Abstract
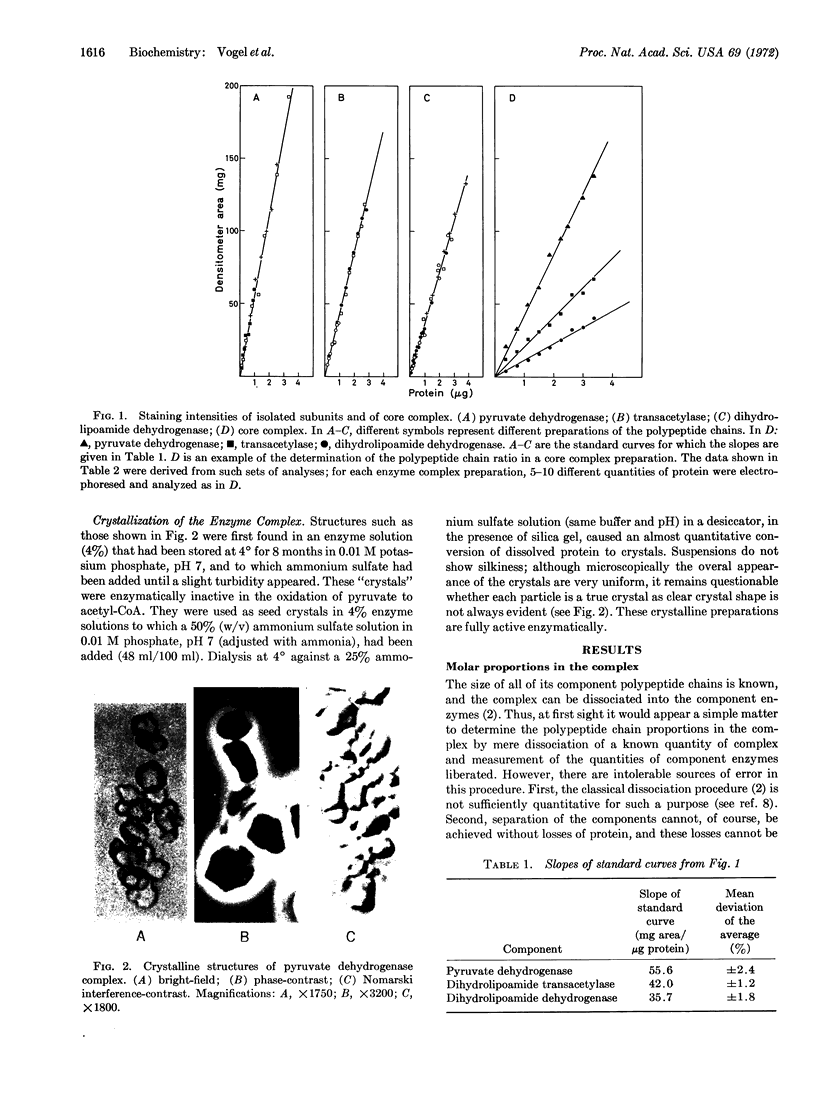
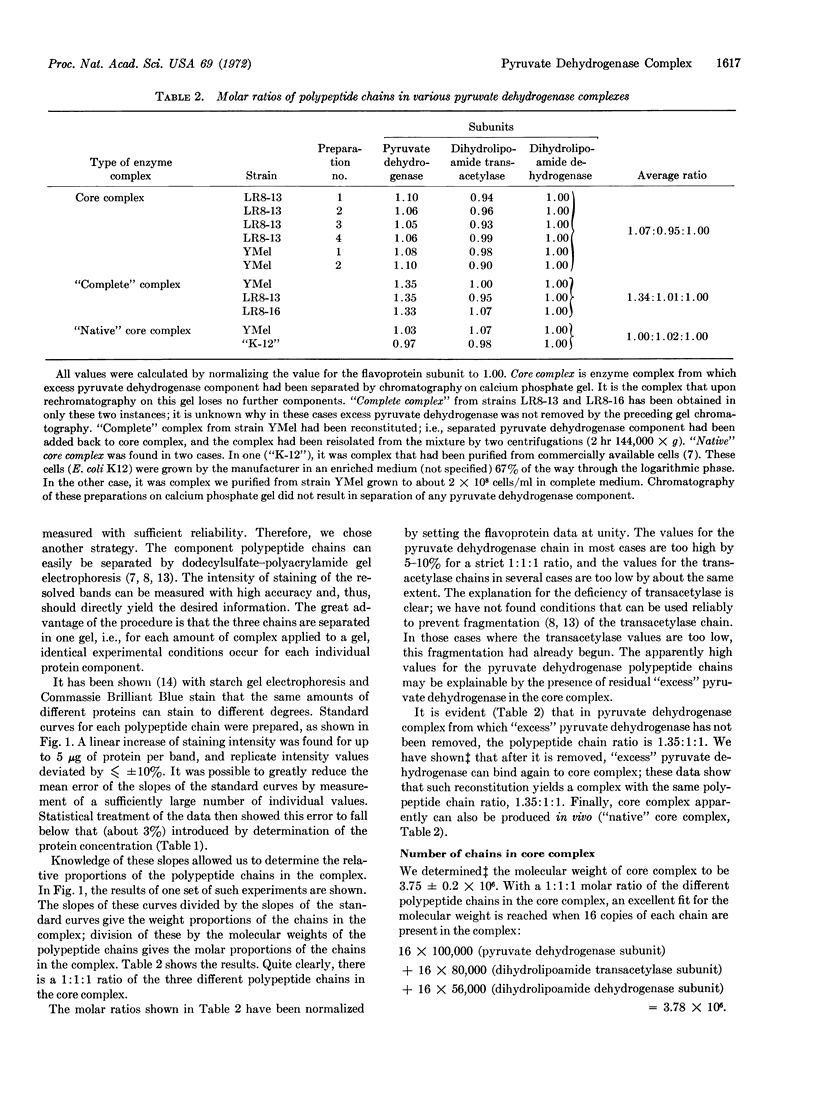
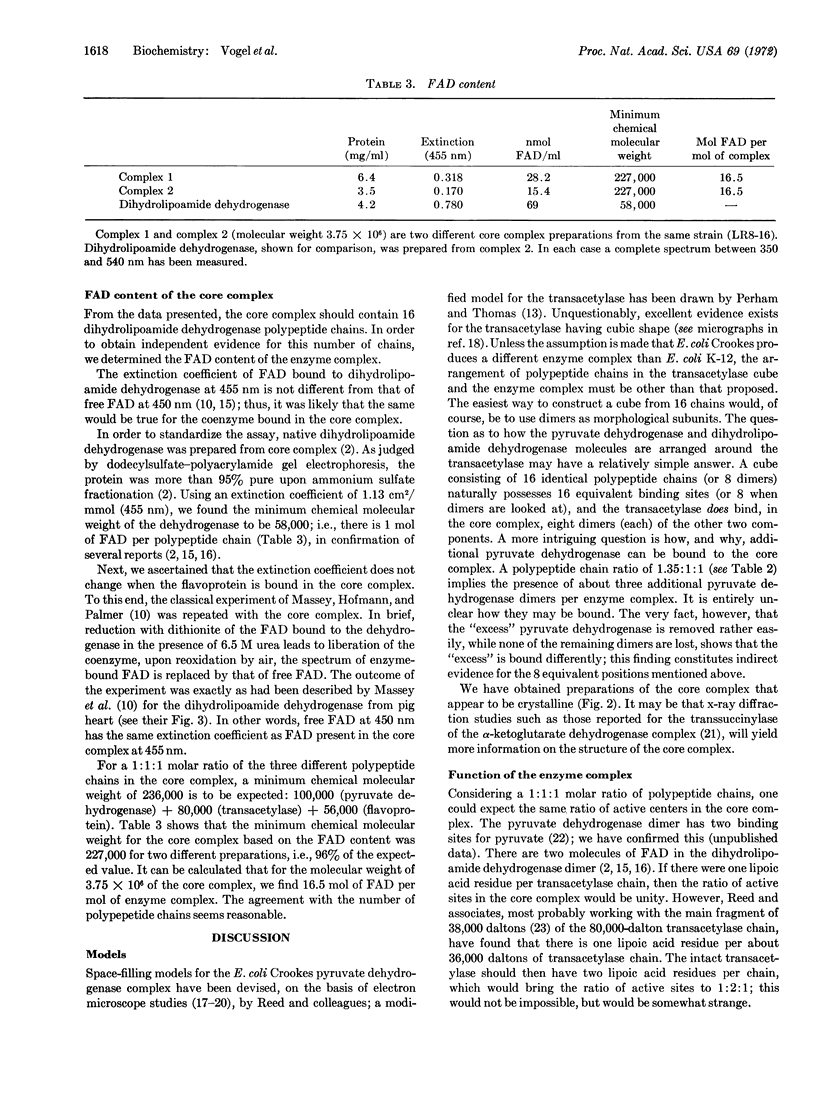
The pyruvate dehydrogenase core complex from E. coli K-12, defined as the multienzyme complex that can be obtained with a unique polypeptide chain composition, has a molecular weight of 3.75 × 106. All results obtained agree with the following numerology. The core complex consists of 48 polypeptide chains. There are 16 chains (molecular weight = 100,000) of the pyruvate dehydrogenase component, 16 chains (molecular weight = 80,000) of the dihydrolipoamide dehydrogenase component, and 16 chains (molecular weight = 56,000) of the dihydrolipoamide dehydrogenase component. Usually, but not always, pyruvate dehydrogenase complex is produced in vivo containing at least 2-3 mol more of dimers of the pyruvate dehydrogenase component than the stoichiometric ratio with respect to the core complex. This “excess” component is bound differently than are the eight dimers in the core complex.
Keywords: multienzyme complex, three different polypeptide chains, 1:1:1 molar ratio, 48 total chains
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRAVEN G. R., STEERS E., Jr, ANFINSEN C. B. PURIFICATION, COMPOSITION, AND MOLECULAR WEIGHT OF THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2468–2477. [PubMed] [Google Scholar]
- Derosier D. J., Oliver R. M., Reed L. J. Crystallization and preliminary structural analysis of dihydrolipoyl transsuccinylase, the core of the 2-oxoglutarate dehydrogenase complex. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1135–1137. doi: 10.1073/pnas.68.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J., Henning U. Regulation of pyruvate dehydrogenase complex synthesis in Escherichia coli K 12. Identification of the inducing metabolite. Eur J Biochem. 1970 Jun;14(2):258–269. doi: 10.1111/j.1432-1033.1970.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Flatgaard J. E., Hoehn B., Henning U. Mutants of Escherichia coli K-12 which synthesize the pyruvate dehydrogenase complex constitutively. Arch Biochem Biophys. 1971 Apr;143(2):461–470. doi: 10.1016/0003-9861(71)90231-1. [DOI] [PubMed] [Google Scholar]
- Henney H. R., Jr, Willms C. R., Muramatsu T., Mukherjee B. B., Reed L. J. Alpha-keto acid dehydrogenase complexes. VII. Isolation and partial characterization of the polypeptide chains in the dihydrolipoyl transacetylase of Escherichia coli. J Biol Chem. 1967 Mar 10;242(5):898–901. [PubMed] [Google Scholar]
- JOLLES J., JAUREGUI ADELL J., BERNIER I., JOLLES P. LA STRUCTURE CHIMIQUE DU LYSOZYME DE BLANC D'OEUF DE POULE: 'ETUDE D'ETAILL'EE. Biochim Biophys Acta. 1963 Dec 13;78:668–689. doi: 10.1016/0006-3002(63)91033-3. [DOI] [PubMed] [Google Scholar]
- KOIKE M., REED L. J., CARROLL W. R. Molecular weight and FAD content of dihydrolipoic dehydrogenase from Escherichia coli. Biochem Biophys Res Commun. 1962 Feb 20;7:16–17. doi: 10.1016/0006-291x(62)90135-3. [DOI] [PubMed] [Google Scholar]
- KOIKE M., REED L. J., CARROLL W. R. alpha-Keto acid dehydrogenation complexes. IV. Resolution and reconstitution of the Escherichia coli pyruvate dehydrogenation complex. J Biol Chem. 1963 Jan;238:30–39. [PubMed] [Google Scholar]
- MASSEY V., HOFMANN T., PALMER G. The relation of function and structure in lipoyl dehydrogenase. J Biol Chem. 1962 Dec;237:3820–3828. [PubMed] [Google Scholar]
- Perham R. N., Thomas J. O. The subunit molecular weights of the alpha-ketoacid dehydrogenase multienzyme complexes from E. coli. FEBS Lett. 1971 Jun 2;15(1):8–12. doi: 10.1016/0014-5793(71)80066-2. [DOI] [PubMed] [Google Scholar]
- REED L. J., FERNANDEZ-MORAN H., KOIKE M., WILLMS C. R. ELECTRON MICROSCOPIC AND BIOCHEMICAL STUDIES OF PYRUVATE DEHYDROGENASE COMPLEX OF ESCHERICHIA COLI. Science. 1964 Aug 28;145(3635):930–932. doi: 10.1126/science.145.3635.930. [DOI] [PubMed] [Google Scholar]
- Reed L. J., Oliver R. M. The multienzyme alpha-keto acid dehydrogenase complexes. Brookhaven Symp Biol. 1968 Jun;21(2):397–412. [PubMed] [Google Scholar]
- SUND H., WEBER K. Studies on the lactose-splitting enzyme. XIII. Quantity and configuration of beta-galactosidase from E. Coli. Biochem Z. 1963;337:24–34. [PubMed] [Google Scholar]
- Schwartz E. R., Reed L. J. Alpha-keto acid dehydrogenase complexes. 13. Reaction of sulfhydryl groups in pyruvate dehydrogenase with organic mercurials. J Biol Chem. 1970 Jan 10;245(1):183–187. [PubMed] [Google Scholar]
- Schwartz E. R., Reed L. J. Alpha-keto acid dehydrogenase complexes. XII. Effects of acetylation on the activity and structure of the dihydrolipoyl transacetylase of Escherichia coli. J Biol Chem. 1969 Nov 25;244(22):6074–6079. [PubMed] [Google Scholar]
- Vogel O., Beikirch H., Müller H., Henning U. The subunit structure of the Escherichia coli K-12 pyruvate dehydrogenase complex. The dihydrolipoamide transacetylase component. Eur J Biochem. 1971 May 28;20(2):169–178. doi: 10.1111/j.1432-1033.1971.tb01375.x. [DOI] [PubMed] [Google Scholar]
- Vogel O., Henning U. Pyruvate dehydrogenase component subunit structure of the Escherichia coli K 12 pyruvate dehydrogenase complex. Eur J Biochem. 1971 Jan 1;18(1):103–115. doi: 10.1111/j.1432-1033.1971.tb01220.x. [DOI] [PubMed] [Google Scholar]
- WHITBY L. G. A new method for preparing flavin-adenine dinucleotide. Biochem J. 1953 Jun;54(3):437–442. doi: 10.1042/bj0540437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Williams C. H., Jr Studies on lipoyl dehydrogenase from Escherichia coli. J Biol Chem. 1965 Dec;240(12):4793–4800. [PubMed] [Google Scholar]
- Willms C. R., Oliver R. M., Henney H. R., Jr, Mukherjee B. B., Reed L. J. Alpha-keto acid dehydrogenase complexes. VI. Dissociation and reconstitution of the dihydrolipoyl transacetylase of Escherichia coli. J Biol Chem. 1967 Mar 10;242(5):889–897. [PubMed] [Google Scholar]



