Abstract
Neuromuscular blocking agents are used to facilitate tracheal intubation in patients undergoing ambulatory surgery. The use of high-dose neuromuscular blocking agents to achieve muscle paralysis throughout the case carries an increased risk of residual post-operative neuromuscular blockade, which is associated with increased respiratory morbidity. Visually monitoring the train-of-four (TOF) fade is not sensitive enough to detect a TOF fade between 0.4 and 0.9. A ratio <0.9 indicates inadequate recovery. Quantitative neuromuscular transmission monitoring (e.g., acceleromyography) should be used to exclude residual neuromuscular blockade at the end of the case. Residual neuromuscular blockade needs to be reversed with neostigmine, but it’s use must be guided by TOF monitoring results since deep block cannot be reversed, and neostigmine administration after complete recovery of the TOF-ratio can induce muscle weakness. The development and use of new selectively binding reversal agents (sugammadex and calabadion) warrants reevaluation of this area of clinical practice.
Keywords: NMBA, Ambulatory surgery, Residual paralysis, PORC, Respiratory complications, Neostigmine, Sugammadex, Calabadion
Introduction
In the USA, it is estimated that over 60 % of surgical and non-surgical procedures are now performed as day-cases [1, 2], with approximately 40 % of these occurring in free-standing ambulatory surgery centers [3]. For anesthesiologists, the challenge of ambulatory surgery lies in balancing good anesthesia with a safe and rapid recovery to a level of minimal or no residual cognitive and psychomotor impairment [4].
Muscle relaxation is an important component of good anesthesia, and is achieved by the use of neuromuscular blocking agents (NMBA). These drugs are used by anesthesiologists to facilitate tracheal intubation [5••] and enhance surgical exposure [6]. The use of high-doses of NMBAs to achieve deep neuromuscular blockade has been shown to increase the risk of post-operative complications (Table 1) [7–19, 20•, 21, 22]. Residual paralysis is the term applied to the persistence of muscle weakness post-operatively following NMBA administration. Clinically significant muscle paralysis occurs at train-of-four ratios (TOFR) < 0.9 [23••, 24, 25, 26••]. Residual paralysis is common when NMBAs are administered, occurring in up to two-thirds of patients [27, 28], and is often undetected in the immediate post-operative period [20•, 29].
Table 1.
Clinical trials on NMBA use for outpatient surgery and reported side effects
| Authors | Title | Year | Number of patients |
Surgery | NMBA and dose | Induction agents | Reversal | Side effect |
|---|---|---|---|---|---|---|---|---|
| Raeder et al. [7] |
Outpatient laparoscopy in general anaesthesia with alfentanil and atracurium. A comparison with fentanyl and pancuronium |
1986 | 62 | Outpatient sterilization by laparoscopy |
Atracurium (0.5 mg/kg) or pancuronium (0.07 mg/kg) |
Fentanyl, alfentanyl |
Used, but drug was not specified |
Worst functionality after reversal of anaesthesia, in the afternoon after the procedure, and at home in the evening in patients with pancuronium. |
| Sengupta et al. [8] |
Post-operative morbidity associated with the use of atracurium and vecuronium in day-case laparoscopy |
1987 | Not available | Day-case gynaecological laparoscopy |
Atracurium or vecuronium |
Not available | Not available | Greater number of patients in the vecuronium group were able to resume normal activity 24 h after laparoscopy; significantly higher incidence of abdominal pain in the vecuronium group. |
| Melnick et al. [9] |
Decreasing post-succinylcholine myalgia in outpatients |
1987 | 395 | Healthy outpatients |
Succinylcholine (1.5 mg/ kg) |
Lidocaine and d- tubocurarine as premedication |
None | Succinylcholine without premedication is associated with a higher incidence of postoperative myalgia. |
| Sosis et al. [10] |
Comparison of atracurium and d tubocurarine for prevention of succinylcholine myalgia |
1987 | 44 | Outpatient females undergoing laparoscopy |
Atracurium and d tubocurarine |
Thiopental | None | Fasciculations occurred in 79 % of patients given saline, in 46 % of those receiving atracurium, and in 12 % of those given d-tubocurarine. 85 % of atracurium patients were free of postoperative myalgia on postoperative day 1. |
| Zuurond et al. [11] |
Atracurium versus vecuronium: a comparison of recovery in outpatient arthroscopy |
1988 | 40 | Outpatient arthroscopy of the knee under general anaesthesia |
Atracurium (0.5 mg/kg) and vecuronium (0.1 mg/kg) |
Methohexitone, isoflurane, N2O/O2 |
None | All but one patient (95 %) in the atracurium group required neostigmine versus nine patients in the vecuronium group (45 %). |
| Trépanier et al. [12] |
Myalgia in outpatient surgery: comparison of atracurium and succinylcholine |
1988 | 60 | Outpatient surgery |
Succinylcholine (1.5 mg/ kg) and atracurium (350 mg/kg) |
Isofluorane, fentanyl, and thiopentone |
None | Myalgia was present in 76 % of the succinylcholine patients compared to 23 % in the atracurium group. 50 % of the patients in the succinylcholine group had myalgia necessitating bed rest or analgesics compared to 23 % in the atracurium group |
| Zahl et al. [13] |
Muscle pain occurs after outpatient laparoscopy despite the substitution of vecuronium for succinylcholine |
1989 | 28 | Outpatient laparoscopy |
Succinylcholine (1.5 mg/ kg), vecuronium (50 mcg/kg) |
N2O, thiopental, and fentanyl. |
Glycopyrrolate (7 mcg/kg) and edrophonium (0.5 mg/kg). |
Rocuronium and succinylcholine are related with postoperative myalgia |
| Luyk et al. [14] |
Comparative trial of succinylcholine vs low dose atracurium-lidocaine combination for intubation in short outpatient procedures |
1990 | 40 | Surgical removal of molar teeth |
Atracurium (0.2 mg/kg), succinylcholine (1 mg/ kg) |
Halothane, lidocaine |
Edrophonium (0.5 mg/kg) and atropine (0.6 mg) |
Succinylcholine was related with significantly more myalgia. Spontaneous respiration was slower after low dose atracurium/lidocaine relative to succinylcholine |
| Poler et al. [15] |
Mivacurium as an alternative to succinylcholine during outpatient laparoscopy |
1992 | 60 | Female healthy outpatients |
Succinylcholine and mivacurium (0.15 mg/ kg) |
Thiopental sodium, alfentani |
Neostigmine | Side effects with mivacurium included flushing and occasional wheezing |
| Laxenaire [16] |
Drugs and other agents involved in anaphylactic shock occurring during anaesthesia. A French multicenter epidemiological inquiry |
1993 | 1585 | Outpatient clinics with allergo- anaesthesia unit |
Not available | Not available | Not available | Succinylcholine was responsible for 43 % of the IgE-dependent reactions involving a muscle relaxant, vecuronium for 37 %, pancuronium for 13 %, alcuronium for 7.6 %, atracurium for 6.8 % and gallamine for 5.6 % |
| Tang et al. [17] |
Comparison of rocuronium and mivacurium to succinylcholine during outpatient laparoscopic surgery |
1996 | 100 | Healthy women undergoing outpatient laparoscopic surgery |
Succinylcholine (1 mg/ kg), rocuronium (0.6 mg/kg), or mivacurium (0.2 mg/ kg) |
Midazolam, fentanyl and thiopental |
Edrophonium (0.5 mg/kg) and atropine (10 mcg/kg) |
One patient with succinylcholine and six patients with mivacurium displayed erythema on the upper body. Postoperative myalgia was experienced by 16 % of the patients with succinylcholine compared to none with rocuronium and mivacurium |
| Whalley et al. [18] |
Comparison of neuromuscular effects, efficacy and safety of rocuronium and atracurium in ambulatory anaesthesia |
1998 | 41 | Laparoscopic gynaecological surgery |
Rocuronium (0.6 mg/kg) or atracurium (0.5 mg/ kg) |
Propofol, alfentanil, N2O/O2 |
Used, but drug was not specified |
One patient in the atracurium group experienced transient flushing of the head and neck. The most frequent adverse event was nausea and vomiting (two patients in the rocuronium group; three patients in the atracurium group) |
| Savaresev et al. [19] |
The clinical neuromuscular pharmacology of mivacurium chloride (BW B 109 OU): a short- acting nondepolarizing ester neuromuscular blocking drug |
1998 | 72 | Healthy volunteers |
Mivacurium 0.03–0.30 mg/kg in boluses, and continuous infusions from 35 to 324 min in length |
N2O, narcotic and thiopental |
Neostigmine (0.06 mg/kg) and atropine (0.03 mg/kg) |
Long onset time (even when doses ED95×3 are used) and prolonged duration of action when higher doses are administered. In patients with atypical plasma cholinesterase, NMB may be prolonged |
| Debaene et al. [20•] |
Residual paralysis in the PACU after a single intubating dose of nondepolarizing muscle relaxant with an intermediate duration of action |
2003 | 526 | Gynecologic and plastic surgery |
Rocuronium (0.58 ± 0.08 mg/kg), atracurium (0.55 ± 0.08 mg/kg), and vecuronium (0.09 ± 0.02 mg/kg) |
Not reported | None used | After vecuronium, atracurium or rocuronium single intubating dose ED95×2 and no reversal, residual paralysis was still present 2 h after administration in almost 50 % of patients |
| Cammu et al. [21] |
Postoperative residual paralysis in outpatients versus inpatients |
2006 | 640 (320 inpatients and 320 outpatients) |
Outpatient surgery, not specified |
Mivacurium (50 %) for outpatients and, rocuronium (44 %) and atracurium (36 %) for inpatients |
Not reported | Used, but drug was not specified |
Postoperative respiratory complications: 38 % in surgical outpatients and 47 % inpatients. Mivacurium was used frequently for outpatients |
| Pendeville et al. [22] |
A comparison of intubation conditions and time-course of action with rocuronium and mivacurium for day case anaesthesia |
2007 | 50 | Outpatient surgery, not specified |
Mivacurium (0.15 mg kg−1) or rocuronium (0.3 mg kg−1) |
Propofol, sulfentanyl, N2O |
None used | Mivacurium has the risk of unexpected prolonged relaxation due to a possible defect in plasma cholinesterase |
Residual paralysis delays the recovery of the patient and the efficiency of ambulatory care centers [30]. Crucially, it also compromises patient safety putting patients at increased risk of post-operative complications such as weakness, hypoxia and respiratory failure [31, 32] thus making it an important patient safety issue.
In ambulatory surgery, residual paralysis occurs less frequently, however the proportion of patients affected is still significant at 38 % [21]. The overall risk of perioperative morbidity and mortality in day-case surgeries is low [33] with one recent study quoting the risk to be as low as 0.1 % [2]. By these observations the effect of residual paralysis on postoperative outcomesin day case surgery must be very minimal. Despite this, concerns over patient safety remain as more procedures are carried out on an ageing population in ambulatory care centers with fewer resources to rapidly identify and treat post-operative complications. This review will consider current evidence on neuromuscular management, strategies aimed at preventing residual paralysis, and the effects on post-operative outcomes. The development and use of new selectively binding reversal agents warrants revaluation of this area of clinical practice.
Residual Paralysis and Outcomes
Respiratory Complications
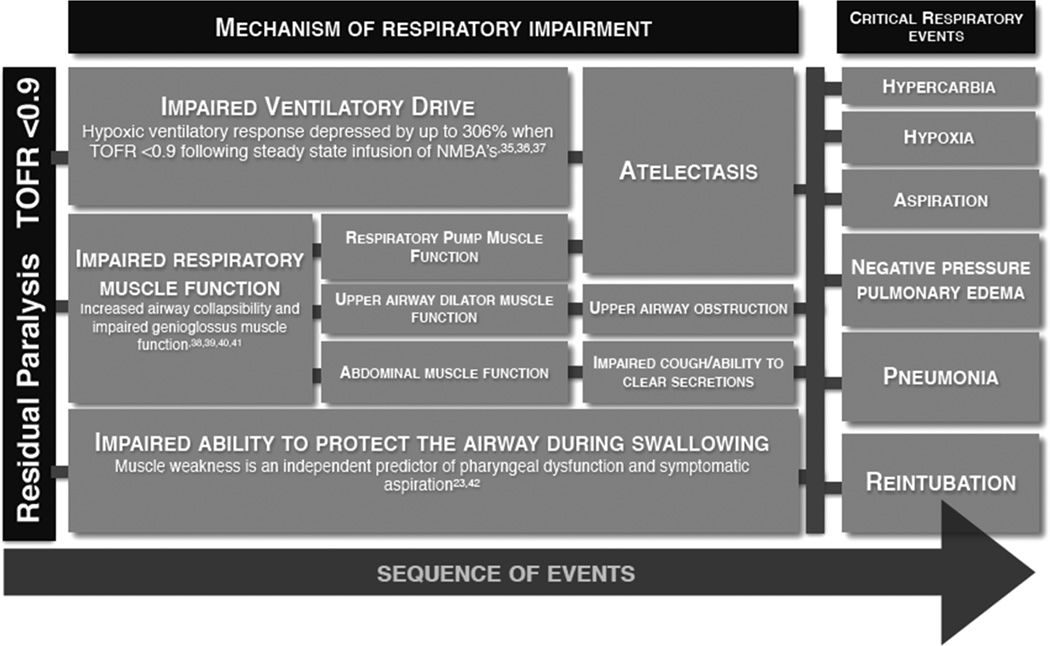
Respiratory events are the most common complication of residual paralysis. They are also the most common reason for post-surgical unplanned admission to intensive care, and the mortality rate for these patients is high [34]. Respiratory events include airway obstruction, hypoxia, atelectasis, aspiration, pneumonia and negative pressure pulmonary edema Respiratory events are a common complication of residual paralysis due to impairments in several important facets of normal respiratory physiology, as illustrated in Fig. 1.
Fig. 1.
Mechanisms of residual paralysis induced postoperative respiratory failure. A small level of residual paralysis that cannot be detected without quantitative monitoring of the train-of-four (TOF) response impairs several important facets of respiratory function. The main mechanisms of respiratory failure induced by minimal, residual neuromuscular blockade (TOF: 0.5–0.9) are impairments of hypoxic ventilatory response, and respiratory muscle function. In addition, the coordination between breathing and swallowing is impaired, leading to the inability to protect the airway during swallowing. Consequently, paralysis increases the vulnerability to hypoxia, symptomatic aspiration, pulmonary edema, and reintubation
Impairment in Hypoxic Ventilatory Response
At TOFR < 0.7 the hypoxic ventilatory response (HVR) is significantly impaired [35, 36]. This is thought to be due to NMBA effects on carotid body chemosensitivity, causing a reduction in nerve discharge in response to hypoxia [35, 37]. Volunteer studies have shown that this effect can be reproduced with both long and intermediate acting NMBAs [35, 36]. HVR was depressed by up to 306 % when train-of-four ratios were maintained at 0.7 by continuous infusion of either atracurium, pancuronium or vecuronium [36]. This innate response to hypoxia was only found to return to normal at TOFR > 0.9 [36, 37]. Partial paralysis thus interferes with ventilatory responses to hypoxia putting patients at risk of hypoxic injury. This impaired ventilation can lead to atelectasis, which in turn predisposes patients to post-operative pneumonias.
Impaired Respiratory Muscle Function
Airway patency is governed by complex interactions between neuromuscular function and inherent airway structural properties [38, 39]. Upper airway integrity is impaired by minimal levels of neuromuscular blockade due to the susceptibility of the upper airway muscles to the effects of NMBAs [6, 40]. The lingering effects of NMBAs can thus have profound and potentially serious consequences on airway patency. Upper airway dilator muscles counter-act the negative intraluminal pressure generated by the respiratory pump muscles to maintain airway patency during inspiration [40, 41]. With residual paralysis however, the efficacy of these dilator muscles is markedly impaired, enough to significantly increase airway collapsibility [40, 41]. By measuring pharyngeal and pulmonary function, we observed that upper airway obstruction occurs commonly during inspiration, even at minimal levels of neuromuscular block [40]. Increased airway vulnerability was detected at TOFR 0.5–0.8 as assessed by pressure-flow analysis, MRI, flow-volume curves and pressure-flow analysis (upper airway closing pressure calculation). This vulnerability to airway obstruction during inspiration predisposes patients to postoperative respiratory failure [32].
Impaired Ability to Protect the Airway from Aspiration
Weakness of the airway muscles results in pharyngeal dysfunction, which impairs innate protective mechanisms during swallowing [23••, 24]. This puts patients at increased risk of aspiration [42••]. Using video fluoroscopy, Sundman et al. [23••] observed that pharyngeal dysfunction was evident in 28 % of volunteers with a TOFR 0.6, compared to only 6 % in the control recordings (P < 0.05). Pharyngeal dysfunction was found to occur due to impaired initiation of swallowing, impaired co-ordination of the pharyngeal muscles and a reduction in tone of the upper oesophageal sphincter [23••]. 80 % of swallows in those with pharyngeal dysfunction resulted in laryngeal penetration [23••]. Mirzakhani et al. [42••] observed that 70 % of critically ill patients with muscle weakness developed symptomatic aspirations. Moreover, muscle weakness was found to increase the risk of symptomatic aspiration almost 10-fold.
Critical Respiratory Events
Multiple studies have shown an association between partial paralysis and critical respiratory events (CRE). Berg et al. [31] observed that patients randomized to receive pancuronium were more likely to become hypoxic on emergence and require supplemental oxygen to maintain saturations >90 % than those randomized to atracurium (intermediate NMBA). Bissenger et al. [43] have demonstrated that TOF ratios <0.7 were associated with significantly higher incidences of hypoxemia (60 %) in comparison to TOF ratios of >0.7 (10 % P<0.05). In a case–control study, Murphy et al. assessed whether residual paralysis contributed to CRE. The most common CRE’s were severe hypoxemia (59.0 %) and upper airway obstruction (34.4 %). 78.3 % of the CRE case group had a TOF ratio <0.7, whereas none of the patients in the control group has a TOFR <0.7 [32]. More recently, Grosse-Sundrup et al. demonstrated that intermediate NMBAs significantly increased risk of oxygen desaturation to both <80 and <90 % in the first 20 min after extubation. The risk of postoperative reintubation and unplanned intensive care admission was also significantly higher in patients who had received intermediate NMBAs intra-operatively. Patients undergoing shorter operations (<2 h) were found to be at increased risk of severe post-operative respiratory complications, a finding of particular significance to ambulatory surgery [44••].
Although minimal neuromuscular transmission failure (TOF-ratio: 0.5–0.8) is a significant contributing risk factor for post-operative respiratory complications, partial paralysis alone does not typically translate to morbidity in healthy patients undergoing minor surgical procedures, but a combination of multiple hits increase the risk of upper airway related respiratory complications in the perioperative period. Repeated airway interventions, upper airway surgery and excessive fluid administration can all result in tissue swelling that may increase airflow resistance [39]. During anesthesia there is also a reduction in the functional residual capacity of the lung, which can increase airway collapsibility, especially in obese patients [45]. Other contributing factors to postoperative airway collapse include the supine position versus elevated back position, increased respiratory pump muscle activity (sepsis), postoperative pain relief with opioid drugs, concomitant use of other anesthetic agents such as benzodiazepines and co-morbidities such as obstructive sleep apnea and obesity [39, 46, 47]. This airway obstruction can go undetected until severe desaturation occurs because reliable and continuous monitoring with capnography and tidal volume measurements ceases following extubation [39].
Skeletal Muscle Weakness
Patients are at increased risk of falls due to skeletal muscle weakness as a result of residual neuromuscular blockade [48•]. Significant skeletal muscle weakness occurs even after recovery of the TOFR to unity. This is especially pertinent in ambulatory cases where patients are expected to mobilize shortly after surgery.
Residual Paralysis: Costs
Residual paralysis is costly to both patients and healthcare institutions. Discharge times were significantly longer in patients with a TOFR < 0.9 on arrival to PACU [30]. This was found to reduce PACU throughput, and incur additional costs as staff recover patients beyond allocated times [30]. Residual paralysis is associated with postoperative respiratory complications. The average cost of treating respiratory complications following surgery is $62,704, versus $5,015 for uncomplicated surgery [49, 50]. In the USA alone, it is estimated that postoperative pulmonary complications lead to an additional 92,000 ICU admissions and incur a cost of $3.42 billion each year [49]. Strategies to prevent residual paralysis and its complications thus have great economic benefit.
Strategies to Prevent Residual Paralysis
Techniques to Avoid or Reduce NMBA Use
Regional Anesthesia
The balanced technique has long been used by anesthesiologists to achieve adequate anesthesia with lower doses of multiple agents, thereby limiting the risk of side effects from each agent. The motor block that accompanies local anesthesia can reduce or negate the need for NMBAs [51]. Combining regional anesthesia with NMBAs however has been shown to delay both the spontaneous and facilitated recovery from NMBA induced muscle paralysis [52]. This is only the case when NMBAs are given at unchanged doses, not taking into account the reduced doses required to achieve surgical relaxation when combined with regional anesthesia.
Laryngeal Mask Airway
Laryngeal mask airways (LMAs) do not require NMBAs for insertion. The use of LMAs allows for spontaneous breathing to be maintained during surgery. This can lead to shortened duration of surgery without affecting surgical procedures or views. Williams et al. compared use of LMA with maintained spontaneous breathing to the use of tracheal intubation with mechanical ventilation in laparoscopic gynecological day-case procedures. Although the initial intra-abdominal pressure in the LMA group was significantly higher, there was no significant difference in the volume of insufflated CO2 or the time taken to reach a steady state pneumoperitoneum [53]. Surgical views were reported as similar for both groups and complications did not differ. Operation time was significantly shorter in the LMA group [53]. For many elective surgical procedures in ambulatory anesthesia, LMAs can thus entirely negate the need for NMBAs without affecting the quality of surgical conditions.
NMBA Dose & Duration of Action
The choice of NMBA can influence post-operative outcomes. The longer the duration of action of NMBAs, the greater the risk of residual paralysis and its complications [54–56]. Intermediate acting NMBAs improve patient safety compared to the use of long-acting NMBAs, but residual paralysis is still present in up to two-thirds of patients given an intermediate NMBA intra-operatively [27, 28]. Benzylisoquinoline NMBAs such as cisatracurium have been demonstrated to have a more predictable duration of action than steroidal NMBAs such as rocuronium, particularly in elderly patients [28]. However, recent data suggests that risk of pulmonary complications increases in a dose-dependent manner, regardless of NMBA group [57], probably because anesthesiologists take these pharmacokinetics into account and may believe its safe to use benzylisoquinoline NMBAs later during cases.
Succinylcholine, a short acting depolarizing NMBA, has a rapid onset and short duration of action [58, 59] and higher doses carry little risk of prolonged paralysis, unless a patient carries a mutation for choline esterase. The use of short acting NMBAs such as succinylcholine and mivacurium may also translate to economical advantages in the context of ambulatory surgery [17, 60]. Succinylcholine can however have serious side effects and has considerably more contraindications than intermediate NMBAs, thus limiting its use [59]. Moreover, the metabolism of succinylcholine and mivacurium is cholinesterase-dependent and any quantitative or qualitative abnormality of this enzyme extends the duration of the neuromuscular blockade [61], which affects safety and represents a logistic challenge in ambulatory surgery centers.
Optimizing Surgical Conditions
Anesthesiologists aim to provide adequate muscle relaxation for tracheal intubation and surgery whilst also ensuring complete recovery of muscle strength at the end of the procedure. Complete recovery means that the patient can independently maintain a patent airway during wakefulness and sleep, breathe normally, prevent aspiration, clear secretions, cough, smile and talk [62••].
NMBAs facilitate tracheal intubation and reduce the incidence of airway tissue injury, leading to fewer upper airway symptoms post-operatively [5••, 63]. Neuromuscular transmission blockade also helps to improve surgical exposure and performance by blocking reflexive movements to surgical stimuli intra-operatively [6].
Determining how much muscle relaxation is required for optimal surgical conditions can be difficult for several reasons. There is considerable inter-individual variability in patient response to NMBAs, making it difficult for anesthesiologists to predict peak effect and recovery times, which is reflected clinically by the high incidence of residual paralysis [6]. The effects of NMBAs are also muscle dependent. For example, the diaphragm is less sensitive to NMBAs than upper airway muscles [64]. After total block of adductor pollicis (AP) function as assessed by TOF ratio, the diaphragm might still be able to contract in a clinically meaningful fashion [64–67]. Almost twice the dose of NMBAs is required for diaphragmatic relaxation than for AP relaxation [64]. The duration of action and the recovery of the twitch height are also faster in the diaphragm than at the AP [65–67]. Even when evaluating muscle strength in a well-defined muscle such as the adductor pollicis muscle, at absence of TOF count, a strong tetanic stimulus can lead to a muscle contraction. Accordingly, it is impossible to ensure complete blockade of neuromuscular transmission of all muscles during the entire surgical procedure. Anesthesiologists need to apply a variable combination of anesthetics, opioids, local anesthetics and NMBA in order to provide optimal surgical conditions throughout the surgical procedure.
Submaximal Block
Complete and continuous neuromuscular block is rarely indicated in surgery, indeed some operations can be performed without any neuromuscular block at all, when the airway is secured with a supraglottic device. The requirement for choosing whether to use neuromuscular blockade or not, varies according to the type of surgical procedure. NMBAs are helpful to achieve optimal conditions for intubation and surgery. Adequate relaxation of the upper airway muscles is important for tracheal intubation, reducing the incidence of hoarseness and vocal cord sequelae; a common postoperative patient complaint and source of litigation against anesthesiologists [5••]. Incision and closure of open abdominal surgery may also require a degree of paralysis in order to prevent reflexive movements to surgical stimuli [6]. Upon exposure of the abdominal cavity however, very little block is required. Even with surgeon-controlled muscle relaxation, surgeons did not request complete neuromuscular block during open abdominal procedures [68] demonstrating that ideal surgical conditions can be accomplished in the absence of NMBAs.
Laparoscopic gynecological surgery for example can be performed safely and adequately without neuromuscular blockade [53]. In cardiac surgery, the lack of continuous neuromuscular blockade had no influence on intraoperative patient movement observed [69]. Moreover, patients who did not receive continuous NMBAs recovered more quickly post-operatively.
Maintenance of Muscle Paralysis
Administering NMBAs by boluses as opposed to a continuous infusion in order to maintain neuromuscular block significantly reduces the likelihood of residual paralysis [70]. Fawcett et al. [70] observed that 24 % of patients receiving NMBA as an infusion arrived at the PACU with residual paralysis and this persisted in 12 % of this patient group when reassessed 15 min later. Of the patients who were given boluses, 12 % had clinically significant muscle weakness on arrival to PACU and only 2 % had not fully recovered at 15 min. The authors of this paper believe that infusions of non-depolarizing NMBAs should not be applied in ambulatory anesthesia. Continuous deep paralysis (i.e. maintaining one twitch on the TOF response) is not necessary for most surgical procedures performed in the outpatient setting. By administering NMBAs when clinically necessary rather than on TOF response, and by also avoiding deep paralysis, the total dose of NMBAs used in surgery is likely to be reduced which may subsequently reduce the risk of postoperative residual paralysis. It might also be reasonable to consider using a low dose of the short acting NMBA succinylcholine at the end of the case, for example to facilitate closure of the peritoneum.
Neuromuscular Transmission Monitoring
Perioperative neuromuscular monitoring by train-of-four stimulation can help guide timing and dosage of NMBAs and their antagonists, helping to reduce the incidence of residual paralysis. Multiple studies have shown that qualitative assessment of TOFR by visual or tactile palpation is an unreliable and inaccurate method of identifying residual paralysis, as evaluators are often unable to detect fade when TOFR > 0.3 [4, 71–75, 76••] thus residual paralysis may go undetected when actual TOFR is between 0.4 and 0.9 [20•, 74]. For this reason detectable fade on TOF stimulation indicates that NMB reversal is required.
Quantitative measurements of TOFR, such as acceleromyography (ACM) are used to aid titration of NMBAs and their antagonists. ACM is more reliable at identifying residual paralysis [75]. Murphy et al. [76••, 77] has demonstrated in 2 randomized trials, that the use of ACM significantly reduces the incidence of residual paralysis. In the first trial, residual paralysis was observed in the PACU in only 4.5 % of patients monitored with ACM, whereas 30 % of patients monitored with conventional TOF had residual neuromuscular blockade. More importantly, the incidence, severity and duration of hypoxemic events in the first 30 min in PACU were less in the ACM group [76••].
Surveys of clinical practice in the US and Europe have shown that neuromuscular monitoring is performed in approximately 50 % of surgeries only, perhaps explaining the high incidence of residual paralysis [78, 79]. Proposals to rectify this inadequacy in patient perioperative care include better education and the development of formal best practice guidelines encouraging the use of quantitative measurement [80].
Reversal
Residual blockade promotes postoperative complications increasing morbidity and mortality. Reversal agents are used to accelerate and facilitate neuromuscular recovery to avoid future complications. Currently available reversal agents are Neostigmine and Sugammadex.
Neostigmine
Reversal of shallow levels of paralysis following administration of a nondepolarizing NMBA can be achieved with an acetylcholinesterase inhibitor combined with an antimuscarinic agent [81, 82]. Neostigmine is commonly used in the United States to antagonize the effects of NMBAs and expedite restoration of muscle function post-operatively [83•].
Acetylcholinesterase inhibitors are ineffective at reversing deep neuromuscular blockade [84]. The pharmacological efficacy of acetylcholinesterase inhibitors is limited as their maximum effect is reached when the enzyme is 100 % inhibited, at which point administering further antagonist does not increase the concentrations of acetylcholine. This means that during deep neuromuscular blockade, when NMBA concentration is high, it is not possible to fully reverse paralysis with acetylcholinesterase inhibitors. In a prospective randomized trial, Kopman et al. attempted reversal of neuromuscular block 1 min after a continuous infusion of either cisatracurium or rocuronium, i.e. at the point of deep neuromuscular block. After 20 min, only 11 of 40 patients had recovered to a TOFR > 0.9 [74]. This finding was more recently reproduced by Della Rocca et al. [85] who also found that in some patients residual paralysis persisted more than 20 min after reversal with neostigmine following deep neuromuscular block. To avoid suboptimal reversal, neostigmine should only be administered when there is a degree of spontaneous recovery, or following shallow block. Practically, this translates to a TOF level of 2 or 3 [83•].
In addition, if neostigmine is used for reversal in the OR, total twitch suppression should ideally be avoided, unless essential for the particular surgical procedure. The dose of neostigmine should be based upon the degree of blockade at the time of reversal. Failure to detect tactile or visual TOF fade does not mean that reversal can be spared. However, in these circumstances doses of neostigmine as low as 0.015 mg/kg are often sufficient [82, 83•].
The use of neostigmine/atropine or glycopyrrolate can have undesirable side effects such as arrhythmias, dry mouth [86], bronchospasm [87], and even asystole [88, 89]. As well as these side effects, neostigmine can also cause neuromuscular transmission failure when given to patients who have already recovered from muscle paralysis [90, 91•]. Furthermore, in the absence of neuromuscular block, neostigmine can impair upper airway dilator volume, genioglossus muscle function and diaphragmatic function, thus putting the patient at risk of respiratory complications [91•, 92•].
Sugammadex
Sugammadex is a γ-cyclodextrin that binds selectively to the NMBAs rocuronium and vercuronium [93]. It works by encapsulating and inactivating these NMBAs directly. When dosed appropriately, sugammadex rapidly reverses any level of neuromuscular block without the autonomic effects seen with neostigmine [85, 93]. These findings are particularly relevant in the context of ambulatory surgery where a rapid and full recovery from neuromuscular block is essential to a safe discharge.
Sugammadex has also been shown to improve recovery times and peri-operative safety in groups of patients who are classically at greater risk of residual paralysis and its complications. Rocuronium’s duration of action can vary with organ dysfunction, age and when larger doses are used. This becomes less relevant when rapid and reliable reversal can be achieved with sugammadex [93]. Morbidly obese patients for example are at increased risk of residual paralysis and critical respiratory events in the post-operative period [94]. Morbidly obese patients receiving sugammadex however, recover to a TOFR 0.9 or greater 3.5 times faster than those given neostigmine [94]. The rapid recovery seen with sugammadex use even in co-morbid patients has the potential to open up safe access to ambulatory surgery to a larger group of patients. However, sugammadex does not eliminate residual neuromuscular blockade in a real world scenario and it is important to conclude that neuromuscular transmission monitoring needs to be applied to all patients who have received NMBAs.
Calabadion
Currently available reversal agents have undesirable side effects. Calabadion is an acyclic member of the cucurbit[n]uril family of molecular containers [95•] that, similar to Sugammadex, forms host–guest complexes with specific targets and thereby modifies the properties of drugs bound within its interior.
Calabadion is derived from urea and its C-shaped structure promotes the ability to flex the glycoluril oligomer backbone, expand its cavity, and accommodate guests of a wide range of sizes. The structural features also enhance the solubility of Calabadion, which caused by additional electrostatic interactions (p–p interactions and hydrophobic effect). Carbonyl portals promote the binding affinity of Calabadion towards the positively charged ammonium ions of NMBAs. The distance between anionic SO3-solubilizing groups of Calabadions selectively complement the N–N separation within Rocuronium, Vecuronium and Pancuronium.
Calabadion I, the first member of this family, was found to have effects on reversal of non-depolarizing NMB in rats [96•]. However, its binding affinity to NMBAs was lower than Sugammadex. Preclinical studies have shown that Calabadion II promotes earlier recovery of muscle function as expressed by time to recovery of righting reflex and to TOF>0.9, compared to Neostigmine, Sugammadex, Calabadion I and placebo [97]. Calabadion II is also more potent than Sugammadex, as it requires a lower number of molecules to promote faster recovery of neuromuscular function, compared to what would be needed of Sugammadex. Moreover, Calabadions are broad-spectrum reversal agents, which also reverse NMBAs with a benzylisoquinoline-structure. This is important, as the market volume of benzylisoquinoline NMBAs is significant: around 20 % of all NMBA. Calabadion II does not affect subsequent Succinylcholine-induced NMB, promoting a safe relaxation in cases where re-intubation would be needed after reversal [97]. Calabadions are well tolerated and do not affect heart rate, mean arterial blood pressure, pH, carbon dioxide pressure, and oxygen tension. Due to its structure and lack of a sugar moiety, it has been speculated that Calabadion II may have a lower allergenic profile compared to Sugammadex. Unlike Sugammadex, Calabadion II does not appear to affect PTT and activated factor X. All these reasons place Calabadion as a promising agent for reversal of NMBAs.
Conclusion
Although the risk of perioperative morbidity and mortality in ambulatory surgery is low [33], residual paralysis occurs frequently following NMBA use, even in day-case surgeries [21]. The most common and serious consequences of residual paralysis are postoperative respiratory complications. Freestanding ambulatory surgery centers may not be adequately equipped or staffed to manage these complications. Outcomes can be improved by employing techniques to minimize the risk of residual paralysis such as neuromuscular transmission monitoring and judicious use of low-dose neuromuscular blocking drugs. Neuromuscular blocking agent effects should be reversed. Neostigmine reversal improves muscle strength in patients with residual neuromuscular blockade, but carries risks related to incomplete reversal and unspecific effects of the reversal agent. New reversal agents that are so far not approved by the FDA such as calabadion and sugammadex may allow for a better control of surgical conditions whilst also improving patient safety.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest Hassan Farhan, Ingrid Moreno-Duarte and Duncan McLean declare that they have no conflict of interest. Matthias Eikermann has received financial support through a grant from Merck, and has submitted a patent application for Calabadion to reverse NMBA and anesthesia.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Hassan Farhan, Department of Anesthesia, Critical Care and Pain Medicine, Harvard Medical School, Massachusetts General Hospital, 55, Fruit Street, Boston, MA 02115, USA.
Ingrid Moreno-Duarte, Email: ingrid.md.8@gmail.com, Department of Anesthesia, Critical Care and Pain Medicine, Harvard Medical School, Massachusetts General Hospital, 55, Fruit Street, Boston, MA 02115, USA.
Duncan McLean, Department of Anesthesia, Critical Care and Pain Medicine, Harvard Medical School, Massachusetts General Hospital, 55, Fruit Street, Boston, MA 02115, USA.
Matthias Eikermann, Email: meikermann@partners.org, Department of Anesthesia, Critical Care and Pain Medicine, Harvard Medical School, Massachusetts General Hospital, 55, Fruit Street, Boston, MA 02115, USA; Universitaet Duisburg-Essen, Essen, Germany.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. [Accessed 6 June 2014];National health statistics reports. 2009 (11):1–25. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=19294964&retmode=ref&cmd=prlinks. [PubMed]
- 2.Mathis MR, Naughton NN, Shanks AM, et al. Patient selection for day case-eligible surgery. Anesthesiology. 2013;119(6):1310–1321. doi: 10.1097/ALN.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 3.Urman RD, Desai SP. History of anesthesia for ambulatory surgery. Curr Opin Anaesthesiol. 2012:1. doi: 10.1097/ACO.0b013e3283593100. [DOI] [PubMed] [Google Scholar]
- 4.Chung F. Discharge criteria—a new trend. Can J Anaesth. 1995;42(11):1056–1058. doi: 10.1007/BF03011083. [DOI] [PubMed] [Google Scholar]
- 5. Mencke T, Echternach M, Kleinschmidt S, et al. Laryngeal morbidity and quality of tracheal intubation: a randomized controlled trial. [Accessed 6 June 2014];Anesthesiology. 2003 98(5):1049–1056. doi: 10.1097/00000542-200305000-00005. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=12717124&retmode=ref&cmd=prlinks. This report was the first to demonstrate in a ran- domized controlled trial that neuromuscular blocking agents improve not only intubating conditions but also laryngeal outcome.
- 6.Alfille PH, Merritt C, Chamberlin NL, Eikermann M. Control of perioperative muscle strength during ambulatory surgery. Curr Opin Anaesthesiol. 2009;22(6):730–737. doi: 10.1097/ACO.0b013e328331d545. [DOI] [PubMed] [Google Scholar]
- 7.Raeder JC, Hole A. Out-patient laparoscopy in general anaesthesia with alfentanil, atracurium. A comparison with fentanyl and pancuronium. Acta Anaesthesiol Scand. 1986;30(1):30–34. doi: 10.1111/j.1399-6576.1986.tb02362.x. [DOI] [PubMed] [Google Scholar]
- 8.Sengupta P, Skacel M, Plantevin OM. Post-operative morbidity associated with the use of atracurium and vecuronium in day-case laparoscopy. Eur J Anaesthesiol. 1987;4(2):93–99. [PubMed] [Google Scholar]
- 9.Melnick B, Chalasani J, Uy NT, Phitayakorn P, Mallett SV, Rudy TE. Decreasing post-succinylcholine myalgia in outpatients. Can J Anaesth. 1987;34(3 (Pt 1)):238–241. doi: 10.1007/BF03015159. [DOI] [PubMed] [Google Scholar]
- 10.Sosis M, Broad T, Larijani GE, Marr AT. Comparison of atracurium and D-tubocurarine for prevention of succinylcholine myalgia. Anesth Analg. 1987;66(7):657–659. [PubMed] [Google Scholar]
- 11.Zuurmond WW, van Leeuwen L. Atracurium versus vecuronium: a comparison of recovery in outpatient arthroscopy. Can J Anaesth. 1988;35(2):139–142. doi: 10.1007/BF03010653. [DOI] [PubMed] [Google Scholar]
- 12.Trépanier CA, Brousseau C, Lacerte L. Myalgia in outpatient surgery: comparison of atracurium and succinylcholine. Can J Anaesth. 1988;35(3 (Pt 1)):255–258. doi: 10.1007/BF03010619. [DOI] [PubMed] [Google Scholar]
- 13.Zahl K, Apfelbaum JL. Muscle pain occurs after outpatient laparoscopy despite the substitution of vecuronium for succinylcholine. Anesthesiology. 1989;70(3):408–11. doi: 10.1097/00000542-198903000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Luyk NH, Weaver JM, Quinn C, Wilson S, Beck FM. Comparative trial of succinylcholine versus low dose atracurium-lidocaine combination for intubation in short outpatient procedures. Anesth Prog. 1990;37(5):238–243. [PMC free article] [PubMed] [Google Scholar]
- 15.Poler SM, Watcha MF, White PF. Mivacurium as an alternative to succinylcholine during outpatient laparoscopy. J Clin Anesth. 1992;4(2):127–133. doi: 10.1016/0952-8180(92)90029-z. [DOI] [PubMed] [Google Scholar]
- 16.Laxenaire MC. Drugs other agents involved in anaphylactic shock occurring during anaesthesia. A French multicenter epidemiological inquiry. Ann Fr Anesth Reanim. 1993;12(2):91–96. doi: 10.1016/s0750-7658(05)81015-9. [DOI] [PubMed] [Google Scholar]
- 17.Tang J, Joshi GP, White PF. Comparison of rocuronium and mivacurium to succinylcholine during outpatient laparoscopic surgery. Anesth Analg. 1996;82(5):994–998. doi: 10.1097/00000539-199605000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Whalley DG, Maurer WG, Knapik AL, Estafanous FG. Comparison of neuromuscular effects, efficacy and safety of rocuronium and atracurium in ambulatory anaesthesia. Can J Anaesth. 1998;45(10):954–959. doi: 10.1007/BF03012303. [DOI] [PubMed] [Google Scholar]
- 19.Savarese JJ, Ali HH, Basta SJ, et al. The clinical neuromuscular pharmacology of mivacurium chloride (BW B1090U). A shortacting nondepolarizing ester neuromuscular blocking drug. Anesthesiology. 1988;68(5):723–732. doi: 10.1097/00000542-198805000-00010. [DOI] [PubMed] [Google Scholar]
- 20. Debaene B, Plaud B, Dilly M-P, Donati F. Residual paralysis in the PACU after a single intubating dose of nondepolarizing muscle relaxant with an intermediate duration of action. Anesthesiology. 2003;98(5):1042–1048. doi: 10.1097/00000542-200305000-00004. This landmark paper showed that residual paralysis may still be relevant 2 h after injection of a single dose of intermediate-acting neuromuscular blocking agents.
- 21.Cammu G, De Witte J, De Veylder J, et al. Postoperative residual paralysis in outpatients versus inpatients. Anesth Analg. 2006;102(2):426–429. doi: 10.1213/01.ane.0000195543.61123.1f. [DOI] [PubMed] [Google Scholar]
- 22.Pendeville PE, Lois F, Scholtes J-L. A comparison of intubation conditions and time-course of action with rocuronium and mivacurium for day case anaesthesia. Eur J Anaesthesiol. 2007;24(6):546–550. doi: 10.1017/S0265021506002341. [DOI] [PubMed] [Google Scholar]
- 23. Sundman E, Witt H, Olsson R, Ekberg O, Kuylenstierna R, Eriksson LI. The incidence and mechanisms of pharyngeal and upper esophageal dysfunction in partially paralyzed humans: pharyngeal videoradiography and simultaneous manometry after atracurium. [Accessed 6 June 2014];Anesthesiology. 2000 92(4):977–984. doi: 10.1097/00000542-200004000-00014. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=10754616&retmode=ref&cmd=prlinks. This milestone paper identified impaired pharyngeal contraction coordination as a mechanism of residual paralysis associated aspiration.
- 24.Eriksson LI, Sundman E, Olsson R, et al. Functional assessment of the pharynx at rest and during swallowing in partially paralyzed humans: simultaneous videomanometry and mechanomyography of awake human volunteers. Anesthesiology. 1997;87(5):1035–1043. doi: 10.1097/00000542-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Eikermann M, Groeben H, Hüsing J, Peters J. Accelerometry of adductor pollicis muscle predicts recovery of respiratory function from neuromuscular blockade. Anesthesiology. 2003;98(6):1333–1337. doi: 10.1097/00000542-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 26. Murphy GS, Szokol JW, Avram MJ, et al. Postoperative Residual Neuromuscular Blockade Is Associated with Impaired Clinical Recovery. Anesth Analg. 2013;117(1):133–141. doi: 10.1213/ANE.0b013e3182742e75. This milestone paper closed the gap between respiratory medicine studies conducted in partially paralyzed volunteers and outcomes research trials, demonstrating that residual paralysis translates to impaired postoperative respiratory function.
- 27.Hayes AH, Mirakhur RK, Breslin DS, Reid JE, McCourt KC. Postoperative residual block after intermediate-acting neuromuscular blocking drugs. Anaesthesia. 2001;56(4):312–318. doi: 10.1046/j.1365-2044.2001.01921.x. [DOI] [PubMed] [Google Scholar]
- 28.Maybauer DM, Geldner G, Blobner M, et al. Incidence and duration of residual paralysis at the end of surgery after multiple administrations of cisatracurium and rocuronium. Anaesthesia. 2007;62(1):12–17. doi: 10.1111/j.1365-2044.2006.04862.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim KS, Lew SH, Cho HY, Cheong AMA. Residual paralysis induced by either vecuronium or rocuronium after reversal with pyridostigmine. Anesth Analg. 2002;95(6):1656–1660. doi: 10.1097/00000539-200212000-00033. [DOI] [PubMed] [Google Scholar]
- 30.Butterly A, Bittner EA, George E, Sandberg WS, Eikermann M, Schmidt U. Postoperative residual curarization from intermediate-acting neuromuscular blocking agents delays recovery room discharge. Br J Anaesth. 2010;105(3):304–309. doi: 10.1093/bja/aeq157. [DOI] [PubMed] [Google Scholar]
- 31.Berg H, Roed J, Viby-Mogensen J, et al. Residual neuromuscular block is a risk factor for postoperative pulmonary complications. [Accessed 6 June 2014];A prospective, randomised, and blinded study of postoperative pulmonary complications after atracurium, vecuronium and pancuronium. Acta Anaesthesiol Scand. 1997 41(9):1095–1103. doi: 10.1111/j.1399-6576.1997.tb04851.x. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=9366929&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 32.Murphy GS, Szokol JW, Marymont JH, Greenberg SB, Avram MJ, Vender JS. Residual neuromuscular blockade and critical respiratory events in the postanesthesia care unit. Anesth Analg. 2008;107(1):130–137. doi: 10.1213/ane.0b013e31816d1268. [DOI] [PubMed] [Google Scholar]
- 33.Warner MA, Shields SE, Chute CG. Major morbidity and mortality within 1 month of ambulatory surgery and anesthesia. [Accessed 6 June 2014];JAMA. 1993 270(12):1437–1441. doi: 10.1001/jama.270.12.1437. Available at: http://eutils.ncbi.nlmnih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=8371443&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 34.Chan K-S, Tan C-K, Fang C-S, et al. Readmission to the intensive care unit: an indicator that reflects the potential risks of morbidity and mortality of surgical patients in the intensive care unit. Surg Today. 2009;39(4):295–299. doi: 10.1007/s00595-008-3876-6. [DOI] [PubMed] [Google Scholar]
- 35.Eriksson LI, Sato M, Severinghaus JW. Effect of a vecuronium-induced partial neuromuscular block on hypoxic ventilatory response. [Accessed 6 June 2014];Anesthesiology. 1993 78(4):693–699. doi: 10.1097/00000542-199304000-00012. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=8096684&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 36.Eriksson LI. Reduced hypoxic chemosensitivity in partially paralysed man. A new property of muscle relaxants? Acta Anaes-thesiol Scand. 1996;40(5):520–523. doi: 10.1111/j.1399-6576.1996.tb04482.x. [DOI] [PubMed] [Google Scholar]
- 37.Eriksson LI. The effects of residual neuromuscular blockade and volatile anesthetics on the control of ventilation. Anesth Analg. 1999;89(1):243–251. doi: 10.1097/00000539-199907000-00045. [DOI] [PubMed] [Google Scholar]
- 38.Isono S. Obstructive sleep apnea of obese adults: pathophysiology and perioperative airway management. Anesthesiology. 2009;110(4):908–921. doi: 10.1097/ALN.0b013e31819c74be. [DOI] [PubMed] [Google Scholar]
- 39.Isono S, Greif R, Mort TC. Airway research: the current status and future directions. Anaesthesia. 2011;66(Suppl 2):3–10. doi: 10.1111/j.1365-2044.2011.06928.x. [DOI] [PubMed] [Google Scholar]
- 40.Eikermann M, Vogt FM, Herbstreit F, Vahid-Dastgerdi M, Zenge MO, Ochterbeck C, de Greiff A, et al. The predisposition to inspiratory upper airway collapse during partial neuromuscular blockade. Am J Respir Crit Care Med. 2007;175(1):9–15. doi: 10.1164/rccm.200512-1862OC. [DOI] [PubMed] [Google Scholar]
- 41.Herbstreit F, Peters J, Eikermann M. Impaired upper airway integrity by residual neuromuscular blockade: increased airway collapsibility and blunted genioglossus muscle activity in response to negative pharyngeal pressure. Anesthesiology. 2009;110(6):1253–1260. doi: 10.1097/ALN.0b013e31819faa71. [DOI] [PubMed] [Google Scholar]
- 42. Mirzakhani H, Williams J-N, Mello J, et al. Muscle weakness predicts pharyngeal dysfunction and symptomatic aspiration in long-term ventilated patients. Anesthesiology. 2013;119(2):389–397. doi: 10.1097/ALN.0b013e31829373fe. This paper demonstrates that muscle weakness is an independent predictor or symptomatic aspiration in the intensive care unit.
- 43.Bissinger U, Schimek F, Lenz G. Postoperative residual paralysis and respiratory status: a comparative study of pancuronium and vecuronium. [Accessed 6 June 2014];Physiol Res/Acad Sci Bohemoslovaca. 2000 49(4):455–462. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=11072806&retmode=ref&cmd= prlinks. [PubMed] [Google Scholar]
- 44. Grosse-Sundrup M, Henneman JP, Sandberg WS, et al. Intermediate acting non-depolarizing neuromuscular blocking agents and risk of postoperative respiratory complications: prospective propensity score matched cohort study. BMJ. 2012 Oct 15;345(5):e6329–e6329. doi: 10.1136/bmj.e6329. This paper showed that the use of intermediate-acting muscle relaxants is an independent predictor of severe postoperative respiratory complications. The data suggest that meticulous titration of neuromuscular blocking agents and reversal drugs is required to improve respiratory safety of patients who received NMBAs.
- 45.Tagaito Y, Isono S, Remmers JE, Tanaka A, Nishino T. Lung volume and collapsibility of the passive pharynx in patients with sleep-disordered breathing. J Appl Physiol. 2007;103(4):1379–1385. doi: 10.1152/japplphysiol.00026.2007. [DOI] [PubMed] [Google Scholar]
- 46.Isono S. Obesity and obstructive sleep apnoea: mechanisms for increased collapsibility of the passive pharyngeal airway. Respirology. 2012;17(1):32–42. doi: 10.1111/j.1440-1843.2011.02093.x. [DOI] [PubMed] [Google Scholar]
- 47.Dhonneur G, Combes X, Leroux B, Duvaldestin P. Postoperative obstructive apnea. Anesth Analg. 1999;89(3):762–767. doi: 10.1097/00000539-199909000-00045. [DOI] [PubMed] [Google Scholar]
- 48. Eikermann M, Gerwig M, Hasselmann C, Fiedler G, Peters J. Impaired neuromuscular transmission after recovery of the train-of-four ratio. Acta Anaesthesiol Scand. 2007;51(2):226–234. doi: 10.1111/j.1399-6576.2006.01228.x. This study shows that even after recovery of the train-of-four ration to 0.9, postoperative skeletal muscle strength is substantially impaired postoperatively as a consequence of lingering effects of neuromuscular blocking agents and anesthetics which induce a tetanic fade.
- 49.Dimick JB, Chen SL, Taheri PA, Henderson WG, Khuri SF, Campbell DA. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199(4):531–537. doi: 10.1016/j.jamcollsurg.2004.05.276. [DOI] [PubMed] [Google Scholar]
- 50.Ramachandran SK, Nafiu OO, Ghaferi A, Tremper KK, Shanks A, Kheterpal S. Independent predictors and outcomes of unanticipated early postoperative tracheal intubation after nonemergent, noncardiac surgery. Anesthesiology. 2011;115(1):44–53. doi: 10.1097/ALN.0b013e31821cf6de. [DOI] [PubMed] [Google Scholar]
- 51.Agarwal A, Pandey R, Dhiraaj S, et al. The effect of epidural bupivacaine on induction and maintenance doses of propofol (evaluated by bispectral index) and maintenance doses of fentanyl and vecuronium. Anesth Analg. 2004;99(6):1684–1688. doi: 10.1213/01.ANE.0000136422.70531.5A. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki T, Mizutani H, Ishikawa K, Miyake E, Saeki S, Ogawa S. Epidurally administered mepivacaine delays recovery of train-of-four ratio from vecuronium-induced neuromuscular block. Br J Anaesth. 2007;99(5):721–725. doi: 10.1093/bja/aem253. [DOI] [PubMed] [Google Scholar]
- 53.Williams MT, Rice I, Ewen SP, Elliott SM. A comparison of the effect of two anaesthetic techniques on surgical conditions during gynaecological laparoscopy. Anaesthesia. 2003;58(6):574–578. doi: 10.1046/j.1365-2044.2003.03150.x. [DOI] [PubMed] [Google Scholar]
- 54.Viby-Mogensen J, Jørgensen BC, Ording H. Residual curarization in the recovery room. [Accessed 6 June 2014];Anesthesiology. 1979 50(6):539–541. doi: 10.1097/00000542-197906000-00014. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elinkfcgi?dbfrom=pubmed&id=156513&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 55.Naguib M, Kopman AF, Ensor JE. Neuromuscular monitoring and postoperative residual curarisation: a meta-analysis. Br J Anaesth. 2007;98(3):302–316. doi: 10.1093/bja/ael386. [DOI] [PubMed] [Google Scholar]
- 56.Bevan DR, Smith CE, Donati F. Postoperative neuromuscular blockade: a comparison between atracurium, vecuronium, and pancuronium. [Accessed 6 June 2014];Anesthesiology. 1988 69(2):272–276. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=2900612&retmode=ref&cmd=prlinks. [PubMed] [Google Scholar]
- 57.McLean D, Igumenscheva A, Ladha K, et al. Dose-dependent association between intermediate-acting neuromuscular blocking agents and postoperative respiratory complications: a prospective analysis of data on file. ASA abstract. doi: 10.1097/ALN.0000000000000674. abstract no. JS09. [DOI] [PubMed] [Google Scholar]
- 58.Karcioglu O, Arnold J, Topacoglu H, Ozucelik DN, Kiran S, Sonmez N. Succinylcholine or rocuronium? A meta-analysis of the effects on intubation conditions. Int J Clin Pract. 2006;60(12):1638–1646. doi: 10.1111/j.1742-1241.2005.00685.x. [DOI] [PubMed] [Google Scholar]
- 59.Perry JJ, Lee JS, Sillberg VAH, Wells GA. Rocuronium versus succinylcholine for rapid sequence induction intubation. Cochrane Database Syst Rev. 2008;16(2):CD002788. doi: 10.1002/14651858.CD002788.pub2. [DOI] [PubMed] [Google Scholar]
- 60.Puura AI, Rorarius MG, Manninen P, Hoppu S, Hopput S, Baer GA. The costs of intense neuromuscular block for anesthesia during endolaryngeal procedures due to waiting time. [Accessed 6 June 2014];Anesth Analg. 1999 88(6):1335–1339. doi: 10.1097/00000539-199906000-00026. Available at: http://eutils.ncbinlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=10357341&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 61.Østergaard D, Viby-Mogensen J, Rasmussen SN, Gätke MR, Varin F. Pharmacokinetics and pharmacodynamics of mivacurium in patients phenotypically homozygous for the atypical plasma cholinesterase variant: effect of injection of human cholinesterase. Anesthesiology. 2005;102(6):1124–1132. doi: 10.1097/00000542-200506000-00011. [DOI] [PubMed] [Google Scholar]
- 62. Murphy GS, Brull SJ. Residual neuromuscular block: lessons unlearned part i: definitions, incidence, and adverse physiologic effects of residual neuromuscular block. Anesth Analg. 2010;111(1):120–128. doi: 10.1213/ANE.0b013e3181da832d. Outstanding review article demonstrating the “blind spot” in Anesthesiologists perception of an adequate recovery from NMBA effects.
- 63.Lundstrøm LH, Møller AM, Rosenstock C, et al. Avoidance of neuromuscular blocking agents may increase the risk of difficult Atracheal intubation: a cohort study of 103 812 consecutive adult patients recorded in the Danish Anaesthesia Database. Br J Anaesth. 2009;103(2):283–290. doi: 10.1093/bja/aep124. [DOI] [PubMed] [Google Scholar]
- 64.Hemmerling TM, Donati F. Neuromuscular blockade at the larynx, the diaphragm and the corrugator supercilii muscle: a review. Can J Anaesth. 2003;50(8):779–94. doi: 10.1007/BF03019373. [DOI] [PubMed] [Google Scholar]
- 65.Lebrault C, Chauvin M, Guirimand F, Duvaldestin P. Relative potency of vecuronium on the diaphragm and the adductor pollicis. Br J Anaesth. 1989;63(4):389–392. doi: 10.1093/bja/63.4.389. [DOI] [PubMed] [Google Scholar]
- 66.Cantineau JP, Porte F, Dhonneur G, Duvaldestin P. Neuromuscular effects of rocuronium on the diaphragm and adductor pol-licis muscles in anesthetized patients. Anesthesiology. 1994;81(3):585–590. doi: 10.1097/00000542-199409000-00010. [DOI] [PubMed] [Google Scholar]
- 67.Ibebunjo C, Hall LW. Succinylcholine and vecuronium blockade of the diaphragm, laryngeal and limb muscles in the anaesthetized goat. Can J Anaesth. 1994;41(1):36–42. doi: 10.1007/BF03009659. [DOI] [PubMed] [Google Scholar]
- 68.Abdulatif M, Taylouni E. Surgeon-controlled mivacurium administration during elective Caesarean section. Can J Anaesth. 1995;42(2):96–102. doi: 10.1007/BF03028259. [DOI] [PubMed] [Google Scholar]
- 69.Gueret G, Rossignol B, Kiss G, et al. Is muscle relaxant necessary for cardiac surgery? Anesth Analg. 2004;99(5):1330–1333. doi: 10.1213/01.ANE.0000132984.56312.FF. [DOI] [PubMed] [Google Scholar]
- 70.Fawcett WJ, Dash A, Francis GA, Liban JB, Cashman JN. Recovery from neuromuscular blockade: residual curarisation following atracurium or vecuronium by bolus dosing or infusions. [Accessed 6 June 2014];Acta Anaesthesiol Scand. 1995 39(3):288–293. doi: 10.1111/j.1399-6576.1995.tb04063.x. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=7793202&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 71.Viby-Mogensen J, Jensen NH, Engbaek J, Ording H, Skovgaard LT, Chraemmer-Jørgensen B. Tactile and visual evaluation of the response to train-of-four nerve stimulation. [Accessed 6 June 2014];Anesthesiology. 1985 63(4):440–443. doi: 10.1097/00000542-198510000-00015. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=4037404&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 72.Tactile evaluation of the response to double burst stimulation decreases, but does not eliminate, the problem of postoperative residual paralysis. [Accessed 6 June 2014];Acta Anaesthesiol Scand. 1998 42(10):1168–1174. doi: 10.1111/j.1399-6576.1998.tb05271.x. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=9834799&retmode=ref&cmd= prlinks. [DOI] [PubMed] [Google Scholar]
- 73.Capron F, Fortier L-P, Racine SB, Donati FO. Tactile fade detection with hand or wrist stimulation using train-of-four, double-burst stimulation, 50-Hertz tetanus, 100-Hertz tetanus, and acceleromyography. Anesth Analg. 2006;102(5):1578–1584. doi: 10.1213/01.ane.0000204288.24395.38. [DOI] [PubMed] [Google Scholar]
- 74.Kopman AF, Kopman DJ, Ng J, Zank LM. Antagonism of profound cisatracurium and rocuronium block: the role of objective assessment of neuromuscular function. J Clin Anesth. 2005;17(1):30–35. doi: 10.1016/j.jclinane.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 75.Claudius C, Viby-Mogensen J. Acceleromyography for use in scientific and clinical practice. Anesthesiology. 2008;108(6):1117–1140. doi: 10.1097/ALN.0b013e318173f62f. [DOI] [PubMed] [Google Scholar]
- 76. Murphy GS, Szokol JW, Marymont JH, et al. Intraoperative Acceleromyographic Monitoring Reduces the Risk of Residual Neuromuscular Blockade and Adverse Respiratory Events in the Postanesthesia Care Unit. Anesthesiology. 2008;109(3):389–398. doi: 10.1097/ALN.0b013e318182af3b. This paper demonstrated first time that rigorous application of quantitative NMT monitoring reduces the likelyhood of postoperative residual neuromuscular blockade and associated respiratory side effects.
- 77.Murphy GS, Szokol JW, Avram MJ, et al. Intraoperative acceleromyography monitoring reduces symptoms of muscle weakness and improves quality of recovery in the early postoperative period. Anesthesiology. 2011;115(5):946–954. doi: 10.1097/ALN.0b013e3182342840. [DOI] [PubMed] [Google Scholar]
- 78.Fuchs-Buder T, Sirieix D, Schmartz D, Plaud B. Monitoring of neuromuscular block by acceleromyography: concepts, applications and limits of use. Ann Fr Anesth Reanim. 2012;31(11):922–925. doi: 10.1016/j.annfar.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 79.Naguib M, Kopman AF, Lien CA, Hunter JM, Lopez A, Brull SJ. A survey of current management of neuromuscular block in the United States and Europe. Anesth Analg. 2010;111(1):110–119. doi: 10.1213/ANE.0b013e3181c07428. [DOI] [PubMed] [Google Scholar]
- 80.El-Orbany M, Ali HH, Baraka A, Salem MR. Residual neuromuscular block should, and can, be a “never event”. Anesth Analg. 2014;118(3):691. doi: 10.1213/ANE.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 81.Bevan DR, Donati F, Kopman AF. Reversal of neuromuscular blockade. [Accessed 6 June 2014];Anesthesiology. 1992 77(4):785–805. doi: 10.1097/00000542-199210000-00025. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=1416176&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 82.Fuchs-Buder T, Baumann C, De Guis J, Guerci P, Meistelman C. Low-dose neostigmine to antagonise shallow atracurium neuromuscular block during inhalational anaesthesia: a prospective randomised controlled trial. Eur J Anaesthesiol. 2013;30(10):594–598. doi: 10.1097/EJA.0b013e3283631652. [DOI] [PubMed] [Google Scholar]
- 83. Kopman AF, Eikermann M. Antagonism of non-depolarising neuromuscular block: current practice. Anaesthesia. 2009;64(Suppl 1):22–30. doi: 10.1111/j.1365-2044.2008.05867.x. This preclinical paper demonstrated that neostigmine administration following recovery from neuromuscular blockade dose-dependently impairs respiratory muscle function and breathing.
- 84.Della Rocca G, Pompei L, Pagan DE, Paganis C, Tesoro S, Mendola C, Boninsegni P, et al. Reversal of rocuronium induced neuromuscular block with sugammadex or neostigmine: a large observational study. Acta Anaesthesiol Scand. 2013;57(9):1138–1145. doi: 10.1111/aas.12155. [DOI] [PubMed] [Google Scholar]
- 85.Della Rocca G, DI Marco P, Beretta L, De Gaudio AR, Ori C, Mastronardi P. Do we need to use sugammadex at the end of a general anesthesia to reverse the action of neuromuscular bloking agents? Position paper on sugammadex use. [Accessed 6 June 2014];Minerva Anestesiol. 2013 79(6):661–666. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=23192221&retmode= ref&cmd=prlinks. [PubMed] [Google Scholar]
- 86.Sacan O, White PF, Tufanogullari B, Klein K. Sugammadex reversal of rocuronium-induced neuromuscular blockade: a comparison with neostigmine-glycopyrrolate and edrophonium-atropine. Anesth Analg. 2007;104(3):569–74. doi: 10.1213/01.ane.0000248224.42707.48. [DOI] [PubMed] [Google Scholar]
- 87.Hazizaj A, Hatija A. Bronchospasm caused by neostigmine. Eur J Anaesthesiol. 2006;23(1):85–86. doi: 10.1017/S0265021505241820. [DOI] [PubMed] [Google Scholar]
- 88.Bjerke RJ, Mangione MP. Asystole after intravenous neostigmine in a heart transplant recipient. Can J Anaesth. 2001;48(3):305–307. doi: 10.1007/BF03019764. [DOI] [PubMed] [Google Scholar]
- 89.Sawasdiwipachai P, Laussen PC, McGowan FX, Smoot L, Casta A. Cardiac arrest after neuromuscular blockade reversal in a heart transplant infant. Anesthesiology. 2007;107(4):663–665. doi: 10.1097/01.anes.0000282140.68060.fa. [DOI] [PubMed] [Google Scholar]
- 90.Caldwell JE. Reversal of residual neuromuscular block with neostigmine at one to four hours after a single intubating dose of vecuronium. [Accessed 6 June 2014];Anesth Analg. 1995 80(6):1168–1174. doi: 10.1097/00000539-199506000-00018. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=7762847&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 91. Eikermann M, Fassbender P, Malhotra A, et al. Unwarranted administration of acetylcholinesterase inhibitors can impair genioglossus and diaphragm muscle function. Anesthesiology. 2007;107(4):621–629. doi: 10.1097/01.anes.0000281928.88997.95. This paper provides a tentative algorithm on how to titrate neostigmine dose to the magnitude of residual blockade quantified by train-of-four monitoring.
- 92. Herbstreit F, Zigrahn D, Ochterbeck C, Peters J, Eikermann M. Neostigmine/glycopyrrolate administered after recovery from neuromuscular block increases upper airway collapsibility by decreasing genioglossus muscle activity in response to negative pharyngeal pressure. Anesthesiology. 2010;113(6):1280–1288. doi: 10.1097/ALN.0b013e3181f70f3d. This paper demonstrates that neostigmine given following recovery from neuromuscular blockade impairs upper airway muscle function and increases airway collapsibility.
- 93.Caldwell JE, Miller RD. Clinical implications of sugammadex. Anaesthesia. 2009;64(s1):66–72. doi: 10.1111/j.1365-2044.2008.05872.x. [DOI] [PubMed] [Google Scholar]
- 94.Gaszynski T, Szewczyk T, Gaszynski W. Randomized comparison of sugammadex and neostigmine for reversal of rocuronium-induced muscle relaxation in morbidly obese undergoing general anaesthesia. Br J Anaesth. 2012;108(2):236–239. doi: 10.1093/bja/aer330. [DOI] [PubMed] [Google Scholar]
- 95. Ma D, Zhang B, Hoffmann U, Sundrup MG, Eikermann M, Isaacs L. Acyclic cucurbit[n]uril-type molecular containers bind neuromuscular blocking agents in vitro and reverse neuromuscular block in vivo. Angew Chem Int Ed Engl. 2012;51(45):11358–11362. doi: 10.1002/anie.201206031. First description of the development of calabadions, acyclic cucurbit[n]uril-type molecular containers which bind neuromuscular blocking agents in vitro and reverse neuromuscular block in vivo.
- 96. Hoffmann U, Grosse-Sundrup M, Eikermann-Haerter K, et al. Calabadion: a new agent to reverse the effects of benzylisoquinoline and steroidal neuromuscular-blocking agents. Anesthesiology. 2013;119(2):317–325. doi: 10.1097/ALN.0b013e3182910213. Describes calabadion, a new broad-spectum drug to reverse the effects of cisatracurium and rocuronium.
- 97.Moreno-Duarte I, Haerter F, Simons J, et al. Calabadion II reverses steroidal neuromuscular blocking agents faster than Sugammadex and reverses the effects of benzylisoquinolines, without altering the effects of succinylcholine. IARS Abstract presented in May 2014. Anesth Analg. 2014;118:S1–S139. (abstract no. S-50) http://www.iars.org/assets/1/7/AM14_AbstractSupp_Fpdf. [Google Scholar]