Abstract
The prevention of obesity in children with DD is a pressing public health issue, with implications for health status, independent living, and quality of life. Substantial evidence suggests that children with developmental disabilities (DD), including those with intellectual disabilities (ID) and autism spectrum disorder (ASD), have a prevalence of obesity at least as high if not higher than their typically developing peers. The paper reviews what is known about the classic and unique risk factors for childhood obesity in these groups of children, including dietary, physical activity, sedentary behavior, and family factors, as well as medication use. We use evidence from the literature to make the case that primary prevention at the individual/family, school and community levels will require tailoring of strategies and adapting existing intervention approaches.
Keywords: Developmental disabilities, autism spectrum disorder, special health care needs, obesity, overweight, weight, obesity prevention, antipsychotic medication
Introduction
Obesity in children is a significant health concern, with the prevalence of childhood obesity having tripled over the last two decades in the United States (U.S.) [1] and increased considerably worldwide [2]. Essentially all population subgroups are affected by obesity (with the exception of sub-Saharan Africa) [3], with substantial evidence of persistent health disparities by race/ethnicity and socioeconomic status [4]. Evidence from clinic-based studies and nationally representative surveys suggests that children with developmental disabilities (DD), including those with intellectual disabilities (ID) and autism spectrum disorder (ASD), have a prevalence of obesity at least as high if not higher than their typically developing peers [5–7]. These groups may be disproportionately impacted by the adverse health outcomes associated with obesity by virtue of their primary disability and associated factors at the individual, family, community, and societal levels. Obesity prevention research specific to these populations is in its infancy.
The objective of this review is to contribute to the foundation from which a prevention research agenda can arise. We limit our discussion to primary prevention of obesity, i.e. efforts undertaken to prevent the condition from occurring. We outline what is known about the extent of the problem of obesity in children with DD, summarize research on putative risk factors, highlight gaps in knowledge, and then suggest practice and research efforts aimed at primary prevention measures organized in an ecologic framework.
Developmental Disabilities: Definitions and Prevalence
In the U.S., DD are defined as severe, often chronic disabilities that arise before the age of 22 and result in substantial functional limitations in three or more major life activities such as self-care, receptive and expressive language, learning, mobility, self-direction, independent living, and economic self-sufficiency [8]. Estimates of the prevalence of DD in the U.S. indicate 1 in 7 children are affected [9]. This review focuses on the primary prevention of obesity among children with ID and ASD because they are common diagnoses among DD and appear to be associated with elevated obesity risk. The population of children with DD is heterogeneous along multiple dimensions, including the presence of one or more cognitive, behavioral, physical, and/or sensory impairments. The severity of disability can vary greatly and may have an impact on learning and adaptive functioning. Although approximately one-third of children (38%) with ASD also have an intellectual disability, an equal proportion has average or above-average intellectual ability [10]. Regardless of their intellectual ability, children with ASD experience difficulties with social interaction and communication. In contrast, children with ID who do not have ASD may have well-developed social interaction skills but have difficulties in other areas of adaptive functioning. Children with ID include those with Down syndrome, fetal alcohol syndrome, Prader Willi, fragile X, and many other syndromes; however, the etiology of ID is often unknown. Some syndromes are associated with obesity, and some children with ID also have physical disabilities which limit their physical activity. The heterogeneous nature of impairments in children with DD presents challenges to research as well as to the development of childhood obesity prevention programs.
Attention in the U.S. to the health needs of persons with DD is growing, spurred in part by a report of the Surgeon General in 2002 [11]that emphasized the health care disparities experienced by persons with ID and called for increased research efforts and implementation of health promotion programs. Research in adults with ID suggests that this population may have elevated rates of diabetes [12], cardiovascular disease [13], and shorter life expectancies [14] than the general population. Given that childhood obesity tends to persist into adulthood and excess weight increases risk for most chronic diseases [15], it is reasonable to expect that these health consequences manifest in children with DD as well. Thus, the prevention of obesity and the secondary conditions associated with obesity in children with DD is a pressing public health issue, with implications for health status, independent living, and quality of life.
Obesity Prevalence in Children with Developmental Disabilities
Overweight and obesity are classifications used to characterize the presence of excess adipose tissue and are generally based on body mass index (BMI) in kg/m2. The definitions currently in use in the U.S. and in some other parts of the world are based on sex-specific BMI-for-age percentiles calculated relative to the 2000 U.S. growth reference developed by the Centers for Disease Control and Prevention [16]. Children aged 2 to 20 are considered to be overweight when their BMI-for-age is greater than or equal to the 85th percentile, and are classified as obese when BMI-for-age is at or above the 95th percentile. Although these BMI cut-offs were developed for populations without disabilities, one study of adolescents with Down syndrome supports the validity of their use as indicators for overweight and obesity in that population [17]. Several other reference populations are in use around the world [18–20], including a WHO international reference [21].
Mounting evidence suggests that children with DD are at greater risk for obesity than their typically developing peers, though estimates vary, including data from nationally representative samples. Using self-reported height and weight data from the 2003 U.S. National Survey of Children’s Health, the adjusted prevalence of obesity was 22% in children with developmental and physical disabilities combined and 23% in children with ASD [6]. These obesity rates are significantly elevated compared to the prevalence of 14.8% in children overall. Using more recent data from the 2007 National Survey of Children’s Health, children with ASD were 42% more likely to be obese than children without ASD [7]. Data from the Longitudinal Study of Australian Children estimated a prevalence of obesity of 8.5% in six to seven year-old children with ID, a rate that is significantly higher than the prevalence of 5.4% seen in children without ID [22].
Estimates derived from convenience samples generally indicate a higher prevalence of obesity in children with DD than is seen in representative samples. For example a high prevalence of obesity has been reported among youth with ID [23], Down syndrome [24], and ASD [25, 26]. The prevalence of obesity in children with ID from around the world ranges from 3% to 37% [27–31] depending upon the sampling frame and the characteristics of the study population.
Risk Factors for Obesity in Children with Developmental Disabilities
Many of the risk factors for children with DD are likely the same as for typically developing children, especially within the context of an obesogenic or obesity-promoting environment. However, these children may also experience additional challenges that increase their susceptibility to typical risk factors, and they may also experience a unique set of risk factors not shared by children in the general population.
Body composition and energy needs
Some children with DD have altered body composition that may increase their risk of obesity. Studies in typically developing youth have shown that fat free mass is highly correlated with resting metabolic rate, a major contributor to daily energy expenditure [32]. Because fat-free mass is the greatest contributor to resting metabolic rate, lower levels of fat-free mass are likely to result in lower resting metabolic rate and decreased energy requirements.
Despite considerable research on body composition and energy expenditure in typically developing children, only a few studies have used state-of-the art measurement techniques (i.e., indirect calorimetry and doubly labeled water) in children with DD. Adolescents with Down syndrome have been shown to have lower fat-free mass [33] and lower resting metabolic rates than typically developing children [34, 35], although in one study lower resting metabolic rate was unrelated to fat accretion prospectively [34]. Children with DD who also have physical disabilities and are non-ambulatory may have reduced energy needs associated with lower activity energy expenditure, thereby increasing their risk of obesity.
Body composition represents an important consideration when developing prevention and intervention programs for affected children and adolescents. The caloric needs of these children are likely to be lower, and thus they may face challenges around feeding and satiety, particularly within an environment that is plentiful in low nutrient/energy-dense foods.
Dietary Factors
Obesity arises when energy intake exceeds energy expenditure over time. Major dietary contributors to energy imbalance among the general pediatric population include sub-optimal intakes of fruits and vegetables [36–38], excessive consumption of sugar-sweetened beverages [39, 40]and over-consumption of energy-dense, nutrient-poor sweets and snack foods [41, 42]. Although studies of these factors among children with DD are few, they appear to be equally or more susceptible to consuming poor quality diets. For example, several studies have found that children with ASD do not meet recommendations for fruit and vegetable intake [43–45], and some research has documented that children with ID consume fewer daily servings of dairy, fruits, and vegetables than recommended [46] and at lower amounts than their typically developing peers [47].
Children with DD, especially those with ASD, are more often described as “picky eaters” than typically developing children [48, 49] and appear to have aversions to specific textures, colors, smells, temperatures, and brand names of foods. An estimated 50–90% of children with ASD have feeding problems, which includes unusual eating patterns, rituals, and food selectivity [50, 51]. Food selectivity, a phenomenon that includes high levels of food refusal and/or consuming a limited repertoire of foods, may have origins in sensory processing impairments, which are common in children with ASD [52]. Children with ASD often have diets that are lacking in variety [53–55, 48], which is generally considered to be indicative of a healthful diet. They also consume fewer fruits and vegetables and more sugar-sweetened beverages [44] than typically developing children, and tend to prefer energy-dense/nutrient-poor foods within food groups (e.g., chicken nuggets, hot dogs, and peanut butter in the protein group; cake, french fries, macaroni, and pizza in the starch group; and ice cream in the dairy group) [56].
Physical Activity
Compared to the dearth research on dietary factors, the physical activity patterns in children with DD have received more attention. They may be particularly challenged to engage in physical activity due to motor skill difficulties [57–61] that may compromise endurance, balance, motor planning, and successful participation. The participation of children with DD in physical activity may also be impeded because of difficulties with social skills and communication. For example, motor planning difficulties in children with ASD were found to be strongly correlated with the social, communicative, and behavioral impairments that define ASD [62]. The need for close supervision may also hamper children’s participation.
Evidence on the extent to which children with ID participate in sufficient amounts of physical activity is equivocal [63–67]. The proportion of youth with ID who meet the prevailing recommendations for daily PA each day ranges from zero to 42%, depending on the study [68–71]. Similar to their typically-developing peers, physical activity levels in children with ID appear to decline with age and girls are less active than boys [71].
While less is known about physical activity levels of children with ASD, physical activity levels among school aged children with ASD do not appear to differ from those of typically developing children [72, 73]. However, as in the general population, physical activity levels in children with ASD are greater in younger compared to older children [74–77], and children with ASD appear to fall short of recommendations for daily physical activity [17] or physical activity during recess [78].
Barriers and facilitators to participation in physical activity must be better understood in order to address low physical activity levels in youth with DD through preventive intervention. Barriers identified to date include lack of accessible programs, lack of child’s interest, physical/motor challenges, behavioral difficulties, lack of time, no place in which to engage in activity, and transportation barriers [79]. Factors that appear to be positively associated with engagement in physical activity among youth with ID include both youth and caregiver preferences for physical activity as well as educational level of caregivers [80–83]. Barriers to physical activity cited by parents of children with ASD specifically include the child’s need for supervision, adults lacking skills needed to include their child, and the child’s lack of friends and/or exclusion by peers [84]. Further, the number of reported barriers has been positively related to total screen time [84]. Participants with ASD who met the recommended guidelines of 60 minutes of moderate-to-vigorous physical activity per day generally cited more facilitators to physical activity than barriers in one study [85]. Activity levels of youth with ASD during physical education classes were positively related to their interactions with their peers, the physical environment, and instructor-related characteristics, such as gender and specialized training in physical education [85]. Interviews with parents of children with ASD suggested that problematic behaviors among young children with ASD are a common reason for non-participation, rather than limited opportunity for activity [85]. Not only might child behavior problems interfere with children’s ability to participate in group-based physical activities, it is also possible that child behavior problems may lead to increased parental stress, which in turn may affect child participation [86]. This pathway should be explored in future studies, as failure to understand the impact of family functioning on child participation could undermine the success of preventive interventions.
Sedentary behavior and screen time
Sedentary behavior can be defined as “any waking behavior characterized by energy expenditures ≤1.5 metabolic equivalents (METs), while in a sitting or reclining posture” [87]. For youth, the major contributor to sedentary behavior outside of time spent in school is screen time, i.e., time spent watching television, viewing videos, playing video games (on large and small screens), and using a computer. Screen time likely contributes to obesity because it displaces other activities requiring greater energy expenditure, has been associated with snacking, and with increased exposure to advertisements that promote foods of limited nutritional value [88, 89]. Frequent mealtime television viewing has also been associated with lower intake of fruits and vegetables and greater intake of red and processed meats among typically developing children [88].
Although children in the general population exhibit high levels of television viewing, children with ASD appear to have particularly high levels based on both nationally representative [90] and non-representative samples [91, 92]and appear to start viewing at a younger age [93]. Children with ASD are often particularly visually oriented, which may manifest as a high interest in television and computers, and have shown better responses to verbal directives delivered via video clips than by live human presentations [92]. While an affinity for visual material has been used successfully to increase communication and adapt learning strategies for children with ASD [94, 95], these strategies may also have an unintended negative consequences of increasing sedentary time. Children with ASD who spent three or more hours per day viewing television/movies did so to the exclusion of other leisure-time activities, such as playing [92]. Parents of children with ASD report using television for its calming effect on their children and as a respite from the challenges of caring for them [96]. However, parental disagreements around child viewing patterns have been reported as a source of family stress, and electronic media use may be viewed negatively by extended family or by child welfare and child development professionals [96].
Studies of children with ID also generally report higher levels of sedentary behavior and greater use of electronic media, compared with typically developing children. More passive and socially isolating leisure activities were commonly identified by students with ID attending a special school in Ireland [81]. For children with ID who also have physical impairments, higher levels of sedentary activity likely reflect barriers to participation in physical activities discussed above.
Family Factors
Eating patterns and physical activity habits often arise within the context of the family, with parents playing a key role in influencing child feeding and eating patterns, mealtime structures, and physical activity [97–99]. Substantial evidence supports the important role of family meals in obesity prevention [100–102]. Associations between children’s weight status and adverse family dynamics, including family stress, maternal depression, and poor family cohesion have also been observed [103, 104]. The stress experienced by families of children with DD has been documented extensively [105–112], but to our knowledge none have examined the association between family stress and obesity.
The needs and behaviors of children with DD can pose unique and significant challenges to parents and may have an impact on parental feeding practices. Children with ID have more feeding and mealtime behavior problems than typically developing children [113]. One early study found that compared to children without feeding challenges, young children with feeding challenges had more problematic mealtime behaviors and their parents used more coercive methods, employed more aversive direction, and made more negative comments to their children [114]. Research in the general population shows that over-control of a child’s eating may promote the development of obesity [115] and that parenting styles that emphasize structure, responsiveness, and warmth yield healthier child eating and activity habits [98]. Although research on parental feeding practices in children with DD is scant, it warrants investigation, given the challenges that these children often pose at mealtimes. One study of young children with Down syndrome and their siblings found that parents took greater responsibility for feeding and had more concern about the weight status of children with Down syndrome compared to siblings without Down syndrome [116]. Provision of special meals was identified as a parental mealtime action positively associated with weight status in a study of 236 young children, approximately half of whom had DD [117]. A 2013 systematic review of the limited literature on parental factors associated with obesity in children with disability concluded that parents’ socioeconomic status, parental BMI, parents’ perceptions and attitudes toward their children’s weight status, and both parents’ and children’s physical activity levels are associated with obesity in youth with DD [118].
Psychotropic Medications
The best understood risk factor for obesity in children with DD is likely the adverse effects of psychotropic medications that are often prescribed for this population. Approximately 30% – 60% of children with ASD are prescribed at least one psychotropic medication, and 10% are prescribed more than one [119]. Prescribing rates have increased since the US Food and Drug Administration issued indications for risperidone (2006) and aripiprazole (2009) for the treatment of irritability associated with ASD, and data suggest that symptomatic benefits persist with longer-term treatment [120–126]. There is no evidence to suggest that children with DD are less susceptible to developing obesity in association with antipsychotic treatment than other youth [127].
Although atypical antipsychotics reduce the frequency of extrapyramidal side effects, they are much more likely to cause weight gain [128–131] and metabolic syndrome [132, 133]. Studies of children with ASD treated with risperidone have found weight gain to be significant in treatment versus placebo groups [133, 134]. Aripiprazole has been associated with significant weight gain over placebo [135, 136]. The diverse mechanisms by which antipsychotic medications cause weight gain are not fully understood, however it has been hypothesized that weight gain is tied to increased appetite associated with interactions among the medications and neuronal dopamine, serotonin, and histamine receptors [137].
Primary obesity prevention
The socioecological framework is commonly used to examine opportunities for childhood obesity prevention from a multilevel perspective, and includes modifying the risk factors at the individual, interpersonal (e.g., family), school, community, and policy levels of influence [138, 139]. We focus here on three primary venues for obesity prevention: the individual/family, school, and community (Figure 1). Evidence-based obesity prevention programs for children with DD are notably absent for all of these venues. The mechanisms by which the home, school, and community environments influence the onset of obesity in children with DD may differ compared to typically developing children. Further, the policies aimed to reduce the inequity in obesity between children with DD and their typically developing peers must address the inequalities that contribute to obesity at these multiple levels. This will require a sustained commitment from multiple stakeholders working in unison with parents. Adaptations to existing programs, such as tailoring to enhance their accessibility, relevance, and inclusivity, and a high level of engagement from the child’s family, are needed to address disparities in the availability of obesity prevention options for children with DD [140, 29].
Fig 1.
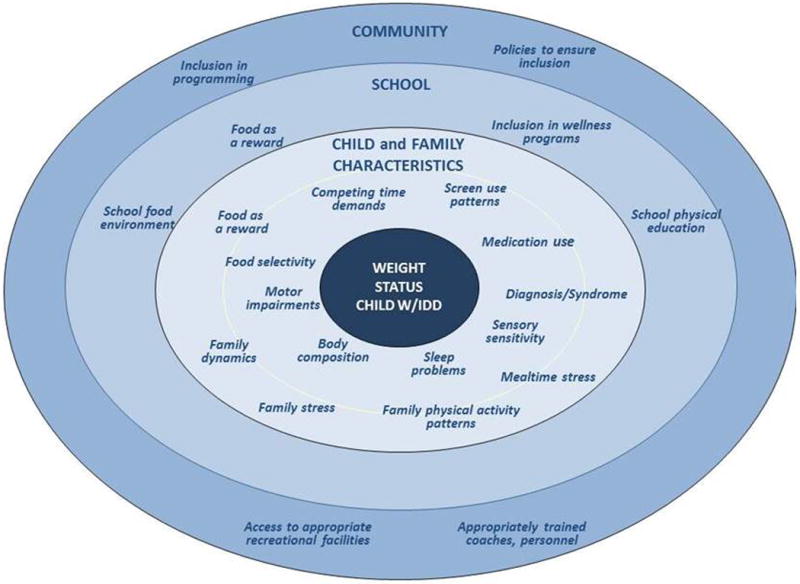
Socioecologic framework related to risk factors for obesity in children with developmental disabilities
Individual/Family Environment
Research to establish obesity prevention guidelines specific to children with DD is extremely limited and, to the extent they exist, are based on those established for the general pediatric population. The American Academy of Pediatrics’ expert recommendations for the prevention of childhood obesity includes universal assessment of obesity risk of all children through BMI screening, encouragement of diets with recommended quantities of fruits and vegetables, limits on consumption of sugar-sweetened beverages, limits on screen time to less than 2 hours daily, and promotion of moderate-to-vigorous physical activity for at least 60 minutes daily [141]. Little is known about the extent to which primary prevention initiatives to promote these behaviors are relevant and accessible to children with DD and their families [142].
At the individual and family levels, consistent assessment of obesity risk should be conducted at well-child visits by the health care provider. BMI assessment is known to vary widely among healthcare professionals [143, 144], and limited evidence suggests that routine recording of weight and height and guidance on nutrition, physical activity, and sedentary activity may not be complete in compliance with these recommendations for children with DD, in particular [145]. Practitioners also need to be aware of and screen for genetically-based syndromes that are associated with both ID and obesity such as Prader Willi, Bardet Biedl, Alstrom syndromes, and others. Weight screening, relevant anticipatory guidance, and parent support should be an established standard of care for all children and integrated fully with a family-centered approach. Specific examples of tailored guidance include providing support to encourage breast feeding for children with a DD at birth, and, for children with altered body composition that may reduce energy requirements, guidance to moderate energy intake.
Primary prevention efforts in home settings should include anticipatory guidance and tailored interventions around parent feeding practices that can foster healthy dietary intake and address problematic eating patterns, as well as promote children’s ability to self-regulate, and engage in physical activity. Setting limits around specific health-related behaviors in the home setting (e.g., the consumption of sugar-sweetened beverages, screen time) may not be readily achieved via the same strategies that parents use with typically developing children. For example, strategies associated with high parental responsiveness (e.g. conversations about limits) may not be effective if the child has a communication disorder, difficulty generalizing social rules, or limited knowledge/understanding about health and well-being.
Interventions to address the restricted eating patterns of children with DD, especially those with ASD, are needed, and represent an important step in improving their diets to promote maintenance of a healthy weight. Use of behavior modification techniques to increase food acceptance has shown some success [146]. However, the majority of studies have been case reports, which do not permit treatment comparisons or conclusions to be drawn about generalizability to the larger population of children with ASD [146]. The link between food selectivity and obesity has yet to be firmly established and further work in this area is warranted and shows promise as part of an obesity prevention effort.
Barriers to engagement in physical activity are real, but in some contexts attributes of the disability may provide opportunity. For example, initiating a structured physical activity routine for the child and family may provide an opportunity for physical activity that meets the child’s need for sameness and repetition, particularly if delivered using a video format. Such guidance must be tailored to meet the needs of parents of children with DD, who will likely need additional support and specific resources to manage eating patterns and help their children develop an interest and motivation to participate in physical activity. Documented benefits of physical activity programming specifically for youth with DD include improvements in aerobic capacity, gross motor function, and high levels of satisfaction for both youth participants and parents [147]. To our knowledge, no prospective studies examining the effects of such programming on weight outcomes for youth with DD have been conducted, but the benefits of physical activity overall are well-established, and underscores the pressing need for further work in this area.
The impact of family context on obesogenic behaviors of children with DD is understudied, but may be significant given this population’s higher dependence on parents, siblings, and caretakers to accommodate their food and physical activity preferences [148], adapt mealtime, and feeding routines [149], and to role model and reinforce food rules and social norms around eating [150]. The family is increasing recognized as the locus of many modifiable risk factors for childhood obesity [99] and functions as the primary “social institution” influencing young children. In the absence of studies of parenting styles and practices specific to children with DD, it is critical to consider that the demands of caring for these children may have differential impacts on family dynamics.
Parents of children with DD experience challenges that are not faced by parents of typically developing children [105]. The obesogenic effects of medications prescribed for behavioral disorders pose a tremendous challenge for families. Given that up to 60% of children with ASD may be prescribed at least one antipsychotic medication, there is an urgent need for effective interventions to combat medication-induced weight gain [119]. Some strategies that reduce antipsychotic-associated weight gain in other patient populations may be useful in youth with DD who are taking such medications. The primary strategies studied to date have been 1) lifestyle modification often facilitated by motivational interviewing, 2) alternative medications with lower risk of weight gain (e.g., aripiprazole rather than risperidone), or 3) addition of metformin, or other adjunctive anti-obesity medications. A motivational interviewing technique for preventing medication-induced weight gain in children with bipolar disorder, which could be helpful to some older children with less severe DD, was described in a case report [151]. One small 8-week study compared risperidone directly with aripiprazole in children with ASD and found no difference in weight over time [152]. However, a much larger observational study clearly suggested aripiprazole is associated with less weight gain in youth [153]. Few adjunctive medications have been systematically studied. In a case series, stimulants were not found to have any protective effects against anti-psychotic related weight gain [154].
Several studies have found that concurrent metformin treatment was associated with a reduction in BMI in youth and adults treated with weight-promoting antipsychotics [155–157]. No comprehensive studies have yet been completed examining the effect of lifestyle interventions for youth with antipsychotic associated weight gain. Such interventions have been shown to be effective in adults with schizophrenia, however. Figure 2 summarizes the results of two studies that attempted to moderate weight gain in adults [158] and children taking antipsychotic medication [156]. The adult study used behavioral intervention and the child study used metformin to address weight gain. As seen in Figure 2 both interventions lead to significant changes in BMI compared to the control groups, in part because the control groups, particularly in the pediatric population, show steady increases in BMI. There are also reports that adjunctive topiramate, zonisamide, amantadine, or sibutramine (no longer marketed due to cardiac concerns), may be useful in limiting antipsychotic-associated weight gain [159, 152, 160–162]. It should be noted that all of these treatments typically lead to very modest weight loss of ~1–3 kg, rather than normalizing an individual’s weight status.
Fig 2.
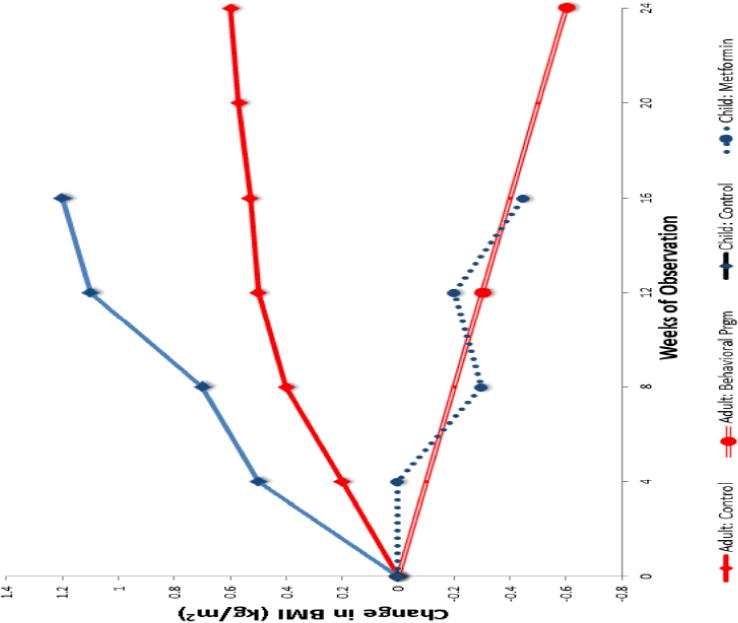
Effect of behavioral intervention (adult) or metformin (child) on antipsychotic-associated weight gain in two studies. Weight trajectory (grey lines) over 24 weeks in adults taking antipsychotic medication for schizophrenia who initiated behavioral intervention for weight (n=59) and controls (n=51) who did not receive intervention [158]. Weight trajectory (solid and dotted black dotted lines) over 16 weeks in children taking antipsychotic medication for 38 also prescribed metformin as adjunctive therapy (n=18) compared to controls not receiving metformin (n=20) [156].
Pharmacologic strategies that reduce weight gain in the general pediatric population may also be instructive [163, 164]. The only medication currently indicated for the long term treatment of obesity in youth is orlistat, which is generally well tolerated but associated with only 0.7kg/m2 loss [164]. Promising novel treatments include long-chain polyunsaturated fatty acid, zinc supplementation, and octreotide [165–168]. The American Academy of Child and Adolescent Psychiatry (AACAP) has developed practice parameters to address the use of antipsychotic medication in children and the risk for weight gain [169].
The additional risk factors of financial hardship [170–172], time constraints [79], and stressful family mealtimes [173–175] are common among families of children with DD. In this context, obesity prevention may or may not be a parental priority, but nevertheless may be a positive outcome with the provision of services and resources that support and enhance family functioning and economic well-being. The complex relationships among financial hardship, family stress, and mealtime behaviors and their relationship to obesity in families caring for children with DD are poorly understood but warrant further attention.
School Environment
The 2012 Institute of Medicine report, Accelerating Progress in Obesity Prevention, Solving the Weight of the Nation, recommended schools be the national focal point for obesity prevention, with strategic partnerships among multiple levels of government, parents, teachers, and the private sector [176]. Research supports schools as a venue to prevent obesity [177] and to improve child dietary intake and physical activity [178]. In the U.S., the Individuals with Disabilities Education Act mandates that obesity prevention efforts in school settings be inclusive of children with DD. Inclusion presents challenges because it requires skilled personnel who must consider the cognitive and physical functioning of an often heterogeneous group of children to adapt evidence-based programs to their unique needs, maximize their participation, and support self-determined health behaviors [140].
Schools have the potential to be an effective venue for obesity prevention among children with DD to the extent that they can properly address the learning needs and additional supports required for meaningful participation [179]. Results from the few studies that have examined participation in school-based obesity prevention activities in the U.S. suggest that children with DD do not participate to their maximum ability nor at levels comparable to typically developing children [179, 180]. School-based obesity prevention programs are often centered on intensive health behavior curricula implemented by teachers in classrooms, with low to moderate family involvement outside of the school environment [181–184]. However, the lack of adapted and tailored curricula for youth with DD may prevent their full participation [185] and may place significant burdens on special education teachers or school nurses to adapt and implement programs [186, 187]. Many schools do not employ adaptive physical education teachers or have adaptive equipment, which may make it difficult for children with physical or motor impairments to engage in physical activity at the same levels as their typically developing peers. In addition, nutrition concepts are abstract and may be difficult for children with DD to apply in real-world contexts without adapted curricula to facilitate their learning [188]. Parent participation is essential, and school-based initiatives that fail to include family outreach may have limited capacity to improve nutrition and fitness behaviors of children with DD [188].
Although widespread efforts are being made to include children with DD in school within their communities, some children both in the U.S. and worldwide live in residential or congregate-care settings. Although well documented for adults with DD, few studies have examined the unique obesity risk factors within such settings for children with DD or have intervened to improve them. Three recent studies in special education schools have evaluated the effects of school-based interventions on dietary intake of children and adolescents with DD. A Smarter Lunchroom intervention based on behavioral economic principles and adapted for youth aged 11–22 years with ID to improve food choices and dietary intake was evaluated in 43 students with ID enrolled in a special education school. The intervention significantly increased whole grain selection and consumption, reduced refined grain selection and consumption, increased fruit consumption, and reduced fruit and vegetable plate waste [189]. A multifactorial school-based intervention (i.e. nutrition policies, daily physical activity, lunch intervention, home newsletter, summer camp) was implemented at an upper secondary school for youth aged 16 to 21 years with DD in Sweden. The lunch intervention, based on a modified Plate Model to teach healthy food selection and portion sizes, resulted in fewer participants consuming extraneous portions (i.e. second helpings), lower fat intake, and less plate waste [190]. Effects of the 2-year intervention were also evaluated among a smaller group of participants who demonstrated improvements in several cardiovascular risk factors compared to historic controls [191]. A third study examined a weight management intervention that used direct caregivers to implement the AAP’s obesity prevention principles (e.g. limited screen time, 60 minutes of physical activity) in a residential/congregate care context [192]. The prevention principles, communication tools, and caregiver education were adapted to support the weight management of 40 youth aged 8–20 years with DD living in residential or congregate care settings in the Midwestern U.S. Although no significant improvements in BMI percentile or physical activity were seen, positive results were achieved with respect to children meeting their personal weight goals as well as improvements in mean number of fruits and vegetables consumed [192].
Results of these studies suggest that school environments have a critical role in the prevention of obesogenic behaviors among children with DD. Given that a proportion of children may continue to need more intensive residential programming, interventions adapted for youth living in these settings are needed.
Fortunately, it is likely that the majority of children with DD in the U.S. have benefited from the policy changes that accompanied the Healthy, Hunger Free Kids Act. These include school wellness policies and updated nutrition standards for the foods and beverages served in the National School Breakfast and Lunch Programs and those available as competitive foods (e.g. vending machines, school stores). Inclusion of children with DD and their families on school wellness committees can help ensure that policies are responsive to their needs. In some cases, obesity prevention approaches may need to be specifically targeted to underserved families and communities where children with DD reside.
Community
While significant efforts have aimed to improve school wellness environments, there are many opportunities to expose children to healthy foods and physical activity in the out-of-school time environment. Community gardening programs represent one promising strategy to improve dietary intake of families of children with DD through improved access and influencing motivation to try new foods. Gardening also provides opportunities for physical activity. Gardening programs have been used successfully in therapeutic contexts with persons with DD [193], and could be adapted to include nutrition and health targets. With respect to increasing levels physical activity in youth with DD, community-based efforts to sustain the higher levels observed at younger ages represents a promising obesity prevention strategy. Special Olympics is one well-known national program that engages youth with DD in physical activity and sports [194]. Although largely unexplored, other after-school, weekend, and summer activities via national organizations such as 4-H and the YMCA may have the potential to reach critical numbers of children with DD and their families by integrating obesity prevention principles into their programs. Having them do so will require commitment to inclusion and the requisite staffing and staff training.
As the health disparities experienced by persons with disabilities are increasingly recognized and a focus of concern, so too is the importance of providing opportunities for children with DD in recreational programming. The Office for Civil Rights in the U.S. Department of Education issued guidance in 2013 that clarified the obligation of schools to provide students with disabilities an equal opportunity to participate alongside their peers in after-school athletics and clubs [195]. The overall goal of the guidance was to help schools ensure that children with disabilities have an equal opportunity to benefit from the many life lessons experienced through extracurricular athletics. Additional research and professional training will be needed to understand how children with DD can engage successfully in physical activity and extramural sports.
Conclusion
Children with DD live in the same obesogenic environments as typically developing children but may be at elevated obesity risk due to additional factors that arise from their specific limitations and social circumstances. The many gaps in our understanding must be filled to develop meaningful preventive interventions and advance policy approaches to address these factors at the individual, family, community, and societal levels. The heterogeneity of the population and complex nature of the developmental conditions themselves will require interdisciplinary research efforts. As conceptualized by the National Academy of Science, interdisciplinary approaches “integrate information, data, techniques, tools, perspectives, concepts, and/or theories from two or more disciplines or bodies of specialized knowledge to advance fundamental understanding or to solve problems whose solutions are beyond the scope of a single discipline or field of research practice [196].” The MCH Research Network on Promoting Healthy Weight among Children with ASD and Developmental Disabilities, funded in 2013 by the Maternal and Child Health Bureau of the Health Resources and Services Administration, was established as an interdisciplinary research network to address the need for obesity prevention in these groups. Its mission is to advance the understanding of obesity risk factors in children with ASD and other developmental disabilities, to promote the development of evidence-based solutions to achieve healthy weight in this population, and to disseminate research findings to broad and diverse audiences. Through its conduct of research, identification of surveillance opportunities, and dissemination efforts, the network hopes to provide scientific leadership and vision in ensuring that the needs of children with DD and their families are addressed in research and clinical endeavors to promote healthy weight and enhanced quality of life in this population.
Acknowledgments
The authors thank the members of the MCH Research Network on Promoting Healthy Weight among Children with Autism Spectrum Disorder and Developmental Disabilities for their many suggestions and Sarah Phillips for her editorial assistance.
Funding sources: This effort was supported by cooperative agreement UA3MC25735 Maternal and Child Health Research Program, Maternal and Child Health Bureau (Title V, Social Security Act), Health Resources and Services Administration, Department of Health and Human Services; the Boston Nutrition Obesity Research Center NIHDK046200; and Interdisciplinary Research in Intellectual and Developmental Disabilities 2P30HD004147-33A2
Contributor Information
Aviva Must, Email: aviva.must@tufts.edu, Department of Public Health and Community Medicine, Tufts University School of Medicine, 136 Harrison Avenue, Boston, MA 02111.
Carol Curtin, Email: carol.curtin@umassmed.edu, Eunice Kennedy Shriver Center, University of Massachusetts Medical School, 465 Medford Street, Suite 500, Charlestown, MA 02129.
Kristie Hubbard, Email: kristie.hubbard@tufts.edu, Friedman School of Nutrition Science and Policy, Tufts University, 75 Kneeland Street, 8th Floor, Boston, MA 02111.
Linmarie Sikich, Email: lsikich@med.unc.edu, Department of Psychiatry, University of North Carolina at Chapel Hill, CB 7167 UNC-CH, Chapel Hill, NC 27599-7167.
James Bedford, Email: jbedford@unch.unc.edu, Department of Psychiatry, University of North Carolina at Chapel Hill, CB 7160 UNC-CH, Chapel Hill, NC 27599-7160.
Linda Bandini, Email: linda.bandini@umassmed.edu, Eunice Kennedy Shriver Center, University of Massachusetts Medical School, 465 Medford Street, Suite 500, Charlestown, MA 02129; Department of Health Sciences, Boston University, 635 Commonwealth Ave. Boston, MA 02115.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA – Journal of the American Medical Association. 2012;307(5):483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Obesity and overweight. Fact sheet No. 311. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/2013.
- 3.Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, et al. The global obesity pandemic: Shaped by global drivers and local environments. The Lancet. 2011;378(9793):804–14. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 4.Cohen AK, Rai M, Rehkopf DH, Abrams B. Educational attainment and obesity: A systematic review. Obesity Reviews. 2013;14(12):989–1005. doi: 10.1111/obr.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandini LG, Curtin C, Hamad C, Tybor DJ, Must A. Prevalence of overweight in children with developmental disorders in the continuous National Health And Nutrition Examination Survey (NHANES) 1999–2002. Journal of Pediatrics. 2005;146(6):738–43. doi: 10.1016/j.jpeds.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 6.Chen AY, Kim SE, Houtrow AJ, Newacheck PW. Prevalence of obesity among children with chronic conditions. Obesity. 2009;18:210–3. doi: 10.1038/oby.2009.185. [DOI] [PubMed] [Google Scholar]
- 7.Curtin C, Anderson SE, Must A, Bandini L. The prevalence of obesity in children with autism: A secondary data analysis using nationally representative data from the National Survey of Children’s Health. BMC Pediatrics. 2010;10 doi: 10.1186/1471-2431-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Developmental Disabilities Assistance and Bill of Rights Act 2000 Public Law 106–402. Section. 102(8) [Google Scholar]
- 9.Boyle CA, Boulet S, Schieve LA, Cohen RA, Blumberg SJ, Yeargin-Allsopp M, et al. Trends in the prevalence of developmental disabilities in US Children, 1997–2008. Pediatrics. 2011;127(6):1034–42. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- 10.Baio J, Prevalence of autism spectrum disorders . National Center on Birth Defects and Developmental Disabilities. Centers for Disease Control; Atlanta, GA: 2012. Autism and Developmental Disabilities Monitoring Network, Surveillance Summaries. http://www.cdc.gov/mmwr/preview/mmwrhtml/ss6103a1.htm?s_cid=ss6103a1_w. Accessed Jan 3 2013. [Google Scholar]
- 11.US Public Health Service. Report of the Surgeon General’s Conference on Health Disparities in Mental Retardation. Washington, D.C.: US Department of Health and Human Services; 2002. Closing the Gap: A National Blueprint for Improving the Health of Individuals with Mental Retardation. [Google Scholar]
- 12.Havercamp SM, Scandlin D, Roth M. Health disparities among adults with developmental disabilities, adults with other disabilities, and adults not reporting disability in North Carolina. Public Health Reports. 2004;119(4):418–26. doi: 10.1016/j.phr.2004.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haveman M, Heller T, Lee L, Maaskant M, Shooshtari S, Strydom A. Major health risks in aging persons with intellectual disabilities: An overview of recent studies. Journal of Policy and Practice in Intellectual Disabilities. 2010;7(1):59–69. [Google Scholar]
- 14.Melville CA, Hamilton S, Hankey CR, Miller S, Boyle S. The prevalence and determinants of obesity in adults with intellectual disabilities. Obesity Reviews. 2007;8(3):223–30. doi: 10.1111/j.1467-789X.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- 15.Must A, Strauss RS. Risks and consequences of childhood and adolescent obesity. International Journal of Obesity. 1999;23(SUPPL. 2):S2–S11. doi: 10.1038/sj.ijo.0800852. [DOI] [PubMed] [Google Scholar]
- 16.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. CDC growth charts for the United States: methods and development. Vital and health statistics Series 11 Data from the national health survey. 2000;246:1–190. [PubMed] [Google Scholar]
- 17.Bandini LG, Fleming RK, Scampini R, Gleason J, Must A. Is body mass index a useful measure of excess body fatness in adolescents and young adults with Down syndrome? Journal of Intellectual Disability Research. 2013;57(11):1050–7. doi: 10.1111/j.1365-2788.2012.01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cacciari E, Milani S, Balsamo A, Dammacco F, De Luca F, Chiarelli F, et al. Italian cross-sectional growth charts for height, weight and BMI (6–20y) European Journal of Clinical Nutrition. 2002;56(2):171–80. doi: 10.1038/sj.ejcn.1601314. [DOI] [PubMed] [Google Scholar]
- 19.Cole TJ, Freeman JV, Preece MA. Body mass index reference curves for the UK, 1990. Archives of Disease in Childhood. 1995;73(1):25–9. doi: 10.1136/adc.73.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inokuchi M, Hasegawa T, Anzo M, Matsuo N. Standardized centile curves of body mass index for Japanese children and adolescents based on the 1978–1981 national survey data. Annals of Human Biology. 2006;33(4):444–53. doi: 10.1080/03014460600802353. [DOI] [PubMed] [Google Scholar]
- 21.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bulletin of the World Health Organization. 2007;85:660–7. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emerson E, Robertson J. Obesity in young children with intellectual disabilities or borderline intellectual functioning. International Journal of Pediatric Obesity. 2010;5(4):320–6. doi: 10.3109/17477160903473713. [DOI] [PubMed] [Google Scholar]
- 23.Rimmer JH, Yamaki K, Lowry BMD, Wang E, Vogel LC. Obesity and obesity-related secondary conditions in adolescents with intellectual/developmental disabilities. Journal of Intellectual Disability Research. 2010;54(9):787–94. doi: 10.1111/j.1365-2788.2010.01305.x. [DOI] [PubMed] [Google Scholar]
- 24.Van Gameren-Oosterom HBM, Van Dommelen P, Schönbeck Y, Oudesluys-Murphy AM, Van Wouwe JP, Buitendijk SE. Prevalence of overweight in Dutch children with Down syndrome. Pediatrics. 2012;130(6):e1520–e6. doi: 10.1542/peds.2012-0886. [DOI] [PubMed] [Google Scholar]
- 25.Curtin C, Bandini LG, Perrin EC, Tybor DJ, Must A. Prevalence of overweight in children and adolescents with attention deficit hyperactivity disorder and autism spectrum disorders: A chart review. BMC Pediatrics. 2005;5 doi: 10.1186/1471-2431-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egan AM, Dreyer ML, Odar CC, Beckwith M, Garrison CB. Obesity in young children with autism spectrum disorders: Prevalence and associated factors. Childhood Obesity. 2013;9(2):125–31. doi: 10.1089/chi.2012.0028. [DOI] [PubMed] [Google Scholar]
- 27.Lin JD, Yen CF, Li CW, Wu JL. Patterns of obesity among children and adolescents with intellectual disabilities in Taiwan. Journal of Applied Research in Intellectual Disabilities. 2005;18(2):123–9. [Google Scholar]
- 28.Mikulovic J, Marcellini A, Compte R, Duchateau G, Vanhelst J, Fardy PS, et al. Prevalence of overweight in adolescents with intellectual deficiency. Differences in socio-educative context, physical activity and dietary habits. Appetite. 2011;56(2):403–7. doi: 10.1016/j.appet.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Rimmer JH, Yamaki K, Davis BM, Wang E, Vogel LC. Obesity and overweight prevalence among adolescents with disabilities. Preventing Chronic Disease. 2011;8(2) [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart L, Van de Ven L, Katsarou V, Rentziou E, Doran M, Jackson P, et al. High prevalence of obesity in ambulatory children and adolescents with intellectual disability. Journal of Intellectual Disability Research. 2009;53(10):882–6. doi: 10.1111/j.1365-2788.2009.01200.x. [DOI] [PubMed] [Google Scholar]
- 31.Bégarie J, Maïano C, Leconte P, Ninot G. The prevalence and determinants of overweight and obesity among French youths and adults with intellectual disabilities attending special education schools. Research in Developmental Disabilities. 2013;34(5):1417–25. doi: 10.1016/j.ridd.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Bandini LG, Must A, Spadano JL, Dietz WH. Relation of body composition, parental overweight, pubertal stage, and race-ethnicity to energy expenditure among premenarcheal girls. American Journal of Clinical Nutrition. 2002;76(5):1040–7. doi: 10.1093/ajcn/76.5.1040. [DOI] [PubMed] [Google Scholar]
- 33.González-Agüero A, Ara I, Moreno LA, Vicente-Rodríguez G, Casajús JA. Fat and lean masses in youths with Down syndrome: Gender differences. Research in Developmental Disabilities. 2011;32(5):1685–93. doi: 10.1016/j.ridd.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 34.Hill DL, Parks EP, Zemel BS, Shults J, Stallings VA, Stettler N. Resting energy expenditure and adiposity accretion among children with Down syndrome: A 3-year prospective study. European Journal of Clinical Nutrition. 2013;67(10):1087–91. doi: 10.1038/ejcn.2013.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luke A, Sutton M, Schoeller DA, Roizen NJM. Nutrient intake and obesity in prapubescent children with Down syndrome. Journal of the American Dietetic Association. 1996;96(12):1262–7. doi: 10.1016/S0002-8223(96)00330-6. [DOI] [PubMed] [Google Scholar]
- 36.Conrey EJ, Welsh J, Sherry B, Rockett H, Mehrle D, Grummer-Strawn L. Association between overweight among low-income preschoolers and fruit and vegetable consumption. American Journal of Epidemiology. 2004;1589(11):S75. [Google Scholar]
- 37.Newby PK, Peterson KE, Berkey CS, Leppert J, Willett WC, Colditz GA. Dietary composition and weight change among low-income preschool children. Archives of Pediatrics & Adolescent Medicine. 2003;157(8):759–64. doi: 10.1001/archpedi.157.8.759. [DOI] [PubMed] [Google Scholar]
- 38.Siega-Riz AM, Popkin BM. US adolescent food intake trends from 1965 to 1996. Western Journal of Medicine. 2000;173(6):378–83. doi: 10.1136/ewjm.173.6.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: A prospective, observational analysis. Lancet. 2001;357(9255):505–8. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 40.Wang YC, Bleich SN, Gortmaker SL. Increasing caloric contribution from sugar-sweetened beverages and 100% fruit juices among US children and adolescents, 1988–2004. Pediatrics. 2008;121(6):E1604–E14. doi: 10.1542/peds.2007-2834. [DOI] [PubMed] [Google Scholar]
- 41.Piernas C, Popkin BM. Trends in snacking among US children. Health Affairs. 2010;29(3):398–404. doi: 10.1377/hlthaff.2009.0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reedy J, Krebs-Smith SM. Dietary Sources of Energy, Solid Fats, and Added Sugars among Children and Adolescents in the United States. Journal of the American Dietetic Association. 2010;110(10):1477–84. doi: 10.1016/j.jada.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emond A, Emmett P, Steer C, Golding J. Feeding symptoms, dietary patterns, and growth in young children with autism spectrum disorders. Pediatrics. 2010;126(2):e337–e42. doi: 10.1542/peds.2009-2391. [DOI] [PubMed] [Google Scholar]
- 44.Evans EW, Must A, Anderson SE, Curtin C, Scampini R, Maslin M, et al. Dietary patterns and body mass index in children with autism and typically developing children. Research in Autism Spectrum Disorders. 2012;6(1):399–405. doi: 10.1016/j.rasd.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lukens CT, Linscheid TR. Development and validation of an inventory to assess mealtime behavior problems in children with autism. Journal of Autism and Developmental Disorders. 2008;38(2):342–52. doi: 10.1007/s10803-007-0401-5. [DOI] [PubMed] [Google Scholar]
- 46.Curtin C, Bandini LG, Must A, Gleason J, Lividini K, Phillips S, et al. Parent support improves weight loss in adolescents and young adults with down syndrome. Journal of Pediatrics. 2013;163(5):1402–8e1. doi: 10.1016/j.jpeds.2013.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yen CF, Lin JD. Factors for healthy food or less-healthy food intake among Taiwanese adolescents with intellectual disabilities. Research in Developmental Disabilities. 2010;31(1):203–11. doi: 10.1016/j.ridd.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Schreck KA, Williams K, Smith AF. A comparison of eating behaviors between children with and without autism. Journal of Autism and Developmental Disorders. 2004;34(4):433–8. doi: 10.1023/b:jadd.0000037419.78531.86. [DOI] [PubMed] [Google Scholar]
- 49.Williams KE, Gibbons BG, Schreck KA. Comparing selective eaters with and without developmental disabilities. Journal of Developmental and Physical Disabilities. 2005;17(3):299–309. [Google Scholar]
- 50.Ledford JR, Gast DL. Feeding problems in children with autism spectrum disorders: a review. Focus on Autism and Other Developmental Disabilities. 2004;21:153–66. [Google Scholar]
- 51.Sharp WG, Berry RC, McCracken C, Nuhu NN, Marvel E, Saulnier CA, et al. Feeding Problems and Nutrient Intake in Children with Autism Spectrum Disorders: A Meta-analysis and Comprehensive Review of the Literature. J Autism Dev Disord. 2013;43(9):2159–73. doi: 10.1007/s10803-013-1771-5. [DOI] [PubMed] [Google Scholar]
- 52.Tomchek SD, Dunn W. Sensory processing in children with and without autism: a comparative study using the short sensory profile. American Journal of Occupational Therapy. 2007;61(2):190–200. doi: 10.5014/ajot.61.2.190. [DOI] [PubMed] [Google Scholar]
- 53●.Bandini LG, Anderson SE, Curtin C, Cermak S, Evans EW, Scampini R, et al. Food selectivity in children with autism spectrum disorders and typically developing children. Journal of Pediatrics. 2010;157(2):259–64. doi: 10.1016/j.jpeds.2010.02.013. This paper establishes a operational definition of food selectivity based on dietary assessment and provides a comparison of food selectivity in children with ASD and typically developing children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cornish E. A balanced approach towards healthy eating in autism. Journal of Human Nutrition and Dietetics. 1998;11(6):501–9. [Google Scholar]
- 55.Schmitt L, Heiss CJ, Campbell EE. A comparison of nutrient intake and eating behaviors of boys with and without autism. Topics in Clinical Nutrition. 2008;23(1):23–31. [Google Scholar]
- 56.Schreck KA, Williams K. Food preferences and factors influencing food selectivity for children with autism spectrum disorders. Research in Developmental Disabilities. 2006;27(4):353–63. doi: 10.1016/j.ridd.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 57.Dewey D, Cantell M, Crawford SG. Motor and general performance in children with autism spectrum disorders, developmental coordination disorder, and/or attention deficit hyperactivity disorder. Journal of the International Neuropsychological Society. 2007;13(2):246–56. doi: 10.1017/S1355617707070270. [DOI] [PubMed] [Google Scholar]
- 58.Minshew NJ, Sung K, Jones BL, Furman JM. Underdevelopment of the postural control system in autism. Neurology. 2004;63(11):2056–61. doi: 10.1212/01.wnl.0000145771.98657.62. [DOI] [PubMed] [Google Scholar]
- 59.Molloy CA, Dietrich KN, Bhattacharya A. Postural Stability in Children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders. 2003;33(6):643–52. doi: 10.1023/b:jadd.0000006001.00667.4c. [DOI] [PubMed] [Google Scholar]
- 60.Provost B, Lopez BR, Heimerl S. A comparison of motor delays in young children: Autism spectrum disorder, developmental delay, and developmental concerns. Journal of Autism and Developmental Disorders. 2007;37(2):321–8. doi: 10.1007/s10803-006-0170-6. [DOI] [PubMed] [Google Scholar]
- 61.Westendorp M, Houwen S, Hartman E, Visscher C. Are gross motor skills and sports participation related in children with intellectual disabilities? Research in Developmental Disabilities. 2011;32(3):1147–53. doi: 10.1016/j.ridd.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 62.Dziuk MA, Larson JCG, Apostu A, Mahone EM, Denckla MB, Mostofsky SH. Dyspraxia in autism: Association with motor, social, and communicative deficits. Dev Med Child Neurol. 2007;49(10):734–9. doi: 10.1111/j.1469-8749.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- 63.Faison-Hodge J, Porretta DL. Physical Activity Levels of Students with Mental Retardation and Students Without Disabilities. Adapted Physical Activity Quarterly. 2004;21(2):139–52. [Google Scholar]
- 64.Lorenzi DG, Horvat M, Pellegrini AD. Physical activity of children with and without mental retardation in inclusive recess settings. Education and Training in Mental Retardation and Developmental Disabilities. 2000;35(2):160–7. [Google Scholar]
- 65.Sharav T, Bowman T. Dietary practices, physical activity, and body-mass index in a selected population of Down syndrome children and their siblings. Clinical Pediatrics. 1992;31(6):341–4. doi: 10.1177/000992289203100605. [DOI] [PubMed] [Google Scholar]
- 66.Suzuki M, Saitoh S, Tasaki Y, Shimomura Y, Makishima R, Hosoya N. Nutritional status and daily physical activity of handicapped students in Tokyo metropolitan schools for deaf, blind, mentally retarded, and physically handicapped individuals. American Journal of Clinical Nutrition. 1991;54(6):1101–11. doi: 10.1093/ajcn/54.6.1101. [DOI] [PubMed] [Google Scholar]
- 67.Whitt-Glover MC, O’Neill KL, Stettler N. Physical activity patterns in children with and without Down syndrome. Pediatric Rehabilitation. 2006;9(2):158–64. doi: 10.1080/13638490500353202. [DOI] [PubMed] [Google Scholar]
- 68.Lin JD, Lin PY, Lin LP, Chang YY, Wu SR, Wu JL. Physical activity and its determinants among adolescents with intellectual disabilities. Research in Developmental Disabilities. 2010;31(1):263–9. doi: 10.1016/j.ridd.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 69.Matute-Llorente A, González-Agüero A, Gómez-Cabello A, Vicente-Rodríguez G, Casajús JA. Decreased levels of physical activity in adolescents with down syndrome are related with low bone mineral density: A cross-sectional study. BMC Endocrine Disorders. 2013;13 doi: 10.1186/1472-6823-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Phillips AC, Holland AJ. Assessment of objectively measured physical activity levels in individuals with intellectual disabilities with and without Down’s syndrome. PLoS ONE. 2011;6(12) doi: 10.1371/journal.pone.0028618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shields N, Dodd KJ, Abblitt C. Do children with down syndrome perform sufficient physical activity to maintain good health? A pilot study. Adapted Physical Activity Quarterly. 2009;26(4):307–20. doi: 10.1123/apaq.26.4.307. [DOI] [PubMed] [Google Scholar]
- 72.Bandini LG, Gleason J, Curtin C, Lividini K, Anderson SE, Cermak SA, et al. Comparison of physical activity between children with autism spectrum disorders and typically developing children. Autism : the international journal of research and practice. 2013;17(1):44–54. doi: 10.1177/1362361312437416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosser-Sandt DD, Frey GC. Comparison of physical activitly levels between children with and without autism spectrum disorders. Adapted Physical Activity Quarterly. 2005;22:146–59. [Google Scholar]
- 74.MacDonald M, Esposito P, Ulrich D. The physical activity patterns of children with autism. BMC Research Notes. 2011;4 doi: 10.1186/1756-0500-4-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Memari AH, Ghaheri B, Ziaee V, Kordi R, Hafizi S, Moshayedi P. Physical activity in children and adolescents with autism assessed by triaxial accelerometry. Pediatric Obesity. 2013;8(2):150–8. doi: 10.1111/j.2047-6310.2012.00101.x. [DOI] [PubMed] [Google Scholar]
- 76.Pan CY, Frey GC. Physical activity patterns in youth with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2006;36(5):597–606. doi: 10.1007/s10803-006-0101-6. [DOI] [PubMed] [Google Scholar]
- 77.Pan CY, Tsai CL, Hsieh KW, Chu CH, Li YL, Huang ST. Accelerometer-determined physical activity among elementary school-aged children with autism spectrum disorders in Taiwan. Research in Autism Spectrum Disorders. 2011;5(3):1042–52. [Google Scholar]
- 78.Pan CY. Objectively measured physical activity between children with autism spectrum disorders and children without disabilities during inclusive recess settings in Taiwan. Journal of Autism and Developmental Disorders. 2008;38(7):1292–301. doi: 10.1007/s10803-007-0518-6. [DOI] [PubMed] [Google Scholar]
- 79.Obrusnikova I, Miccinello DL. Parent perceptions of factors influencing after-school physical activity of children with autism spectrum disorders. Adapted Physical Activity Quarterly. 2012;29(1):63–80. doi: 10.1123/apaq.29.1.63. [DOI] [PubMed] [Google Scholar]
- 80.Barr M, Shields N. Identifying the barriers and facilitators to participation in physical activity for children with Down syndrome. Journal of Intellectual Disability Research. 2011;55(11):1020–33. doi: 10.1111/j.1365-2788.2011.01425.x. [DOI] [PubMed] [Google Scholar]
- 81.Buttimer J, Tierney E. Patterns of leisure participation among adolescents with a mild intellectual disability. Journal of Intellectual Disabilities. 2005;9(1):25–42. doi: 10.1177/1744629505049728. [DOI] [PubMed] [Google Scholar]
- 82.Sayers Menear K. Parents’ perceptions of health and physical activity needs of children with Down syndrome. Down’s syndrome, research and practice : the journal of the Sarah Duffen Centre / University of Portsmouth. 2007;12(1):60–8. doi: 10.3104/reports.1996. [DOI] [PubMed] [Google Scholar]
- 83.Yazdani S, Yee CT, Chung PJ. Factors predicting physical activity among children with special needs. Preventing Chronic Disease. 2013;10(7) doi: 10.5888/pcd10.120283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Must A, Phillips S, Curtin C, Bandini L. Barriers to physical activity in children with autism spectrum disorders: relationship to physical activity and screen time. Journal of Physical Activity and Health. 2014 doi: 10.1123/jpah.2013-0271. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Obrusnikova I, Cavalier AR. Perceived barriers and facilitators of participation in after-school physical activity by children with autism spectrum disorders. Journal of Developmental and Physical Disabilities. 2011;23:195–211. doi: 10.1007/s10882-010-9215-z. [DOI] [Google Scholar]
- 86.King G, Law M, King S, Rosenbaum P, Kertoy MK, Young NL. A conceptual model of the factors affecting the recreation and leisure participation of children with disabilities. Physical & Occupational Therapy in Pediatrics. 2003;23(1):63–90. [PubMed] [Google Scholar]
- 87.Sedentary Behaviour Research Network. Standardized use of the terms “sedentary” and “sedentary behaviours”. Appl Physiol Nutr Metab. 2012;37:540–2. doi: 10.1139/h2012-024. [DOI] [PubMed] [Google Scholar]
- 88.Coon KA, Goldberg J, Rogers BL, Tucker KL. Relationships between use of television during meals and children’s food consumption patterns. Pediatrics. 2001;107(1) doi: 10.1542/peds.107.1.e7. [DOI] [PubMed] [Google Scholar]
- 89.Jordan AB, Robinson TN. Children, television viewing, and weight status: Summary and recommendations from an expert panel meeting. Annals of the American Academy of Political and Social Science. 2008;615(1):119–32. [Google Scholar]
- 90●.Mazurek MO, Shattuck PT, Wagner M, Cooper BP. Prevalence and correlates of screen-based media use among youths with autism spectrum disorders. Journal of Autism & Developmental Disorders. 2012;42(8):1757–67. doi: 10.1007/s10803-011-1413-8. Thestudy characterizes screen-based media use amonga large, nationally representative sample of youths participating in the National Longitudinal Transition Study 2(NLTS2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Orsmond GI, Kuo H-Y. The daily lives of adolescents with an Autism spectrum disorder. Discretionary time use and activity partners. Autism : the international journal of research and practice. 2011;15(5):579–99. doi: 10.1177/1362361310386503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shane HC, Albert PD. Electronic screen media for persons with autism spectrum disorders: results of a survey. Journal of Autism & Developmental Disorders. 2008;38(8):1499–508. doi: 10.1007/s10803-007-0527-5. [DOI] [PubMed] [Google Scholar]
- 93.Chonchaiya W, Nuntnarumit P, Pruksananonda C. Comparison of television viewing between children with autism spectrum disorder and controls. Acta Pædiatrica. 2011;100(7):1033–7. doi: 10.1111/j.1651-2227.2011.02166.x. [DOI] [PubMed] [Google Scholar]
- 94.Charlop-Christy M, Carpenter M, Le L, LeBlanc L, Kellet K. Using the picture-exchange communication system (PECS) with children with autism: assessment of pecs acquisition, speech, social-communicative behavior, and problem behavior. Journal of Applied Behavior Analysis. 2002;35(3):213–31. doi: 10.1901/jaba.2002.35-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Corbett B, Abdullah M. Video Modeling: Why Does It Work for Children with Autism? Journal of Early and Intensive Behavior Intervention. 2005;2(1):2–8. [Google Scholar]
- 96.Nally B, Houlton B, Ralph S, Mudford O. Researches in Brief: The management of television and video by parents of children with autism. Autism : the international journal of research and practice. 2000;4(3):331–8. [Google Scholar]
- 97.Birch LL, Davison KK. Family environmental factors influencing the developing behavioral controls of food intake and childhood overweight. Pediatric Clinics of North America. 2001;48(4):893–907. doi: 10.1016/s0031-3955(05)70347-3. [DOI] [PubMed] [Google Scholar]
- 98.Golan M, Crow S. Parents Are Key Players in the Prevention and Treatment of Weight-related Problems. Nutrition Reviews. 2004;62(1):39–50. doi: 10.1111/j.1753-4887.2004.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 99.Ventura AK, Birch LL. Does parenting affect children’s eating and weight status? International Journal of Behavioral Nutrition and Physical Activity. 2008;5 doi: 10.1186/1479-5868-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gillman MW, Rifas-Shiman SL, Frazier AL, Rockett HRH, Camargo CA, Field AE, et al. Family dinner and diet quality among older children and adolescents. Arch Fam Med. 2000;9(3):235–40. doi: 10.1001/archfami.9.3.235. [DOI] [PubMed] [Google Scholar]
- 101.Larson NI, Neumark-Sztainer D, Hannan PJ, Story M. Family Meals during Adolescence Are Associated with Higher Diet Quality and Healthful Meal Patterns during Young Adulthood. Journal of the American Dietetic Association. 2007;107(9):1502–10. doi: 10.1016/j.jada.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 102.Sen B. Frequency of Family Dinner and Adolescent Body Weight Status: Evidence from the National Longitudinal Survey of Youth, 1997. Obesity. 2006;14(12):2266–76. doi: 10.1038/oby.2006.266. [DOI] [PubMed] [Google Scholar]
- 103.Mendelson BK, White DR, Schliecker E. Adolescents’ weight, sex, and family functioning. International Journal of Eating Disorders. 1995;17(1):73–9. doi: 10.1002/1098-108x(199501)17:1<73::aid-eat2260170110>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 104.Zeller MH, Reiter-Purtill J, Modi AC, Gutzwiller J, Vannatta K, Davies WH. Controlled study of critical parent and family factors in the obesigenic environment. Obesity. 2007;15(1):126–36. doi: 10.1038/oby.2007.517. [DOI] [PubMed] [Google Scholar]
- 105.Abbeduto L, Seltzer MM, Shattuck P, Krauss MW, Orsmond G, Murphy MM. Psychological well-being and coping in mothers of youths with autism, Down syndrome, or fragile X syndrome. American Journal on Mental Retardation. 2004;109(3):237–54. doi: 10.1352/0895-8017(2004)109<237:PWACIM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 106.Gray DE. Gender and coping: The parents of children with high functioning autism. Social Science and Medicine. 2003;56(3):631–42. doi: 10.1016/s0277-9536(02)00059-x. [DOI] [PubMed] [Google Scholar]
- 107.Hastings R, Brown T. Behavior problems of children with autism, parental self-efficacy, and mental health. American Journal of Mental Retardation. 2002;107:222–32. doi: 10.1352/0895-8017(2002)107<0222:BPOCWA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 108.Hastings RP, Kovshoff H, Ward NJ, Degli Espinosa F, Brown T, Remington B. Systems analysis of stress and positive perceptions in mothers and fathers of pre-school children with autism. Journal of Autism and Developmental Disorders. 2005;35(5):635–44. doi: 10.1007/s10803-005-0007-8. [DOI] [PubMed] [Google Scholar]
- 109.Hutton A, Caron S. Experiences of families with children with autism in rural New England. Focus on Autism and Other Developmental Disabilities. 2005;20:108–89. [Google Scholar]
- 110.Little L. Differences in stress and coping for mothers and fathers of children with Asperger’s syndrome and nonverbal learning disorders. Pediatric nursing. 2002;28(6):565–70. [PubMed] [Google Scholar]
- 111.Montes G, Halterman JS. Psychological functioning and coping among mothers of children with autism: A population-based study. Pediatrics. 2007;119(5):e1040–e6. doi: 10.1542/peds.2006-2819. [DOI] [PubMed] [Google Scholar]
- 112.Pisula E. A comparative study of stress profiles in mothers of children with autism and those of children with down’s syndrome. Journal of Applied Research in Intellectual Disabilities. 2007;20(3):274–8. [Google Scholar]
- 113.Field D, Garland M, Williams K. Correlates of specific childhood feeding problems. Journal of Paediatrics and Child Health. 2003;39(4):299–304. doi: 10.1046/j.1440-1754.2003.00151.x. [DOI] [PubMed] [Google Scholar]
- 114.Sanders MR, Patel RK, Le Grice B, Shepherd RW. Children With Persistent Feeding Difficulties: An Observational Analysis of the Feeding Interactions of Problem and Non-Problem Eaters. Health Psychology. 1993;12(1):64–73. doi: 10.1037//0278-6133.12.1.64. [DOI] [PubMed] [Google Scholar]
- 115.Fisher JO, Birch LL. Restricting access to palatable foods affects children’s behavioral response, food selection, and intake. American Journal of Clinical Nutrition. 1999;69(6):1264–72. doi: 10.1093/ajcn/69.6.1264. [DOI] [PubMed] [Google Scholar]
- 116.O’Neill KL, Shults J, Stallings VA, Stettler N. Child-feeding practices in children with down syndrome and their siblings. Journal of Pediatrics. 2005;146(2):234–8. doi: 10.1016/j.jpeds.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 117.Hendy HM, Williams KE, Riegel K, Paul C. Parent mealtime actions that mediate associations between children’s fussy-eating and their weight and diet. Appetite. 2010;54(1):191–5. doi: 10.1016/j.appet.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 118.McGillivray J, McVilly K, Skouteris H, Boganin C. Parental factors associated with obesity in children with disability: a systematic review. Obesity Reviews. 2013;14(7):541–54. doi: 10.1111/obr.12031. [DOI] [PubMed] [Google Scholar]
- 119.Siegel M. Psychopharmacology of Autism Spectrum Disorder. Evidence and Practice. Child and Adolescent Psychiatric Clinics of North America. 2012;21(4):957–73. doi: 10.1016/j.chc.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 120.FDA. First drug to treat irritability associated with autism. FDA Consumer. 2007;41(1):4. [PubMed] [Google Scholar]
- 121.Logan SL, Nicholas JS, Carpenter LA, King LB, Garrett-Mayer E, Charles JM. High prescription drug use and associated costs among medicaid-eligible children with autism spectrum disorders identified by a population-based surveillance network. Annals of Epidemiology. 2012;22(1):1–8. doi: 10.1016/j.annepidem.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marcus RN, Owen R, Manos G, Mankoski R, Kamen L, McQuade RD, et al. Aripiprazole in the treatment of irritability in pediatric patients (aged 6–17 years) with autistic disorder: results from a 52-week, open-label study. Journal of Child & Adolescent Psychopharmacology. 2011;21(3):229–36. doi: 10.1089/cap.2009.0121. [DOI] [PubMed] [Google Scholar]
- 123.Research Units on Pediatric Psychopharmacology Autism N. Risperidone treatment of autistic disorder: longer-term benefits and blinded discontinuation after 6 months. American Journal of Psychiatry. 2005;162(7):1361–9. doi: 10.1176/appi.ajp.162.7.1361. [DOI] [PubMed] [Google Scholar]
- 124.Troost PW, Lahuis BE, Steenhuis M-P, Ketelaars CEJ, Buitelaar JK, van Engeland H, et al. Long-term effects of risperidone in children with autism spectrum disorders: a placebo discontinuation study. Journal of the American Academy of Child & Adolescent Psychiatry. 44(11):1137–44. doi: 10.1097/01.chi.0000177055.11229.76. [DOI] [PubMed] [Google Scholar]
- 125.Waknine Y. FDA Approves Aripiprazole to Treat Irritability in Autistic Children. Medscape. 2009 http://www.medscape.com/viewarticle/713006.
- 126.Zito JM, Burcu M, Ibe A, Safer DJ, Magder LS. Antipsychotic use by medicaid-insured youths: impact of eligibility and psychiatric diagnosis across a decade. Psychiatric Services. 2013;64(3):223–9. doi: 10.1176/appi.ps.201200081. [DOI] [PubMed] [Google Scholar]
- 127.Demb H, Valicenti-Mcdermott M, Navarro A, Ayoob KT. The effect of long-term use of risperidone on body weight of children with an autism spectrum disorder. Journal of Clinical Psychopharmacology. 2011;31(5):669–70. doi: 10.1097/JCP.0b013e31822befa9. [DOI] [PubMed] [Google Scholar]
- 128.Basile VS, Masellis M, McIntyre RS, Meltzer HY, Lieberman JA, Kennedy JL. Genetic dissection of atypical antipsychotic-induced weight gain: Novel preliminary data on the pharmacogenetic puzzle. Journal of Clinical Psychiatry. 2001;62(SUPPL. 23):45–66. [PubMed] [Google Scholar]
- 129.Crilly J. The history of clozapine and its emergence in the US market: A review and analysis. History of Psychiatry. 2007;18(1):39–60. doi: 10.1177/0957154X07070335. [DOI] [PubMed] [Google Scholar]
- 130.Scheltema Beduin A, De Haan L. Off-label second generation antipsychotics for impulse regulation disorders: A review. Psychopharmacology Bulletin. 2010;43(3):45–81. [PubMed] [Google Scholar]
- 131.Simpson MM, Goetz RR, Devlin MJ, Goetz SA, Walsh BT. Weight gain and antipsychotic medication: Differences between antipsychotic-free and treatment periods. Journal of Clinical Psychiatry. 2001;62(9):694–700. doi: 10.4088/jcp.v62n0906. [DOI] [PubMed] [Google Scholar]
- 132.Grundy SM, Hansen B, Smith Jr SC, Cleeman JI, Kahn RA. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Arteriosclerosis, thrombosis, and vascular biology. 2004;24(2):e19–24. doi: 10.1161/01.ATV.0000112379.88385.67. [DOI] [PubMed] [Google Scholar]
- 133.Maayan L, Correll CU. Weight gain and metabolic risks associated with antipsychotic medications in children and adolescents. Journal of Child and Adolescent Psychopharmacology. 2011;21(6):517–35. doi: 10.1089/cap.2011.0015. [DOI] [PubMed] [Google Scholar]
- 134.Posey DJ, Stigler KA, Erickson CA, McDougle CJ. Antipsychotics in the treatment of autism. Journal of Clinical Investigation. 2008;118(1):6–14. doi: 10.1172/JCI32483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Marcus RN, Owen R, Kamen L, Manos G, McQuade RD, Carson WH, et al. A Placebo-Controlled, Fixed-Dose Study of Aripiprazole in Children and Adolescents With Irritability Associated With Autistic Disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(11):1110–9. doi: 10.1097/CHI.0b013e3181b76658. [DOI] [PubMed] [Google Scholar]
- 136.Owen R, Sikich L, Marcus RN, Corey-Lisle P, Manos G, McQuade RD, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics. 2009;124(6):1533–40. doi: 10.1542/peds.2008-3782. [DOI] [PubMed] [Google Scholar]
- 137.Baptista T. Body weight gain induced by antipsychotic drugs: Mechanisms and management. Acta Psychiatrica Scandinavica. 1999;100(1):3–16. doi: 10.1111/j.1600-0447.1999.tb10908.x. [DOI] [PubMed] [Google Scholar]
- 138.Bronfenbrenner U. Ecological models of human development. In: Postlethwaite TN, Husen T, editors. International Encyclopedia of Education. 1994. pp. 1643–7. [Google Scholar]
- 139.Sallis JF, Owen N, Fisher EB. Ecologic models of health behavior. In: Glanz K, Rimer BK, Viswanath K, editors. Health Behavior and Health Education: Theory, Practice and Research. New York: Jossey-Bass; 2008. pp. 465–82. [Google Scholar]
- 140●.Rimmer JH. Promoting Inclusive Community-Based Obesity Prevention Programs for Children and Adolescents with Disabilities: The Why and How. Childhood Obesity. 2011;7(3):177–84. This paper provides a framework for the systematic adaptation of existing community-based obesity interventions for children with disabilities. [Google Scholar]
- 141.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–92. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 142.Mudge S, Kayes N, Stavric V, Channon A, Kersten P, McPherson K. Living well with disability: needs, values and competing factors. International Journal of Behavioral Nutrition and Physical Activity. 2013;10(1):100. doi: 10.1186/1479-5868-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huang JS, Donohue M, Golnari G, Fernandez S, Walker-Gallego E, Galvan K, et al. Pediatricians’ weight assessment and obesity management practices. BMC Pediatrics. 2009;9 doi: 10.1186/1471-2431-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Patel AI, Madsen KA, Maselli JH, Cabana MD, Stafford RS, Hersh AL. Underdiagnosis of Pediatric Obesity during Outpatient Preventive Care Visits. Acad Pediatr. 2010;10(6):405–9. doi: 10.1016/j.acap.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McPherson AC, Swift JA, Yung E, Lyons J, Church P. The assessment of weight status in children and young people attending a spina bifida outpatient clinic: a retrospective medical record review. Disability and Rehabilitation. 2013;35(25):2123–31. doi: 10.3109/09638288.2013.771705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Matson JL, Fodstad JC. The treatment of food selectivity and other feeding problems in children with autism spectrum disorders. Research in Autism Spectrum Disorders. 2009;3(2):455–61. [Google Scholar]
- 147.Johnson CC. The benefits of physical activity for youth with developmental disabilities: A systematic review. American Journal of Health Promotion. 2009;23(3):157–67. doi: 10.4278/ajhp.070930103. [DOI] [PubMed] [Google Scholar]
- 148.Nadon G, Feldman DE, Dunn W, Gisel E. Mealtime problems in children with autism spectrum disorder and their typically developing siblings: a comparison study. Autism : the international journal of research and practice. 2011;15(1):98–113. doi: 10.1177/1362361309348943. [DOI] [PubMed] [Google Scholar]
- 149.Reilly S, Skuse D. Characteristics and Management of Feeding Problems of Young-Children with Cerebral-Palsy. Dev Med Child Neurol. 1992;34(5):379–88. doi: 10.1111/j.1469-8749.1992.tb11449.x. [DOI] [PubMed] [Google Scholar]
- 150.Rhee KE, Lumeng JC, Appugliese DP, Kaciroti N, Bradley RH. Parenting styles and overweight status in first grade. Pediatrics. 2006;117(6):2047–54. doi: 10.1542/peds.2005-2259. [DOI] [PubMed] [Google Scholar]
- 151.Goldstein TR, Goldstein BI, Mantz MB, Bailey B, Douaihy A. A brief motivational intervention for preventing medication-associated weight gain among youth with bipolar disorder: Treatment development and case report. Journal of Child and Adolescent Psychopharmacology. 2011;21(3):275–80. doi: 10.1089/cap.2010.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ghanizadeh A, Sahraeizadeh A, Berk M. A Head-to-Head Comparison of Aripiprazole and Risperidone for Safety and Treating Autistic Disorders, a Randomized Double Blind Clinical Trial. Child Psychiatry and Human Development. 2013:1–8. doi: 10.1007/s10578-013-0390-x. [DOI] [PubMed] [Google Scholar]
- 153.Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA – Journal of the American Medical Association. 2009;302(16):1765–73. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Penzner JB, Dudas M, Saito E, Olshanskiy V, Parikh UH, Kapoor S, et al. Lack of effect of stimulant combination with second-generation antipsychotics on weight gain, metabolic changes, prolactin levels, and sedation in youth with clinically relevant aggression or oppositionality. Journal of Child and Adolescent Psychopharmacology. 2009;19(5):563–73. doi: 10.1089/cap.2009.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jarskog LF, Hamer RM, Catellier DJ, Stewart DD, LaVange L, Ray N, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. American Journal of Psychiatry. 2013;170(9):1032–40. doi: 10.1176/appi.ajp.2013.12010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Klein DJ, Cottingham EM, Sorter M, Barton BA, Morrison JA. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. American Journal of Psychiatry. 2006;163(12):2072–9. doi: 10.1176/ajp.2006.163.12.2072. [DOI] [PubMed] [Google Scholar]
- 157.Morrison JA, Cottingham EM, Barton BA. Metformin for weight loss in pediatric patients taking psychotropic drugs. American Journal of Psychiatry. 2002;159(4):655–7. doi: 10.1176/appi.ajp.159.4.655. [DOI] [PubMed] [Google Scholar]
- 158.Poulin MJ, Chaput JP, Simard V, Vincent P, Bernier J, Gauthier Y, et al. Management of antipsychotic-incluced weight gain: Prospective naturalistic study of the effectiveness of a supervised exercise programme. Australian and New Zealand Journal of Psychiatry. 2007;41(12):980–9. doi: 10.1080/00048670701689428. [DOI] [PubMed] [Google Scholar]
- 159.Deberdt W, Winokur A, Cavazzoni PA, Trzaskoma QN, Carlson CD, Bymaster FP, et al. Amantadine for weight gain associated with olanzapine treatment. European Neuropsychopharmacology. 2005;15(1):13–21. doi: 10.1016/j.euroneuro.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 160.Henderson DC, Copeland PM, Daley TB, Borba CP, Cather C, Nguyen DD, et al. A double-blind, placebo-controlled trial of sibutramine for olanzapine-associated weight gain. American Journal of Psychiatry. 2005;162(5):954–62. doi: 10.1176/appi.ajp.162.5.954. [DOI] [PubMed] [Google Scholar]
- 161.Mahmood S, Booker I, Huang J, Coleman CI. Effect of topiramate on weight gain in patients receiving atypical antipsychotic agents. Journal of Clinical Psychopharmacology. 2013;33(1):90–4. doi: 10.1097/JCP.0b013e31827cb2b7. [DOI] [PubMed] [Google Scholar]
- 162.McElroy SL, Frye MA, Altshuler LL, Suppes T, Hellemann G, Black D, et al. A 24-week, randomized, controlled trial of adjunctive sibutramine versus topiramate in the treatment of weight gain in overweight or obese patients with bipolar disorders. Bipolar Disorders. 2007;9(4):426–34. doi: 10.1111/j.1399-5618.2007.00488.x. [DOI] [PubMed] [Google Scholar]
- 163.McGovern L, Johnson JN, Paulo R, Hettinger A, Singhal V, Kamath C, et al. Clinical review: treatment of pediatric obesity: a systematic review and meta-analysis of randomized trials. Journal of Clinical Endocrinology & Metabolism. 2008;93(12):4600–5. doi: 10.1210/jc.2006-2409. [DOI] [PubMed] [Google Scholar]
- 164.Whitlock EP, O’Connor EA, Williams SB, Beil TL, Lutz KW. Effectiveness of weight management interventions in children: A targeted systematic review for the USPSTF. Pediatrics. 2010;125(2):e396–e418. doi: 10.1542/peds.2009-1955. [DOI] [PubMed] [Google Scholar]
- 165.Kelishadi R, Hashemipour M, Adeli K, Tavakoli N, Movahedian-Attar A, Shapouri J, et al. Effect of zinc supplementation on markers of insulin resistance, oxidative stress, and inflammation among prepubescent children with metabolic syndrome. Metabolic Syndrome and Related Disorders. 2010;8(6):505–10. doi: 10.1089/met.2010.0020. [DOI] [PubMed] [Google Scholar]
- 166.López-Alarcón M, Martínez-Coronado A, Velarde-Castro O, Rendón-Macías E, Fernández J. Supplementation of n3 Long-chain Polyunsaturated Fatty Acid Synergistically Decreases Insulin Resistance with Weight Loss of Obese Prepubertal and Pubertal Children. Archives of Medical Research. 2011;42(6):502–8. doi: 10.1016/j.arcmed.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 167.Lustig RH, Greenway F, Velasquez-Mieyer P, Heimburger D, Schumacher D, Smith D, et al. A multicenter, randomized, double-blind, placebo-controlled, dose-finding trial of a long-acting formulation of octreotide in promoting weight loss in obese adults with insulin hypersecretion. International Journal of Obesity. 2006;30(2):331–41. doi: 10.1038/sj.ijo.0803074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lustig RH, Hinds PS, Ringwald-Smith K, Christensen RK, Kaste SC, Schreiber RE, et al. Octreotide therapy of pediatric hypothalamic obesity: A double-blind, placebo-controlled trial. Journal of Clinical Endocrinology and Metabolism. 2003;88(6):2586–92. doi: 10.1210/jc.2002-030003. [DOI] [PubMed] [Google Scholar]
- 169.AACAP. Practice Parameter for the use of Atypical Antipsychotic Medications in Children and Adolescents. 2012 [Google Scholar]
- 170.Kuhlthau K, Hill KS, Yucel R, Perrin JM. Financial burden for families of children with special health care needs. Maternal and Child Health Journal. 2005;9(2):207–18. doi: 10.1007/s10995-005-4870-x. [DOI] [PubMed] [Google Scholar]
- 171.Meyers MK, Lukemeyer A, Smeeding T. The cost of caring: Childhood disability and poor families. Soc Serv Rev. 1998;72(2):209–33. doi: 10.1086/515751. [DOI] [Google Scholar]
- 172.Newacheck PW, Inkelas M, Kim SE. Health services use and health care expenditures for children with disabilities. Pediatrics. 2004;114(1):79–85. doi: 10.1542/peds.114.1.79. [DOI] [PubMed] [Google Scholar]
- 173.Ausderau K, Juarez M. The Impact of Autism Spectrum Disorders and Eating Challenges on Family Mealtimes. ICAN: Infant, Child, & Adolescent Nutrition. 2013;5(5):315–23. doi: 10.1177/1941406413502808. [DOI] [Google Scholar]
- 174.Collins MSR, Kyle R, Smith S, Laverty A, Roberts S, Eaton-Evans J. Coping with the Usual Family Diet: Eating Behaviour and Food Choices of Children with Down’s Syndrome, Autistic Spectrum Disorders or Cri du Chat Syndrome and Comparison Groups of Siblings. Journal of Learning Disabilities. 2003;7(2):137–55. doi: 10.1177/1469004703007002004. [DOI] [Google Scholar]
- 175.Sullivan PB, Lambert B, Rose M, Ford-Adams M, Johnson A, Griffiths P. Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford Feeding Study. Dev Med Child Neurol. 2000;42(10):674–80. doi: 10.1017/s0012162200001249. [DOI] [PubMed] [Google Scholar]
- 176.Institute of Medicine. Accelerating Progress in Obesity Prevention, Solving the Weight of the Nation. Washington, D.C.: National Academies Press; 2012. [Google Scholar]
- 177.Wang Y, Wu Y, Wilson RF, Bleich S, Cheskin L, Weston C, et al. Review and Meta-Analysis. Rockville, MD: Agency for Healthcare Research and Quality; 2013. Childhood Obesity Prevention Programs: Comparative Effectiveness. (Contract No.: AHRQ Publication No. 13-EHC081-EF). [PubMed] [Google Scholar]
- 178.Hoelscher DM, Kirk S, Ritchie L, Cunningham-Sabo L, Academy Positions C. Position of the academy of nutrition and dietetics: interventions for the prevention and treatment of pediatric overweight and obesity. Journal of the Academy of Nutrition and Dietetics. 2013;113(10):1375–94. doi: 10.1016/j.jand.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 179.Munoz-Baell IM, Alvarez-Dardet C, Ruiz MT, Ferreiro-Lago E, Aroca-Fernandez E. Setting the stage for school health-promoting programmes for Deaf children in Spain. Health Promotion International. 2008;23(4):311–27. doi: 10.1093/heapro/dan026. [DOI] [PubMed] [Google Scholar]
- 180.Scott J, Wishart J, Currie C. Including children with intellectual disabilities/special educational needs into national child health surveys: A pilot study. Journal of Applied Research in Intellectual Disabilities. 2011;24(5):437–49. [Google Scholar]
- 181.Gortmaker SL, Cheung LWY, Peterson KE, Chomitz G, Cradle JH, Dart H, et al. Impact of a school-based interdisciplinary intervention on diet and physical activity among urban primary school children – Eat well and keep moving. Archives of Pediatrics & Adolescent Medicine. 1999;153(9):975–83. doi: 10.1001/archpedi.153.9.975. [DOI] [PubMed] [Google Scholar]
- 182.Gortmaker SL, Peterson K, Wiecha J, Sobol AM, Dixit S, Fox MK, et al. Reducing obesity via a school-based interdisciplinary intervention among youth – Planet health. Archives of Pediatrics & Adolescent Medicine. 1999;153(4):409–18. doi: 10.1001/archpedi.153.4.409. [DOI] [PubMed] [Google Scholar]
- 183.Lytle LA, Stone EJ, Nichaman MZ, Perry CL, Montgomery DH, Nicklas TA, et al. Changes in nutrient intakes of elementary school children following a school-based intervention: results from the CATCH Study. Preventive medicine. 1996;25(4):465–77. doi: 10.1006/pmed.1996.0078. [DOI] [PubMed] [Google Scholar]
- 184.Nicklas TA, Johnson CC, Myers L, Farris RP, Cunningham A. Outcomes of a high school program to increase fruit and vegetable consumption: Gimme 5–a fresh nutrition concept for students. The Journal of school health. 1998;68(6):248–53. doi: 10.1111/j.1746-1561.1998.tb06348.x. [DOI] [PubMed] [Google Scholar]
- 185.Minihan PM, Fitch SN, Must A. What does the epidemic of childhood obesity mean for children with special health care needs? Journal of Law, Medicine and Ethics. 2007;35(1):61–77. doi: 10.1111/j.1748-720X.2007.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Alexandropoulou M, Sourtzi P, Kalokerinou A. Health promotion practices and attitudes among nurses in special education schools in Greece. The Journal of School Nursing. 2010;26(4):278–88. doi: 10.1177/1059840510374879. [DOI] [PubMed] [Google Scholar]
- 187.Hinckson EA, Dickinson A, Water T, Sands M, Penman L. Physical activity, dietary habits and overall health in overweight and obese children and youth with intellectual disability or autism. Research in Developmental Disabilities. 2013;34(4):1170–8. doi: 10.1016/j.ridd.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 188.Simpson CG, Swicegood PR, Gaus MD. Nutrition and Fitness Curriculum: Designing Instructional Interventions for Children with Developmental Disabilities. TEACHING Exceptional Children. 2006;38(6):50–3. [Google Scholar]
- 189.Hubbard K, Bandini L, Folta S, Wansink B, Eliasziw MMA. Impact of a Smarter Lunchroom Intervention on Food Selection and Consumption among Adolescents and Young Adults with Intellectual and Developmental Disabilities in a Residential School Setting. Presented at the 141st American Public Health Association Annual Meeting; Boston, MA. November, 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Flygare Wallén E, Müllerdorf M, Christensson K, Marcus C. Eating patterns among students with intellectual disabilities after a multifactorial school intervention using the plate model. Journal of Policy and Practice in Intellectual Disabilities. 2013;10(1):45–53. [Google Scholar]
- 191.Wallén EF, Müllersdorf M, Christensson K, Marcus C. A school-based intervention associated with improvements in cardiometabolic risk profiles in young people with intellectual disabilities. Journal of Intellectual Disabilities. 2013;17(1):38–50. doi: 10.1177/1744629512472116. [DOI] [PubMed] [Google Scholar]
- 192.Gephart EF, Loman DG. Use of Prevention and Prevention Plus Weight Management Guidelines for Youth With Developmental Disabilities Living in Group Homes. Journal of Pediatric Health Care. 2013;27(2):98–108. doi: 10.1016/j.pedhc.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 193.Relf D, Dorn S. Horticulture: Meeting the needs of special populations. Hort Technology. 1995;5(2):94–103. [Google Scholar]
- 194.Siperstein GN, Harada CM, Parker RC, Hardman ML, McGuire J. A Comprehensive National Study of Special Olympics Programs in the United States. 2005 http://www.specialolympics.org/uploadedFiles/LandingPage/WhatWeDo/Research_Studies_Desciption_Pages/A%20Comprehensive%20National%20Study%20of%20Special%20Olympics%20Programs%20in%20the%20United%20States.pdf.
- 195.United States Government Accountability Office. Students with Disabilities: More Information and Guidance Could Improve Opportunities in Physical Education and Athletics. 2013 http://www.gao.gov/assets/310/30577.pdf.
- 196.National Academies, Committee on Facilitating Interdisciplinary Research, Committee on Science PPU. Facilitating interdisciplinary research. National Academy Press; 2005. p. 24. [Google Scholar]


