Abstract
OBJECTIVE
Intensive insulin therapy (IIT) in the critically ill reduces mortality but carries the risk of increased hypoglycemia. Point-of-care (POC) blood glucose analysis is standard; however anemia causes falsely high values and potentially masks hypoglycemia. Permissive anemia is routinely practiced in most intensive care units (ICUs). We hypothesized that POC glucometer error due to anemia is prevalent, can be mathematically corrected, and correction uncovers occult hypoglycemia during IIT.
DESIGN
The study has both retrospective and prospective phases. We reviewed data to verify the presence of systematic error, determine the source of error, and establish the prevalence of anemia. We confirmed our findings by reproducing the error in an in-vitro model. Prospective data was used to develop a correction formula validated by the Monte Carlo method. Correction was implemented in a burn ICU and results evaluated after nine months.
SETTING
Burn and trauma ICUs at a single research institution.
PATIENTS/SUBJECTS
Samples for in-vitro studies were taken from healthy volunteers. Samples for formula development were from critically ill patients on IIT.
INTERVENTIONS
Insulin doses were calculated based on predicted serum glucose values from corrected POC glucometer measurements.
MEASUREMENTS
Time-matched POC glucose, laboratory glucose, and hematocrit values.
MAIN RESULTS
We previously found that anemia (HCT<34%) produces systematic error in glucometer measurements. The error was correctible with a mathematical formula developed and validated using prospectively collected data. Error of uncorrected POC glucose ranged from 19% to 29% (p<0.001), improving to ≤5% after mathematical correction of prospective data. Comparison of data pairs before and after correction formula implementation demonstrated a 78% decrease in the incidence of hypoglycemia in critically ill and anemic patients treated with insulin and tight glucose control (p<0.001).
CONCLUSIONS
A mathematical formula that corrects erroneous POC glucose values due to anemia in ICU patients reduces the incidence of hypoglycemia during IIT.
Keywords: glucose, insulin, point-of-care systems, hematocrit, glucose oxidase, critical care, ICU, glucometer, glucose measurement: arterial, venous, capillary glucose measurement, anemia, capillary
Introduction
Inaccuracy of glucometer measurement is an oft discussed but poorly understood phenomenon, in part because of the plethora of variables that can affect performance. Many of these exert effects only under specialized circumstances and relevance to the majority of intensive care unit (ICU) patients is unknown. Here we identify the single most important factor affecting glucometer performance in hemodynamically stable ICU patients and we describe the impact of glucometer error on hypoglycemia.
Glucometer performance is an important issue in the care of ICU patients because, while the demonstrated benefits of intensive insulin therapy (IIT) changed therapy around the world (1, 2), problems with implementation are generating controversy. Hypoglycemia is a recognized complication of insulin treatment and, as we previously showed, can be related to the effects of anemia on glucometer performance (2). This is not a trivial problem; in severe cases, hypoglycemia can lead to seizures, coma, and death (3, 4). Recent European trials (GLUCONTROL and VISEP) designed to confirm and extend the findings of the original Van den Berghe study were closed prior to completion due to unacceptable increases in incidence of hypoglycemia (5, 6).
IIT requires frequent glucose measurement to maintain patient safety; single-channel point-of-care whole blood glucometers (POC) are almost universally used to direct care as they are inexpensive, require small blood volumes, and have rapid response times compared to laboratory analysis (7). Recent studies, however, question whether they are sufficiently accurate and reliable for use in critically ill patients (8, 9). According to ADA guidelines (10, 11), error rates in glucose measurement should not exceed 5%, but actual rates greater than 25% have been reported (9, 12, 13). Given the narrow glucose target of 80-110 mg/dL associated with IIT, this degree of measurement error can have a significant clinical impact.
Error in POC analysis is multifactorial and the literature describing causative factors is extensive, yet prior to this study the relative clinical contribution of individual sources of error was unclear. Reported causes of poor glucometer performance include abnormal hematocrit, low oxygen tension, acetaminophen, uric acid, ascorbic acid, maltose, galactose, xylose, lactose, operator inexperience, age of strips, heat, and humidity. Anemia results in error because the estimated volume of plasma equivalent used to calculate glucose concentrations are based on expected plasma displacement associated with normal erythrocyte content (12, 14). In anemic samples the degree of displacement is overestimated, the plasma volume is underestimated, and the reported glucose concentration is thus artificially high. Laboratory analyzers are not subject to this error as plasma rather than whole blood is measured.
Given the effect of anemia on glucometers and their widespread use, implementation of IIT has resulted in a potentially dangerous clinical scenario due to the coincident adoption of restrictive blood transfusion therapies in response to the landmark work of Hebert and his colleagues (15). Lower transfusion thresholds have increased the prevalence and depth of anemia in the ICU (15-20), but the impact on glucometer performance is poorly recognized (12, 14). We previously showed that significant error is found in four of the most widely used glucometers and that the error was associated with anemia (2, 6). This study examines causation and seeks to identify the most important source of glucometer error in hemodynamically stable patients. We further studied the effect of glucometer error correction on occult hypoglycemia and hypoglycemia frequency, and its role in preventing excessive insulin administration in anemic patients on IIT.
Materials and Methods
This study was conducted at Brooke Army Medical Center/United States Army Institute of Surgical Research (USAISR), San Antonio, Texas. This study was approved by the Institutional Review Boards (IRB) from Brooke Army Medical Center and the University of Texas Health Science Center – San Antonio. The risk of study participation was considered minimal and formal consent was waived by the IRB, except in the case of healthy volunteers who signed consents prior to enrollment.
All POC whole blood glucometer measurements were made with the SureStepFlexx™ glucometer (Lifescan, Milpitas, CA) except as noted, and laboratory glucose values were obtained from plasma samples using the Vitros Fusion analyzer, (Ortho Clinical Diagnostics, Rochester, NY). The intra- and inter-assay coefficient of variations for the Vitros analyzer range from 0.5-1.2% and 1.2-3.5%, respectively (21). The POC glucometer uses reflectance-based glucose oxidase technology, as does the laboratory analyzer (14). Glucometer measurements were made according to manufacturer's specifications on undiluted whole blood. Samples for glucose analysis were sent to the laboratory in additive-free, or sodium fluoride and potassium oxalate-containing evacuated tubes filled with whole blood.
Frequency of Glucometer Error
Our first task was to establish the frequency of systematic glucometer error in ICU patients. To do so, we analyzed glucometer and laboratory glucose (used as the reference in our study) paired by recorded collection time. Data from nineteen ICU subjects over four consecutive months were analyzed to determined the prevalence of error. The source of blood could not be determined in this retrospective review. Error rates found in the retrospective data were confirmed with prospective data. POC glucometer glucose measurements from patients in the medical, surgical, and burn intensive care units (MICU, SICU and BICU, respectively) were collected and compared to reference.
Cause of Glucometer Error
Once glucometer error was defined and found to be consistently present, we conducted a literature search which identified multiple potential sources of error. These included suboptimal environmental and operator-related conditions, sample degradation, interfering substances, and low oxygen tension, which were eliminated as confounders (data not shown).
We assessed hematocrit effect due to anemia as a causative factor by adding time-matched hematocrit values to the retrospective data from the nineteen ICU subjects described above, almost all of whom were anemic. The association of glucometer error with abnormal hematocrit was examined by comparison to data from hospitalized patients over the same time period with normal hematocrits, and confirmed with an in-vitro model in which five glucose and three hematocrit concentrations were artificially constructed from the blood of healthy volunteers stored in sodium heparin containing evacuated tubes. POC glucometer, reference laboratory, and hematocrit analysis was conducted on the resulting samples.
Once anemia was confirmed as a significant cause of glucometer error in our ICU populations, the additional contribution of interfering substances and low oxygen tension was evaluated by comparing results from a traditional single-channel glucometer corrected for error due to anemia alone (for details regarding development of the correction formula, see below), to measurement of the same samples using a new, commercially-available multi-channel glucometer (StatStrip™, Nova Biomedical, Waltham, MA) that corrects for the effects of anemia, low oxygen tension, acetaminophen, uric acid, ascorbic acid, maltose, galactose, xylose, and lactose. Whole blood samples prospectively collected from anemic critically ill patients in the MICU, SICU, and BICU were used for testing. Arterial blood was preferentially used unless arterial access was unavailable; in these cases the research team used careful collection techniques in obtaining venous blood to avoid contamination with glucose containing intravenous infusions. Capillary blood was not used. Error rates for each meter model were calculated by comparing POC results to reference laboratory values.
Hematocrit Threshold of Error
The threshold of low hematocrit giving rise to measurable differences in POC and laboratory glucose testing was determined through analysis of a large number of retrospectively collected hematocrit, laboratory glucose and point-of-care glucose measurements. We plotted the ratio of laboratory to glucometer glucose versus hematocrit, and filter curve analysis defined the lower limit of the 95% confidence interval where glucometer error reached statistical significance. The percentage of POC glucometer measurements in the burn (BICU), surgical (SICU) and medical (MICU) units associated with hematocrit below this level defined level of risk to patients in each unit. The upper limit was not assessed as polycythemic patients are rare in the ICUs studied and available data was insufficient for meaningful analysis.
Formula Development
Blood samples (n=196 measurements) from ICU patients were collected in evacuated tubes containing sodium fluoride and potassium oxalate and sent for laboratory glucose analysis. Results were matched with complete blood count (CBC) quantification from evacuated tubes in ethylenediaminetetraacetic acid (EDTA) sent within 12 hours of glucose sample collection. Additive-free whole blood from the same collection specimen was simultaneously used for single-channel POC glucometer measurement. Arterial blood was used preferentially and venous blood used if arterial access was unavailable. Capillary blood was not used for point-of-care glucose measurements, as several studies have demonstrated that glucometer capillary blood measurement is less reliable than arterial (22-25). Glucometer linearity tests were performed according to manufacturers’ instructions every twenty four hours using high and low glucose control solutions. No glucometer used in the study failed the linearity test at any time. Glucometer analysis was performed by operators trained in use and maintenance of the device. The mathematical relationship between POC glucometer, laboratory glucose, and hematocrit values was defined using regression analysis of a logarithmic-based model. The predictive value of the resulting equation was validated according to the Monte Carlo method (26). Percent error of glucometer data relative to laboratory glucose analyzer results was also tested both before and after correction using POC glucose values from samples not used in the correction formula derivation.
Effect of Correction on Incidence of Hypoglycemia
The research team initiated a staff education program to disseminate information among intensive care providers regarding the risk of hypoglycemia in ICU patients receiving intensive insulin therapy managed by single channel glucometers. We analyzed the clinical impact of the model by comparing the incidence of hypoglycemia in the BICU before and after formula implementation. Correction was not instituted in the SICU, which provided a negative control. Differences solely reflected the effect of the formula, because staff education in both units was similar. Matched sets of glucometer and laboratory glucose measurements were collected over four-month periods before and after implementation of the formula, and the delta in hypoglycemic episodes was calculated.
Data Analysis
Data were analyzed using linear and logarithmic regression analysis. Error was defined as percent deviation in glucometer values from laboratory analyzer quantification of glucose. Quantitative variables were analyzed with t-test, and qualitative with chi-square. Significance was set at p < 0.05. Variability in sample sets was reported as standard deviation (SD) except where specifically noted.
Results
Frequency of Glucometer Error
We quantified glucometer error in 300 glucometer and laboratory (reference) glucose data pairs from patients admitted to the BICU. Glucometer values were on average 21%±16% higher than reference. The regression equation from a random subset of patients (n=9 subjects, 154 data pairs) was applied to glucometer measurements for the second subset (n=10 subjects, 146 data pairs), correcting the error in the latter group (data not shown). This analysis served to show that the error in this population was systematic, reproducible, and consistently in the direction of glucometer overestimation. Linear models including subject as a random effect revealed no significant variation in the slope (p=0.64) or intercept (p=0.43) within subjects. To confirm the findings, glucometer and reference glucose samples were prospectively collected from 41 hemodynamically stable subjects (n=196 samples). Glucometers overestimated the reference value on average by 19±7%, confirming the reproducibility and direction of systematic error.
Cause of Glucometer Error
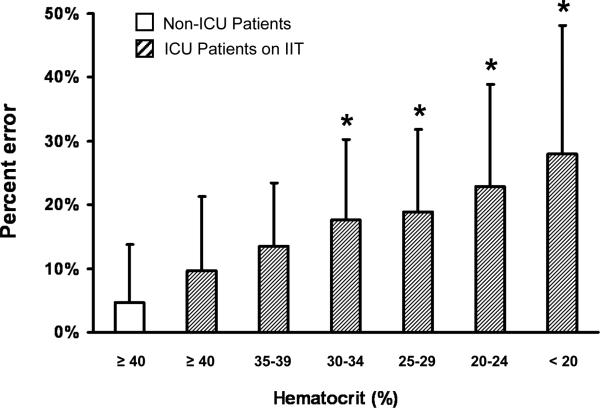
To examine the effect of anemia on glucometer accuracy, we re-analyzed the initial retrospective data set with regard to hematocrit and compared percent error from ICU samples to that from non-ICU patients of normal hematocrit (n=56, 75 data pairs). Percent error was inversely correlated with hematocrit (Fig. 1); effects of polycythemia were not assessed due to insufficient data. Average hematocrit in the ICU group was 25%±7% compared to 42%±2% in the non-ICU group (selected for normal hematocrit). Hematocrit analysis yielded similar results in the prospectively collected sample set (data not shown). Anemia was confirmed to be a significant source of error by reproducing it in an in-vitro model that tested the effect of different hematocrit concentrations on glucometer performance. As expected, low hematocrit resulted in inappropriately high glucometer values (data not shown).
Figure 1.
Percent error in glucometer measurements (reference = laboratory glucose) is inversely correlated with degree of anemia in ICU patients and, is the lowest hematocrit groups, significantly differs from that of normal hematocrit non-ICU patient samples collected during same time period (p<0.001).
The effects of interfering substances (acetaminophen, uric acid, ascorbic acid, maltose, galactose, xylose, lactose) and low oxygen tension were eliminated as significant contributors to error by comparison of glucometer results corrected for anemia alone (for formula development and validation, see below) with those from a new 4-channel glucometer which corrects for hematocrit effect and all substances listed above. The average hematocrit for samples tested was 26.6%±5.2%. Uncorrected single-channel glucose measurement error was 22%±9.4% compared to reference, however correction improved the error to levels similar to that from the multi-channel device (4.36%± 5.6% versus -4.25%±5.3%, p=0.88). The two analyzers were within the set zone of indifference of ±5% (-0.67%, CI: -1.79% to 0.45%). The demonstrated non-inferiority between methods is evidence that contributions to single-channel glucometer error were primarily due to the effect of anemia.
Hematocrit threshold of error
A very large retrospective data set (n=12,800 measurements) was analyzed to determine the level of hematocrit at which correction for anemia becomes necessary. The ratio of laboratory to glucometer glucose was plotted against hematocrit; error was significant at hematocrit levels of less than 34% (p = 0.05). A subanalysis of measurements from ICU patients undergoing frequent glucose quantification revealed that 64%, 79%, and 92% of glucometer measurements in the MICU, SICU, and BICU were associated with a hematocrit below the threshold 34%, posing a significant risk of hematocrit error and occult hypoglycemia to patients in those units.
Formula Development
Samples were prospectively collected from 41 hemodynamically stable ICU subjects (n=196 measurements) using hematocrit measurement within six hours of phlebotomy for analysis. POC glucometer error rates ±SD in the prospective data were 19±7% (average hematocrit = 25%). This was similar to error found in the retrospective samples (21±16% error ±SD, average hematocrit = 25%±7%). The linear equation derived from regression analysis was:
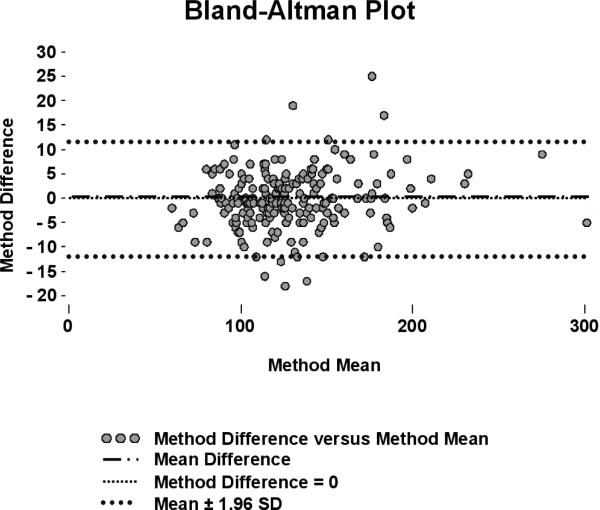
where LGP is the laboratory glucose predicted by correction, POCG is the whole blood glucose measurement prior to correction, LN is the natural log, and HCT is the hematocrit. The predictive validity of the formula was tested with randomly extracted subsets of data; the formula reduced average error to -0.02±4.78%. Corrected POC glucometer data were highly correlated with laboratory glucose measurements (r2=0.97) and conformed closely to the line of identity. An additional 205 prospectively collected, matched glucometer and reference values (average hematocrit = 23%±5%) were used to test adequacy of correction. Application of the formula improved the error rate in this dataset from 29±13% (p<0.001) to 5±11%, resulting in corrected glucometer values that did not differ statistically from laboratory analysis (p=0.43). Bland-Altman analysis revealed negligible measurement size effect on error after correction (Fig. 2).
Figure 2.
Bland-Altman plot with laboratory glucose as the reference measurement and corrected glucometer glucose as the test measurement. Units on the x and y axes are mg/dL. SD = standard deviation.
Effect of Correction on Incidence of Hypoglycemia
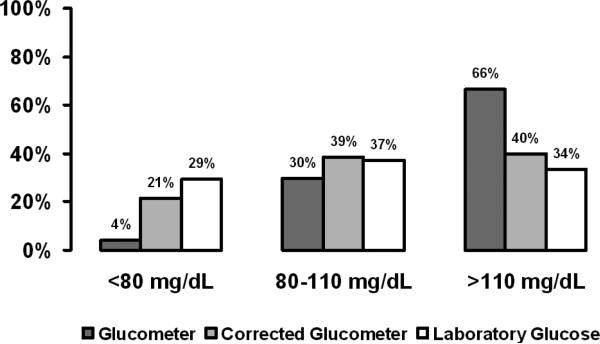
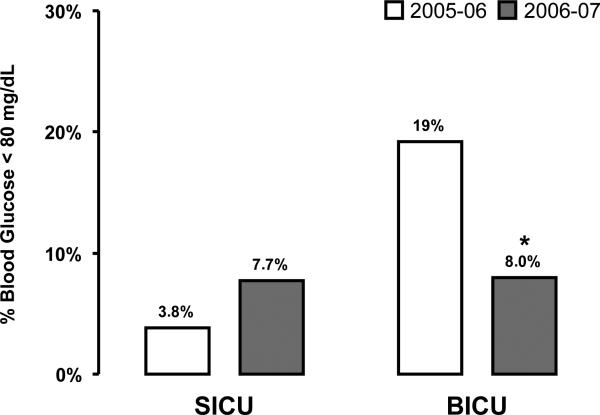
Uncorrected glucometer analysis underestimated the number of glucose values below target (80 -110 mg/dL) in the above dataset by 86% and overestimated those above target by 93%, undoubtedly leading to excessive insulin infusion (Fig. 3). To determine whether correction of glucometer results decreased the frequency of low glucose measurements, we reviewed data from four-month periods before and after the formula was implemented in the BICU. The SICU served as a control in this analysis. Laboratory measurements in the low (<80 mg/dL) and hypoglycemic (<60 mg/dL) glucose ranges were reduced by 58% (Fig. 4) and 78%, respectively in the BICU (p<0.001), but not in the SICU.
Figure 3.
Percent measurement of glucose values below, in, and above the glucose target as measured by glucometer, corrected glucometer, and laboratory analysis. Glucometer measurement significantly underestimates glucose below target compared to laboratory measurement (*p<0.001), but improves with correction. Data were collected prior to implementation of the glucometer correction formula.
Figure 4.
Difference in percent measurements requiring a reduction or discontinuation of intravenous insulin infusions (less than 80 mg/dL) before and after implementation of the glucometer correction formula (burn intensive care unit, *p<0.001) compared to no implementation of glucometer correction (surgical intensive care unit, p=NS). SICU = surgical intensive care unit, BICU = burn intensive care unit.
Discussion
Anemia is common in the ICU, and low hematocrit significantly affects the accuracy of POC glucose measurement (8, 22, 27). In our study, hematocrit effect was the overriding cause for glucometer error, and the false results masked hypoglycemia. Furthermore, we demonstrated that a mathematical formula corrects this error within clinically acceptable limits. Lastly, application of this formula significantly decreased the incidence of hypoglycemia in critically ill patients treated with insulin.
Previous studies cautioned critical care specialists on the risks of using glucometers for IIT (4, 8, 9), however, a low cost, practical alternative offering ease of testing and minimal blood volume was not available. When institutions simultaneously adopted IIT and restrictive transfusion strategies (1, 3, 15), the likelihood of concomitant hypoglycemia and anemia increased. Glucometer error drives glucose to a lower range by reporting glucose concentrations that are higher than actual. The likely result is an increase in administered insulin. Hypoglycemia is associated with higher mortality and other complications in the ICU, and its association with IIT is increasingly recognized (4, 28). Our low-cost mathematical formula produces results equivalent to those from a multi-channel glucometer. Regardless of the method used, eliminating low hematocrit error improves patient safety by reducing hypoglycemia and its attendant risks.
This study had several limitations. Patient diagnosis and the percentage of samples derived from arterial versus venous blood were not evaluated, and these are variables which can affect POC measurements. The level of oxygenation was not recorded, however no significant difference was found between results from a four-channel glucometer that corrects for low oxygen levels and the single-channel glucometer/mathematical correction method. This finding suggests that low oxygen levels are not a significant source of error in hemodynamically stable ICU patients such as those in our study. Retrospective data was used to identify potential causes of error, however since all findings were confirmed with prospective and in-vitro data, this is not considered a limitation of the study.
The focus of this paper is not to advocate one method over the other but rather to highlight the prevalence of anemia in ICU patients receiving IIT and emphasize the risk of glucometer error in this population. In a previous paper, we showed that anemia is a significant cause of glucometer error (2). Here we further demonstrate for the first time that anemia is the primary cause of glucometer error in hemodynamically stable adult ICU patients and that eliminating hematocrit error decreases the frequency of hypoglycemia.
Hypoglycemia can cause severe injury or death and the association with strict glucose control has become a major source of concern (9, 29). Given that impact of anemia on glucometer performance (Fig 3) remains largely unaddressed, the true prevalence of hypoglycemia in patients treated with IIT is likely even higher than generally feared. We previously quantified error in four widely-used POC glucometers and found that error rates between models were comparable (2), and thus concluded that glucometer error likely poses a risk to anemic patients on IIT at multiple institutions beyond our own.
The emphasis of the work described here was to raise clinician awareness that single-channel glucometers currently in use do not correct for hematocrit, with the consequence that the data used to drive patient care overestimate the actual glucose concentration and may contribute to the higher rates of hypoglycemia associated with IIT. Hematocrit effect may be the reason why the GLUCONTROL and VISEP studies reported high rates of hypoglycemia (5, 6), eventually leading to the discontinuation of both trials. The inability to reproduce the benefits reported by Van den Berghe and her associates has been widely discussed, and the root cause may lie in glucose measurement error due to anemia. In this study, we found that four-channel glucometers, which use proprietary software to correct hematocrit error prior to reporting a result, perform at least well as our correction formula. Critical care providers should be aware of the potential for unrecognized hypoglycemia with the use of single channel glucometers, and that four-channel glucometers do not pose the same risks to anemic patients.
Conclusions
Anemia is the major cause of glucometer error in ICU patients on IIT, and correction with a mathematical formula decreased the frequency of low glucose values. This provides evidence that low hematocrit indirectly results in hypoglycemia, which if severe could reduce or even negate the known benefits of IIT. Clinicians should be aware that the use of single-channel glucometers is contraindicated in anemic patients and consider using mathematical correction or multi-channel analyzers to manage IIT in this population.
Acknowledgements
The authors would like to thank Joel Michalek, PhD and John Jones, BS for help with statistical analysis, as well as to the following individuals for their invaluable assistance in the execution of this study:
Nikolaos Kypreos
Jesus Morales
Roger Price
Claudia Romero
Virgil Moore
Glen Rossman
Linda Speights
Esther Juarez
David Zahn
Nancy Molter, PhD
Elizabeth Frail
Peggy Bielke
Kari Williams
Juliette Castillo
Annette McClinton, MA
Todd Silliman
Amy Newland
John Jones
Supported by grants from:
The National Institutes of Health (1 R01 GM063120-04) and The Technologies for Metabolic Monitoring (TMM)/Julia Weaver Fund, A Congressionally Directed Program Jointly Managed by the USA MRMC, NIH, NASA and the Juvenile Diabetes Research Foundation and Combat Casualty Care Division United States Army Medical Research and Materiel Command
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of Defense or United States Government. The authors are affiliated with or employed by the U.S. government. This work was prepared as part of their official duties and, as such, there is no copyright to be transferred.
Footnotes
Clinical Trials Registration Number: NCT00464386
Conflict of interest statements: the authors do not have competing interests to disclose.
References
- 1.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 2.Mann EA, Salinas J, Pidcoke HF, Wolf SE, Holcomb JB, Wade CE. Error rates resulting from anemia can be corrected in multiple commonly used point-of-care glucometers. J Trauma. 2008;64:15–20. doi: 10.1097/TA.0b013e318160b9e4. [DOI] [PubMed] [Google Scholar]
- 3.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 4.Wintergerst KA, Buckingham B, Gandrud L, Wong BJ, Kache S, Wilson DM. Association of hypoglycemia, hyperglycemia, and glucose variability with morbidity and death in the pediatric intensive care unit. Pediatrics. 2006;118:173–179. doi: 10.1542/peds.2005-1819. [DOI] [PubMed] [Google Scholar]
- 5.Devos P, Preiser JC. Current controversies around tight glucose control in critically ill patients. Curr Opin Clin Nutr Metab Care. 2007;10:206–209. doi: 10.1097/MCO.0b013e3280147d2d. [DOI] [PubMed] [Google Scholar]
- 6.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K. German Competence Network Sepsis (SepNet): Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–39. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 7.Mann EA, Pidcoke HF, Wade CE, Salinas J, Holcomb JB, Wolf SE. The impact of intensive insulin protocols and restrictive blood transfusion strategies on glucose measurement in American Burn Association (ABA) verified burn centers. J Burn Care Res. doi: 10.1097/BCR.0b013e3181848c74. in press. [DOI] [PubMed] [Google Scholar]
- 8.Finkielman J, Oyen L, Afessa B. Agreement between bedside blood and plasma glucose measurement in the ICU setting. Chest. 2005;127:1749–1751. doi: 10.1378/chest.127.5.1749. [DOI] [PubMed] [Google Scholar]
- 9.Kanji S, Buffie J, Hutton B, Bunting PS, Singh A, McDonald K, Fergusson D, McIntyre LA, Hebert PC. Reliability of point-of-care testing for glucose measurement in critically ill adults. Crit Care Med. 2005;33:2778–2785. doi: 10.1097/01.ccm.0000189939.10881.60. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association Consensus statement on self-monitoring of blood glucose. Diabetes Care. 1987;10:95–99. [PubMed] [Google Scholar]
- 11.American Diabetes Association Self-monitoring of blood glucose. Diabetes Care. 1995;18:47–52. [Google Scholar]
- 12.Barreau PB, Buttery JE. The effect of the haematocrit value on the determination of glucose levels by the reagent-strip methods. Med J Aust. 1987;147:286–288. doi: 10.5694/j.1326-5377.1987.tb133457.x. [DOI] [PubMed] [Google Scholar]
- 13.Bekefi D, Szolnoki J, Koranyi J, Ferencz A. Reflectometric blood glucose determination in the neonatalogical intentive care unit: haematocrit dependence. Exp Clin Endocrinol. 1984;83:178–183. doi: 10.1055/s-0029-1210328. [DOI] [PubMed] [Google Scholar]
- 14.Singh Dhatt G, Agarwal M, Bishawi B. Evaluation of a glucose meter against analytical quality specifications for hospital use. Clin Chim Acta. 2004;343:S217–S221. doi: 10.1016/j.cccn.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care: Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 16.Moore FD, Peacock WC, Blakely E, Cope O. The anemia of thermal burns. Ann Surg. 1946;124:811–839. [PMC free article] [PubMed] [Google Scholar]
- 17.Hebert PC, Fergusson DA, Stather D, McIntyre L, Martin C, Doucette S, Blajchman M, Graham ID. Canadian Critical Care Trials Group: Revisiting transfusion practices in critically ill patients. Crit Care Med. 2005;33:7–12. doi: 10.1097/01.ccm.0000151047.33912.a3. [DOI] [PubMed] [Google Scholar]
- 18.Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345:1230–1236. doi: 10.1056/NEJMoa010615. [DOI] [PubMed] [Google Scholar]
- 19.Rao SV, Jollis JG, Harrington RA, Granger CB, Newby LK, Armstrong PW, Moliterno DJ, Lindblad L, Pieper K, Topol EJ, Stamler JS, Califf RM. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292:1555–1562. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 20.Lacroix J, Hebert PC, Hutchison JS, Hume HA, Tucci M, Ducruet T, Gauvin F, Collet JP, Toledano BJ, Robillard P, Joffe A, Biarent D, Meert K, Peters MJ. TRIPICU Investigators; Canadian Critical Care Trials Group; Pediatric Acute Lung Injury and Sepsis Investigators Network: Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–1619. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 21.Soldin SJ, Devairakkam PD, Agarwalla PK. Evaluation of the Abbott PCx (R) point of care glucose analyzer in a pediatric hospital. Clin Biochem. 2000;33:319–21. doi: 10.1016/s0009-9120(00)00075-8. [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni A, Saxena M, Price G, O'Leary MJ, Jacques T, Myburgh JA. Analysis of blood glucose measurements using capillary and arterial blood samples in intensive care patients. Intensive Care Med. 2005;31:142–145. doi: 10.1007/s00134-004-2500-5. [DOI] [PubMed] [Google Scholar]
- 23.Slater-Maclean L, Cembrowski G, Chin D, Shalapay C, Binette T, Hegadoren K, Newburn-Cook C. Accuracy of glycemic measurements in the critically ill. Diabetes Technol Ther. 2008;10:169–77. doi: 10.1089/dia.2008.0263. [DOI] [PubMed] [Google Scholar]
- 24.Arias-Rivera S, Copete-Vega A, Vadillo-Obesso P, Corrochano-Varas S, Sánchez-Izquierdo R, Sánchez-Sánchez MM, Sáiz-Sanz AI, Frutos-Vivar F. Pascual-Durán T: Reliability of the measurement of glucose at the bedside of critical patients. Enferm Intensiva. 2007;18:15–28. doi: 10.1016/s1130-2399(07)74385-7. [DOI] [PubMed] [Google Scholar]
- 25.Critchell CD, Savarese V, Callahan A, Aboud C, Jabbour S, Marik P. Accuracy of bedside capillary blood glucose measurements in critically ill patients. Intensive Care Med. 2007;33:2079–84. doi: 10.1007/s00134-007-0835-4. [DOI] [PubMed] [Google Scholar]
- 26.Metropolis N, Ulam S. The Monte Carlo method. J Am Stat Assoc. 1949;44:335–341. doi: 10.1080/01621459.1949.10483310. [DOI] [PubMed] [Google Scholar]
- 27.Corwin HL, Parsonnet KC, Gettinger A. RBC transfusion in the ICU: Is there a reason? Chest. 1995;108:767–771. doi: 10.1378/chest.108.3.767. [DOI] [PubMed] [Google Scholar]
- 28.Krinsley JS, Preiser JC. Moving beyond tight glucose control to safe effective glucose control. Crit Care. 2008;12:149. doi: 10.1186/cc6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mechanick JI, Handelsman Y, Bloomgarden ZT. Hypoglycemia in the intensive care unit. Curr Opin Clin Nutr Metab Care. 2007;10:193–196. doi: 10.1097/MCO.0b013e32802b7016. [DOI] [PubMed] [Google Scholar]