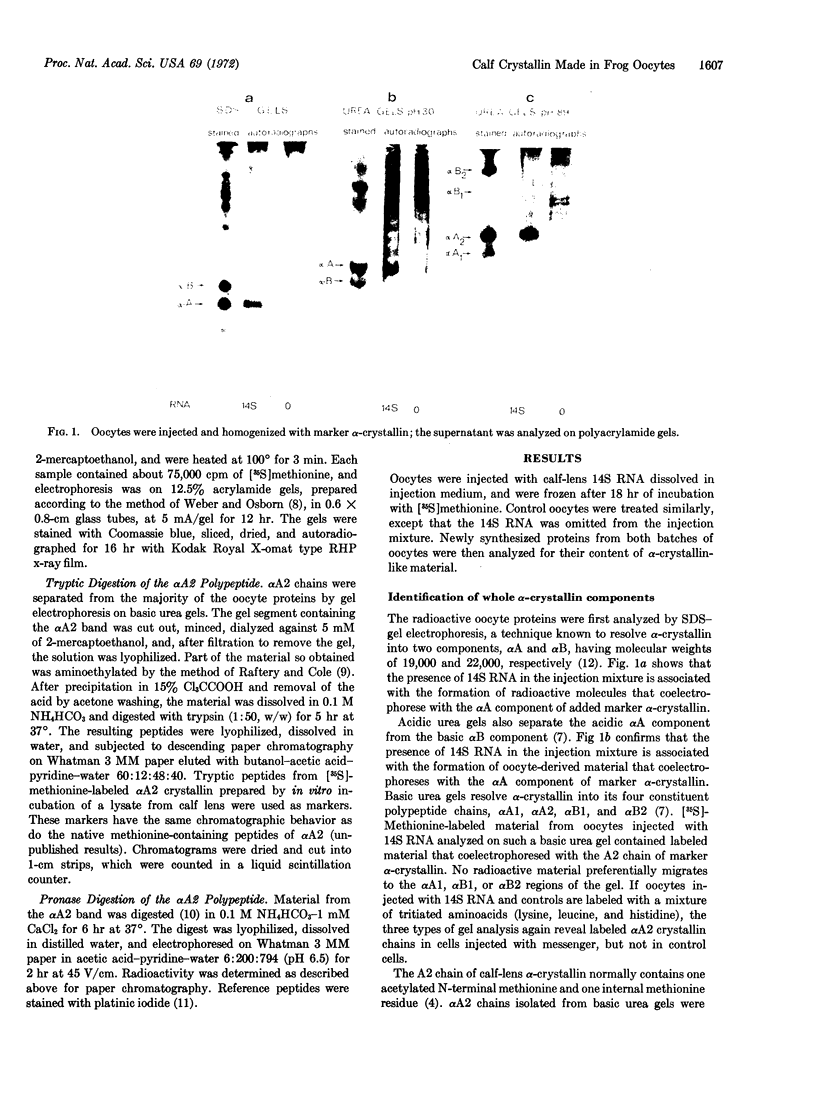
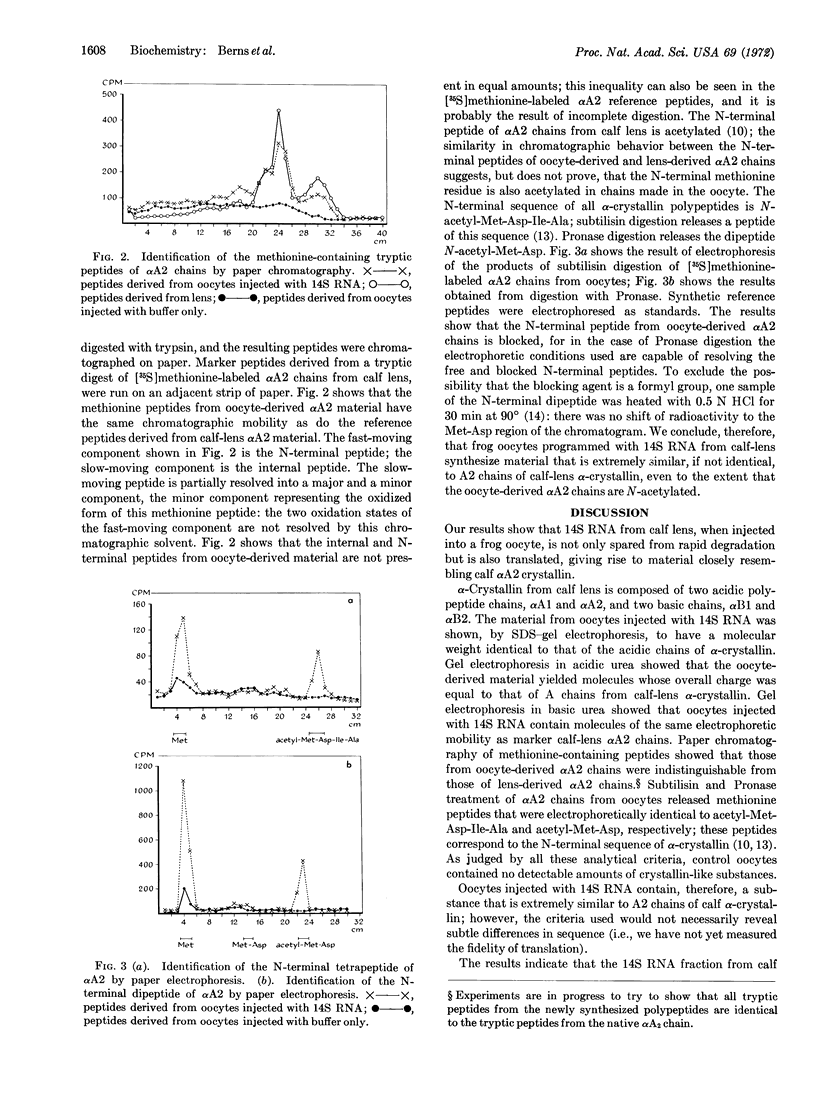
Abstract
14S RNA isolated from calf-lens polyribosomes was injected into oocytes of the frog Xenopus laevis. Oocytes injected with 14S RNA and buffer contained a protein resembling the A2 chain of calf α-crystallin; oocytes injected with buffer alone contained no crystallin-like material. αA2 crystallin polypeptides were identified by various criteria: urea-gel electrophoresis under acidic and basic conditions, gel electrophoresis in sodium dodecyl sulfate, N-terminal analysis, and paper chromatography of methionine-containing tryptic peptides.
It is concluded that when it is injected into a living frog oocyte, the 14S RNA from lens tissue is reasonably stable and has the properties of an αA2 crystallin messenger. The messenger requires no lens cell-specific components for translation within the oocyte, and the translational machinery of the frog cell will accept messenger RNA from a totally different cell type from another species.
The A2 chains of α-crystallin extracted from lens tissue possess an acetylated N-terminal methionine residue; the N-terminal methionine of αA2 chains derived from frog oocytes injected with 14S RNA was also acetylated.
Keywords: messenger RNA, initiation factors, protein synthesis, acetyl methionine
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berns A. J.M., De Abreu R. A., Van Kraaikamp M., Benedetti E. L., Bloemendal H. Synthesis of lens protein in vitro V. Isolation of messenger-like RNA from lens by high resolution zonal centrifugation. FEBS Lett. 1971 Oct 15;18(1):159–163. doi: 10.1016/0014-5793(71)80434-9. [DOI] [PubMed] [Google Scholar]
- Berns A. J., Strous G. J., Bloemendal H. Heterologous in vitro synthesis of lens -crystallin polypeptide. Nat New Biol. 1972 Mar 1;236(61):7–9. doi: 10.1038/newbio236007a0. [DOI] [PubMed] [Google Scholar]
- Bloemendal H., Schoenmakers J., Zweers A., Matze R., Benedetti E. L. Polyribosomes from calf-lens epithelium. Biochim Biophys Acta. 1966 Jul 20;123(1):217–220. doi: 10.1016/0005-2787(66)90179-1. [DOI] [PubMed] [Google Scholar]
- Easley C. W. Combinations of specific color reactions useful in the peptide mapping technique. Biochim Biophys Acta. 1965 Sep 13;107(2):386–388. doi: 10.1016/0304-4165(65)90147-9. [DOI] [PubMed] [Google Scholar]
- Heywood S. M. Specificity of mRNA binding factor in eukaryotes. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1782–1788. doi: 10.1073/pnas.67.4.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenders H. J., Schoenmakers J. G., Gerding J. J., Tesser G. I., Bloemendal H. N-terminus of alpha-crystallin. Exp Eye Res. 1968 Apr;7(2):291–300. doi: 10.1016/s0014-4835(68)80080-6. [DOI] [PubMed] [Google Scholar]
- Hoenders H. J., van Tol J., Bloemendal H. Release of an N-terminal tetrapeptide from alpha-crystallin. Biochim Biophys Acta. 1968 Jun 26;160(2):283–285. doi: 10.1016/0005-2795(68)90106-2. [DOI] [PubMed] [Google Scholar]
- Housman D., Jacobs-Lorena M., Rajbhandary U. L., Lodish H. F. Initiation of haemoglobin synthesis by methionyl-tRNA. Nature. 1970 Aug 29;227(5261):913–918. doi: 10.1038/227913a0. [DOI] [PubMed] [Google Scholar]
- Hunter A. R., Jackson R. J. The origin and nature of the methionine residue initiating the synthesis of haemoglobin in vivo and in vitro. Eur J Biochem. 1971 Apr;19(3):316–322. doi: 10.1111/j.1432-1033.1971.tb01321.x. [DOI] [PubMed] [Google Scholar]
- Lane C. D., Marbaix G., Gurdon J. B. Rabbit haemoglobin synthesis in frog cells: the translation of reticulocyte 9 s RNA in frog oocytes. J Mol Biol. 1971 Oct 14;61(1):73–91. doi: 10.1016/0022-2836(71)90207-5. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Lingrel J. B. Identification of mouse haemoglobin messenger RNA. Nature. 1971 Oct 13;233(5320):204–206. [PubMed] [Google Scholar]
- Mathews M. B., Osborn M., Berns A. J., Bloemendal H. Translation of two messenger RNAs from lens in a cell free system from Krebs II ascites cells. Nat New Biol. 1972 Mar 1;236(61):5–7. doi: 10.1038/newbio236005a0. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Osborn M., Lingrel J. B. Translation of globin messenger RNA in a heterologous cell-free system. Nature. 1971 Oct 13;233(5320):206–209. [PubMed] [Google Scholar]
- Prichard P. M., Picciano D. J., Laycock D. G., Anderson W. F. Translation of exogenous messenger RNA for hemoglobin on reticulocyte and liver ribosomes. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2752–2756. doi: 10.1073/pnas.68.11.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery M. A., Cole R. D. On the aminoethylation of proteins. J Biol Chem. 1966 Aug 10;241(15):3457–3461. [PubMed] [Google Scholar]
- Schoenmakers J. G., Gerding J. J., Bloemendal H. The subunit structure of alpha-crystallin. Isolation and characterization of the S-carboxymethylated acidic subunits from adult and embryonic origin. Eur J Biochem. 1969 Dec;11(3):472–481. doi: 10.1111/j.1432-1033.1969.tb00797.x. [DOI] [PubMed] [Google Scholar]
- Schoenmakers J. G., Matze R., Van Poppel M., Bloemendal H. Isolation of non-identical polypeptide chains of -crystallin. Int J Protein Res. 1969;1(1):19–27. doi: 10.1111/j.1399-3011.1969.tb01623.x. [DOI] [PubMed] [Google Scholar]
- Spector A., Li L. K., Augusteyn R. C., Schneider A., Freund T. -Crystallin. The isolation and characterization of distinct macromolecular fractions. Biochem J. 1971 Sep;124(2):337–343. doi: 10.1042/bj1240337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavnezer J., Huang R. C. Synthesis of a mouse immunoglobulin light chain in a rabbit reticulocyte cell-free system. Nat New Biol. 1971 Apr 7;230(14):172–176. doi: 10.1038/newbio230172a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]