI] [PubMed] [Google Scholar]
- 70.Rappolee DA. (1999). It's not just baby's babble/Babel: recent progress in understanding the language of early mammalian development: a minireview. Mol Reprod Dev 52:234–240 [DOI] [PubMed] [Google Scholar]
- 71.Rappolee DA, Zhou S, Puscheck EE. and Xie Y. (2013). Stress responses at the endometrial-placental interface regulate labyrinthine placental differentiation from trophoblast stem cells. Reproduction 145:R139–R155 [DOI] [PubMed] [Google Scholar]
- 72.McLaren A. (1976). Growth from fertilization to birth in the mouse. In: Embryogenesis in Mammals. Elsevier, Amsterdam, New York [Google Scholar]
- 73.Smith MHL. (1976). Embryo growth during the immediate postimplantation period. In: Embryogenesis in Mammals. Elsevier, Amsterdam, New York [Google Scholar]
- 74.Kang J, Shakya A. and Tantin D. (2009). Stem cells, stress, metabolism and cancer: a drama in two Octs. Trends Biochem Sci 34:491–499 [DOI] [PubMed] [Google Scholar]
- 75.Kang J, Gemberling M, Nakamura M, Whitby FG, Handa H, Fairbrother WG. and Tantin D. (2009). A general mechanism for transcription regulation by Oct1 and Oct4 in response to genotoxic and oxidative stress. Genes Dev 23:208–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tantin D, Schild-Poulter C, Wang V, Hache RJ. and Sharp PA. (2005). The octamer binding transcription factor Oct-1 is a stress sensor. Cancer Res 65:10750–10758 [DOI] [PubMed] [Google Scholar]
- 77.Riley JK, Carayannopoulos MO, Wyman AH, Chi M, Ratajczak CK. and Moley KH. (2005). The PI3K/Akt pathway is present and functional in the preimplantation mouse embryo. Dev Biol 284:377–386 [DOI] [PubMed] [Google Scholar]
- 78.Mao L, Hartl D, Nolden T, Koppelstatter A, Klose J, Himmelbauer H. and Zabel C. (2008). Pronounced alterations of cellular metabolism and structure due to hyper- or hypo-osmosis. J Proteome Res 7:3968–3983 [DOI] [PubMed] [Google Scholar]
- 79.Naujokat C. and Saric T. (2007). Concise review: role and function of the ubiquitin-proteasome system in mammalian stem and progenitor cells. Stem Cells 25:2408–2418 [DOI] [PubMed] [Google Scholar]
- 80.Vilchez D, Boyer L, Morantte I, Lutz M, Merkwirth C, Joyce D, Spencer B, Page L, Masliah E, et al. (2012). Increased proteasome activity in human embryonic stem cells is regulated by PSMD11. Nature 489:304–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hernebring M, Brolen G, Aguilaniu H, Semb H. and Nystrom T. (2006). Elimination of damaged proteins during differentiation of embryonic stem cells. Proc Natl Acad Sci U S A 103:7700–7705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saxe JP, Tomilin A, Scholer HR, Plath K. and Huang J. (2009). Post-translational regulation of Oct4 transcriptional activity. PLoS One 4:e4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wei F, Scholer HR. and Atchison ML. (2007). Sumoylation of Oct4 enhances its stability, DNA binding, and transactivation. J Biol Chem 282:21551–21560 [DOI] [PubMed] [Google Scholar]
- 84.Taranger CK, Noer A, Sorensen AL, Hakelien AM, Boquest AC. and Collas P. (2005). Induction of dedifferentiation, genomewide transcriptional programming, and epigenetic reprogramming by extracts of carcinoma and embryonic stem cells. Mol Biol Cell 16:5719–5735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Niwa H, Toyooka Y, Shimosato D, Strumpf D, Takahashi K, Yagi R. and Rossant J. (2005). Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell 123:917–929 [DOI] [PubMed] [Google Scholar]
- 86.Arcuri F, Sestini S, Paulesu L, Bracci L, Carducci A, Manzoni F, Cardone C. and Cintorino M. (1998). 11Beta-hydroxysteroid dehydrogenase expression in first trimester human trophoblasts. Mol Cell Endocrinol 141:13–20 [DOI] [PubMed] [Google Scholar]
- 87.Ma YG, Rosfjord E, Huebert C, Wilder P, Tiesman J, Kelly D. and Rizzino A. (1992). Transcriptional regulation of the murine k-FGF gene in embryonic cell lines. Dev Biol 154:45–54 [DOI] [PubMed] [Google Scholar]
- 88.Cho LT, Wamaitha SE, Tsai IJ, Artus J, Sherwood RI, Pedersen RA, Hadjantonakis AK. and Niakan KK. (2012). Conversion from mouse embryonic to extra-embryonic endoderm stem cells reveals distinct differentiation capacities of pluripotent stem cell states. Development 139:2866–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krawchuk D, Honma-Yamanaka N, Anani S. and Yamanaka Y. (2013). FGF4 is a limiting factor controlling the proportions of primitive endoderm and epiblast in the ICM of the mouse blastocyst. Dev Biol 384:65–71 [DOI] [PubMed] [Google Scholar]
- 90.Ramakrishna S, Suresh B, Lim KH, Cha BH, Lee SH, Kim KS. and Baek KH. (2011). PEST motif sequence regulating human NANOG for proteasomal degradation. Stem Cells Dev 20:1511–1519 [DOI] [PubMed] [Google Scholar]
- 91.Gontan C, Achame EM, Demmers J, Barakat TS, Rentmeester E, van IW, Grootegoed JA. and Gribnau J. (2012). RNF12 initiates X-chromosome inactivation by targeting REX1 for degradation. Nature 485:386–390 [DOI] [PubMed] [Google Scholar]
- 92.Pelton TA, Sharma S, Schulz TC, Rathjen J. and Rathjen PD. (2002). Transient pluripotent cell populations during primitive ectoderm formation: correlation of in vivo and in vitro pluripotent cell development. J Cell Sci 115:329–339 [DOI] [PubMed] [Google Scholar]
- 93.Thompson JR. and Gudas LJ. (2002). Retinoic acid induces parietal endoderm but not primitive endoderm and visceral endoderm differentiation in F9 teratocarcinoma stem cells with a targeted deletion of the Rex-1 (Zfp-42) gene. Mol Cell Endocrinol 195:119–133 [DOI] [PubMed] [Google Scholar]
- 94.Zhou S, Xie Y, Puscheck EE. and Rappolee DA. (2011). Oxygen levels that optimize TSC culture are identified by maximizing growth rates and minimizing stress. Placenta 32:475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ying QL, Nichols J, Chambers I. and Smith A. (2003). BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 115:281–292 [DOI] [PubMed] [Google Scholar]
- 96.Do DV, Ueda J, Messerschmidt DM, Lorthongpanich C, Zhou Y, Feng B, Guo G, Lin PJ, Hossain MZ, et al. (2013). A genetic and developmental pathway from STAT3 to the OCT4-NANOG circuit is essential for maintenance of ICM lineages in vivo. Genes Dev 27:1378–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.