Abstract
When Trypanosoma cruzi are treated with Berenil, a trypanocide, their kinetoplast DNA contains an increased proportion of double-branched circular molecules. These replicating molecules have closed-circular template strands; their decrease in density when complexed by ethidium bromide in a cesium chloride gradient is proportional to the length of the replicated segments. Replication seems to be blocked at specific points, which are equidistantly spaced along the circular kinetoplast DNA molecules. Analysis of about 800 replicating forms showed that the lengths of the replicated branches are not distributed at random, but into several populations, which correspond to multiples of 15% of the total contour length of 0.5 μm. This distribution evokes a discontinuous replication process. The problem of whether kinetoplast DNA is synthesized by successive replication units, or whether and how Berenil might induce specific blocking of DNA replication, is discussed.
Keywords: electron microscopy, dye equilibrium centrifugation, replicating units, trypanocidal drugs, Berenil
Full text
PDF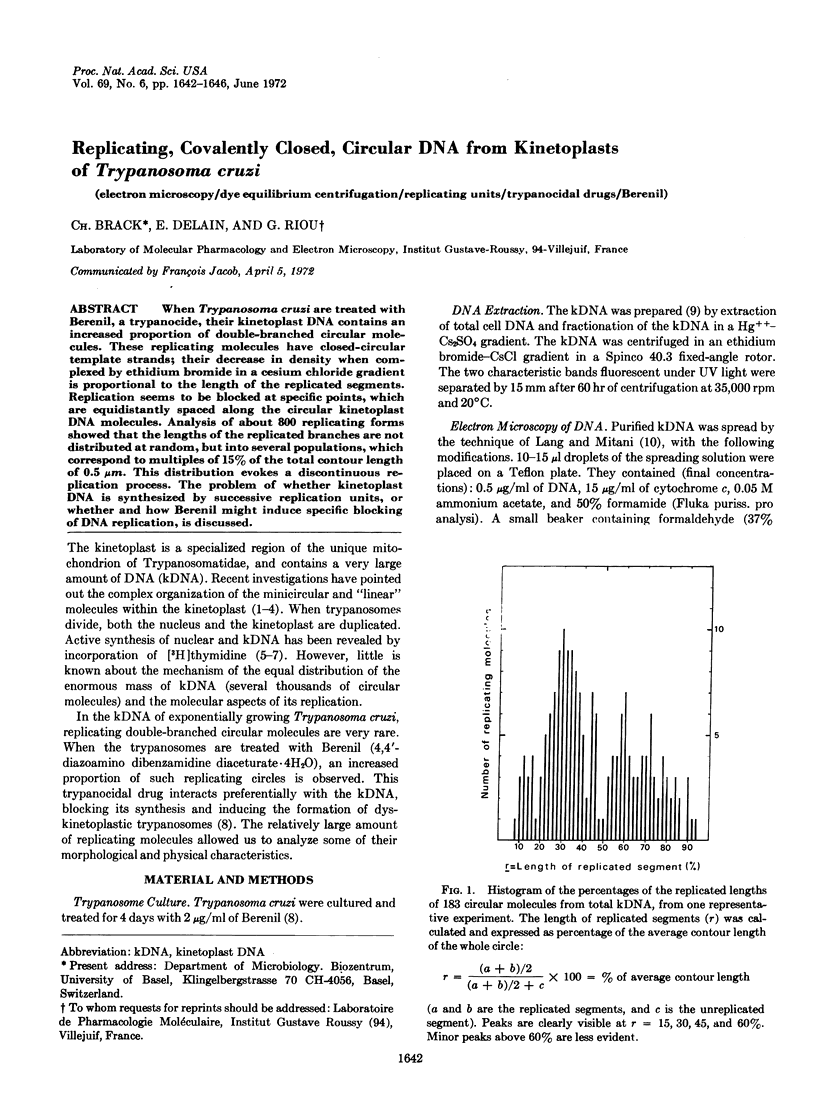
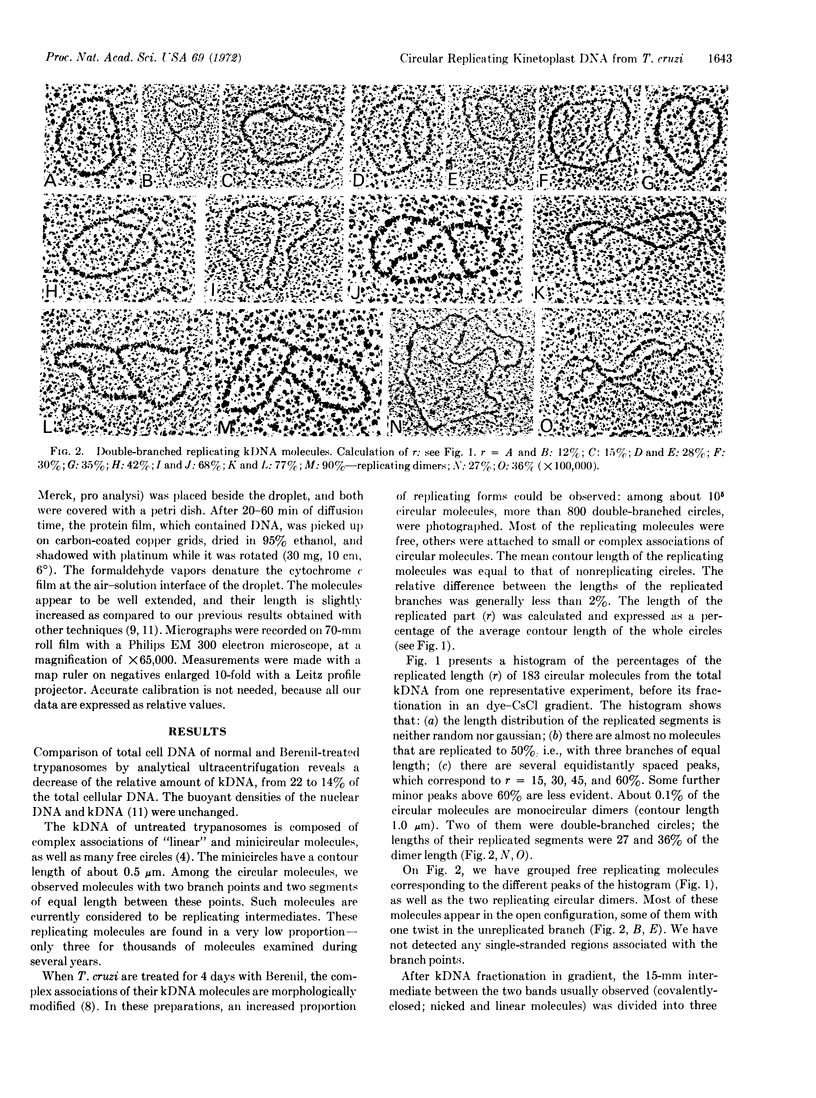
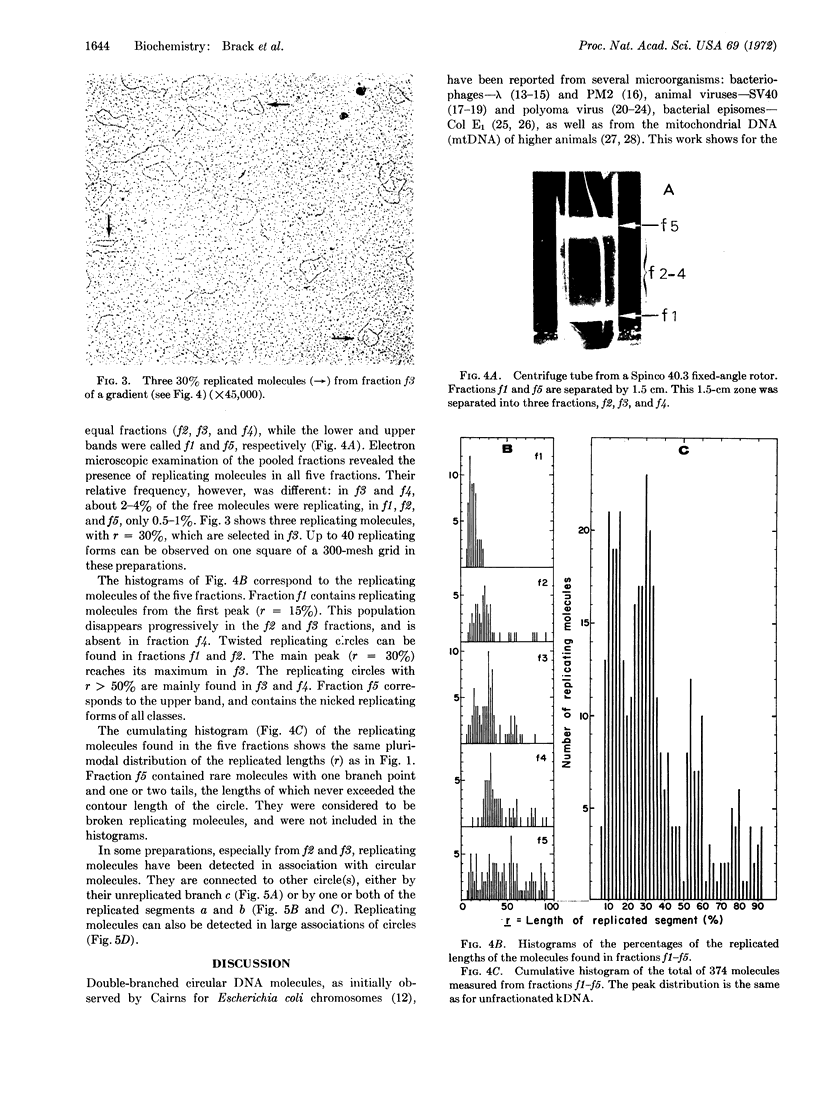
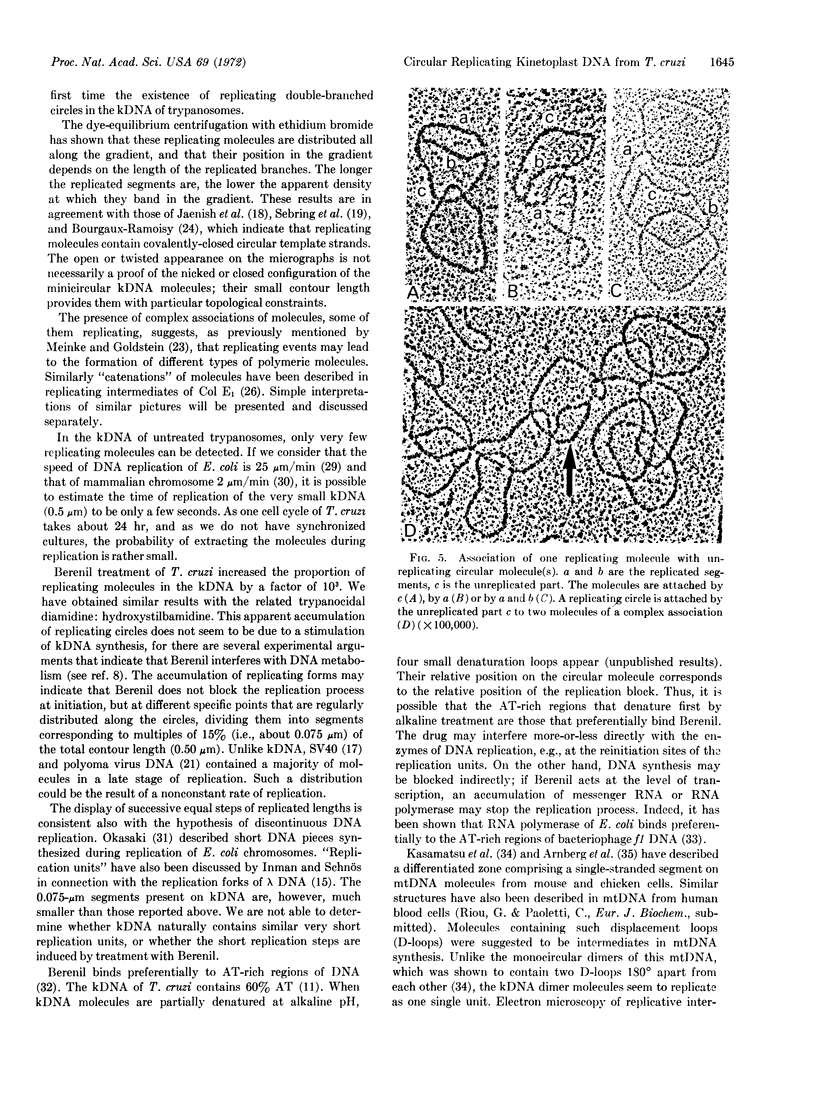

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W., Hill G. C. Division and DNA synthesis in the kinetoplast ofCrithidia fasciculata. J Cell Sci. 1969 May;4(3):611–620. doi: 10.1242/jcs.4.3.611. [DOI] [PubMed] [Google Scholar]
- Arnberg A., van Bruggen E. F., Borst P. The presence of DNA molecules with a displacement loop in standard mitochondrial DNA preparations. Biochim Biophys Acta. 1971 Aug 26;246(2):353–357. doi: 10.1016/0005-2787(71)90147-x. [DOI] [PubMed] [Google Scholar]
- Bourgaux-Ramoisy D. The secondary structure of replicating polyoma virus DNA. Biochim Biophys Acta. 1971 Dec 30;254(3):412–414. doi: 10.1016/0005-2787(71)90873-2. [DOI] [PubMed] [Google Scholar]
- Bourgaux P., Bourgaux-Ramoisy D. A symmetrical model for polyoma virus DNA replication. J Mol Biol. 1971 Dec 28;62(3):513–524. doi: 10.1016/0022-2836(71)90152-5. [DOI] [PubMed] [Google Scholar]
- Bourgaux P., Bourgaux-Ramoisy D., Seiler P. The replication of the ring-shaped DNA of polyoma virus. II. Identification of molecules at various stages of replication. J Mol Biol. 1971 Jul 14;59(1):195–206. doi: 10.1016/0022-2836(71)90421-9. [DOI] [PubMed] [Google Scholar]
- Burton P. R., Dusanic D. G. Fine structure and replication of the kinetoplast of Trypanosoma lewisi. J Cell Biol. 1968 Nov;39(2):318–331. doi: 10.1083/jcb.39.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAIRNS J. The bacterial chromosome and its manner of replication as seen by autoradiography. J Mol Biol. 1963 Mar;6:208–213. doi: 10.1016/s0022-2836(63)80070-4. [DOI] [PubMed] [Google Scholar]
- Delius H., Howe C., Kozinski A. W. Structure of the replicating DNA from bacteriophage T4. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3049–3053. doi: 10.1073/pnas.68.12.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. Replication of bacteriophage PM2 deoxyribonucleic acid: a closed circular double-stranded molecule. J Mol Biol. 1971 Mar 28;56(3):597–621. doi: 10.1016/0022-2836(71)90404-9. [DOI] [PubMed] [Google Scholar]
- Festy B., Lallemant A. M., Riou G., Brack C., Delain E. Mécanisme d'action des diamidines trypanocides. Importance de la composition en bases dans l'association berenil-polynucléotides. C R Acad Sci Hebd Seances Acad Sci D. 1970 Aug 17;271(7):684–687. [PubMed] [Google Scholar]
- Fuke M., Inselburg J. Electron microscopic studies of replicating and catenated colicin factor E1 DNA isolated from minicells (DNA replication). Proc Natl Acad Sci U S A. 1972 Jan;69(1):89–92. doi: 10.1073/pnas.69.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter C., Cooper S., Pierucci O., Revelas E. On the bacterial life sequence. Cold Spring Harb Symp Quant Biol. 1968;33:809–822. doi: 10.1101/sqb.1968.033.01.093. [DOI] [PubMed] [Google Scholar]
- Hirt B. Replicating molecules of polyoma virus DNA. J Mol Biol. 1969 Feb 28;40(1):141–144. doi: 10.1016/0022-2836(69)90302-7. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Riggs A. D. On the mechanism of DNA replication in mammalian chromosomes. J Mol Biol. 1968 Mar 14;32(2):327–341. doi: 10.1016/0022-2836(68)90013-2. [DOI] [PubMed] [Google Scholar]
- Inman R. B., Schnös M. Structure of branch points in replicating DNA: presence of single-stranded connections in lambda DNA branch points. J Mol Biol. 1971 Mar 14;56(2):319–325. doi: 10.1016/0022-2836(71)90467-0. [DOI] [PubMed] [Google Scholar]
- Inselburg J., Fuke M. Replicating DNA: structure of colicin factor E1. Science. 1970 Aug 7;169(3945):590–592. doi: 10.1126/science.169.3945.590. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Mayer A., Levine A. Replicating SV40 molecules containing closed circular template DNA strands. Nat New Biol. 1971 Sep 15;233(37):72–75. doi: 10.1038/newbio233072a0. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Robberson D. L., Vinograd J. A novel closed-circular mitochondrial DNA with properties of a replicating intermediate. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2252–2257. doi: 10.1073/pnas.68.9.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiger J. A., Jr, Sinsheimer R. L. DNA of vegetative bacteriophage lambda. VI. Electron microscopic studies of replicating lambda DNA. Proc Natl Acad Sci U S A. 1971 Jan;68(1):112–115. doi: 10.1073/pnas.68.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner R. H., Wolstenholme D. R., Gross N. J. Replicating molecules of circular mitochondrial DNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1466–1472. doi: 10.1073/pnas.60.4.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D., Mitani M. Simplified quantitative electron microscopy of biopolymers. Biopolymers. 1970;9(3):373–379. doi: 10.1002/bip.1970.360090310. [DOI] [PubMed] [Google Scholar]
- Laurent M., Steinert M. Electron microscopy of kinetoplastic DNA from Trypanosoma mega. Proc Natl Acad Sci U S A. 1970 Jun;66(2):419–424. doi: 10.1073/pnas.66.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Kang H. S., Billheimer F. E. DNA replication in SV40 infected cells. I. Analysis of replicating SV40 DNA. J Mol Biol. 1970 Jun 14;50(2):549–568. doi: 10.1016/0022-2836(70)90211-1. [DOI] [PubMed] [Google Scholar]
- Meinke W., Goldstein D. A. Studies on the structure and formation of polyoma DNA replicative intermediates. J Mol Biol. 1971 Nov 14;61(3):543–563. doi: 10.1016/0022-2836(71)90064-7. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Tomizawa J. I., Fuke M. Replication of bacteriophage DNA, II. Structure of replicating DNA of phage lambda. Proc Natl Acad Sci U S A. 1968 Jul;60(3):861–865. doi: 10.1073/pnas.60.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti C., Riou G., Pairault J. Circular oligomers in mitochondrial DNA of human and beef nonmalignant thyroid glands. Proc Natl Acad Sci U S A. 1972 Apr;69(4):847–850. doi: 10.1073/pnas.69.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renger H. C., Wolstenholme D. R. Kinetoplast and other satellite DNAs of kinetoplastic and dyskinetoplastic strains of Trypanosoma. J Cell Biol. 1971 Aug;50(2):533–540. doi: 10.1083/jcb.50.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou G., Delain E. Electron microscopy of the circular kinetoplastic DNA from Trypanosoma cruzi: occurrence of catenated forms. Proc Natl Acad Sci U S A. 1969 Jan;62(1):210–217. doi: 10.1073/pnas.62.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou G., Paoletti C. Preparation and properties of nuclear and satellite deoxyribonucleic acid of Trypanosoma cruzi. J Mol Biol. 1967 Sep 14;28(2):377–382. doi: 10.1016/s0022-2836(67)80017-2. [DOI] [PubMed] [Google Scholar]
- Riou G. Specific inhibition by ethidium bromide of the incorporation of 3 H thymidine into the kinetoplastic DNA of Trypanosoma cruzi. Biochem Pharmacol. 1970 Apr;19(4):1524–1526. doi: 10.1016/0006-2952(70)90074-2. [DOI] [PubMed] [Google Scholar]
- Sebring E. D., Kelly T. J., Jr, Thoren M. M., Salzman N. P. Structure of replicating simian virus 40 deoxyribonucleic acid molecules. J Virol. 1971 Oct;8(4):478–490. doi: 10.1128/jvi.8.4.478-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishido K., Ikeda Y. Preferential binding of E. coli RNA polymerase to the AT-rich regions of bacteriophage f1 DNA. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1420–1428. doi: 10.1016/s0006-291x(71)80244-9. [DOI] [PubMed] [Google Scholar]
- Simpson L., Da Silva A. Isolation and characterization of kinetoplast DNA from Leishmania tarentolae. J Mol Biol. 1971 Mar 28;56(3):443–473. doi: 10.1016/0022-2836(71)90394-9. [DOI] [PubMed] [Google Scholar]






