Abstract
Exposure to drugs of abuse can result in profound structural modifications on neurons in circuits involved in addiction that may contribute to drug dependence, withdrawal and related processes. Structural alterations on medium spiny neurons (MSNs) of the nucleus accumbens (NAc) have been observed following exposure to and withdrawal from a variety of drugs, however, relatively little is known about the effects of alcohol exposure and withdrawal on structural alterations of NAc MSNs. In the present study male rats were chronically exposed to vaporized ethanol for 10 days and underwent 1 or 7 days of withdrawal after which the brains were processed for Golgi-Cox staining and analysis of dendritic length, branching and spine density. MSNs of the NAc shell and core underwent different patterns of changes following ethanol exposure and withdrawal. At 1 day of withdrawal there were modest reductions in the dendritic length and branching of MSNs in both the core and the shell compared to control animals exposed only to air. At 7 days of withdrawal the length and branching of shell MSNs was reduced, whereas the length and branching of core MSNs were increased relative to the shell. The density of mature spines was increased in the core at 1 day of withdrawal, whereas the density of less mature spines was increased in both regions at 7 days of withdrawal. Collectively, these observations indicate that MSNs of the NAc core and shell undergo distinct patterns of structural modifications following ethanol exposure and withdrawal suggesting that modifications in dendritic structure in these regions may contribute differentially to ethanol withdrawal.
Keywords: Alcohol, addiction, Golgi, medium spiny neuron, structural plasticity
1. Introduction
Drugs of abuse can cause profound and persistent modifications in dendritic length, branching and spine density on neurons in circuits implicated in drug addiction and reward (Kolb, Gorny, Li, Samaha, & Robinson, 2003; Rice et al., 2012; Robinson & Kolb, 1997, 1999a, 2004; Zhou et al., 2007). These types of morphological changes represent one of the primary mechanisms by which experience modifies the nervous system to facilitate future behavior. Importantly, these modifications can be advantageous or disadvantageous. Studies have found structural alterations in medium spiny neurons (MSNs) of the nucleus accumbens (NAc), a region implicated in drug seeking, reward learning, and reinforcement (Di Chiara, 2002; Everitt & Robbins, 2005; Ikemoto & Panksepp, 1999; Koob et al., 2014; Koob & Volkow, 2010; McFarland, Lapish, & Kalivas, 2003; Robinson & Berridge, 1993), following exposure to various psychoactive drugs. Modifications in dendritic morphology of NAc MSNs include increases in branching, length, spine density and/or spine head diameter following exposure to nicotine (Brown & Kolb, 2001; Gipson, Reissner, et al., 2013; D. A. Hamilton & Kolb, 2005), THC (Kolb, Gorny, Limebeer, & Parker, 2006), cocaine (Gipson, Kupchik, et al., 2013; Kolb et al., 2003; Robinson & Kolb, 1999a), and amphetamines (Kolb et al., 2003; Robinson & Kolb, 1999a, 2004) and decreases following exposure to morphine (Robinson & Kolb, 1999b), haloperidol (Frost, Page, Carroll, & Kolb, 2010), and olanzapine (Frost et al., 2010). Ethanol exposure predominately causes reductions in MSN branching, length, and/or spine density (McMullen, Stcyr, & Carlen, 1984; Rice et al., 2012; Romero et al., 2013; Zhou et al., 2007), however, the direction of morphological changes following ethanol exposure varies (see, e.g. Zhou et al., 2007) perhaps owing to the diversity of exposure paradigms.
The effects of ethanol exposure and withdrawal on the morphology of NAc MSNs are not well represented in the literature, but are critically important for better understanding the neural bases and progression of ethanol addiction and withdrawal. Prenatal ethanol exposure induces long-term modifications in the nervous system associated with increased voluntary ethanol consumption in adulthood (Barbier et al., 2009; Barbier et al., 2008) which may be partially attributed to reductions in dendritic morphology of MSNs (Rice et al., 2012) and/or elevated dopamine in the NAc (Blanchard et al., 1993). Acute analysis following prenatal and perinatal ethanol exposure, however, failed to detect effects on MSN morphology (Lawrence, Otero, & Kelly, 2012), suggesting that morphological changes in the NAc may be time dependent. In adulthood, “alcohol-preferring” (P) rats show reductions in spine density and terminal branching and increases in mushroom and multi-headed spines following chronic ethanol drinking and repeated deprivation (Zhou et al., 2007). Spiga et al. (2014) also recently reported reductions in thin spines within the nucleus accumbens early during alcohol withdrawal in young rats. The comparative lack of literature on the impact of ethanol dependence and withdrawal on dendritic morphology and spine density in the NAc of normal adult rats motivated the present study.
This study sought to characterize the effects of passive, chronic intermittent ethanol exposure and both short-term (1 day) and long-term (7 day) withdrawal on dendritic morphology on MSNs in the NAc core and shell. Examining the possibility of a dissociation between the shell and core was motivated by previous experimental data demonstrating functional dissociations between the NAc core and shell in normal rats (Di Chiara, 2002; Di Chiara & Bassareo, 2007; Horsley, Norman, & Cassaday, 2007) and in relation to drug self-administration (Chaudhri, Sahuque, Schairer, & Janak, 2010; Gonzales, Job, & Doyon, 2004; Meredith, Baldo, Andrezjewski, & Kelley, 2008), as well as identification of the NAc core as part of a broader circuit implicated in reinstatement of drug self-administration (McFarland & Kalivas, 2001). Adult male Sprague-Dawley rats were passively exposed to vaporized ethanol (~37 mg/L; 12 h/day) for ten consecutive days; the control group received no ethanol. This exposure protocol yields blood ethanol concentrations in the 150–200mg/dL (0.15–0.20) range, produces robust physical dependence, and increases in anxiety-like behaviors (Lack, Diaz, Chappell, DuBois, & McCool, 2007) that are accompanied by significant alterations in glutamatergic and GABAergic neurotransmission in the amygdala (Christian, Alexander, Diaz, Robinson, & McCool, 2012; Diaz, Christian, Anderson, & McCool, 2011). Further, passive vaporized ethanol exposure protocols similar to that employed here induce conspicuous signs of withdrawal (e.g., tremor) upon removal of ethanol (Macey, Schulteis, Heinrichs, & Koob, 1996; Roberts, Cole, & Koob, 1996), anxiety (Rassnick, Heinrichs, Britton, & Koob, 1993; Valdez, Sabino, & Koob, 2004), increased tolerance for the hypothermic effects of ethanol (Ristuccia & Spear, 2005), reduced seizure thresholds (Ferko & Bobyock, 1977), and enhancements of subsequent ethanol seeking and operant self-administration (Buck, Malavar, George, Koob, & Vendruscolo, 2014; Roberts, Heyser, Cole, Griffin, & Koob, 2000). Following the withdrawal period (1 day or 7 days) or control exposure the brains were extracted for Golgi-Cox staining (Gibb & Kolb, 1998) and dendritic length, branching, overall spine density, and density of specific spine types on MSNs of the NAc core and shell were quantified.
2. Results
2.1. Length and Branching
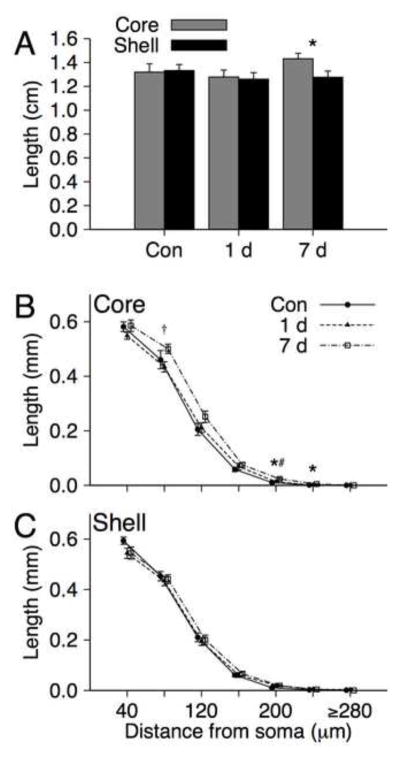
Mean total dendritic length of shell and core MSNs for air control, 1 day and 7 day ethanol withdrawal groups are shown in Figure 2A. An ANOVA with Group and Region (shell v. core) for overall dendritic length revealed a significant Group X Region interaction [F(2, 22) = 3.74, p = 0.04, ]. Neither main effect was significant [both ps > 0.066]. Analysis of simple effects of region within each group indicate that the significant interaction is attributable to a significant region effect for the 7 day withdrawal group [core > shell; F(1, 8) = 8.36, p = 0.02, ] that was not observed for either the control group [p = 0.79, ] or 1 day withdrawal group [p = 0.67, ].
FIGURE 2.
A) Mean (+SEM) dendritic length of medium spiny neurons in the NAc Shell and Core for control (n = 8), 1 day withdrawal (n = 8) and 7 day withdrawal (n = 9) groups. * indicates a significant region effect at p < 0.05. B) Mean (± SEM) dendritic length as a function of distance from soma (in 40μm segments) of medium spiny neurons in the NAc Shell and Core for control, 1 day withdrawal and 7 day withdrawal groups. * indicates a significant group effect at p = 0.02, † indicates a significant contrast (7 day > 1 day withdrawal) at p = 0.019, # indicates a significant contrast (7 day withdrawal > Control) at p = 0.005.
Mean dendritic length based on distance from the soma for each combination of group and region are down in Figure 2B-C. Separate repeated measures multivariate ANOVAs (MANOVAs) with Group and Distance from soma (7 segments in 40μm increments) as factors were conducted each region. There was a significant interaction observed for the NAc core [λ = 0.308, F(12, 34) = 2.27, p = 0.03, ], but not the shell [λ = 0.558, F(12, 34) = 0.96, p = 0.50, ]. Inspection of the group means for each segment obtained for the core indicate that the means for the 7 day withdrawal group were higher than those of the control and 1 day groups. When directly compared the 7 day withdrawal group had greater dendritic length values following Bonferroni correction than controls for segment 5 of the core [F(1, 15) = 10.96, p = 0.005, ]. There was a trend in the same direction for segment 6 [F(1, 15) = 3.75, p = 0.07, ], however, none of the other comparisons were significant [all ps > .15]. The 7 day withdrawal group also had significantly greater dendritic length than the 1 day withdrawal group for core segment 2 following correction [F(1, 15) = 6.88, p = 0.019, ], with similar trends for segments 5 [F(1, 15) = 4.06, p = 0.062, ] and 6 [F(1, 15) = 4.31, p = 0.055, ]. All other comparisons for the 1 day and 7 day withdrawal groups were not significant [all ps > 0.16]. None of the comparisons of the control and 1 day withdrawal group for length were significant [all ps > 0.14].
Mean total branches on NAc core and shell MSNs for control, 1 day and 7 day withdrawal groups are shown in Figure 3A. An ANOVA with Group and Region for total branches yielded a significant effect of region [ core > shell ; F(1, 22) = 4.86, p = 0.038, η2 = 0.181], but failed to yield a significant group main effect [F(1, 22) = 1.09, p = 0.355, ] or Group X Region interaction [F(2, 22) = 2.88, p = 0.078, ]. Although the interaction was not significant, it is noted that a pattern of regional effects within groups was similar to that observed for dendritic length. There was a significant effect of region following Bonferroni correction for the 7 day withdrawal group [core > shell; F(1, 8) = 9.79, p = 0.015, ], whereas there were no significant region effects for the other groups [all ps > 0.60].
FIGURE 3.
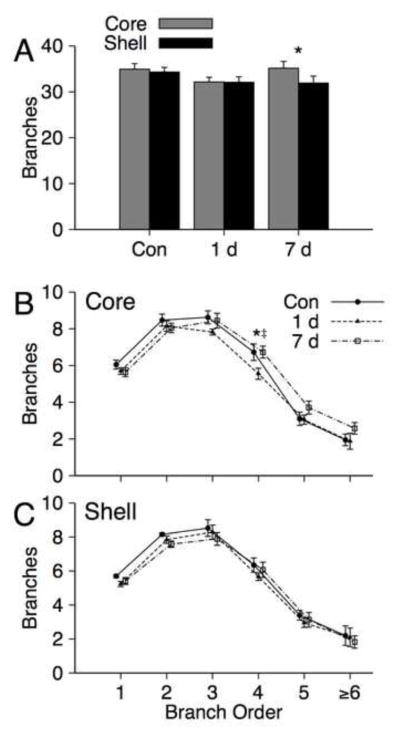
A) Mean (+SEM) branches on medium spiny neurons in the NAc core and shell for control (n = 8), 1 day withdrawal (n = 8) and 7 day withdrawal (n = 9) groups. * indicates a significant region effect at p = 0.015. B) Mean (± SEM) branches as a function of branch order (1–6+) for medium spiny neurons in the NAc core and shell for control, 1 day withdrawal and 7 day withdrawal groups. * indicates a significant group effect at p = 0.049, ‡ indicates significant contrasts (1 day vs. 7 day withdrawal groups) at p = 0.018. The 1 day vs. control comparison was not significant following correction [p = 0.047].
Mean numbers of branches of order 1–6+ are shown in Figure 3B–C. Separate repeated measures MANOVAs with Group and Branch Order (1–6+) as factors were conducted for each region. There was no significant main effect of Group or Group X Branch Order interaction for either region [all ps > 0.21]. We note that there was a group main effect for fourth order branches in the core [F(2, 22) = 3.47, p = 0.049, ] which resulted from decreases in the 1 day group compared to the other two groups [both ps < 0.047], however, only the comparison of the 1 day and 7 day groups was significant following Bonferroni correction F(1, 15) = 7.005, p = 0.018, ]. Further, no other group effects were observed for the frequencies of specific branch orders in the core or shell [all ps > 0.08].
2.2. Spine density and morphology
Means for the density of five spine types (flipodial, thin, stubby, mushroom, multiheaded) within each region (core and shell) for each group are shown in Figure 4A-B. Separate repeated measures MANOVAs were conducted for each region of interest with Group and Spine Type as factors. There was a significant Group X Spine Type interaction for MSNs in the core [λ = 0.422, F(8, 38) = 2.56, p = 0.024, ], whereas the interaction for shell MSNs was not significant [p = 0.98]. Separate univariate ANOVAs for each spine type within the core revealed significant group effects for the density of stubby [F(2, 22) = 3.80, p = 0.038, ] and mushroom spines [F(2, 22) = 3.54, p = 0.046, ]. None the analyses of simple effects survived Bonferroni correction, however, inspection of the individual means suggests a clear pattern of group comparisons. For mushroom spines the 1 day withdrawal group had higher values than the control group [F(1, 14) = 5.03, p = 0.042, ] and 7 day withdrawal group [F(1, 15) = 5.08, p = 0.04, ]. For stubby spines the 7 day withdrawal had higher values than the control group [F(1, 15) = 4.91, p = 0.043, ] and 1 day withdrawal group [F(1, 15) = 5.88, p = 0.028, ]. An additional post-hoc analysis was motivated by inspection of the means for stubby spines in the shell (Figure 4B), which suggested a similar pattern to that observed for the core. The 7 day withdrawal had higher values than the control group [F(1, 15) = 4.60, p = 0.049, ] and 1 day withdrawal group [F(1, 15) = 6.41, p = 0.023, ], however, only the latter was significant following Bonferroni correction.
FIGURE 4.
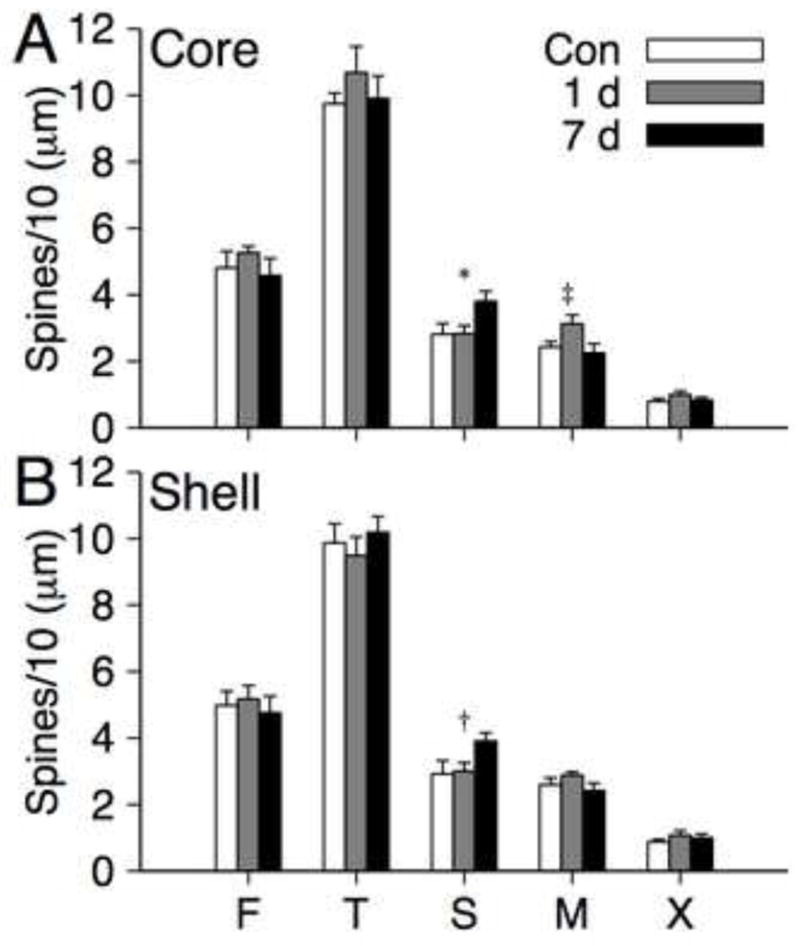
Mean (+SEM) density (spines per 10 μm) of filopodial (F), thin (T), stubby (S), mushroom (M) and multiheaded (X) spines on medium spiny neurons in the NAc core and shell for control (n = 8), 1 day withdrawal (n = 8) and 7 day withdrawal (n = 9) groups. * indicates a significant group effect for stubby spines in the core at p = 0.038 and contrasts [7 day > control, p = 0.043; 7 day > 1 day, p = 0.028]. ‡ indicates a significant group effect for mushroom spines at p = .046 and contrasts [1 day > control, p = 0.042; 7 day > 1 day, p = 0.04]. † indicates a contrast for stubby spines in the shell [7 day > control, p = 0.049; 7 day > 1 day, p = 0.023]. Only the latter contrast was significant following correction.
3. General Discussion
Chronic intermittent exposure to and withdrawal from vaporized ethanol at levels that produce physical dependence yielded several effects on the dendritic morphology of medium spiny neurons (MSNs) in the nucleus accumbens (NAc). Modest, non-significant reductions in dendritic length and branching on MSNs were observed in the NAc shell and core 24 hours following cessation of ethanol exposure. In contrast, at 7 days of withdrawal, dendritic length and branching on NAc core MSNs increased to levels comparable to or greater than that of control animals, whereas dendritic length and branching on shell MSNs remained comparable to the reduced levels observed following 1 day of withdrawal. Quantification of specific spine morphologies at terminal segments revealed increases in the density of mushroom spines on core MSNs following 1 day of withdrawal and increases in the density of stubby spines on core and shell MSNs at 7 days of withdrawal, however, there were no effects of ethanol exposure and cessation on overall spine density. These observations indicate that alterations in dendritic length, branching and spine density in the NAc following ethanol exposure vary as a function of region (core v. shell) and time since cessation of ethanol exposure. Considering that goal-directed behavior (Corbit, Muir, & Balleine, 2001; Ikemoto & Panksepp, 1999; Parkinson, Willoughby, Robbins, & Everitt, 2000) and drug craving (Robinson & Berridge, 2001) have been closely linked with the NAc, the modifications of dendritic structure following ethanol exposure and withdrawal observed here may contribute to important features of withdrawal and drug-seeking.
At 1 day of ethanol withdrawal there were non-significant reductions (~5%–10%) in dendritic length and branching in the NAc shell and core as well as a significant increase in mushroom spines in the core. These alterations occurred in the context of no changes in overall spine density measured at terminal segments in either the core or shell. Although spine density at terminals was not altered, the numerical reductions in dendritic length indicate an overall numerical reduction in spines on MSNs throughout the NAc. Because dendritic spines represent the primary sites of excitatory synaptic connections these changes suggest a modest reduction in the overall amount excitatory synaptic space on NAc MSNs. However, ethanol exposure and dependence have been shown to increase extracellular glutamate levels in the nucleus accumbens (Ding et al., 2013; Griffin, Haun, Hazelbaker, Ramachandra, & Becker, 2014) and to enhance glutamatergic synaptic transmission in a number of brain regions (Christian, Alexander, Diaz, & McCool, 2013; Faingold, Li, & Evans, 2000; Kash, Baucum, Conrad, Colbran, & Winder, 2009; Roberto et al., 2004). The overall loss of spines following 1 day of withdrawal may, therefore, represent ethanol-dependent pruning of spines such that the most active synapses are preserved by ethanol exposure. Because mushroom spines are the most mature and stable types of spine, followed by stubby, thin and filopodial (Hering & Sheng, 2001; Kasai, Matsuzaki, Noguchi, Yasumatsu, & Nakahara, 2003), the increased density of mushroom spines in the NAc core is consistent with this possibility. Further, mushroom spines are associated with high post-synaptic densities (Konur, Rabinowitz, Fenstermaker, & Yuste, 2003) and appear to be the exclusive site of the spine apparatus (Spacek & Harris, 1997), which is implicated in synaptic plasticity (Segal, Vlachos, & Korkotian, 2010). This suggests that the changes observed here may be relevant to withdrawal-related plasticity in comparatively stable dendritic spines. Interpretation of the results at 1 day of withdrawal is, however, not straightforward as disentangling whether the outcomes reflect the direct effects of ethanol exposure, withdrawal, or a combination of both is not possible (see also, Zhou et al., 2007). Future studies to evaluate dendritic morphology and spine density immediately after ethanol is removed are needed to address this issue.
The present findings for 1 day withdrawal can be compared with the results of Zhou et al. (2007). These authors examined the density of dendritic spines in the NAc core and shell in male inbred alcohol preferring (P) rats that voluntarily consumed ethanol for 14 weeks or experienced repeated bouts of deprivation during the final 8 weeks of the 14 week drinking protocol. As in the 1 day withdrawal period utilized here, the brains were extracted at 24 hours following the final exposure session. Spine density was significantly reduced in the shell following repeated deprivation, but was also numerically reduced following chronic exposure. Reductions in spine density in the core were limited to the chronic exposure condition. Zhou et al. (2007) also observed increases in “large” spines in both regions, primarily for the repeated deprivation protocol, consistent with the observations of increased mushroom spines in the NAc core observed here. The lack of comparable effects in the shell across the two studies could be related to several methodological factors including the exposure method, rat strain/line, and the dose and duration of exposure.
Among our most intriguing findings were the distinct effects of 7 days of withdrawal following chronic ethanol exposure on dendritic length and branching in the NAc shell and core. Dendritic length was significantly greater in the core than shell, with length in the core reaching levels greater than that observed in the control group. Inspection of Figure 2B suggests that the increased dendritic length in the core results from increases throughout the dendritic field. In contrast, dendritic branching appears to have returned to control levels by day 7. Inspection of Figure 3B, however, suggests differences between 7 day and control groups, with increased branching observed for higher order (4–6+) branches and numerically lower values for lower order branches relative to the control group. These observations are consistent with a dynamic reconfiguration of the dendritic field on shell MSNs involving emergence of new dendritic growth further from the soma and slight reductions of lower order branches more proximal to the soma. There was also an increase in less mature, stubby spines observed in both regions indicating that both regions of the NAc undergo continuous structural modifications throughout the 7 days of withdrawal. The functions associated with stubby spines are not well understood and are subject to disagreement, however, synapse formation occurs less frequently in them compared to mushroom spines (Fiala, Feinberg, Popov, & Harris, 1998; Harris, 1999) and the lack of a distinctive head has been taken to indicate reduced compartmentalization of Ca2+ signaling from the dendrite (Nimchinsky, Sabatini, & Svoboda, 2002). With respect to ethanol exposure, increases in brain-derived neurotrophic factor (BDNF) in the dorsal striatum have been observed (Logrip, Janak, & Ron, 2008; McGough et al., 2004) which comprises part of a proposed homeostatic process that can break down with extensive exposure (Logrip, Janak, & Ron, 2009). That BDNF exposure is associated with increases in stubby spines (Swanger, Yao, Gross, & Bassell, 2011) suggests a possible link between ethanol exposure, BDNF and alterations in stubby spines, however, firmly establishing such a relationship will require additional studies. Although there were no major ethanol-related changes in overall dendritic spine density observed at terminal segments of MSNs for any withdrawal period, the increased dendritic length in the context of unaltered spine density observed in NAc core MSNs at 7 days of withdrawal indicate an overall increase in dendritic spines.
Understanding the functional implications of the pattern of changes observed here will require additional studies, however, several possibilities are worth discussing. Regarding the increase in dendritic length and mushroom spine density in the core relative to the shell following 7 days of withdrawal, it is tempting to speculate that these alterations could contribute to drug craving and seeking during withdrawal. Baum et al. (2006) observed increased glutamate levels in the NAc core within 1 day of withdrawal, which may contribute to the early stages of withdrawal and may be a precursor to subsequent enhancements in dendritic length and mushroom spines reported here. The NAc shell and core also differentially contribute to cocaine craving during withdrawal, with the core contributing more substantially to craving during the early stages of abstinence (~1 day) and the shell contributing more substantially to craving during later stages of abstinence (~90 days)(Li et al., 2013). Purgianto et al. (2013) also found that Ca2+ permeable AMPA receptors in the NAc core were increased for up to 440 days following cocaine exposure. Such alterations should increase baseline excitatory drive and contribute to increased activity in NAc core MSNs, thereby enhancing the associated behavioral and cognitive functions of this circuitry. The NAc core is a constituent of a broader circuit implicated in the reinstatement of cocaine self administration (McFarland & Kalivas, 2001), thus, the comparative modifications of NAc core and shell neurons at 7 days of withdrawal may contribute to drug craving and seeking during withdrawal and abstinence. Of course, future studies are needed to firmly establish the relationships between dendritic modifications, neural function, and behavioral outcomes. The present findings are at least suggestive that changes in spine density during protracted withdrawal may represent one of the mechanisms contributing to drug seeking during abstinence.
The decreases in dendritic length and branching in the NAc core at 1 day of withdrawal and in the NAc shell at 1 and 7 days of withdrawal are similar to reductions observed in the NAc in a range of ethanol exposure protocols including voluntary drinking in adulthood (Zhou et al., 2007) and moderate prenatal ethanol exposure (Rice et al., 2012). Further, decreases in dendritic length and branching following ethanol exposure have been observed in diverse brain regions including the agranular insular cortex (D. A. Hamilton et al., 2010), medial prefrontal cortex (G. F. Hamilton, Whitcher, & Klintsova, 2010; Whitcher & Klintsova, 2008), visual cortex (Cui et al., 2010), amygdala (Moonat, Sakharkar, Zhang, & Pandey, 2011), the CA1 subfield of the hippocampus (McMullen et al., 1984) and dorsal striatum (Susick, Lowing, Provenzano, Hildebrandt, & Conti, 2014). Increases in spine density have been observed in the basolateral amygdala (Cullen, Burne, Lavidis, & Moritz, 2013), medial prefrontal cortex in the context of reduced dendritic morphology (G. F. Hamilton et al., 2010), and the CA1 of rats after extended ethanol deprivation (McMullen et al., 1984). The results of the present study are closely aligned with the outcomes observed by McMullen et al. (1984) in which trends of reduced branching in CA1 in the short term were followed by increased length in the long term following cessation. Thus, the NAc is not the only region affected ethanol exposure and withdrawal, but may contribute to unique functional consequences associated with ethanol. The pattern of changes observed at 1 day and 7 days of withdrawal in the NAc core are also reminiscent of those reported by Teskey and colleagues (Teskey, Hutchinson, & Kolb, 1999, 2001) in anterior neocortex (Fr1) following kindling. These authors observed initial decreases in branching, length and spine density of layer III pyramidal neurons at 1 day post-kindling followed by increases in the same measures at 21 days post-kindling. Thus, the overall pattern of dendritic modifications (decreases followed by increases) may be observed in affected neural populations following various forms of experience.
The present observations with chronic ethanol exposure and withdrawal can be dissociated from the effects of other classes of drugs on dendritic morphology, particularly psychomotor stimulants that have been shown to elicit increases in dendritic length, branching and spine density on NAc MSNs that are present early following cessation of exposure (Brown & Kolb, 2001; D. A. Hamilton & Kolb, 2005; Kolb et al., 2003) and can persist long after cessation (Robinson & Kolb, 1997). Increases in dendritic length and branching of NAc MSNs has been observed following exposure to THC (Kolb et al., 2006). It is noteworthy that exposure to and withdrawal from morphine yield increases or decreases in spine density in the NAc shell, respectively, (Robinson & Kolb, 2004; Spiga, Puddu, Pisano, & Diana, 2005), whereas withdrawal has been associated with increases in filopodial and mature spines in the NAc (Pal & Das, 2013). Thus, the pattern of observations reported here with ethanol can be distinguished from the effects of a broad range of other psychoactive drugs.
It is important to note that the combination of Golgi-Cox staining and the use of the camera lucida technique for neuron tracing and classification of spines is based on quantification in multiple, contiguous two-dimensional planes. As a result, some percentage of spines, branch points and dendritic segments will be obscured within the plane selected for visualization, and the absolute numbers of dendritic branches, length of dendritic segments, and spine numbers will, therefore, be underestimated. Of course, the underestimation of absolute numbers should not systematically vary between treatment conditions. New technologies such as delivery of diolistics and microinjection of fluorescent agents (see Russo et al. (2010) for review) can be combined with confocal microscopy to provide three-dimensional images of overall neuron morphology and spine morphology and number. More fine-grained assessments of synaptic structure including synaptic alterations related beyond spine morphology would require the use of electron microscopy (Kuwajima, Spacek, & Harris, 2013). Future research using these techniques will be needed to determine how the morphological characteristics of specific spine types on NAc MSNs are altered following ethanol exposure and withdrawal, and how these changes differ from other drugs. For example, Shen, Moussawi, Zhou, Toda, and Kalivas (2011) analyzed diolistic-labeled neurons in the NAc core and found spine enlargements during heroin withdrawal and relapse. Gipson, Kupchik, et al. (2013) observed similar results with cocaine withdrawal and relapse. Thus, evaluation of spine size following chronic intermittent ethanol exposure and withdrawal might provide a promising avenue of research for identifying alterations in spines that hold functional significance for ethanol seeking and self-administration.
Several additional, important questions for future research are motivated by the present observations. Perhaps the most prominent of these is to determine the physiological and related behavioral outcomes associated with the patterns of alterations in dendritic morphology and spine density that follow ethanol exposure and withdrawal. Electrophysiological assessments of glutamatergic synaptic transmission in the basolateral amygdala have been reported following ethanol exposure and cessation (Christian et al., 2013) which may hold significance for withdrawal-anxiety and related symptoms (McCool, 2011; McCool, Christian, Diaz, & Lack, 2010). Thus, an obvious question concerns whether similar relationships between electrophysiological alterations and behavioral processes, including ethanol consumption, can be established for NAc shell and core MSNs. Future studies should also expand upon the methods and parameters of the present study. For example, the present study examined only male rats that were passively exposed to ethanol, thus, future studies to address the generality of the present observations to females and other exposure methods are needed. Further, we only examined outcomes two withdrawal durations (1 day and 7 days), however, a major open question concerns the persistence of the changes observed in the NAc core MSNs. Considering that morphological changes in NAc MSNs can be short-lived or last for the life of the animal (Robinson & Becker, 1986), establishing the persistence of these changes will be important for characterizing the potential significance and long-term consequences of ethanol-related structural modifications of NAc MSNs. Examination of changes at intermediate time points (1–6 days of withdrawal) is also needed to establish the earliest time point following cessation at which increases in NAc core MSNs can be detected. We also note that behavioral outcomes were not assessed in the present study to avoid potentially complicating interpretation of the results, as behavioral testing/experience could influence dendritic morphology and spine density. Passive ethanol exposure via vapor chambers as performed here has repeatedly yielded behavioral signs of withdrawal and physical dependence (Lack et al., 2007; Macey et al., 1996; Roberts et al., 1996) as well as increased ethanol seeking/consumption (Buck et al., 2014; Roberts et al., 2000) and anxiety (Lack et al., 2007; Rassnick et al., 1993; Valdez et al., 2004). Whether these behavioral outcomes are related to the specific patterns of morphological alteration observed here represents an important topic for future research.
In summary, chronic passive exposure to ethanol and withdrawal were associated with several distinct forms of structural alteration on MSNs in the NAc core and shell. The most robust ethanol-related differences were the distinct structural alterations observed in the NAc core relative to the shell after 1 day or 7 days of withdrawal. There were also dissociable alterations in the density of specific spine types, with increases in mushroom spines in the core at 1 day of withdrawal and increases in less mature, stubby spines in the shell and core at 7 days of withdrawal. The present data join a larger body of experimental data indicating that the NAc shell and core can be dissociated (Corbit et al., 2001; Parkinson et al., 2000; Yin, 2008) and motivate future research to identify the functional and behavioral outcomes associated with these morphological alterations, which may be important for understanding the neurobiology of ethanol withdrawal and related processes.
2. Methods and Materials
2.1. Subjects
Male Sprague Dawley rats (120–140 g) were obtained from Harlan Laboratories at the beginning of the experiments described in the following section. A total of 25 rats were assigned to one of three conditions described below. All animals were group-housed in an animal care facility at 23C° with a 12-h light/dark cycle and given food and water ad libitum. Rats were weighed daily to ensure that at least 80% of their free-feeding weight was maintained during vapor chamber ethanol exposure. All animal procedures were performed in accordance with protocols approved by the Wake Forest School of Medicine Animal Care and Use Committee and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Brain tissue was transferred to the University of New Mexico for analysis as approved by the Institutional Animal Care and Use Committee.
2.2. Chronic ethanol exposure
Ethanol exposure was accomplished via an ethanol vapor chamber similar to that used in other studies (Christian et al., 2013). Briefly, animals in their home cages were placed into airtight, Plexiglas enclosures and exposed to either ethanol vapor (~37 mg/L) or room air during the light cycle (12 h/day) for 10 consecutive days. Tail blood was taken from sentinel animals periodically during the exposure to monitor blood ethanol concentration. This exposure produces blood-ethanol concentrations in the range of 150–200mg/dL. Animals were then euthanized with an overdose of sodium pentobarbital followed by a transcardial perfusion (0.9% saline) resulting in exsanguination either 1 day (precisely 24 hours) or 7 days after the last ethanol exposure. Control animals were euthanized two or three days after the final exposure to air. Whole brains were extracted for Golgi-Cox staining and analysis.
2.3. Golgi-Cox Staining and Analysis
Brains were immersed in Golgi-Cox solution (Glaser & van der Loos, 1981) for 14 days and subsequently immersed in 30% (wt/vol) sucrose for at least 3 days. The brains were then cut in coronal sections (200 μm thick) on a vibrating microtome, mounted on slides subbed in 2% gelatin, and stained according to the procedures described by (Gibb & Kolb, 1998).
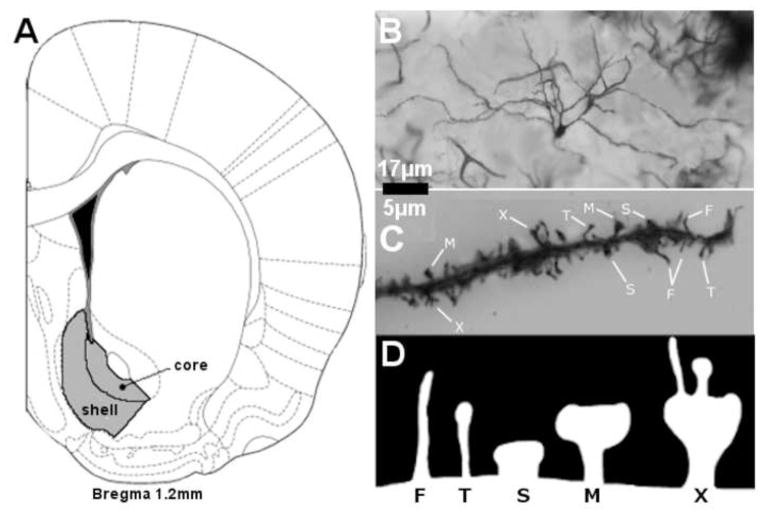
Medium spiny neurons (MSNs) from the NAc shell and core were selected for analysis (see Figure 1A). A representative stained MSN is illustrated in Figure 1B. The brain regions of interest were first identified at low power (200× magnification), and neurons were traced at 500× (final magnification) using the camera lucida technique on an Olympus light microscope (Model BX51) equipped with a drawing attachment. Sampling for all regions included sections ranging from 1.0 to 1.7 mm anterior to Bregma. Selection was limited to neurons that were not obscured by stain precipitate, blood vessels, astrocytes, or other artifacts, and had intact dendritic fields that were well impregnated and visible within a single section. All tracings were performed by an experimenter who was blind to exposure conditions. Ten neurons were drawn for each region of interest (5 per hemisphere) for each animal, totaling twenty samples per brain. Outcome measures were averaged for each rat, collapsing across hemispheres.
FIGURE 1.
A) Regions from which MSNs in the nucleus accumbens shell (AcbSh) and core (AcbC) are indicated in grey. B) A Golgi-Cox stained MSN illustrating the quality of the staining. C) Representative examples of filopodial (F), thin (T), stubby (S), mushroom (M) and multi-headed (X) spines.
Dendritic branching was measured by counting bifurcations for each dendrite (Coleman & Riesen, 1968). Dendritic segments prior to the first bifurcation from the soma were designated as first-order branches and branch order was incremented by 1 for each subsequent bifurcation on a given dendritic branch. The number of first-through sixth-order (and higher) branches were quantified and an estimate of total branches was determined from these values. Dendritic length was measured using a Sholl analysis of ring intersections (Sholl, 1981). A series of concentric rings at 20 μm increments (calibrated to the final magnification of 500×) printed on a transparency was centered over the soma. The total number of intersections between each ring and dendritic branches was counted and converted to estimates of dendritic length as a function of distance from the soma (i.e., for each 20 μm segment) and overall dendritic length.
Spine density was measured by tracing terminal tips (>40 μm in length) of third order branches or higher at high power (2500× final magnification). Spines along the sampled dendritic segment were categorized as filopodial, thin, stubby, mushroom, or multi-headed (see Figure 1C–1D) based on characteristic morphological differences including the combination of spine length, width, the presence of a head, and the relative width of the head (Hering & Sheng, 2001; Nimchinsky et al., 2002). Filopodial spines were those that were comparatively narrow, long, and without a distinctive head. Thin spines were those that were comparatively wider than fliopodial and had a clear head. Stubby spines were those that were comparatively short and wide without a clear head. Mushroom spines were those that were clearly had a comparatively wide head. Multi-headed spines were categorized based on unambiguous presence of 2 or more distinct segments (heads). Representative examples of each spine type are illustrated in Figure 1C–1D. A total of ten segments were sampled per region (NAc core and shell). Selected samples were unobstructed by other dendrites, glial cells, blood vessels or other artifacts.
2.4. Statistical Analyses
All analyses were performed using SPSS version 20 (for Macintosh). For each measure mixed model analyses of variance (ANOVAs) were conducted with group (control, 1 day or 7 day) as a between-subject factor and region as a within-subject factor, with an additional within-subject measure for branching (branch order) and length (distance from soma). For analyses involving dendritic segments, branch order or spine types the multivariate approach to repeated measures ANOVA (MANOVAs) was employed to protect against type I error. Subsequent separate ANOVAs evaluated group effects for individual dendritic segments, branch orders, and each spine type. Significant group main effects were then followed by simple effects analyses using Bonferroni correction. For all reported effects we report the precise p-value and associated effect sizes (partial eta squared, ).
Distinct patterns of dendritic changes on NAc core and shell MSNs during ethanol withdrawal
Length and branching of NAc core and shell MSNs decreased at 1 day of ethanol withdrawal
Length and branching of NAc core MSNs greater than shell MSNs at 7 days of ethanol withdrawal
Increase in mature, mushroom spines in the NAc core at 1 day of ethanol withdrawal
Increase in less stable stubby spines in the NAc core and shell at 7 days of withdrawal
Acknowledgments
We would like to thank Ms. Ann Chappell (WFSM) for her technical assistance with the ethanol vapor inhalation and animal perfusions. Funding provided by grants R01 AA019462 (DAH), R01 AA014445 (BAM), U01 AA020942 (BAM), and 5R25-GM060201
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barbier E, Houchi H, Warnault V, Pierrefiche O, Daoust M, Naassila M. Effects of Prenatal and Postnatal Maternal Ethanol on Offspring Response to Alcohol and Psychostimulants in Long Evans Rats. Neuroscience. 2009;161:427–440. doi: 10.1016/j.neuroscience.2009.03.076. [DOI] [PubMed] [Google Scholar]
- Barbier E, Pierrefiche O, Vaudry D, Vaudry H, Daoust M, Naassila M. Long-Term Alterations in Vulnerability to Addiction to Drugs of Abuse and in Brain Gene Expression after Early Life Ethanol Exposure. Neuropharmacology. 2008;55:1199–1211. doi: 10.1016/j.neuropharm.2008.07.030. [DOI] [PubMed] [Google Scholar]
- Baum SS, Huebner A, Krimphove M, Morgenstern R, Badawy AAB, Spies CD. Nicotine Stimulation on Extracellular Glutamate Levels in the Nucleus Accumbens of Ethanol-Withdrawn Rats in Vivo. Alcoholism-Clinical and Experimental Research. 2006;30:1414–1421. doi: 10.1111/j.1530-0277.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- Blanchard BA, Steindorf S, Wang S, Lefevre R, Mankes RF, Glick SD. Prenatal Ethanol Exposure Alters Ethanol-Induced Dopamine Release in Nucleus-Accumbens and Striatum in Male and Female Rats. Alcoholism-Clinical and Experimental Research. 1993;17:974–981. doi: 10.1111/j.1530-0277.1993.tb05651.x. [DOI] [PubMed] [Google Scholar]
- Brown RW, Kolb B. Nicotine Sensitization Increases Dendritic Length and Spine Density in the Nucleus Accumbens and Cingulate Cortex. Brain Research. 2001;899:94–100. doi: 10.1016/s0006-8993(01)02201-6. [DOI] [PubMed] [Google Scholar]
- Buck CL, Malavar JC, George O, Koob GF, Vendruscolo LF. Anticipatory 50 Khz Ultrasonic Vocalizations Are Associated with Escalated Alcohol Intake in Dependent Rats. Behavioural Brain Research. 2014;271:171–176. doi: 10.1016/j.bbr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Schairer WW, Janak PH. Separable Roles of the Nucleus Accumbens Core and Shell in Context- and Cue-Induced Alcohol-Seeking. Neuropsychopharmacology. 2010;35:783–791. doi: 10.1038/npp.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian DT, Alexander NJ, Diaz MR, McCool BA. Thalamic Glutamatergic Afferents into the Rat Basolateral Amygdala Exhibit Increased Presynaptic Glutamate Function Following Withdrawal from Chronic Intermittent Ethanol. Neuropharmacology. 2013;65:134–142. doi: 10.1016/j.neuropharm.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian DT, Alexander NJ, Diaz MR, Robinson S, McCool BA. Chronic Intermittent Ethanol and Withdrawal Differentially Modulate Basolateral Amygdala Ampa-Type Glutamate Receptor Function and Trafficking. Neuropharmacology. 2012;62:2430–2439. doi: 10.1016/j.neuropharm.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman PD, Riesen AH. Evironmental Effects on Cortical Dendritic Fields. I. Rearing in the Dark. Journal of Anatomy. 1968;102:363–374. [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. The Role of the Nucleus Accumbens in Instrumental Conditioning: Evidence of a Functional Dissociation between Accumbens Core and Shell. Journal of Neuroscience. 2001;21:3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui ZJ, Zhao KB, Zhao HJ, Yu DM, Niu YL, Zhang JS, Deng JB. Prenatal Alcohol Exposure Induces Long-Term Changes in Dendritic Spines and Synapses in the Mouse Visual Cortex. Alcohol and Alcoholism. 2010;45:312–319. doi: 10.1093/alcalc/agq036. [DOI] [PubMed] [Google Scholar]
- Cullen CL, Burne THJ, Lavidis NA, Moritz KM. Low Dose Prenatal Ethanol Exposure Induces Anxiety-Like Behaviour and Alters Dendritic Morphology in the Basolateral Amygdala of Rat Offspring. Plos One. 2013:8. doi: 10.1371/journal.pone.0054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus Accumbens Shell and Core Dopamine: Differential Role in Behavior and Addiction. Behavioural Brain Research. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward System and Addiction: What Dopamine Does and Doesn’t Do (Vol 7, Pg 69, 2007) Current Opinion in Pharmacology. 2007;7:233–233. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Diaz MR, Christian DT, Anderson NJ, McCool BA. Chronic Ethanol and Withdrawal Differentially Modulate Lateral/Basolateral Amygdala Paracapsular and Local Gabaergic Synapses. Journal of Pharmacology and Experimental Therapeutics. 2011;337:162–170. doi: 10.1124/jpet.110.177121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, Bailey JA, Lahiri DK, McBride WJ. Alcohol Drinking and Deprivation Alter Basal Extracellular Glutamate Concentrations and Clearance in the Mesolimbic System of Alcohol-Preferring (P) Rats. Addiction Biology. 2013;18:297–306. doi: 10.1111/adb.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural Systems of Reinforcement for Drug Addiction: From Actions to Habits to Compulsion. Nature Neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Faingold C, Li Y, Evans MS. Decreased Gaba and Increased Glutamate Receptor-Mediated Activity on Inferior Colliculus Neurons in Vitro Are Associated with Susceptibility to Ethanol Withdrawal Seizures. Brain Research. 2000;868:287–295. doi: 10.1016/s0006-8993(00)02342-8. [DOI] [PubMed] [Google Scholar]
- Ferko AP, Bobyock E. Induction of Physical-Dependence in Rats by Ethanol Inhalation without Use of Pyrazole. Toxicology and Applied Pharmacology. 1977;40:269–276. doi: 10.1016/0041-008x(77)90097-7. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis Via Dendritic Filopodia in Developing Hippocampal Area CA1. Journal of Neuroscience. 1998;18:8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost DO, Page SC, Carroll C, Kolb B. Early Exposure to Haloperidol or Olanzapine Induces Long-Term Alterations of Dendritic Form. Synapse. 2010;64:191–199. doi: 10.1002/syn.20715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb R, Kolb B. A Method for Vibratome Sectioning of Golgi-Cox Stained Whole Rat Brain. Journal of Neuroscience Methods. 1998;79:1–4. doi: 10.1016/s0165-0270(97)00163-5. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse Induced by Cues Predicting Cocaine Depends on Rapid, Transient Synaptic Potentiation. Neuron. 2013;77:867–872. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith ACW, Stankeviciute N, Hensley-Simon ME, Kalivas PW. Reinstatement of Nicotine Seeking Is Mediated by Glutamatergic Plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser EM, van der Loos H. Analysis of Thick Brain Sections by Obverse-Reverse Computer Microscopy: Application of a New, High Clarity Golgi-Nissl Stain. Journal of Neuroscience Methods. 1981;4:117–125. doi: 10.1016/0165-0270(81)90045-5. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The Role of Mesolimbic Dopamine in the Development and Maintenance of Ethanol Reinforcement. Pharmacology & Therapeutics. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Griffin WC, III, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC. Increased Extracellular Glutamate in the Nucleus Accumbens Promotes Excessive Ethanol Drinking in Ethanol Dependent Mice. Neuropsychopharmacology. 2014;39:707–717. doi: 10.1038/npp.2013.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Rice JP, Johnson TE, Candelaria-Cook FT, Maes LI, Savage DD. Prenatal Exposure to Moderate Levels of Ethanol Alters Social Behavior in Adult Rats: Relationship to Structural Plasticity and Immediate Early Gene Expression in Frontal Cortex. Behavioural Brain Research. 2010;207:290–304. doi: 10.1016/j.bbr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DA, Kolb B. Differential Effects of Nicotine and Complex Housing on Subsequent Experience-Dependent Structural Plasticity in the Nucleus Accumbens. Behavioral Neuroscience. 2005;119:355–365. doi: 10.1037/0735-7044.119.2.355. [DOI] [PubMed] [Google Scholar]
- Hamilton GF, Whitcher LT, Klintsova AY. Postnatal Binge-Like Alcohol Exposure Decreases Dendritic Complexity While Increasing the Density of Mature Spines in Mpfc Layer Ii/Iii Pyramidal Neurons. Synapse. 2010;64:127–135. doi: 10.1002/syn.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM. Structure, Development, and Plasticity of Dendritic Spines. Current Opinion in Neurobiology. 1999;9:343–348. doi: 10.1016/s0959-4388(99)80050-6. [DOI] [PubMed] [Google Scholar]
- Hering H, Sheng M. Dendritic Spines: Structure, Dynamics and Regulation. Nature Reviews Neuroscience. 2001;2:880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- Horsley RR, Norman C, Cassaday HJ. Lesions of the Nucleus Accumbens Shell Can Reduce Activity in the Elevated Plus-Maze. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2007;31:906–914. doi: 10.1016/j.pnpbp.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The Role of Nucleus Accumbens Dopamine in Motivated Behavior: A Unifying Interpretation with Special Reference to Reward-Seeking. Brain Research Reviews. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-Stability-Function Relationships of Dendritic Spines. Trends in Neurosciences. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Kash TL, Baucum AJ, II, Conrad KL, Colbran RJ, Winder DG. Alcohol Exposure Alters Nmdar Function in the Bed Nucleus of the Stria Terminalis. Neuropsychopharmacology. 2009;34:2420–2429. doi: 10.1038/npp.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Li YL, Samaha AN, Robinson TE. Amphetamine or Cocaine Limits the Ability of Later Experience to Promote Structural Plasticity in the Neocortex and Nucleus Accumbens. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10523–10528. doi: 10.1073/pnas.1834271100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Limebeer CL, Parker LA. Chronic Treatment with Delta-9-Tetrahydrocannabinol Alters the Structure of Neurons in the Nucleus Accumbens Shell and Medial Prefrontal Cortex of Rats. Synapse. 2006;60:429–436. doi: 10.1002/syn.20313. [DOI] [PubMed] [Google Scholar]
- Konur S, Rabinowitz D, Fenstermaker VL, Yuste R. Systematic Regulation of Spine Sizes and Densities in Pyramidal Neurons. Journal of Neurobiology. 2003;56:95–112. doi: 10.1002/neu.10229. [DOI] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, George O. Addiction as a Stress Surfeit Disorder. Neuropharmacology. 2014;76:370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of Addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima M, Spacek J, Harris KM. Beyond Counts and Shapes: Studying Pathology of Dendritic Spines in the Context of the Surrounding Neuropil through Serial Section Electron Microscopy. Neuroscience. 2013;251:75–89. doi: 10.1016/j.neuroscience.2012.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic Ethanol and Withdrawal Differentially Modulate Pre- and Postsynaptic Function at Glutamatergic Synapses in Rat Basolateral Amygdala. Journal of Neurophysiology. 2007;98:3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence RC, Otero NKH, Kelly SJ. Selective Effects of Perinatal Ethanol Exposure in Medial Prefrontal Cortex and Nucleus Accumbens. Neurotoxicology and Teratology. 2012;34:128–135. doi: 10.1016/j.ntt.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, DeJoseph MR, Urban JH, Bahi A, Dreyer JL, Meredith GE, Wolf ME. Different Roles of Bdnf in Nucleus Accumbens Core Versus Shell During the Incubation of Cue-Induced Cocaine Craving and Its Long-Term Maintenance. Journal of Neuroscience. 2013;33:1130–1142. doi: 10.1523/JNEUROSCI.3082-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Dynorphin Is a Downstream Effector of Striatal Bdnf Regulation of Ethanol Intake. FASEB Journal. 2008;22:2393–2404. doi: 10.1096/fj.07-099135. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Escalating Ethanol Intake Is Associated with Altered Corticostriatal Bdnf Expression. Journal of Neurochemistry. 2009;109:1459–1468. doi: 10.1111/j.1471-4159.2009.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey DJ, Schulteis G, Heinrichs SC, Koob GF. Time-Dependent Quantifiable Withdrawal from Ethanol in the Rat: Effect of Method of Dependence Induction. Alcohol. 1996;13:163–170. doi: 10.1016/0741-8329(95)02030-6. [DOI] [PubMed] [Google Scholar]
- McCool BA. Ethanol Modulation of Synaptic Plasticity. Neuropharmacology. 2011;61:1097–1108. doi: 10.1016/j.neuropharm.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Christian DT, Diaz MR, Lack AK. Glutamate Plasticity in the Drunken Amygdala: The Making of an Anxious Synapse. In: Reilly MT, Lovinger DM, editors. Functional Plasticity and Genetic Variation: Insights into the Neurobiology of Alcoholism. Vol. 91. 2010. pp. 205–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The Circuitry Mediating Cocaine-Induced Reinstatement of Drug-Seeking Behavior. Journal of Neuroscience. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal Glutamate Release into the Core of the Nucleus Accumbens Mediates Cocaine-Induced Reinstatement of Drug-Seeking Behavior. Journal of Neuroscience. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough NNH, He DY, Logrip ML, Jeanblanc J, Phamluong K, Luong K, Ron D. Rack1 and Brain-Derived Neurotrophic Factor: A Homeostatic Pathway That Regulates Alcohol Addiction. Journal of Neuroscience. 2004;24:10542–10552. doi: 10.1523/JNEUROSCI.3714-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen PA, Stcyr JA, Carlen PL. Morphological Alterations in Rat CA1 Hippocampal Pyramidal Cell Dendrites Resulting from Chronic Ethanol-Consumption and Withdrawal. Journal of Comparative Neurology. 1984;225:111–118. doi: 10.1002/cne.902250112. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The Structural Basis for Mapping Behavior onto the Ventral Striatum and Its Subdivisions. Brain Structure & Function. 2008;213:17–27. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang HB, Pandey SC. The Role of Amygdaloid Brain-Derived Neurotrophic Factor, Activity-Regulated Cytoskeleton-Associated Protein and Dendritic Spines in Anxiety and Alcoholism. Addiction Biology. 2011;16:238–250. doi: 10.1111/j.1369-1600.2010.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky EA, Sabatini BL, Svoboda K. Structure and Function of Dendritic Spines. Annual Review of Physiology. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- Pal A, Das S. Chronic Morphine Exposure and Its Abstinence Alters Dendritic Spine Morphology and Upregulates Shankl. Neurochemistry International. 2013;62:956–964. doi: 10.1016/j.neuint.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Willoughby PJ, Robbins TW, Everitt BJ. Disconnection of the Anterior Cingulate Cortex and Nucleus Accumbens Core Impairs Pavlovian Approach Behavior: Further Evidence for Limbic Cortical-Ventral Striatopallidal Systems. Behavioral Neuroscience. 2000;114:42–63. [PubMed] [Google Scholar]
- Purgianto A, Scheyer AF, Loweth JA, Ford KA, Tseng KY, Wolf ME. Different Adaptations in Ampa Receptor Transmission in the Nucleus Accumbens after Short Vs Long Access Cocaine Self-Administration Regimens. Neuropsychopharmacology. 2013;38:1789–1797. doi: 10.1038/npp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a Corticotropin-Releasing Factor Antagonist into the Central Nucleus of the Amygdala Reverses Anxiogenic-Like Effects of Ethanol Withdrawal. Brain Research. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Rice JP, Suggs LE, Lusk AV, Parker MO, Candelaria-Cook FT, Akers KG, Hamilton DA. Effects of Exposure to Moderate Levels of Ethanol During Prenatal Brain Development on Dendritic Length, Branching, and Spine Density in the Nucleus Accumbens and Dorsal Striatum of Adult Rats. Alcohol. 2012;46:577–584. doi: 10.1016/j.alcohol.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristuccia RC, Spear LP. Sensitivity and Tolerance to Autonomic Effects of Ethanol in Adolescent and Adult Rats During Repeated Vapor Inhalation Sessions. Alcoholism-Clinical and Experimental Research. 2005;29:1809–1820. doi: 10.1097/01.alc.0000183010.72764.cd. [DOI] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and Chronic Ethanol Alter Glutamatergic Transmission in Rat Central Amygdala: An in Vitro and in Vivo Analysis. Journal of Neuroscience. 2004;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-Amygdala Muscimol Decreases Operant Ethanol Self-Administration in Dependent Rats. Alcoholism-Clinical and Experimental Research. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive Ethanol Drinking Following a History of Dependence: Animal Model of Allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring Changes in Brain and Behavior Produced by Chronic Amphetamine Administration: A Review and Evaluation of Animal Models of Amphetamine Psychosis. Brain Research. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The Neural Basis of Drug Craving - an Incentive-Sensitization Theory of Addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-Sensitization and Addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent Structural Modifications in Nucleus Accumbens and Prefrontal Cortex Neurons Produced by Previous Experience with Amphetamine. Journal of Neuroscience. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the Morphology of Dendrites and Dendritic Spines in the Nucleus Accumbens and Prefrontal Cortex Following Repeated Treatment with Amphetamine or Cocaine. European Journal of Neuroscience. 1999a;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Morphine Alters the Structure of Neurons in the Nucleus Accumbens and Neocortex of Rats. Synapse. 1999b;33:160–162. doi: 10.1002/(SICI)1098-2396(199908)33:2<160::AID-SYN6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural Plasticity Associated with Exposure to Drugs of Abuse. Neuropharmacology. 2004;47:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Romero AM, Renau-Piqueras J, Marin MP, Timoneda J, Berciano MT, Lafarga M, Esteban-Pretel G. Chronic Alcohol Alters Dendritic Spine Development in Neurons in Primary Culture. Neurotoxicity Research. 2013;24:532–548. doi: 10.1007/s12640-013-9409-0. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The Addicted Synapse: Mechanisms of Synaptic and Structural Plasticity in Nucleus Accumbens. Trends in Neurosciences. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M, Vlachos A, Korkotian E. The Spine Apparatus, Synaptopodin, and Dendritic Spine Plasticity. The Neuroscientist. 2010;16:125–131. doi: 10.1177/1073858409355829. [DOI] [PubMed] [Google Scholar]
- Shen HW, Moussawi K, Zhou WH, Toda S, Kalivas PW. Heroin Relapse Requires Long-Term Potentiation-Like Plasticity Mediated by Nmda2b-Containing Receptors. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19407–19412. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl DA. The Organization of the Cerebral Cortex. London: 1981. [Google Scholar]
- Spacek J, Harris KM. Three-Dimensional Organization of Smooth Endoplasmic Reticulum in Hippocampal CA1 Dendrites and Dendritic Spines of the Immature and Mature Rat. Journal of Neuroscience. 1997;17:190–203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga S, Puddu MC, Pisano M, Diana M. Morphine Withdrawal-Induced Morphological Changes in the Nucleus Accumbens. European Journal of Neuroscience. 2005;22:2332–2340. doi: 10.1111/j.1460-9568.2005.04416.x. [DOI] [PubMed] [Google Scholar]
- Spiga S, Talani G, Mulas G, Licheri V, Fois GR, Muggironi G, Diana M. Hampered Long-Term Depression and Thin Spine Loss in the Nucleus Accumbens of Ethanol-Dependent Rats. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E3745–E3754. doi: 10.1073/pnas.1406768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susick LL, Lowing JL, Provenzano AM, Hildebrandt CC, Conti AC. Postnatal Ethanol Exposure Simplifies the Dendritic Morphology of Medium Spiny Neurons Independently of Adenylyl Cyclase 1 and 8 Activity in Mice. Alcoholism: Clinical and Experimental Research. 2014;38:1339–1346. doi: 10.1111/acer.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanger SA, Yao X, Gross C, Bassell GJ. Automated 4d Analysis of Dendritic Spine Morphology: Applications to Stimulus-Induced Spine Remodeling and Pharmacological Rescue in a Disease Model. Molecular Brain. 2011:4. doi: 10.1186/1756-6606-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teskey GC, Hutchinson JE, Kolb B. Sex Differences in Cortical Plasticity and Behavior Following Anterior Cortical Kindling in Rats. Cerebral Cortex. 1999;9:675–682. doi: 10.1093/cercor/9.7.675. [DOI] [PubMed] [Google Scholar]
- Teskey GC, Hutchinson JE, Kolb B. Cortical Layer Iii Pyramidal Dendritic Morphology Normalizes within 3 Weeks after Kindling and Is Dissociated from Kindling-Induced Potentiation. Brain Research. 2001;911:125–133. doi: 10.1016/s0006-8993(01)02702-0. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Sabino V, Koob GF. Increased Anxiety-Like Behavior and Ethanol Self-Administration in Dependent Rats: Reversal Via Corticotropin-Releasing Factor-2 Receptor Activation. Alcoholism-Clinical and Experimental Research. 2004;28:865–872. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- Whitcher LT, Klintsova AY. Postnatal Binge-Like Alcohol Exposure Reduces Spine Density without Affecting Dendritic Morphology in Rat Mpfc. Synapse. 2008;62:566–573. doi: 10.1002/syn.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH. From Actions to Habits Neuroadaptations Leading to Dependence. Alcohol Research & Health. 2008;31:340–344. [PMC free article] [PubMed] [Google Scholar]
- Zhou FC, Anthony B, Dunn KW, Lindquist WB, Xu ZC, Deng P. Chronic Alcohol Drinking Alters Neuronal Dendritic Spines in the Brain Reward Center Nucleus Accumbens. Brain Research. 2007;1134:148–161. doi: 10.1016/j.brainres.2006.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]